Abstract
Transdermal alcohol sensors continuously collect reliable and valid data on alcohol consumption in vivo over the course of hours to weeks. Transdermal alcohol readings are highly correlated with breath alcohol measurements, but transdermal alcohol levels lag behind breath alcohol levels by one or more hours due to the longer time required for alcohol to be expelled through perspiration. By providing objective information about alcohol consumption, transdermal alcohol sensors can validate self-report and provide important information not previously available. In this article we describe the development and evaluation of currently available transdermal alcohol sensors, present the strengths and limitations of the technology, and give examples of recent research using the sensors.
Currently, alcohol use in research and clinical settings is primarily measured via participant or client self-report. Although approaches to collecting self-reported alcohol use are well established and generally considered reliable and valid (Babor, Steinberg, Del Boca, & Anton, 2000; Babor, Stephens, & Marlatt, 1987; Del Boca & Darkes, 2003; Sobell & Sobell, 1990), it is likely that self-report of alcohol use frequency is more reliable and valid than quantity (given higher correspondence with collateral reports; LaForge, Borsari & Baer, 2005), yet quantity is often a more critical variable in clinical and research contexts, particularly those that take a risk-reduction rather than abstinence perspective. Some specific data collection methods maximize the validity and reliability of self-report of alcohol consumption among participants (e.g., timeline follow-back procedures; Sobell & Sobell, 1990; Del Boca & Darkes, 2003), and collateral reporting is sometimes used as an adjunctive measure of self-report, but may be of dubious additive value (Del Boca & Darkes, 2003; Laforge, Borsari, & Baer, 2005, Borsari & Muellerleile, 2009). Even the best attempts to maximize the value of self-report likely fall short of producing data free from method error, intentional or unintentional misrepresentation or reporting biases, and memory artifacts.
Biochemical measures of breath or blood alcohol concentrations only provide indices of very recent consumption given the rapid metabolism of alcohol by the body. Biological assays of alcohol metabolites may provide valid indicators of heavy consumption in recent days, but cannot provide information about the quantity or frequency of drinking episodes. Measuring alcohol consumption among participants in contrived (e.g., “bar lab”) settings maximizes confidence in measurement of consumption quantities, but raises questions of external validity and is of limited value in evaluating drinking over time or across contexts.
A tool is now available that provides valid, reliable, and continuous measurement of both the frequency and quantity of alcohol consumption in a relatively unobtrusive and noninvasive manner and therefore provides valuable objective data about alcohol consumption that is more detailed than other biochemical approaches, and avoids the limitations of self-report. This article describes transdermal alcohol sensors (see also Litten, Bradley & Moss, 2010), and provides examples of recent research projects investigating these devices.
Transdermal Alcohol Measurement
Objective biochemical verification of abstinence from alcohol is difficult, since alcohol is quickly metabolized and excreted. Even daily breath or blood tests may miss episodes of drinking, and more frequent testing is inconvenient, impractical, and intrusive. However, recent technology has been developed that measures the small fraction (approximately 1%) of ingested alcohol that is excreted through the skin via sweat glands and diffusion; this skin surface water vapor is known as insensible perspiration (Swift & Swette, 1992; Swift, 2003). Transdermal alcohol sensors measure the concentration of alcohol in insensible perspiration and provide a continuous estimate of ingested alcohol over extended periods of time (Phillips, Greenberg, & Andrzejewski, 1995; Swift, 1993; Swift & Swette, 1992). Transdermal alcohol concentration (TAC) is closely related, but not identical to circulating blood alcohol levels.
Available transdermal measurement devices
There are two devices that have been developed for detecting transdermal alcohol, the SCRAM (Secure Continuous Remote Alcohol Monitor; Alcohol Monitoring Systems, Inc.) and the WrisTAS (Wrist Transdermal Alcohol Sensor; Giner, Inc).1 The SCRAM bracelet weighs approximately 8 ounces and is locked to the ankle and worn continuously (Figure 1). The device contains three sensors: an electrochemical alcohol sensor that samples the vapor close to the skin once every 30 minutes, and two circumvention detection sensors that measure skin temperature and contact with the skin. The SCRAM bracelet can store several weeks of readings, and offers two different mechanisms for extracting data from the bracelet. The bracelet can transfer the collected data via a wireless radio frequency signal to a modem installed in the wearer’s home; this modem transfers the data to the SCRAM data server through a cellular or landline telephone line. A local computer and USB interface can also be used to download data from the bracelet and upload it via the internet. These two methods transfer data from the bracelet to a secure central server administered by Alcohol Monitoring Systems; the data can then immediately be accessed by the individual monitoring the client through a secure web-based interface called SCRAMNet.
Figure 1.
SCRAM bracelet device.
