Abstract
PAF complex (PAFc) is an RNA polymerase II associated factor that controls diverse steps of transcription. Although it is generally associated with actively transcribed genes, a repressive PAFc has also been suggested. Here, we report that PAFc regulates the transition from transcription initiation to transcription elongation. PAFc repressed IL-6-induced, but not TNF-α-induced, immediate early gene expression. PAFc constitutively associated with the 5′-coding region of the c-Fos locus, then transiently dissociated upon IL-6 stimulation. When CTR9, a component of PAFc, was depleted, higher levels of serine 5-phosphorylated or serine 2-phosphorylated forms of RNA Polymerase II were associated with the unstimulated c-Fos locus. We also observed an increased association of CDK9, a kinase component of the pTEF-b elongation factor, with the c-Fos locus in the CTR9-depleted condition. Furthermore, association of negative elongation factor, NELF, which is required to proceed to the elongation phase, was significantly reduced by CTR9 depletion, whereas elongation factor SPT5 recruitment was enhanced by CTR9 depletion. Finally, the chromatin association of CTR9 was specifically controlled by IL-6-induced kinase activity, because a JAK2 kinase inhibitor, AG-490, blocked its association. In conclusion, our data suggest that PAFc controls the recruitment of NELF and SPT5 to target loci in a signal- and locus-specific manner.
Introduction
Transcription occurs in three phases: initiation, elongation, and termination. General transcription factors recognize promoter sequences and recruit RNA polymerase II (Pol II) to form a preinitiation complex (PIC), the first step of messenger RNA (mRNA) synthesis [1]. Before proceeding to the elongation phase, the functional and structural block created by the pre-initiation complex (PIC), a complex process that is regulated by multiple elongation factors, has to be cleared [2]. Appropriate cellular responses to specific stimuli depend on signaling cascades to activate transcription factors (TFs) that bind to specific gene regulatory regions. Generally, TF binding facilitates chromatin remodeling and the recruitment of eukaryotic RNA Pol II to the transcription start site (TSS) to initiate transcription [3]. However, a genome-wide analysis of eukaryotic RNA Pol II revealed that over 30% of human genes are blocked from proceeding to elongation by an RNA Pol II PIC at the promoter-proximal region [4].
Among these blocked genes are the immediate early genes (IEGs), such as c-Fos, junB, and c-myc, which are rapidly induced by external stimuli [5]–[7]. In contrast to the stress-responsive genes whose transcription is tightly controlled by signal-specific TFs at the pre-initiation step, the promoters of IEGs are pre-occupied by RNA Pol II to facilitate rapid induction, and the signal-induced transcription of IEGs is primarily controlled at the transition from initiation into elongation [8]–[10]. Therefore, the transcriptional machinery has diverse mechanisms to ensure the efficiency and specificity of gene regulation.
While moving through the template DNA, eukaryotic RNA Pol II encounters nucleosomes. The concerted actions of histone modifying enzymes, ATP-dependent chromatin remodelers, and histone chaperones are necessary for the efficient movement of RNA Pol II through nucleosomes [11]. Stepwise transcriptional processes and co-transcriptional pre-mRNA processing require a structural platform to ensure the coordinated regulation of transcription. The C-terminal domain (CTD) of RNA Pol II serves as a major assembly point for the binding of multiple proteins that control transcription, RNA processing, and histone modification [12]. Both genetic and biochemical approaches have been applied to identify the RNA Pol II-associated transcription factors that regulate these processes. Among these transcriptional regulators is the PAFc, which was originally identified in yeast.
PAFc is an evolutionarily conserved RNA Pol II-associated complex that is composed of Paf1, Ctr9, Cdc73, Rtf1, and Leo1 in yeast [13]–[16]. In humans, PAFc contains an additional subunit, SKI8 [17]. PAFc is involved in multiple steps of transcription, including initiation, elongation and mRNA 3′-end-processing [18]. PAFc associates with the chromatin of actively transcribing genes and plays critical roles in the recruitment of the histone modifying machinery to target loci [19], [20]. Furthermore, PAFc interacts with the FACT complex and hDst1/SII, and stimulates transcriptional elongation in vitro [21], [22]. Because PAFc associates with chromatin and physically interact with multiple transcriptional regulators, most work on PAFc has primarily focused on its role in the positive regulation of transcription. However, accumulating evidence indicates that there are groups of genes that are negatively regulated by PAFc [23]–[25], although the molecular mechanisms governing its negative effect are not well understood.
Previously, we reported that PAFc participates in the transcriptional activation of acute phase protein (APP) genes through the direct interaction of CTR9 and STAT3 [26]. In a search for general mechanisms of target gene selection by PAFc, we found that PAFc behaves as a dual regulator; it acts as a transcriptional activator for stimulus-dependent APP gene induction, whereas the transcription of IEGs is negatively controlled by PAFc. In this study, the molecular mechanism governing PAFc-mediated negative regulation of c-Fos transcription was explored.
Materials and Methods
Plasmids
A full-length cDNA of mouse Ctr9 was obtained in the laboratory of S. Desiderio, as previously described [27]. Mouse Paf1 cDNA was obtained by PCR and cloned into pCDNA3.1 myc-His(A) vector (Invitrogen, Clarlsbad, CA). A 2.0 kb promoter fragment of c-Fos gene, 1.3 kb fragment containing 0.4 kb promoter and 1.1 kb coding region of c-Fos, and a total 3.5 kb c-Fos genomic region fragment were obtained by PCR with appropriate primer sets and cloned into pGL3 basic vector (Promega, Madison, WI). 0.9 kb c-Fos first intron was obtained by PCR with appropriate primer sets and cloned into pGL3 control vector (Promega, Madison, WI). The sequences of the primers for PCR are provided in Table S1.
