Abstract
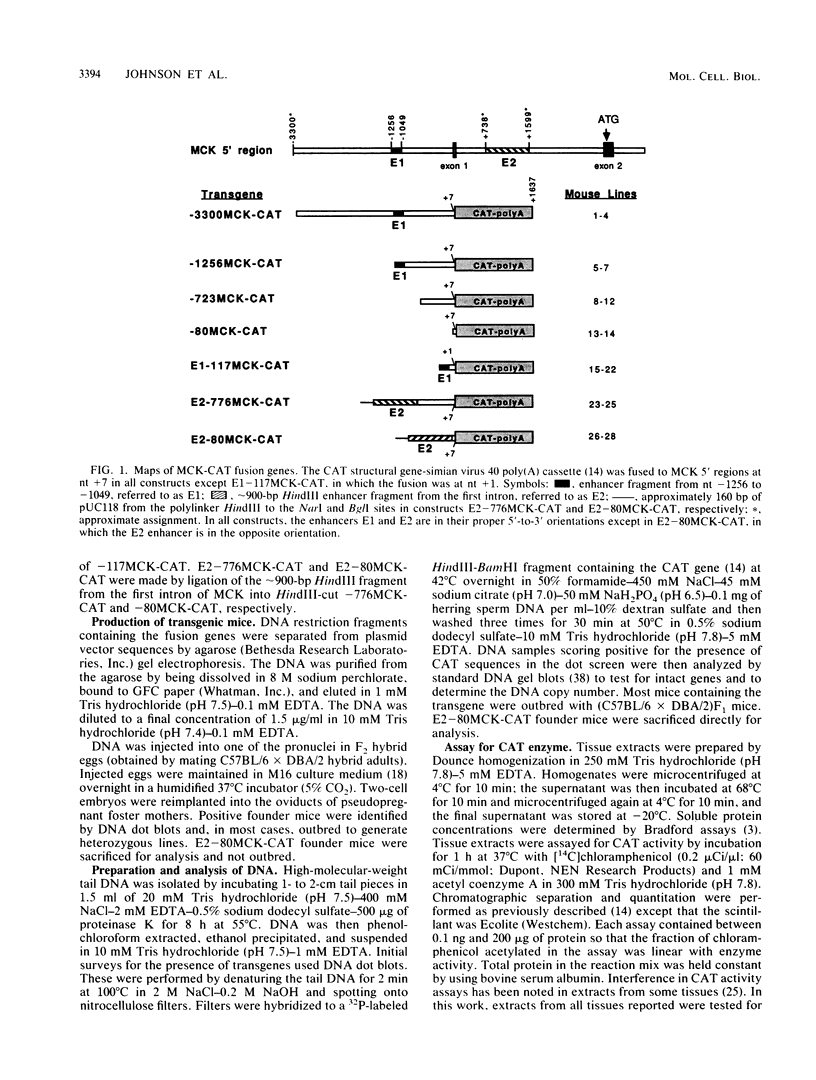
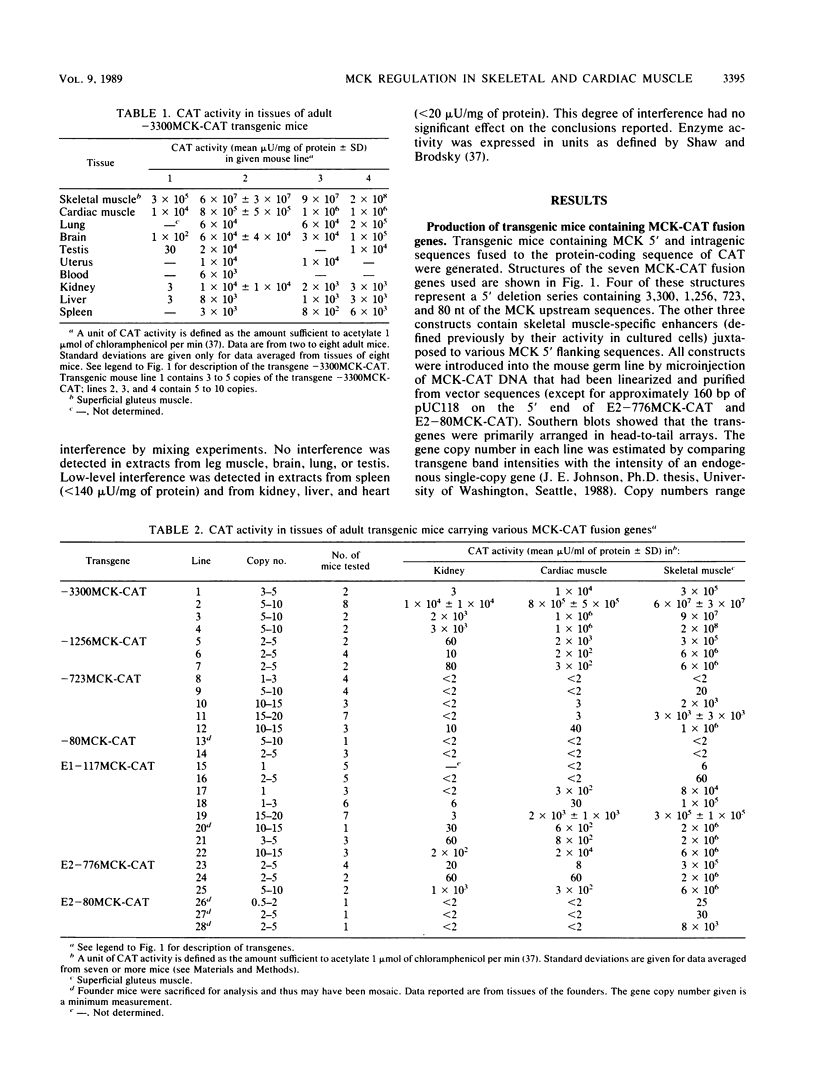
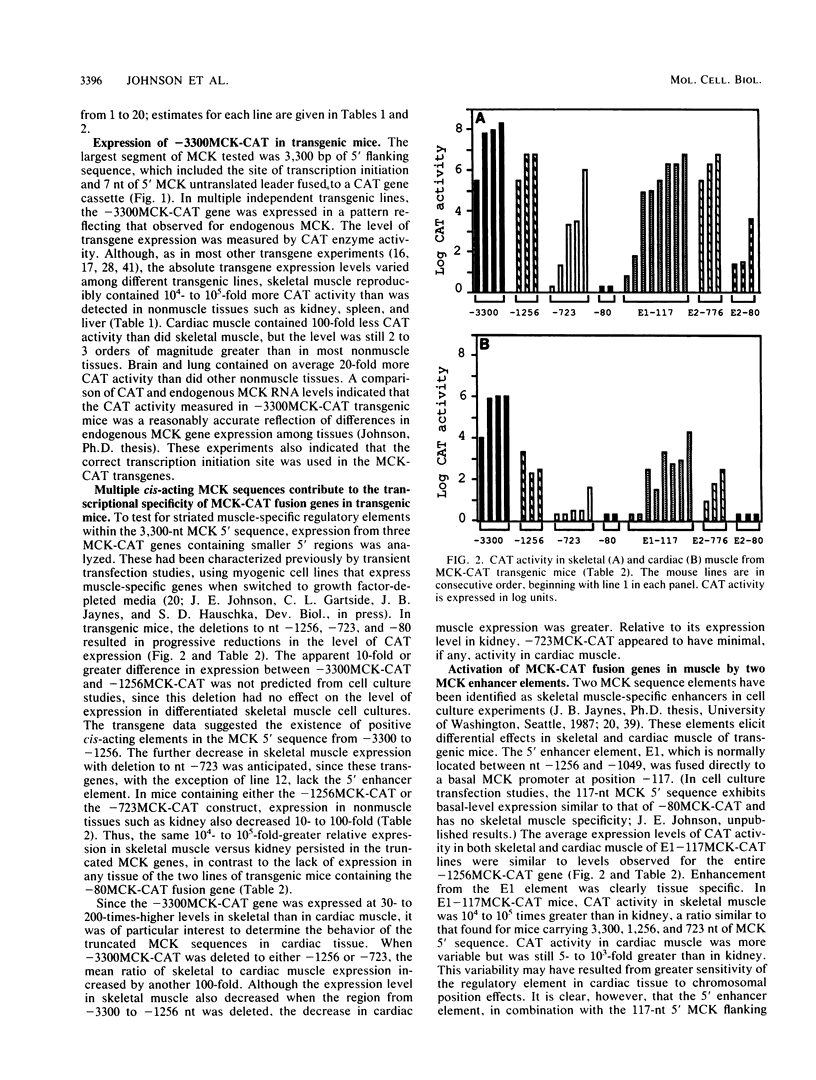
Muscle creatine kinase (MCK) is expressed at high levels only in skeletal and cardiac muscle tissues. Previous in vitro transfection studies of skeletal muscle myoblasts and fibroblasts had identified two MCK enhancer elements and one proximal promoter element, each of which exhibited expression only in differentiated skeletal muscle. In this study, we have identified several regions of the mouse MCK gene that are responsible for tissue-specific expression in transgenic mice. A fusion gene containing 3,300 nucleotides of MCK 5' sequence exhibited chloramphenicol acetyltransferase activity levels that were more than 10(4)-fold higher in skeletal muscle than in other, nonmuscle tissues such as kidney, liver, and spleen. Expression in cardiac muscle was also greater than in these nonmuscle tissues by 2 to 3 orders of magnitude. Progressive 5' deletions from nucleotide -3300 resulted in reduced expression of the transgene, and one of these resulted in a preferential decrease in expression in cardiac tissue relative to that in skeletal muscle. Of the two enhancer sequences analyzed, only one directed high-level expression in both skeletal and cardiac muscle. The other enhancer activated expression only in skeletal muscle. These data reveal a complex set of cis-acting sequences that have differential effects on MCK expression in skeletal and cardiac muscle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsma D. J., Grichnik J. M., Gossett L. M., Schwartz R. J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol Cell Biol. 1986 Jul;6(7):2462–2475. doi: 10.1128/mcb.6.7.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvagnet P. F., Strehler E. E., White G. E., Strehler-Page M. A., Nadal-Ginard B., Mahdavi V. Multiple positive and negative 5' regulatory elements control the cell-type-specific expression of the embryonic skeletal myosin heavy-chain gene. Mol Cell Biol. 1987 Dec;7(12):4377–4389. doi: 10.1128/mcb.7.12.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buskin J. N., Hauschka S. D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989 Jun;9(6):2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. L., Bergsma D. J., Schwartz R. J. A 29-nucleotide DNA segment containing an evolutionarily conserved motif is required in cis for cell-type-restricted repression of the chicken alpha-smooth muscle actin gene core promoter. Mol Cell Biol. 1988 Jan;8(1):241–250. doi: 10.1128/mcb.8.