The SCRAM bracelet was designed to be worn by court-referred alcohol offenders and for this reason the device includes tamper detection features. The temperature and contact sensors aid in detecting interferents (i.e. something blocking the sensors) or breaks in continuity (i.e., device removal). In addition, during installation the bracelet is locked with a special clip that must be broken to remove the bracelet; when the bracelet is uninstalled, a broken clip indicates the bracelet had been tampered with and possibly removed. The SCRAM can be worn while showering, but cannot be immersed in water. It is commercially available and can be worn for as long as six months.
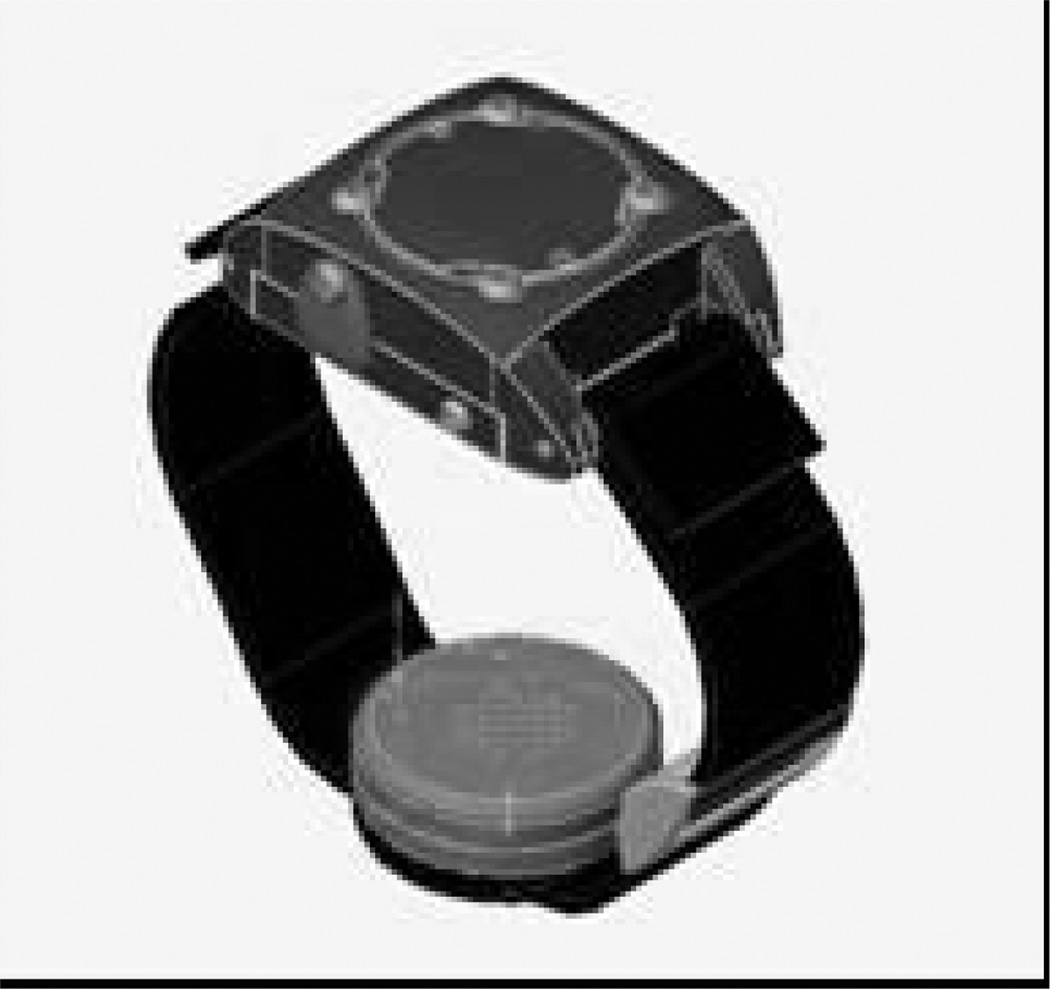
The WrisTAS is worn on the wrist and has the appearance of a wristwatch (Figure 2). The WrisTAS has an electrochemical sensor that detects ethanol vapor near the skin. The sensor continuously samples the ethanol and stores the values, which are downloaded manually to a computer serial port. Similar to the SCRAM, the device has temperature and skin contact sensors that indicate if the WrisTAS is removed, but the WrisTAS retainer strap does not have a lock so can be removed by the user, and must be removed for showering. The WrisTAS is not commercially available but has been tested in research settings. However, the technology in the WrisTAS has been included in a commercially available alcohol monitor called the Transdermal Alcohol Detector (TAD) by BI, Inc. All of the available information about the WrisTAS technology was produced prior to its incorporation in the BI device, so we will use the name WrisTAS below.
Figure 2.