Cell Culture
HepG2 cells were maintained in MEM (Welgene, Korea) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and maintained at 37°C in a 5% CO2 atmosphere. For cytokine stimulation, cells were treated with rhIL-6 (20 ng/ml) plus IL-6sR (20 ng/ml) for indicated times (R&D Systems, Minneapolis, MN). Transient transfection was carried out using Lipofectamine 2000 (Invitrogen, Clarlsbad, CA) according to the manufacturer's instructions.
RNA interference
Oligomer sequences used for RNA interference are as follows; Control: 5′-UUCUCCGAACGUGUCACGUTT-3′, CTR9: 5′-CCGUGUGGCUCCAAACUUUATT-3′, LEO1: 5′-GCCGGUAGCUUCUGAUAAUTT-3′, CDC73: 5′- GGUACAUGGUAAAGCAUAATT-3′, PAF1: 5′-GCAG UUUACCGAGGAAGAATT-3′, NELF-E: 5′-GACCCAGAUUGUCUACAG UTT-3′, SPT5: 5′-GGACUGUCAAAUGUAAGAUTT-3′.
RNA Preparation and Analysis
Total RNA was extracted from cells using the RNAiso Plus (Takara Bio, Shiga, Japan). For conventional or real time PCR, 1 µg of total RNA was reverse-transcribed using oligo-d (T)15 primer or random hexamer (Promega, Madison, WI), respectively, and M-MLV reverse transcriptase (Promega, Madison, WI). For the Nuclear/Cytoplasmic fractionation, PARIS kit (Ambion, Austin, TX) was used, according to the manufacturer's instructions. The sequences of the primers for PCR are provided in Table S1. Presented data are representatives of more than two biological replicates and indicated error bars came from three technical replicates.
Chromatin Immunoprecipitation (ChIP)
ChIP was performed as previously described [26]. Antibodies against CDK9, NELF-E, Pol II N-terminal, and SPT5 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Also, antibodies against Serine 5P and Serine 2P Pol II were purchased from Abcam (Cambridge, MA). The sequences of the primers for PCR are provided in Table S1. Presented data are representatives of more than two biological replicates and indicated error bars came from three technical replicates.
Immunoblotting and Immunoprecipitation
HepG2 cells were lysed in lysis buffer (25 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS, 0.1 mg/ml PMSF, 1 mM DTT, 1 mM sodium orthovanadate, 5 µg/ml aprotinin, 2 µg/ml pepstatin, 5 µg/ml leupeptin, 1 mM benzamidine). Total cell lysates (40 µg per sample) were resolved using SDS-polyacrylamide gels. For immunoprecipitation, 1 mg of lysate was incubated with 2 ug of antibody overnight. NaF (25 mM), β-glycerol phosphate (25 mM) and Na3VO4 (5 mM) were added additionally to detect phosphorylated forms of RNA polymerase II during the immunoprecipitation. Antibodies against CTR9, PAF1, CDC73, and LEO1 were purchased from Bethyl Laboratory (Montgomery, TX). Antibodies against STAT3, phospho-STAT3(Y705), TBP, c-Myc and NELF-E were purchased from Santa Cruz Biotechnology. Antibody against GAPDH was purchased from Chemicon (Chemicon International, Temecula, CA).
Fractionation
Chromatin-bound and –unbound fractions were prepared as previously described [28]. Briefly, HepG2 cells were incubated on ice for 5 min with 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 10 mM PIPES (pH 6.8), 1 mM EGTA, 0.2% Triton X-100, and protease inhibitors (0.1 mg/ml PMSF, 1 mM DTT, 1 mM sodium orthovanadate, 5 µg/ml aprotinin, 2 µg/ml pepstatin, 5 µg/ml leupeptin, 1 mM benzamidine). Cells were then centrifugated, and the supernatant was collected as the “unbound” fraction. The remaining pellet was further incubated with 0.5 U/µl DNaseI containing buffer 37°c for 30 min, centrifugated, and the supernatant was collected as the “bound” fraction.
Results
c-Fos transcription is negatively regulated by PAFc in the nucleus
We have previously shown that CTR9, a component of PAFc, aids the transcription of IL-6-inducible acute phase protein (APP) genes, such as fibrinogen (FGG) and haptoglobin (Hp), by stabilizing the binding between STAT3 and chromatin at promoters [26]. This finding prompted us to investigate whether CTR9 aids the transcription of all genes whose transcription is mediated by STAT3. We studied an immediate early gene, c-Fos, which is transcriptionally induced by IL-6 in hepatocytes. The proto-oncogene c-Fos contains a canonical STAT3 binding site in its promoter region and is known to be directly controlled by IL-6-activated STAT3 [29]. Surprisingly, we found that knockdown of endogenous CTR9 led to a marked increase in c-Fos transcription under both basal and IL-6-induced conditions (Fig. 1A and 1B ). As previously reported, reduced CTR9 expression led to a decrease in the expression of other components of PAFc (Fig. 1A ) [17]. To assess whether the negative effect of PAFc on c-Fos transcription is IL-6-signal specific, we then stimulated cells with TNFα, which induces c-Fos expression through activation of the NFκB signaling pathway (Fig. 1C ). In contrast to the effect of IL-6 stimulation, TNFα-mediated induction of c-Fos was not significantly enhanced in the CTR9-deficient cells. This demonstrates that the negative effect of PAFc on c-Fos transcription occurs in a signal-specific manner.
Figure 1. c-Fos transcription is negatively regulated by PAFc in the nucleus.
