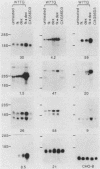
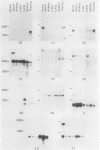
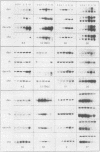
Abstract
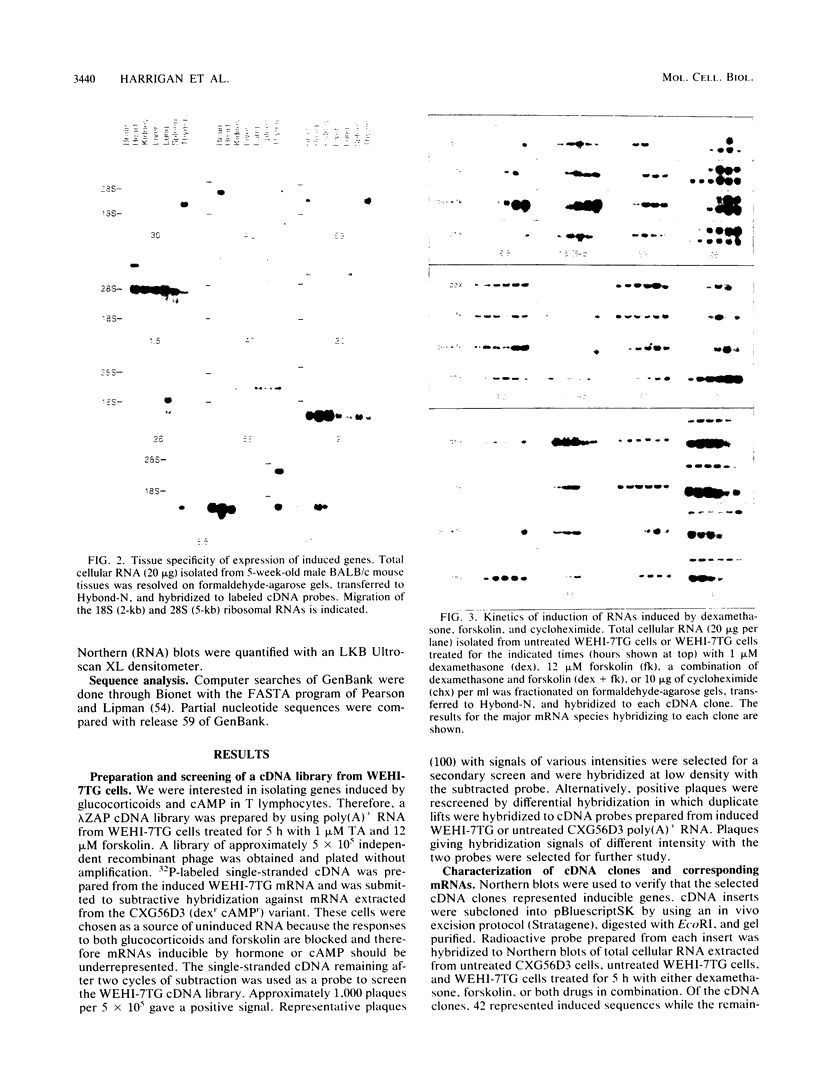
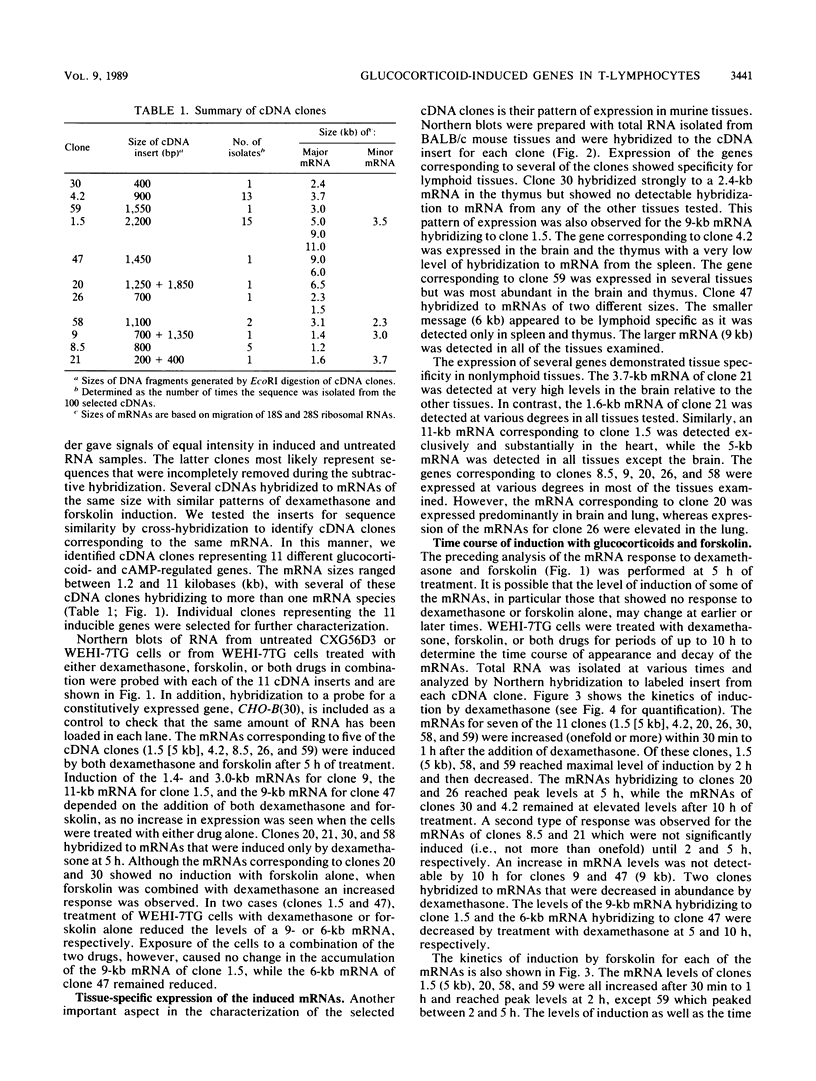
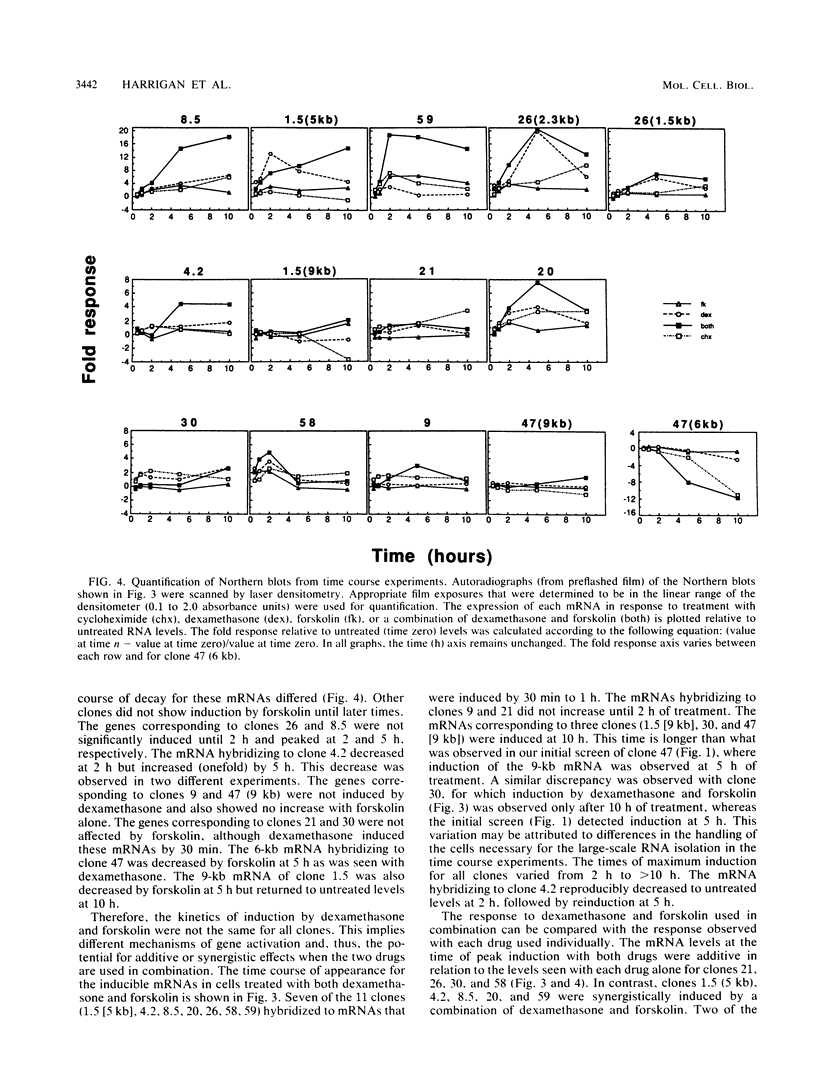
Glucocorticoids and cyclic AMP exert dramatic effects on the proliferation and viability of murine T lymphocytes through unknown mechanisms. To identify gene products which might be involved in glucocorticoid-induced responses in lymphoid cells, we constructed a lambda cDNA library prepared from murine thymoma WEHI-7TG cells treated for 5 h with glucocorticoids and forskolin. The library was screened with a subtracted cDNA probe enriched for sequences induced by the two drugs, and cDNA clones representing 11 different inducible genes were isolated. The pattern of expression in BALB/c mouse tissues was examined for each cDNA clone. We have identified two clones that hybridized to mRNAs detected exclusively in the thymus. Other clones were identified that demonstrated tissue-specific gene expression in heart, brain, brain and thymus, or lymphoid tissue (spleen and thymus). The kinetics of induction by dexamethasone and forskolin were examined for each gene. The majority of the cDNA clones hybridized to mRNAs that were regulated by glucocorticoids and forskolin, two were regulated only by glucocorticoids, and three hybridized to mRNAs that required both drugs for induction. Inhibition of protein synthesis by cycloheximide resulted in the induction of all mRNAs that were inducible by glucocorticoids. Preliminary