WrisTAS device.
Data generated by transdermal sensors
The primary data recorded by transdermal alcohol sensors is Transdermal Alcohol Concentration, or TAC. This value is similar (although not directly equivalent) to Breath Alcohol Concentration, or BrAC. Because TAC readings are recorded continuously (a reading every 30 minutes for the SCRAM and a user-determined interval for the WrisTAS), alcohol consumption curves can be plotted (see Figure 3 for an example from the SCRAM device). A typical alcohol consumption curve includes a rise in TAC levels to a peak value (i.e., absorption), followed by a lesser sloped “burn off” phase with declining TAC levels that reflect the excretion and metabolism of alcohol after consumption ceases (i.e., elimination).
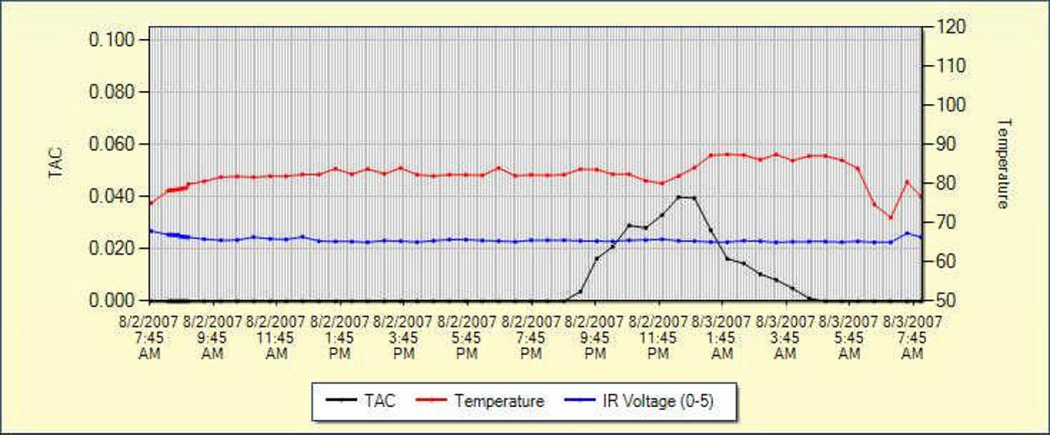
Figure 3.
Example of output from SCRAMNet of recorded data. The black line indicates Transdermal Alcohol Concentration (TAC) readings and demonstrate a typical alcohol consumption curve. The red line indicates skin temperature and the blue line indicates Infrared (IR) voltage, a measure of contact of the device with the skin. The temperature and IR readings provide indications about whether tampering has occurred.
From the TAC data, users can extract several useful variables. The first is simply the presence or absence of consumption. Neither device can reliably detect small alcohol consumption events; both have a reliable lowest threshold of mild intoxication (i.e., blood alcohol levels greater than 0.02). A second variable that can be derived from transdermal sensors is the highest TAC reading. Peak TAC, extracted from any single consumption event reflects peaking blood alcohol concentration and, therefore, level of intoxication. Higher peak TAC readings reflect greater consumption amounts, a higher rate of consumption, or both. Another valuable variable, known as area under the curve or AUC, is the total geometric area beneath the TAC data points. This value is highly correlated with the overall quantity consumed during a consumption event (Barnett et al., 2011; Sakai et al., 2006). Peak TAC and AUC provide useful measures of drinking event intensity. Other measures that can be derived from transdermal alcohol readings include drinking episode length, absorption rate, and elimination rate.
Validity and reliability of transdermal sensors
Several laboratory and field studies have evaluated the performance of the WrisTAS and SCRAM relative to other biological measures and self-report. One study evaluated the WrisTAS with voluntary nonalcoholic participants in a laboratory session in which participants consumed standardized alcohol quantities in a lab setting (Swift et al., 1992). Numerous TAC readings were obtained during the 6–8 hour period that participants remained in the laboratory, and TAC values were compared to BrAC readings from a standard, calibrated breathalyzer. The two primary TAC indices, peak TAC and total area under the TAC curve (representing total alcohol consumed), were highly correlated with the corresponding breath alcohol index across individuals (r = .61 and r = .91, respectively). This study also found that TAC values generated by two devices worn simultaneously were highly correlated (peak TAC r = .71, AUC r = .94).
In a later field trial, 30 participants wore the WrisTAS on the arm or leg for 4 weeks; 15 were alcohol-dependent patients in outpatient treatment and 15 were social drinkers not involved in treatment (Swift et al., 2004). All participants kept a daily drinking diary and returned to the laboratory weekly for data collection and equipment check. Self-reported drinks per drinking episode were highly correlated with TAC estimates of area under the curve (r = .69) and peak BAC (r = .59). Within-subject correlations between self-reported drinks and TAC estimates generally exceeded .80, suggesting that the sensors were very sensitive to the amount of alcohol consumed. Specificity and sensitivity using the participants’ report as the standard were 89.2% and 84.0%, respectively.