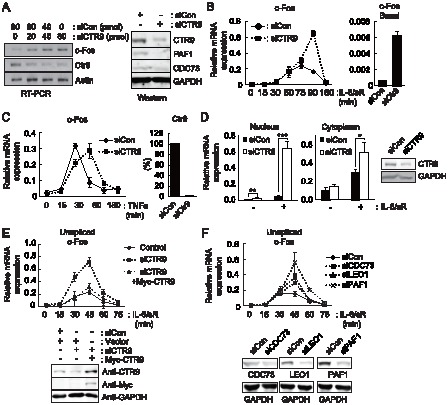
A, Left, transcript levels of c-Fos, Ctr9 and Actin were analyzed by RT-PCR in HepG2 cells transfected with control siRNA (siCon) or CTR9 siRNA (siCTR9), as indicated above. Right, endogenous protein levels of PAFc components in CTR9 knockdown cells were measured by western blot analysis. B, IL-6-induced and basal c-Fos expression was analyzed by Real-time RT-PCR (RT-qPCR). HepG2 cells were transfected with either control or CTR9 siRNA. Approximately 48 hours later, cells were treated with IL-6 plus IL-6sR (20 ng/ml each) for the indicated length of time. Expression levels were normalized to β-Actin. C, Cells were transfected with Control or CTR9 siRNA. 48 hours later, cells were treated with TNFα (20 ng/ml) for the indicated length of time. D, Left, Nuclear and cytoplasmic RNA were prepared using the PARIS kit. The levels of c-Fos mRNA in the nuclear and cytoplasmic fractions were then analyzed by RT-qPCR. Right, endogenous protein levels of CTR9 in control and knockdown cells were measured by western blot analysis. E, Cells were transfected as indicated. 48 hours later, cells were treated with IL-6 plus IL-6sR (20 ng/ml each) for the indicated length of time. The unspliced c-Fos transcripts were analyzed by RT-qPCR. Bottom, endogenous protein levels of CTR9 and exogenous Myc-CTR9 were measured by western blot analysis. F, PAFc components were individually knocked down by transient siRNA transfection. Samples were prepared and analyzed as described in E. Bottom, endogenous protein levels of PAFc components were measured by western blot analysis. **p<0.01, ***p<0.001 by Student's t test. Error bars represents standard deviation(SD) (n = 3).
PAFc is known to be involved in multiple transcriptional steps, including initiation, elongation, and the 3′-end processing of mRNA [18]. Because most of these regulatory processes occur in the nucleus, we harvested mRNAs from the nucleus and the cytoplasm separately and assessed the regulatory effect of CTR9 knockdown on each of these mRNA populations (Fig. 1D ). Although a negative effect of CTR9 was observed in the c-Fos mRNA prepared from the cytoplasmic fraction, the most dramatic effect was observed in the RNA prepared from the nuclear fraction. The knockdown effect of CTR9 on c-Fos transcription was observed in unspliced transcripts, and overexpression of mouse CTR9, which is insensitive to human CTR9 siRNA, successfully reversed the hyper-induction of c-Fos in the CTR9 knockdown condition (Fig. 1E ). However, Myc-CTR9 overexpression alone did not alter the expression of c-Fos significantly (Fig. S1 A). Since CTR9 is relatively abundant than other PAFc components in yeast [30], it is possible that endogenous CTR9 is already saturated to exhibit inhibitory effect. At the same time, it is also possible that CTR9 requires other protein for its inhibitory action. Since CTR9 is reported to act as a component of PAFc (PAFc) [15], [31]–[33], we next examined the contribution of other components of PAFc on c-Fos transcription (Fig. 1F ). Although the effect was not as strong as CTR9, unspliced c-Fos transcripts were increased by individual knockdown of CDC73, LEO1 or PAF1. When individual component of PAFc such as PAF1 or CDC73 was overexpressed, it did not significantly changed c-Fos expression (Fig. S1 B). These data demonstrate that PAFc plays a negative role in the transcriptional control of c-Fos in the nucleus.
PAFc controls the transition from the initiation to elongation phases of transcription at the c-Fos locus
During the transcriptional control of c-Fos, the elongation step is critical [34]. The promoter-proximal region of c-Fos is pre-occupied by RNA Pol II to form a pre-initiation complex that is stalled by an elongation block present in the first intron of c-Fos [35]. The stalled RNA Pol II only enters the elongation phase after stimuli trigger the recruitment of positive elongation factor b (pTEF-b) to the phosphorylated CTD of RNA Pol II; pTEF-b then recruits other elongation factors [36]. Because PAFc is known to associate with elongation factors, such as pTEF-b, SPT4/5, and the FACT complex, and aids RNA Pol II elongation in vitro [16], [21], we examined its effect on the control of elongation during c-Fos transcription.
The association of modified RNA Pol II with chromatin at the c-Fos genomic locus was first examined by ChIP analysis using antibodies that detect the serine 5-phosphorylated or serine 2-phosphorylated forms of RNA Pol II. RNA Pol II phosphorylated on the serine 5 residue of the CTD (Ser5P RNA Pol II) was mainly detected in the TSS region of c-Fos under basal conditions, and this association was further enhanced by IL-6 stimulation (Fig. 2A ). In CTR9-depleted cells, slightly higher levels of Ser5P RNA Pol II were found in the TSS region of c-Fos under basal conditions, indicating that CTR9 plays negative roles in the progression of transcription under basal conditions. Even more interestingly, the CTD of RNA Pol II associated with the coding regions of c-Fos was heavily phosphorylated on serine 2 residues even in the absence of IL-6 stimulation and remained high after IL-6 stimulation (Fig. 2B ). Upon IL-6 stimulation, levels of the phosphorylated serine 5 or serine 2 in the CTR9-depleted cells were either statistically increased or not changed, compared to control cells. Consistent with its role in transcription elongation, more RNA Pol II was found in the 3′- coding region of the c-Fos locus in the CTR9-deficient cells (Fig. 2C ). These results indicate that when CTR9 is limited, stalled RNA Pol II at the c-Fos locus can proceed to the elongation phase without stimulation. In other words, the association of PAFc with c-Fos functions to prevent elongation; dissociation of PAFc from the coding region of c-Fos might therefore be required for transcriptional elongation.