sequence analysis of four of the 11 cDNAs suggests that two cDNAs represent previously undescribed genes while two others correspond to the mouse VL30 retrovirus-like element and the mouse homolog of chondroitin sulfate proteoglycan core protein.
Full text
PDF
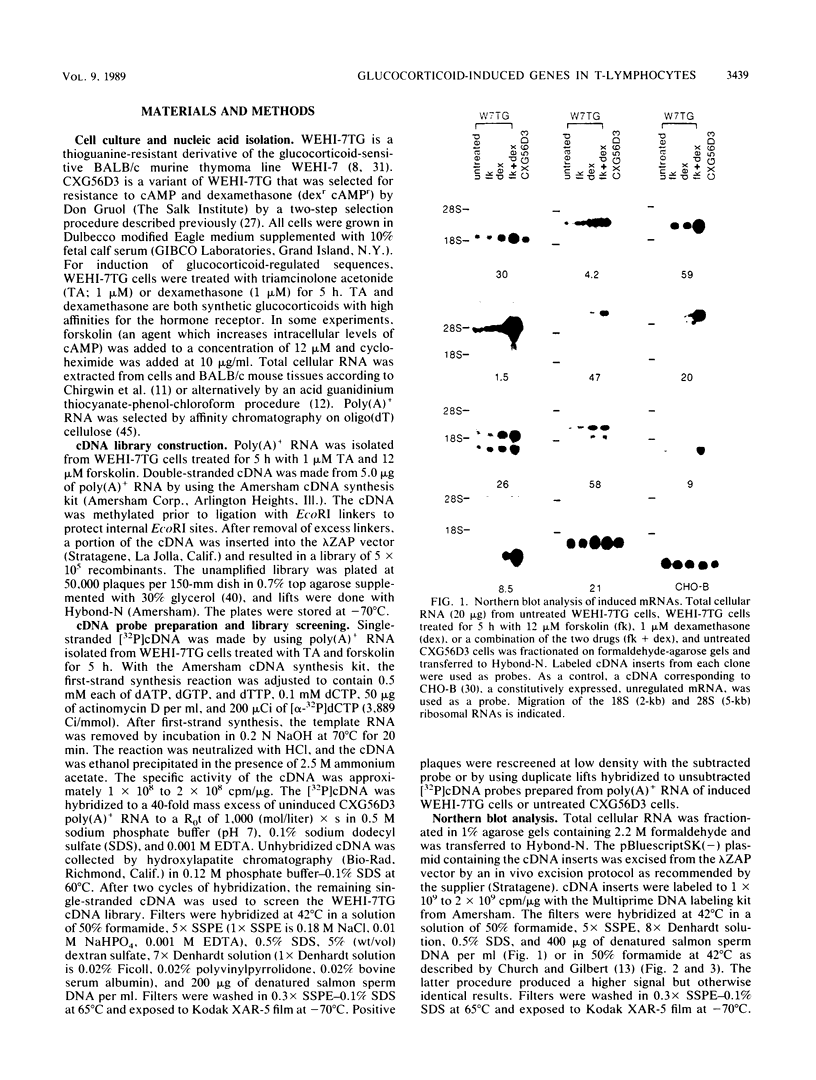




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. E., Rathjen P. D., Stanway C. A., Fulton S. M., Malim M. H., Wilson W., Ogden J., King L., Kingsman S. M., Kingsman A. J. Complete nucleotide sequence of a mouse VL30 retro-element. Mol Cell Biol. 1988 Aug;8(8):2989–2998. doi: 10.1128/mcb.8.8.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahoshi T., Oppenheim J. J., Matsushima K. Induction of high-affinity interleukin 1 receptor on human peripheral blood lymphocytes by glucocorticoid hormones. J Exp Med. 1988 Mar 1;167(3):924–936. doi: 10.1084/jem.167.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour K. W., Berger S. H., Berger F. G., Thompson E. A., Jr Glucocorticoid regulation of the genes encoding thymidine kinase, thymidylate synthase, and ornithine decarboxylase in P1798 cells. Mol Endocrinol. 1988 Jan;2(1):78–84. doi: 10.1210/mend-2-1-78. [DOI] [PubMed] [Google Scholar]
- Beale E. G., Hartley J. L., Granner D. K. N6,O2'-dibutyryl cycle AMP and glucose regulate the amount of messenger RNA coding for hepatic phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1982 Feb 25;257(4):2022–2028. [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bourdon M. A., Shiga M., Ruoslahti E. Identification from cDNA of the precursor form of a chondroitin sulfate proteoglycan core protein. J Biol Chem. 1986 Sep 25;261(27):12534–12537. [PubMed] [Google Scholar]