A study by Greenfield et al. (2005) compared the diary report of alcohol use relative to WrisTAS readings among participants recruited from a random-digit dial sample who wore the WrisTAS for two weeks. The WrisTAS unit was judged as not functioning well (due to sensor failures) 49% of the time. On days with higher quality data, TAC-diary agreement was 83–96%.
The SCRAM has been evaluated in a similar fashion to the WrisTAS. Sakai et al. (2006) conducted a laboratory trial with 24 participants who received doses of 0, 0.28, or 0.56 g/kg of ethanol, followed by breath alcohol (BrAC) and TAC measurements every 15 or 30 minutes. No false-positive or false-negative readings were produced. Correlations were 0.85 for peak alcohol concentration and 0.84 for AUC. In the same study, non-alcohol dependent and alcohol-dependent participants wore the SCRAM for one week and recorded alcohol consumption in a daily log. All individuals who reported drinking during the week had positive TAC readings. Similar to the laboratory study, the individual results were not quantitatively equivalent to breath results.
In a recent report, Marques and McKnight (2009) evaluated both the SCRAM and the WrisTAS in laboratory and field conditions. In the laboratory study, participants were dosed with alcohol to attain a BAC of .08 g/dL. In the self-dosing field trial, participants kept a log of alcohol consumed and recorded breath-test readings after consuming alcohol. The researchers determined that as TAC increased from .02 g/dL to .08 g/dL, sensitivity of SCRAM improved from 65.3% to 86.5%. Specificity was 87.7%. The WrisTAS had generally higher sensitivity (from 84.4% at .02 g/dL to 82.3% at .08 g/dL) but lower specificity (74.1%). Failure of both devices was observed, although the failure rate of WrisTAS was notably higher; 37.6% of the drinking episodes could not be evaluated due to missing data. This malfunction rate was partially responsible for a low rate of performance in the WrisTAS (23.6% of drinking episodes detected). In debriefing interviews participants reported initial discomfort with the devices, but compliance with the 4-week field trial was very good, and no participants dropped out. The circumvention protection system of the SCRAM performed well; the authors report that the communication protocols that require regular upload of data and provide daily alerts make successful circumvention unlikely.
Strengths and Limitations of Current Technology
Transdermal alcohol sensor technology allows continuous remote monitoring of in vivo alcohol use without intrusive daily contact with human monitors. Both available devices reviewed above have alcohol, contact, and temperature sensors, provide a means to extract data regularly, and can be worn continuously for weeks at a time. Laboratory and field tests demonstrate that the transdermal alcohol sensors: (a) provide estimates of BAC that are highly correlated with standard breath and blood alcohol measures collected in laboratory settings, (b) yield estimates that are highly correlated with state-of-the-art self-report measures of alcohol consumption, (c) provide continuous estimates of BACs over extended periods of time in the natural environment, and (d) can be worn unobtrusively without interfering with daily activities. Transdermal devices provide objective, continuous alcohol consumption data, and thus are an important methodological advancement for alcohol-related research and treatment.
However, as introduced above, the longer time required for alcohol to be expelled through perspiration results in a lag in TAC curves relative to breath alcohol (Sakai et al., 2006; Swift, 2003), and TAC peak values tend to be lower than peak BACs (Sakai et al., 2006). Individual differences in metabolism and skin features result in differing discrepancies between transdermal TAC and simultaneous breath alcohol measurements; these discrepancies had previously made it quite difficult to predict the actual BAC of an individual from transdermal readings (Sakai et al., 2006). Recent advances in methodology described later in the paper are making dramatic improvements in predicting BAC based upon TAC readings. Recommendations are to use the TAC readings as a measure of compliance with sobriety or to use semi-quantitative values, as positive/negative ratings are highly accurate. Further, some researchers have found high malfunction rates and low true-positive rates with the WrisTAS (Greenfield et al., 2005; Marques & McKnight, 2009), although more recent models may address earlier issues.
The devices may require some adjustments to daily activities by the wearer. They cannot be submerged in water and the ankle bracelet can be uncomfortable, especially when exercising and sleeping. The WrisTAS can be removed, which allows for flexibility when activities make wearing the device uncomfortable, but may not be appropriate for some users, including those in clinical or research protocols that require objective verification of compliance. The SCRAM bracelet cannot be removed by the user, and circumvention of the sensors is possible but unlikely (Marques & McKnight, 2009). The SCRAM modem method of data transmission requires an analog or fiber optic telephone line or the addition of a cellular card. Remote transmission of the data for the WrisTAS is not fully developed, although as noted above, its sensor is included in a sensor produced by BI, Inc. that has wireless transmission capability.