Figure 2. Elongating RNA Pol II pre-occupies the c-Fos locus in the CTR9-deficient cells.
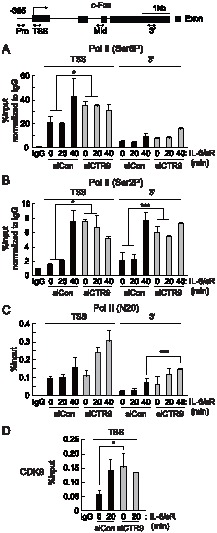
Top, diagram of the c-Fos genomic loci. Every PCR primers used for ChIP analyses in this study are shown as two-sided arrows. HepG2 cells were transfected with control siRNAs or CTR9 siRNAs and treated with IL-6 plus IL-6sR (20 ng/ml each) for the indicated length of time. ChIP analyses were performed with soluble chromatin using antibodies as indicated: anti-Ser5P CTD (A), anti-Ser2P CTD (B), anti-RNA polymerase II N-terminus (C), and anti-CDK9 (D),. Bound DNA was analyzed by quantitative PCR using primers specific to c-Fos loci. *p<0.05, ***p<0.001 by Student's t test. Error bars represents SD (n = 3).
To determine the role of PAFc in the process of elongation, we examined the recruitment of pTEF-b to the c-Fos locus upon stimulation. At basal condition, weak association of CDK9, a kinase component of pTEF-b, was detected, and this association was further increased after IL-6 stimulation (Fig. 2D ). When CTR9 was depleted, CDK9 association with c-Fos TSS region increased significantly, even in the absence of stimulation (Fig. 2D ). Therefore, it is possible that the enhanced phosphorylation of the serine 5 and serine 2 residues of RNA Pol II CTD in the absence of CTR9 might be partially resulted from the increased CDK9 association to the c-Fos locus. These results collectively indicate that the RNA Pol II transition from the initiation phase to the elongation phase was significantly increased at the c-Fos locus in the absence of CTR9.
Recruitment of the negative elongation factor NELF to the c-Fos locus requires CTR9
Our data suggested that CTR9 acts to prevent the transition from transcription initiation to elongation at the c-Fos locus. To investigate the molecular mechanism of PAFc regulation of c-Fos transcription, we examined the recruitment of elongation factors to the c-Fos locus in the presence or absence of PAFc. The negative elongation factor (NELF) complex is known to inhibit elongation of the c-Fos gene [37]. Therefore, we examined the association of NELF-E with the c-Fos locus using the ChIP assay. Under basal conditions, significant amounts of NELF-E were present only in the TSS region, while almost no signal was detected in the 3′ region. Upon IL-6 stimulation, NELF-E rapidly dissociated from the TSS (Fig. 3A ). When CTR9 was depleted, the association of NELF with the TSS of c-Fos was significantly reduced under basal condition (Fig. 3A ). Upon IL-6 stimulation, however, NELF association increased to a level seen after activation in control cells. As a result, similar levels of NELF association were achieved in both cases, although they started from different points. It is noteworthy to mention that NELF is known to be recruited to target locus to prevent re-initiation and premature termination [38], [39]. Therefore we postulate that CTR9 might also function to prevent or delay the re-association of NELF to target locus. This result is in agreement with the increased CDK9 (p-TEFb) recruitment observed at the c-Fos loci after CTR9 knockdown (Fig. 2D ). It also explains why RNA Pol II at the c-Fos locus was heavily phosphorylated and efficiently preceded in the CTR9-deficient basal conditions (Fig. 2B and 2C ). Since the recruitment of NELF was changed by CTR9 knockdown, we examined whether CTR9 physically interacts with NELF. CTR9 protein interacted with NELF-E at basal states and this interaction became weaker after IL-6 stimulation (Fig. 3B ).
Figure 3. Recruitment of the negative elongation factor NELF to the c-Fos locus requires CTR9.
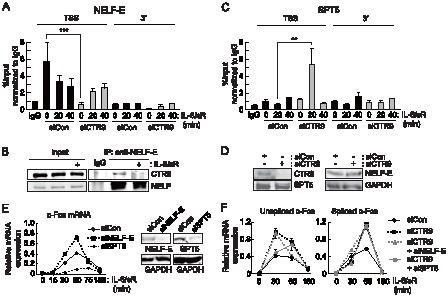
Cells were transfected with control or CTR9 siRNA and treated with IL-6 and IL-6sR (20 ng/ml each) for the indicated length of time. ChIP analyses were performed with soluble chromatin using the antibodies indicated, and the bound DNA was analyzed by quantitative PCR using primers specific to c-Fos. Antibodies specific to NELF-E (A), or SPT5 (C), were used. B, Cells were treated with IL-6 and IL-6sR (20 ng/ml each) for 30 minutes and lysates were immunoprecipitated with anti-NELF-E antibody. Immunoprecipitated proteins were detected by western blot analysis. D, Cells were transfected with either control siRNA or CTR9 siRNA. Approximately 48 hours later, protein levels of CTR9, SPT5, NELF-E, and GAPDH were detected by western blot analysis. E, Cells were transfected with the indicated siRNAs and treated with IL-6 and IL-6sR (20 ng/ml each) for the indicated time period. The c-Fos mRNA induction level was measured by RT-qPCR. E, NELF-E and SPT5 knockdown efficiencies were measured by RT-qPCR (Left) and western blot analysis (Right). mRNA expression levels were normalized to β-Actin. F, Cells were transfected with the indicated siRNAs and treated with IL-6 and IL-6sR (20 ng/ml each) for the indicated time period. Expression levels were normalized to β-Actin. **p<0.01, ***p<0.001 by Student's t test. Error bars represents SD (n = 3).