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. S., Jaynes J. B., Hauschka S. D. Regulation of creatine kinase induction in differentiating mouse myoblasts. Mol Cell Biol. 1985 Mar;5(3):484–492. doi: 10.1128/mcb.5.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubas P., Klarsfeld A., Garner I., Pinset C., Cox R., Buckingham M. Functional activity of the two promoters of the myosin alkali light chain gene in primary muscle cell cultures: comparison with other muscle gene promoters and other culture systems. Nucleic Acids Res. 1988 Feb 25;16(4):1251–1271. doi: 10.1093/nar/16.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- EPPENBERGER H. M., EPPENBERGER M., RICHTERICH R., AEBI H. THE ONTOGENY OF CREATINE KINASE ISOZYMES. Dev Biol. 1964 Aug;10:1–16. doi: 10.1016/0012-1606(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Einat P., Bergman Y., Yaffe D., Shani M. Expression in transgenic mice of two genes of different tissue specificity integrated into a single chromosomal site. Genes Dev. 1987 Dec;1(10):1075–1084. doi: 10.1101/gad.1.10.1075. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Zweig M. H., Carney D. N., Van Steirteghen A. C., Baylin S. B., Minna J. D. Levels of creatine kinase and its BB isoenzyme in lung cancer specimens and cultures. Cancer Res. 1981 Jul;41(7):2773–2777. [PubMed] [Google Scholar]
- Godbout R., Ingram R., Tilghman S. M. Multiple regulatory elements in the intergenic region between the alpha-fetoprotein and albumin genes. Mol Cell Biol. 1986 Feb;6(2):477–487. doi: 10.1128/mcb.6.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall N., DeLuca M. Developmental changes in creatine phosphokinase isoenzymes in neonatal mouse hearts. Biochem Biophys Res Commun. 1975 Oct 6;66(3):988–994. doi: 10.1016/0006-291x(75)90737-8. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Krumlauf R., Camper S. A., Brinster R. L., Tilghman S. M. Diversity of alpha-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science. 1987 Jan 2;235(4784):53–58. doi: 10.1126/science.2432657. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Swift G. H., Ornitz D. M., Quaife C. J., Palmiter R. D., Brinster R. L., MacDonald R. J. The rat elastase I regulatory element is an enhancer that directs correct cell specificity and developmental onset of expression in transgenic mice. Mol Cell Biol. 1987 Aug;7(8):2956–2967. doi: 10.1128/mcb.7.