By providing an objective measure of alcohol use, transdermal alcohol monitors can augment self-report and collateral reports, including as a verification of abstinence during treatment and at follow-up in clinical intervention trials, thereby enhancing current alcohol research and supporting new research endeavors. Examples of how the sensors are being used currently in alcohol research protocols are described in some detail below.
Recent Research: Estimating BACs using TAC
Some current studies are further investigating the reliability and validity of transdermal data, and exploring more sophisticated ways to model the data. In one study, one of the authors (DD) examined the correspondence between self-report data and TAC readings, using calculated estimations of peak BAC levels (using the Widmark equation). Fifty participants wore the SCRAM for 28 days. All participants were told to “drink as usual” and visited the clinic weekly, where timeline follow-back self-reports were used to record self-reported drinking and TAC data was downloaded from the SCRAM. In total there were more than 65,000 TAC readings recorded during the study. For each drinking episode, two methods were used to estimate peak BrAC: Widmark's equation, which is based on self-reported alcohol consumption, and a previously developed predictive model based on data collected from the TAC monitor. Estimates of peak BrAC based on the predictive model were significantly correlated with peak TAC measurements (r = .59). Additionally, the estimated peak BrAC using Widmark's method was significantly correlated with the peak BrAC estimated using the TAC-based predictive model (r = .57). These data provides greater confidence that TAC data can not only be used to identify the time and duration of a drinking episode, but can also be used to estimate the quantity of alcohol consumed.
Two authors of this paper (SL and GR) are pursuing a program of research focused on utilizing transdermal alcohol concentration (TAC) data to obtain semi-quantitative measures of BrAC using sophisticated mathematical modeling. These authors have created the BrAC Estimator Software, a MATLAB program that calibrates TAC models to a particular subject and transdermal device using parameters obtained from a laboratory alcohol administration session and then applies them to field data to produce semi-quantitative estimates of BrACs and alcohol ingestion profiles.
The mathematical models for the BrAC estimate models of the software are established in three steps. First, simultaneous BrAC and TAC data obtained during a laboratory alcohol administration session are used to estimate the forward convolution filter in the form of an impulse response function for an infinite dimensional linear system consisting of a two parameter parabolic diffusion equation with appropriate boundary conditions. Then the alcohol challenge BrAC and TAC data are used to optimize the regularization coefficients of the penalty terms involving the BrAC and its derivative in a least squares performance index. Lastly, the calibrated model is used to de-convolve estimates of field BrAC from the field trial TAC data of each drinking episode (see Dumett et al., 2007for further details).
The BrAC Estimator software program is designed to automatically calibrate the fit models to a particular subject and transdermal device and produce estimates of a subject’s field BrACs, thereby eliminating the dependency of alcohol researchers on mathematicians that is currently required to use the transdermal device as a semi-quantitative measure of BrAC. The software is user friendly and requires only minimal training; it is compatible with both Microsoft Windows and Apple OS X. The basic program requires only the standard installation of MATLAB, although certain advanced capabilities of the code are only available with the Optimization Toolbox. The software consists of two phases, the initial calibration phase that establishes the individualize mathematical parameters for the specific person and transdermal device, and the inversion phase that uses these parameters to estimate BrACs from field trial TAC data. The software computes as output estimates of the peak BrAC, time of peak BrAC, and area under the estimated BrAC curve. It also produces plots of the estimated BrACs and TACs as predicted by the model; measured BrAC from the breath analyzer and TAC from the sensor are included in these plots to compare predicted and actual values.
Early results have been promising. In initial testing, the BrAC Estimator software created consistent models across transdermal devices, despite differences in raw TACs, and was able to compensate for the attenuation of peak BrAC and latency of the time of peak BrAC typically observed in TAC data. The models performed particularly well for both calibration sessions in which quantity and pace were tightly controlled and field drinking episodes in which the episode was relatively uncomplicated. These results suggest the BrAC Estimator software is a relatively effective tool for obtaining semi-quantitative measure of BrACs from transdermal devices. Ongoing work will continue to refine this resource, including incorporating more sophisticated non-linear mathematical modeling and increasing the user friendliness of the software program.
Recent Research: Using Transdermal Sensors in Clinical Interventions
A limitation of intervention research is its use of retrospective self-report of alcohol consumption (days or weeks prior) from participants as the primary dependent variable of interest. These reports are subject to a variety of recall deficits and other experimental effect biases of participants (e.g., Hawthorne effect). Given that alcohol interventions are intended to reduce alcohol use and associated consequences, these biases could tend to show favorable outcomes for the intervention. As an alternative or adjunct to self-report, participants can wear transdermal alcohol monitors over the course of days to weeks before, during, and/or after an intervention, providing an objective measure of alcohol use. Key dependent variables for intervention studies can be derived from TAC values, including number of days of TAC-detected use as an indicator of frequency of use, and Peak TAC and AUC variables as indices of level of intoxication and quantity of alcohol consumed during consumption events.