We next examined the association of SPT5, a component of the DRB sensitivity inducing (DSIF) complex, with the c-Fos locus. Upon depletion of CTR9, more SPT5 was recruited to the TSS region of c-Fos after IL-6 stimulation (Fig. 3C ). However, total protein levels of NELF and SPT5 were not significantly changed by CTR9 knockdown (Fig. 3D ). Based on these data and our data regarding the association of PAFc with c-Fos, we postulated that NELF acts as a negative regulator of c-Fos transcription, and that SPT5 acts as a positive regulator of c-Fos transcription via PAFc. To test this hypothesis, we determined the levels of c-Fos transcripts in NELF-E-depleted and SPT5-depleted cells. In agreement with their physical associations with the locus, the IL-6-induced production of c-Fos transcripts was further enhanced in NELF- depleted cells, whereas it was decreased in SPT5-depleted cells (Fig. 3E ). However, double knockdown of NELF-E or SPT5 along with CTR9 did not amplify the single knockdown effect, indicating that recruitment of NELF or SPT5 and CTR9 are interdependent (Fig. 3F ). Taken together, these results suggest that PAFc functions to recruit negative elongation factors and block positive elongation factor access to target gene loci.
PAFc dissociates from the c-Fos locus after IL-6 stimulation
To determine whether PAFc affects c-Fos transcription directly, we examined the chromatin association patterns of PAFc with the c-Fos locus under basal and IL-6 stimulated conditions. CTR9 was primarily associated with the TSS region of c-Fos in the absence of stimulation (Fig. 4A ). Upon stimulation with IL-6, CTR9 rapidly dissociated from the c-Fos locus. The IL-6-induced dissociation of CTR9 from the coding region of a gene was not a universal event, because the chromatin association of CTR9 with the coding region of the Hp locus was not dramatically altered by the same stimulus (Fig. S2). These results indicate that the association of CTR9 with chromatin is controlled in a locus-specific manner. Like CTR9, CDC73, LEO1, and ectopic myc-PAF1 dissociated from the coding region of the c-Fos locus upon IL-6 stimulation (Fig. 4B–D ). These dissociations are similar to the signal-dependent dissociation of NELF and suggest a possible role of PAFc as a modulator of NELF association with target gene chromatin.
Figure 4. PAFc dissociates from the c-Fos locus after IL-6 stimulation. HepG2 cells were treated with IL-6 plus IL-6sR (20 ng/ml each) for the indicated length of time.
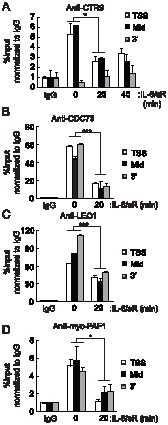
Soluble chromatins were immunoprecipitated with anti-CTR9 antibodies, and the bound DNAs were analyzed by quantitative PCR using primers specific to c-Fos (A). (B,C) CDC73 and LEO1 associations with c-Fos locus were analyzed after IL-6 plus IL-6sR (20 ng/ml each) stimulation. (D) Cells were transfected with Myc-PAF1 and 48 hours later, cells were treated with IL-6 plus IL-6sR (20 ng/ml each) for 20 minutes. ChIP assay was performed with anti-Myc antibody and bound DNAs were analyzed by quantitative PCR using primers specific to c-Fos. *p<0.05, ***p<0.001 by Student's t test. Error bars represents SD (n = 3).
Dissociation of CTR9 from the c-Fos locus requires signal-activated kinase activity
Our data collectively indicate that the negative regulation of c-Fos transcription by PAFc occurs at the post-initiation step; PAFc must be released from a target locus to proceed to elongation, a process that is induced by IL-6 stimulation. Therefore, we hypothesized that the dissociation of CTR9 from a target locus depends on signaling activity mediated by IL-6. To test this hypothesis, we treated cells with AG490, a kinase inhibitor of JAK2, to inhibit the transcriptional induction of c-Fos by IL-6. AG490 significantly reduced IL-6-induced c-Fos expression (Fig. 5A ). We then measured the association of CTR9 with the c-Fos locus in the cells treated with AG490. The association of CTR9 at the c-Fos locus was not altered after IL-6 stimulation in AG490-treated cells (Fig. 5B ). These data suggest that kinase activity related to JAK2 might be responsible for the regulation of the dissociation of CTR9 from the c-Fos locus.
Figure 5. Chromatin association of CTR9 requires kinase activity inhibited by AG490.
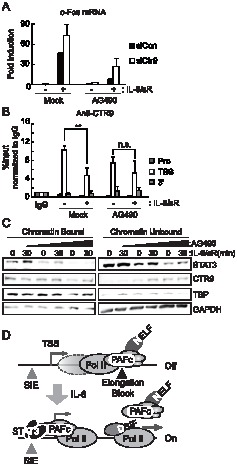
A, Cells were transfected with control siRNA or CTR9 siRNA for 48 hrs and treated with DMSO (mock) or AG490 (100 uM) for 4 hrs prior to IL-6 plus IL-6sR (20 ng/ml each) stimulation for 30 min. The c-Fos mRNA induction level was measured by RT-qPCR. B, Cells were treated as in A and a ChIP assay was performed with an anti-CTR9 antibody. Bound DNAs were analyzed by quantitative PCR using primers specific to c-Fos genomic loci. C, Cells were treated with DMSO (mock), 100 uM or 200 uM of AG490 for 12 hrs prior to IL-6 plus IL-6sR (20 ng/ml each) stimulation for 30 min. Cells were fractionated into chromatin -unbound and -bound fractions and blotted with anti-CTR9, anti-STAT3, anti-TBP, and anti-GAPDH antibodies. D, Negative function of PAFc in IL-6-responsive gene regulation. At TSS of IEG loci in basal condition, PAFc is associated with NELF. Upon IL-6 stimulation, PAFc along with NELF dissociates from the elongation block to allow transcriptional elongation to occur. SIE: Serum Inducible Element (STAT3 binding site). n.s. = not significant, **p<0.01 by Student's t test. Error bars represents SD (n = 3).