- Bourgeois S., Newby R. F. Diploid and haploid states of the glucocorticoid receptor gene of mouse lymphoid cell lines. Cell. 1977 Jun;11(2):423–430. doi: 10.1016/0092-8674(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Vedeckis W. V. Glucocorticoid effects on thymidine incorporation into the DNA of S49 lymphoma cells. J Steroid Biochem. 1983 Jun;18(6):691–698. doi: 10.1016/0022-4731(83)90247-9. [DOI] [PubMed] [Google Scholar]
- Carter A. T., Norton J. D., Avery R. J. A novel approach to cloning transcriptionally active retrovirus-like genetic elements from mouse cells. Nucleic Acids Res. 1983 Sep 24;11(18):6243–6254. doi: 10.1093/nar/11.18.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cidlowski J. A. Glucocorticoids stimulate ribonucleic acid degradation in isolated rat thymic lymphocytes in vitro. Endocrinology. 1982 Jul;111(1):184–190. doi: 10.1210/endo-111-1-184. [DOI] [PubMed] [Google Scholar]
- Cimbala M. A., Lamers W. H., Nelson K., Monahan J. E., Yoo-Warren H., Hanson R. W. Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney. Effects of insulin and cyclic AMP. J Biol Chem. 1982 Jul 10;257(13):7629–7636. [PubMed] [Google Scholar]
- Coffino P., Gray J. W., Tomkins G. M. Cyclic AMP, a nonessential regulator of the cell cycle. Proc Natl Acad Sci U S A. 1975 Mar;72(3):878–882. doi: 10.1073/pnas.72.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Colbert R. A., Young D. A. Glucocorticoid-mediated induction of glucocortin: a rapid primary response common to major target tissues. Proc Natl Acad Sci U S A. 1986 Jan;83(1):72–76. doi: 10.1073/pnas.83.