Contingency management interventions
Contingency management provides a means of enhancing motivation to reduce drinking by providing rewards such as money or vouchers following a period of abstinence that can be verified by a drug test (Higgins et al., 2002; Silverman et al., 1999). Contingency management approaches that seek to reinforce abstinence or reduced levels of alcohol intoxication could benefit from the availability of continuous transdermal alcohol monitoring, specifically because daily breath tests are otherwise necessary to verify abstinence, and even daily tests could miss episodes of drinking. Continuous monitoring provides objective verification of reduced drinking and reduces the possibility of reinforcing intermittent abstinence. The tamper resistant features of the SCRAM device are also ideally suited to this application, as the bracelet cannot be removed without being detected.
A recent study by two of the authors (NB and JM) established the feasibility of using the SCRAM alcohol sensor to verify reductions in alcohol use in a contingency management intervention (Barnett et al., 2011). Thirteen heavy drinkers who were interested in reducing drinking wore the SCRAM bracelet for three weeks. In the first week, no contingencies were provided. In the second and third weeks, participants received daily cash reinforcers of $5 – 17 on an escalating schedule for showing no alcohol consumption on self-report and no detected alcohol use on the SCRAM. Even in this small sample, within-subjects comparisons showed significant reductions in alcohol use as measured by fewer days of detected use, lower self-reported drinks per week, lower average and peak TAC, and less area under the curve.
Following this pilot work, a small randomized controlled trial was conducted (N = 30), in which a contingent reinforcement condition was compared to a (yoked) noncontingent reinforcement condition using one baseline week, three intervention weeks, and a 1-month follow up. Controlling for gender and baseline week values, this study found a significant effect of contingent reinforcement on percent days with no drinking detected and on the longest number of sensor-verified consecutive days abstinent, suggesting that drinking was reduced significantly by the applied contingencies. Group comparisons on self-reported alcohol use at 1-month follow up were not significantly different between conditions. Participants reported moderate physical discomfort and embarrassment about the bracelet but 73% said they would be willing to wear the bracelet longer.
In another recent study conducted by one of the authors (DD), the effects of modest CM-based incentives to reduce risky alcohol consumption among non-treatment seeking alcohol drinkers were explored. More specifically, TAC monitoring was used to determine whether weekly contingencies could be used to moderate alcohol consumption compared to “normal” unrestricted drinking. Twenty-eight men and women (between the ages of 21 and 45 years of age) were recruited from the community through advertisements for problem drinkers. Each wore the SCRAM device during 3 contingency conditions: 4 weeks of unrestricted alcohol consumption (Non-contingency), followed by two 4-week periods of either $25 or $50 weekly contingencies awarded for restricting alcohol consumption. The monetary contingency was given to a participant when TAC readings recorded during the previous week’s monitoring did not exceed .03%. Cash was awarded if the contingency rule was met. Compared to the Non-contingency condition, both the $25 and $50 contingency conditions produced significantly fewer drinking episodes, with the $50 contingency producing more significant reductions in the number of drinking episodes exceeding a TAC of .03%. Future research might incorporate contingent reinforcement with other intervention approaches such as medication, motivational enhancement therapy, and relapse prevention.
Evaluating the value of collateral informants
A widely employed alternative to self-report of alcohol use in clinical trials has been the use of collateral informants. Typically informants are asked to provide information similar to that provided by the subject to augment the subject’s self-report. When compared, these two reports tend to be highly correlated when collected using the best available research practices (Maisto & Conners, 1992). Connors and Maisto (2003) observed that in most of the studies where collaterals were used, participants were aware of the collateral’s involvement, so one explanation for the high degree of consistency between subject and collateral reports may be that subjects’ accuracy or truthfulness is enhanced due to the anticipated comparison with collateral report. In the absence of an independent, objective measure of the subject’s actual alcohol consumption, it is difficult to estimate whether self-report is more accurate when known informants are added.