To confirm the role of JAK2-related kinase activity in the dissociation of CTR9 from chromatin, we fractionated cells into chromatin-bound and chromatin-unbound fractions and analyzed the chromatin association patterns of STAT3 and CTR9 (Fig. 5C ). Upon stimulation with IL-6, more STAT3 was observed in the chromatin-bound fraction. The amount of STAT3 in the chromatin-bound fraction gradually decreased with AG-490 treatment. In contrast, less CTR9 was observed in the chromatin-bound fraction. When cells were treated with AG-490, the association of CTR9 with chromatin decreased, and at the same time, more CTR9 appeared in the chromatin-unbound fraction. This result is in complete agreement with the ChIP data obtained from the experiments on the c-Fos locus described above (Fig. 5B ) and implies that the dissociation of CTR9 from chromatin is a dynamically regulated process that requires JAK2-related kinase activity.
Discussion
Our data suggest that the mammalian PAFc acts as a negative regulator of transcription by aiding recruitment of the negative elongation factor NELF, which blocks the transition of RNA polymerase II-mediated transcription from the initiation to the elongation phase (Fig. 5D ), to target loci. We observed that phosphorylation of RNA Pol II CTD on serine 5 and serine 2 residues were increased by CTR9 knockdown although the recruitment of RNA Pol II was not increased in unstimulated condition. These data indicate that c-Fos transcription relies more heavily on the release of the elongation block than de novo transcription. This finding again emphasizes the importance of elongation control in the transcriptional regulation of immediate early genes.
PAFc was originally identified as a transcriptional activator. However, accumulating evidence suggests that PAFc also has a negative role in gene regulation [23]–[25]. Although a differential effect of H3K4 methylation on different loci has been proposed to explain the dual functions of PAFc [24], it is still not clear how PAFc functions as both a positive and negative factor at the same time. It is well known that PAFc physically interacts with multiple proteins; thus, PAFc might change its interaction partners as transcription proceeds. For example, PAFc recruits H3K4 methyltransferase SET1 to RNA Pol II in the promoter-proximal region, while it recruits SET2, which methylates H3K36, to Pol II in the coding region [19], [20]. It is noteworthy that SET2 recruitment to its target region relies on the phosphorylation status of the RNA Pol II CTD, which is mediated by Ctk1 (a putative P-TEFb homolog in yeast) [40], indicating that P-TEFb may play a role in the SET1/SET2 interactions with PAFc. Here, we showed that recruitment of P-TEFb (CDK9) is also regulated by PAFc at the c-Fos locus (Fig. 2D ).
Originally identified as a negative regulator of elongation, NELF/DSIF also acts as a positive regulator; genome-wide analyses revealed that one-third of NELF associated genes were up-regulated by NELF-depletion, while the remaining two thirds were down-regulated [41]. Similarly, genome-wide analyses of an Spt5 mutant demonstrated that SPT5 has dual functions in transcription regulation [42]. Although NELF is known to differentially affect chromatin architecture [41], it is not clear how NELF/DSIF performs dual roles, or how specific target genes are selected for positive or negative regulation. Therefore, it will be interesting to investigate how PAFc functions as a modulator of NELF/DSIF-mediated gene regulation. In support of this idea, it has recently been suggested that PAFc aids the molecular function of NELF and DSIF through physical interaction [43].
We observed that PAFc dissociated from target loci locus in response to IL-6 signals, and that this behavior was sensitive to AG490, an inhibitor of JAK2 kinase (Fig. 5B, C ). Therefore, our data indicate that the phospho-state of PAFc might be dynamically regulated by external stimuli, which play key roles in the regulation of transcription. So far, neither the signal-dependent regulation of PAFc nor the posttranslational modification of PAFc has been explored in detail. Although PAFc is known to interact with RNA Pol II in many of its states, such as the non-phosphorylated, serine 2-phosphorylated, and serine 5-phosphorylated forms [30], [44]–[46], whether PAFc undergoes posttranslational modification during transcriptional processes is unclear. Interestingly, phosphorylation of CDC73 at the tyrosine residue has recently been reported to function in its physical interaction with β-catenin, suggesting that post-translational modifications of PAFc may play important roles in the regulation of transcription [47].
We previously reported the function of PAFc as a transcriptional activator in APP gene expression. CTR9 regulated APP gene transcription at promoter region by stabilizing STAT3 association with chromatin. In this study, we have demonstrated a novel function of PAFc controlling the elongation block of the c-Fos locus through the regulation of NELF/DSIF recruitment. These two studies suggest a dual function of PAFc in target gene expression. It is interesting to note that although the mode of action is opposite, the end-result is similar. For the transcriptional activation of IL-6-dependent APP gene induction, PAFc is actively recruited to the target locus and aids in gene induction. For the transcriptional activation of IL-6-dependent IEG induction, PAFc is specifically dissociated from the target locus and again, helps gene induction. Therefore it will be interesting to study what determines the locus specificity or specific genomic occupancy of PAFc in the basal condition. Transcriptional potentials of PAFc might be also modulated by its interacting partners, which results in both positive and negative outcomes.
Our results provide new insights that will help improve our understanding of PAFc's mechanism of negative regulation. It will be of great importance to further investigate the signal-dependent regulation of PAFc in relation to its composition and posttranslational modification status to better understand the complexity of the regulation of eukaryotic transcriptional elongation.
Supporting Information
Overexpression of PAFc components did not affect c-Fos expression A . The Myc-CTR9 expression vector was transfected to HepG2 cells. Approximately 48 hours later, cells were treated with IL-6 plus IL-6sR (20 ng/ml each) for the indicated length of time. Left, western blot assay was performed to detect exogenous Myc-CTR9. Right, c-Fos mRNA levels were measured by RT-qPCR analysis. Expression levels were normalized to β-Actin. B. Cells were transfected with Myc-Paf1 or Myc-Cdc73. 48 hours later, cells were treated with IL-6 plus IL-6sR (20 ng/ml each) for 1 hour and PAF1, CDC73 and GAPDH proteins levels were analyzed by westernblot analysis using endogenous antibodies. c-Fos mRNA levels were measured by RT-qPCR analysis.