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J. B., Chamberlain J. S., Buskin J. N., Johnson J. E., Hauschka S. D. Transcriptional regulation of the muscle creatine kinase gene and regulated expression in transfected mouse myoblasts. Mol Cell Biol. 1986 Aug;6(8):2855–2864. doi: 10.1128/mcb.6.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J. B., Johnson J. E., Buskin J. N., Gartside C. L., Hauschka S. D. The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol Cell Biol. 1988 Jan;8(1):62–70. doi: 10.1128/mcb.8.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers-Wretou E., Pfleiderer G. Quantitation of creatine kinase isoenzymes in human tissues and sera by an immunological method. Clin Chim Acta. 1975 Feb 8;58(3):223–232. doi: 10.1016/0009-8981(75)90441-6. [DOI] [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr Complex regulation of the muscle-specific contractile protein (troponin I) gene. Mol Cell Biol. 1987 Sep;7(9):3065–3075. doi: 10.1128/mcb.7.9.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loike J. D., Kozler V. F., Silverstein S. C. Creatine kinase expression and creatine phosphate accumulation are developmentally regulated during differentiation of mouse and human monocytes. J Exp Med. 1984 Mar 1;159(3):746–757. doi: 10.1084/jem.159.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar J. H., Antin P. B., Cooper T. A., Ordahl C. P. Analysis of the upstream regions governing expression of the chicken cardiac troponin T gene in embryonic cardiac and skeletal muscle cells. J Cell Biol. 1988 Aug;107(2):573–585. doi: 10.1083/jcb.107.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercola M., Goverman J., Mirell C., Calame K. Immunoglobulin heavy-chain enhancer requires one or more tissue-specific factors. Science. 1985 Jan 18;227(4684):266–270. doi: 10.1126/science.3917575. [DOI] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat G. E., Kedes L. Multiple 5'-flanking regions of the human alpha-skeletal actin gene synergistically modulate muscle-specific expression. Mol Cell Biol. 1987 Nov;7(11):4089–4099. doi: 10.1128/mcb.7.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriard J. C., Caravatti M., Perriard E. R., Eppenberger H. M. Quantitation of creatine kinase isoenzyme transition in differentiating chicken embryonic breast muscle and myogenic cell cultures by immunoadsorption. Arch Biochem Biophys. 1978 Nov;191(1):90–100. doi: 10.1016/0003-9861(78)90070-x. [DOI] [PubMed] [Google Scholar]