A recent study by two of the authors (NC and TL) examined the effect of a collateral informant on the correspondence between retrospective self-reports and SCRAM alcohol readings. A randomized controlled trial was conducted using a 2×2 (collateral × SCRAM) design. Approximately 100 heavy drinking college students participated in the study which required two weeks of involvement and provided reasonable compensation to participants wearing SCRAM bracelets and to collaterals. Participants were informed at the time of their assignment to experimental conditions whether collaterals would be contacted to provide a report on them. In order to avoid reactivity effects upon drinking and to minimize the extent to which participants might view the device itself as a collateral, mild deception was used as to the nature of the SCRAM. Participants were told only that the device measured and recorded “physiological information” and the SCRAM logo was covered by a label that indicated that the device was lab property. A high-degree of correspondence was found between the self-reported total drinks per drinking episode and the AUC value for each episode derived from SCRAM data (r = 0.76). Participants with or without a collateral did not systematically differ in self-reports of drinking or transdermal measures of drinking, and the presence of a collateral informant did not change the degree of correspondence observed between self-reports and SCRAM data. Taken together, these findings suggest that knowing that the collateral would be contacted did not affect self-reports or drinking behavior, strengthen our confidence in the validity of self-report, and provide further evidence of the questionable added value of collateral informants.
Potential Clinical Uses of Transdermal Alcohol Sensors
Numerous potential opportunities exist for the use of transdermal monitors in the clinical treatment context. The accountability that comes with 24-hour alcohol use monitoring itself can become an important treatment tool, especially for supporting individuals in early abstinence. For example, early identification of lapses or binge episodes could be followed by appropriate treatment adjustments to limit the duration of a lapse or prevent a full-blown relapse and treatment failure. As described earlier, transdermal devices may be ideally suited for contingency management interventions seeking to reinforce non-use of alcohol. Continuous alcohol monitoring may also be quite useful in treatment settings where other substances are the drug of choice, but individuals may turn to alcohol due to rigorous drug testing and the absence of reliable accountability systems for alcohol.
Applications to other settings where psychology services are offered may also be possible. For example, transdermal monitoring may be a useful tool for verifying abstinence among individuals undergoing custody or competency evaluations. Monitoring may also be useful for verifying abstinence among individuals taking medications or being evaluated for medical procedures or treatments for which concurrent alcohol consumption may be contraindicated or dangerous.
Conclusions
Alcohol researchers and treatment professionals share a common interest in valid and reliable measurement of alcohol consumption in real-world settings. Recent advancements in the technology of transdermal measures of alcohol in insensible perspiration have brought a new tool to address this problem. Wearable transdermal alcohol monitors are capable of providing reasonably valid and reliable measurement of real-world alcohol consumption in a relatively nonintrusive manner. The possible applications of this technology to research are widespread, including studies of in vivo human consumption of alcohol and clinical treatment contexts.
Current transdermal technology and available devices do have notable limitations. Embarrassment about the appearance of the SCRAM bracelet has been noted as the most bothersome element of wearing the bracelet (Barnett et al., 2011), and this concern may be greater for women. TAC readings lag behind consumption by up to several hours, making the devices less useful for applications needing more real-time data. Neither the SCRAM or WrisTAS device display data on the device itself, and require download to an external device for data viewing. The SCRAM device adds another layer of complexity by requiring that the data be uploaded to central servers and accessed over the internet. It would be desirable for research and clinical applications to develop devices that display and store data more locally for both convenience and security reasons. Although preliminary data is promising, more research on the reliability and validity of the devices is also warranted. Additional data on the within-subject test-retest reliability of similar drinking episodes across time would be especially helpful. Nevertheless, the development of transdermal alcohol sensor technology brings a highly useful tool for research and clinical uses.
Acknowledgments
The subject of this mini-review has been presented in a symposium held at the Scientific Meeting of the Research Society on Alcoholism (RSA), June 25 to 29, 2011 (Atlanta, GA). Organizer and Chair of the symposium was Nancy Barnett. Speakers were Robert Swift, Susan Luczak, Donald Dougherty, and Nancy Barnett, and the discussant was Raye Litten.
Footnotes
As of this writing, the current version of the SCRAM bracelet is the SCRAMx and the current version of the WrisTAS is version 7. For ease in reporting, and because some investigations include more than one version of their respective sensors, we will refer to the devices as SCRAM and WrisTAS.