(EPS)
Top, diagram of the Hp genomic loci. PCR primers used for ChIP analyses are shown as two-sided arrows. HepG2 cells were treated with IL-6 plus IL-6sR (20 ng/ml each) for the indicated length of time. Soluble chromatins were immunoprecipitated with anti-CTR9 antibodies, and the bound DNAs were analyzed by quantitative PCR using primers specific to Hp loci. Error bars represents SD (n = 3).
(EPS)
Primer sequences used for PCR experiments.
(DOC)
Funding Statement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NO. 2012R1A2A2A01007525 and KRF-2008-313-C00604). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Roeder RG (1991) The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci 16: 402–408. [DOI] [PubMed] [Google Scholar]
- 2. Saunders A, Core LJ, Lis JT (2006) Breaking barriers to transcription elongation. Nat Rev Mol Cell Biol 7: 557–567. [DOI] [PubMed] [Google Scholar]
- 3. Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128: 707–719. [DOI] [PubMed] [Google Scholar]
- 4. Core LJ, Waterfall JJ, Lis JT (2008) Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322: 1845–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cochran BH (1993) Regulation of immediate early gene expression. NIDA Res Monogr 125: 3–24. [PubMed] [Google Scholar]
- 6. Gess B, Wolf K, Kurtz A (1997) Lack of control by immediate early response genes in the oxygen regulation of erythropoietin gene expression. Pflugers Arch 433: 827–831. [DOI] [PubMed] [Google Scholar]
- 7. Nakajima K, Kusafuka T, Takeda T, Fujitani Y, Nakae K, et al. (1993) Identification of a novel interleukin-6 response element containing an Ets-binding site and a CRE-like site in the junB promoter. Mol Cell Biol 13: 3027–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Core LJ, Lis JT (2008) Transcription regulation through promoter-proximal pausing of RNA polymerase II. Science 319: 1791–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Price DH (2008) Poised polymerases: on your mark…get set…go!. Mol Cell 30: 7–10. [DOI] [PubMed] [Google Scholar]
- 10. Wade JT, Struhl K (2008) The transition from transcriptional initiation to elongation. Curr Opin Genet Dev 18: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Selth LA, Sigurdsson S, Svejstrup JQ (2010) Transcript Elongation by RNA Polymerase II. Annu Rev Biochem 79: 271–293. [DOI] [PubMed] [Google Scholar]
- 12. Buratowski S (2009) Progression through the RNA polymerase II CTD cycle. Mol Cell 36: 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi X, Finkelstein A, Wolf AJ, Wade PA, Burton ZF, et al. (1996) Paf1p, an RNA polymerase II-associated factor in Saccharomyces cerevisiae, may have both positive and negative roles in transcription. Mol Cell Biol 16: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi X, Chang M, Wolf AJ, Chang CH, Frazer-Abel AA, et al. (1997) Cdc73p and Paf1p are found in a novel RNA polymerase II-containing complex distinct from the Srbp-containing holoenzyme. Mol Cell Biol 17: 1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mueller CL, Jaehning JA (2002) Ctr9, Rtf1, and Leo1 are components of the Paf1/RNA polymerase II complex. Mol Cell Biol 22: 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krogan NJ, Kim M, Ahn SH, Zhong G, Kobor MS, et al. (2002) RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol Cell Biol 22: 6979–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu B, Mandal SS, Pham AD, Zheng Y, Erdjument-Bromage H, et al. (2005) The human PAF complex coordinates transcription with events downstream of RNA synthesis. Genes Dev 19: 1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jaehning JA (2010) The Paf1 complex: platform or player in RNA polymerase II transcription? Biochim Biophys Acta 1799: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ng HH, Robert F, Young RA, Struhl K (2003) Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol Cell 11: 709–719. [DOI] [PubMed] [Google Scholar]
- 20. Krogan NJ, Kim M, Tong A, Golshani A, Cagney G, et al. (2003) Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol Cell Biol 23: 4207–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Squazzo SL, Costa PJ, Lindstrom DL, Kumer KE, Simic R, et al. (2002) The Paf1 complex physically and functionally associates with transcription elongation factors in vivo. EMBO J 21: 1764–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim J, Guermah M, Roeder RG (2010) The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell 140: 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bai X, Kim J, Yang Z, Jurynec MJ, Akie TE, et al. (2010) TIF1gamma controls erythroid cell fate by regulating transcription elongation. Cell 142: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crisucci EM, Arndt KM (2011) The Paf1 complex represses ARG1 transcription in Saccharomyces cerevisiae by promoting histone modifications. Eukaryot Cell 10: 712–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pruneski JA, Hainer SJ, Petrov KO, Martens JA (2011) The Paf1 complex represses SER3 transcription in Saccharomyces cerevisiae by facilitating intergenic transcription-dependent nucleosome occupancy of the SER3 promoter. Eukaryot Cell 10: 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Youn MY, Yoo HS, Kim MJ, Hwang SY, Choi Y, et al. (2007) hCTR9, a component of Paf1 complex, participates in the transcription of interleukin 6-responsive genes through regulation of STAT3-DNA interactions. J Biol Chem 282: 34727–34734. [DOI] [PubMed] [Google Scholar]
- 27. Malek SN, Yang CH, Earnshaw WC, Kozak CA, Desiderio S (1996) p150TSP, a conserved nuclear phosphoprotein that contains multiple tetratricopeptide repeats and binds specifically to SH2 domains. J Biol Chem 271: 6952–6962. [DOI] [PubMed] [Google Scholar]