- Perriard J. C. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Levels of mRNA for creatine kinase subunits M and B. J Biol Chem. 1979 Aug 10;254(15):7036–7041. [PubMed] [Google Scholar]
- Pieper F. R., Slobbe R. L., Ramaekers F. C., Cuypers H. T., Bloemendal H. Upstream regions of the hamster desmin and vimentin genes regulate expression during in vitro myogenesis. EMBO J. 1987 Dec 1;6(12):3611–3618. doi: 10.1002/j.1460-2075.1987.tb02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg U. B., Kunz G., Frischauf A., Lehrach H., Mähr R., Eppenberger H. M., Perriard J. C. Molecular cloning and expression during myogenesis of sequences coding for M-creatine kinase. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6589–6592. doi: 10.1073/pnas.79.21.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M. A., Ruben M., McBurney M. W. Regulated expression of a transfected human cardiac actin gene during differentiation of multipotential murine embryonal carcinoma cells. Mol Cell Biol. 1988 Jan;8(1):406–417. doi: 10.1128/mcb.8.1.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M. Tissue-specific and developmentally regulated expression of a chimeric actin-globin gene in transgenic mice. Mol Cell Biol. 1986 Jul;6(7):2624–2631. doi: 10.1128/mcb.6.7.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M. Tissue-specific expression of rat myosin light-chain 2 gene in transgenic mice. Nature. 1985 Mar 21;314(6008):283–286. doi: 10.1038/314283a0. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg E. A., Spizz G., Perry W. M., Vizard D., Weil T., Olson E. N. Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine kinase gene. Mol Cell Biol. 1988 Jul;8(7):2896–2909. doi: 10.1128/mcb.8.7.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANZER M. L., GILVARG C. Creatine and creatine kinase measurement. J Biol Chem. 1959 Dec;234:3201–3204. [PubMed] [Google Scholar]
- Trudel M., Costantini F. A 3' enhancer contributes to the stage-specific expression of the human beta-globin gene. Genes Dev. 1987 Nov;1(9):954–961. doi: 10.1101/gad.1.9.954. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Ziter F. A. Creatine kinase in developing skeletal and cardiac muscle of the rat. Exp Neurol. 1974 Jun;43(3):539–546. doi: 10.1016/0014-4886(74)90193-9. [DOI] [PubMed] [Google Scholar]