Contributor Information
Thad R. Leffingwell, Oklahoma State University
Nathaniel J. Cooney, Oklahoma State University
James G. Murphy, University of Memphis
Susan Luczak, University of Southern California
Gary Rosen, University of Southern California
Donald M. Dougherty, University of Texas Health Sciences Center – San Antonio
Nancy P. Barnett, Brown University
References
- Babor TF, Brown J, Del Boca FK. Validity of self-reports in applied research on addictive behaviors: Fact or fiction? Beh Assmtt. 1990;12:5–31. [Google Scholar]
- Babor TF, Steinberg K, Del Boca FK, Anton R. Talk is cheap: Measuring drinking outcomes in clinical trials. J Stud Alc. 2000;61:55–63. doi: 10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- Babor TF, Stephens RS, Marlatt GA. Verbal report methods in clinical research on alcoholism: Response bias and its minimization. J Stud Alc. 1987;48:410–424. doi: 10.15288/jsa.1987.48.410. [DOI] [PubMed] [Google Scholar]
- Barnett N, Tidey J, Murphy J, Swift R, Colby S. Contingency management for alcohol use reduction: A pilot study using a transdermal alcohol sensor. Drug And Alc Dep. 2011;118(2–3):391–399. doi: 10.1016/j.drugalcdep.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsari B, Muellerleile P. Collateral reports in the college setting: a meta-analytic integration. Alc: Clin and Exp Rsrch. 2009;33:826–838. doi: 10.1111/j.1530-0277.2009.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors G, Maisto S. Drinking reports from collateral individuals. Addiction. 2003;98(Suppl2):21–29. doi: 10.1046/j.1359-6357.2003.00585.x. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction. 2003;98(Suppl. 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- Dumett M, Rosen G, Sabat J, Shaman A, Tempelman L, Wang C, Swift RM. Deconvolving an estimate of breath measured blood alcohol concentration from biosensor collected transdermal ethanol data. Appl Math and Comp. 2007;196:724–743. doi: 10.1016/j.amc.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield TK, Tujague J, Bond J, Kerr W. A pilot study of self-report alcohol consumption diary vs. transdermal alcohol sensor accuracy. Alc: Clin & Exp Rsrch. 2005;29(Suppl S):103A–130A. [Google Scholar]
- Higgins S, Sigmon S, Budney A. Treating chronic and severe mental disorders: A handbook of empirically supported interventions. Guilford, New York: 2002. Psychosocial treatment for cocaine dependence: The community reinforcement plus vouchers approach; pp. 296–313. [Google Scholar]
- Laforge RG, Borsari B, Baer JS. The utility of collateral informant assessment in college alcohol research: Results from a longitudinal prevention trial. J Stud Alc. 2005;66:479–487. doi: 10.15288/jsa.2005.66.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Bradley AM, Moss HB. Alcohol biomarkers in applied settings: recent advances and future research opportunities. Alc: Clin Exp Res. 2010;34:955–967. doi: 10.1111/j.1530-0277.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- Maisto S, Connors G. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ US: Humana Press; 1992. Using subject and collateral reports to measure alcohol consumption; pp. 73–96. [Google Scholar]
- Marques PR, McKnight AS. Evaluating transdermal alcohol measuring devices. Washington, DC: National Highway Traffic Safety Administration; 2007. (DOT HS 810 875) [Google Scholar]
- Marques PR, McKnight AS. Field and laboratory alcohol detection with 2 types of transdermal devices. Alc: Clin & Exp Rsrch. 2009;33:703–711. doi: 10.1111/j.1530-0277.2008.00887.x. [DOI] [PubMed] [Google Scholar]
- Phillips M, Greenberg J, Andrzejewski J. Evaluation of the Alcopatch, a transdermal dosimeter for monitoring alcohol consumption. Alc: Clin & Exp Rsrch. 1995;19:1547–1549. doi: 10.1111/j.1530-0277.1995.tb01022.x. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Mikulich-Gilbertson SK, Long RJ, Crowley TJ. Validity of transdermal alcohol monitoring: fixed and self-regulated dosing. Alc: Clin. Exp Res. 2006;30:26–33. doi: 10.1111/j.1530-0277.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Preston K, Stitzer M, Schuster C. Motivating behavior change among illicit-drug abusers: Research on contingency management interventions. Washington DC: American Psychological Association; 1999. Efficacy and versatility of voucher-based reinforcement in drug abuse treatment; pp. 163–181. [Google Scholar]
- Sobell L, Sobell M. Self-report issues in alcohol abuse: State of the art and future directions. Beh Assmnt. 1990;12:77–90. [Google Scholar]
- Sobell L, Sobell M. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ US: Humana Press; 1992. Timeline follow-back: A technique for assessing self-reported alcohol consumption; pp. 41–72. [Google Scholar]
- Swift RM. Transdermal measurement of alcohol consumption. Addiction. 1993;88:1037–1039. doi: 10.1111/j.1360-0443.1993.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Swift RM. Direct measurement of alcohol and its metabolites. Addiction. 2003;98(suppl 2):73–80. doi: 10.1046/j.1359-6357.2003.00605.x. [DOI] [PubMed] [Google Scholar]
- Swift RM, Martin C, Swette L, LaConti A, Kackley N. Studies on a wearable, electronic, transdermal alcohol sensor. Alc: Clin & Exp Rsrch. 1992;16:721–725. doi: 10.1111/j.1530-0277.1992.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Swift R, Swette L. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ US: Humana Press; 1992. Assessment of ethanol consumption with a wearable, electronic ethanol sensor/recorder; pp. 189–202. [Google Scholar]