- 28. Shema E, Kim J, Roeder RG, Oren M (2011) RNF20 inhibits TFIIS-facilitated transcriptional elongation to suppress pro-oncogenic gene expression. Mol Cell 42: 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang E, Lerner L, Besser D, Darnell JE Jr (2003) Independent and cooperative activation of chromosomal c-fos promoter by STAT3. J Biol Chem 278: 15794–15799. [DOI] [PubMed] [Google Scholar]
- 30. Mueller CL, Porter SE, Hoffman MG, Jaehning JA (2004) The Paf1 complex has functions independent of actively transcribing RNA polymerase II. Mol Cell 14: 447–456. [DOI] [PubMed] [Google Scholar]
- 31. Koch C, Wollmann P, Dahl M, Lottspeich F (1999) A role for Ctr9p and Paf1p in the regulation G1 cyclin expression in yeast. Nucleic Acids Res 27: 2126–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Penheiter KL, Washburn TM, Porter SE, Hoffman MG, Jaehning JA (2005) A posttranscriptional role for the yeast Paf1-RNA polymerase II complex is revealed by identification of primary targets. Mol Cell 20: 213–223. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Y, Sikes ML, Beyer AL, Schneider DA (2009) The Paf1 complex is required for efficient transcription elongation by RNA polymerase I. Proc Natl Acad Sci U S A 106: 2153–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fort P, Rech J, Vie A, Piechaczyk M, Bonnieu A, et al. (1987) Regulation of c-fos gene expression in hamster fibroblasts: initiation and elongation of transcription and mRNA degradation. Nucleic Acids Res 15: 5657–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blanchard JM, Piechaczyk M, Fort P, Bonnieu A, Mechti N, et al. (1988) The regulatory strategies of c-myc and c-fos proto-oncogenes share some common mechanisms. Biochimie 70: 877–884. [DOI] [PubMed] [Google Scholar]
- 36. Peterlin BM, Price DH (2006) Controlling the elongation phase of transcription with P-TEFb. Mol Cell 23: 297–305. [DOI] [PubMed] [Google Scholar]
- 37. Fujita T, Piuz I, Schlegel W (2009) Negative elongation factor NELF controls transcription of immediate early genes in a stimulus-specific manner. Exp Cell Res 315: 274–284. [DOI] [PubMed] [Google Scholar]
- 38. Renner DB, Yamaguchi Y, Wada T, Handa H, Price DH (2001) A highly purified RNA polymerase II elongation control system. J Biol Chem 276: 42601–42609. [DOI] [PubMed] [Google Scholar]
- 39. Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, et al. (1999) NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell 97: 41–51. [DOI] [PubMed] [Google Scholar]
- 40. Li B, Howe L, Anderson S, Yates JR 3rd, Workman JL (2003) The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J Biol Chem 278: 8897–8903. [DOI] [PubMed] [Google Scholar]
- 41. Gilchrist DA, Nechaev S, Lee C, Ghosh SK, Collins JB, et al. (2008) NELF-mediated stalling of Pol II can enhance gene expression by blocking promoter-proximal nucleosome assembly. Genes Dev 22: 1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krishnan K, Salomonis N, Guo S (2008) Identification of Spt5 target genes in zebrafish development reveals its dual activity in vivo. PLoS One 3: e3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen Y, Yamaguchi Y, Tsugeno Y, Yamamoto J, Yamada T, et al. (2009) DSIF, the Paf1 complex, and Tat-SF1 have nonredundant, cooperative roles in RNA polymerase II elongation. Genes Dev 23: 2765–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wade PA, Werel W, Fentzke RC, Thompson NE, Leykam JF, et al. (1996) A novel collection of accessory factors associated with yeast RNA polymerase II. Protein Expr Purif 8: 85–90. [DOI] [PubMed] [Google Scholar]
- 45. Jones JC, Phatnani HP, Haystead TA, MacDonald JA, Alam SM, et al. (2004) C-terminal repeat domain kinase I phosphorylates Ser2 and Ser5 of RNA polymerase II C-terminal domain repeats. J Biol Chem 279: 24957–24964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Phatnani HP, Jones JC, Greenleaf AL (2004) Expanding the functional repertoire of CTD kinase I and RNA polymerase II: novel phosphoCTD-associating proteins in the yeast proteome. Biochemistry 43: 15702–15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takahashi A, Tsutsumi R, Kikuchi I, Obuse C, Saito Y, et al. (2011) SHP2 tyrosine phosphatase converts parafibromin/Cdc73 from a tumor suppressor to an oncogenic driver. Mol Cell 43: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overexpression of PAFc components did not affect c-Fos expression A . The Myc-CTR9 expression vector was transfected to HepG2 cells. Approximately 48 hours later, cells were treated with IL-6 plus IL-6sR (20 ng/ml each) for the indicated length of time. Left, western blot assay was performed to detect exogenous Myc-CTR9. Right, c-Fos mRNA levels were measured by RT-qPCR analysis. Expression levels were normalized to β-Actin. B. Cells were transfected with Myc-Paf1 or Myc-Cdc73. 48 hours later, cells were treated with IL-6 plus IL-6sR (20 ng/ml each) for 1 hour and PAF1, CDC73 and GAPDH proteins levels were analyzed by westernblot analysis using endogenous antibodies. c-Fos mRNA levels were measured by RT-qPCR analysis.
(EPS)
Top, diagram of the Hp genomic loci. PCR primers used for ChIP analyses are shown as two-sided arrows. HepG2 cells were treated with IL-6 plus IL-6sR (20 ng/ml each) for the indicated length of time. Soluble chromatins were immunoprecipitated with anti-CTR9 antibodies, and the bound DNAs were analyzed by quantitative PCR using primers specific to Hp loci. Error bars represents SD (n = 3).
(EPS)
Primer sequences used for PCR experiments.
(DOC)