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote G. J., Gagel R. F. Dexamethasone differentially affects the levels of calcitonin and calcitonin gene-related peptide mRNAs expressed in a human medullary thyroid carcinoma cell line. J Biol Chem. 1986 Nov 25;261(33):15524–15528. [PubMed] [Google Scholar]
- Daniel V., Litwack G., Tomkins G. M. Induction of cytolysis of cultured lymphoma cells by adenosine 3':5'-cyclic monophosphate and the isolation of resistant variants. Proc Natl Acad Sci U S A. 1973 Jan;70(1):76–79. doi: 10.1073/pnas.70.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. C., Newby R. F., Bourgeois S. Regulation of fibronectin biosynthesis by dexamethasone, transforming growth factor beta, and cAMP in human cell lines. J Cell Biol. 1988 Jun;106(6):2159–2170. doi: 10.1083/jcb.106.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelhorst C. W. Glucocorticosteroids induce DNA fragmentation in human lymphoid leukemia cells. Blood. 1988 Oct;72(4):1305–1309. [PubMed] [Google Scholar]
- Eastman-Reks S. B., Vedeckis W. V. Glucocorticoid inhibition of c-myc, c-myb, and c-Ki-ras expression in a mouse lymphoma cell line. Cancer Res. 1986 May;46(5):2457–2462. [PubMed] [Google Scholar]
- Gasson J. C., Bourgeois S. A new determinant of glucocorticoid sensitivity in lymphoid cell lines. J Cell Biol. 1983 Feb;96(2):409–415. doi: 10.1083/jcb.96.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J. C., Ryden T., Bourgeois S. Role of de novo DNA methylation in the glucocorticoid resistance of a T-lymphoid cell line. Nature. 1983 Apr 14;302(5909):621–623. doi: 10.1038/302621a0. [DOI] [PubMed] [Google Scholar]
- Gruol D. J., Campbell N. F., Bourgeois S. Cyclic AMP-dependent protein kinase promotes glucocorticoid receptor function. J Biol Chem. 1986 Apr 15;261(11):4909–4914. [PubMed] [Google Scholar]
- Harmon J. M., Norman M. R., Fowlkes B. J., Thompson E. B. Dexamethasone induces irreversible G1 arrest and death of a human lymphoid cell line. J Cell Physiol. 1979 Feb;98(2):267–278. doi: 10.1002/jcp.1040980203. [DOI] [PubMed] [Google Scholar]
- Harmon J. M., Thompson E. B. Glutamine synthetase induction by glucocorticoids in the glucocorticoid-sensitive human leukemic cell line CEM-C7. J Cell Physiol. 1982 Feb;110(2):155–160. doi: 10.1002/jcp.1041100208. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Harris A. W., Bankhurst A. D., Mason S., Warner N. L. Differentiated functions expressed by cultured mouse lymphoma cells. II. Theta antigen, surface immunoglobulin and a receptor for antibody on cells of a thymoma cell line. J Immunol. 1973 Feb;110(2):431–438. [PubMed] [Google Scholar]
- Higuchi H., Yang H. Y., Sabol S. L. Rat neuropeptide Y precursor gene expression. mRNA structure, tissue distribution, and regulation by glucocorticoids, cyclic AMP, and phorbol ester. J Biol Chem. 1988 May 5;263(13):6288–6295. [PubMed] [Google Scholar]
- Hoshikawa Y., Izawa M., Ichii S. Cloning of glucocorticoid-responsive mRNA in the rat thymus. Endocrinol Jpn. 1988 Jun;35(3):429–437. doi: 10.1507/endocrj1954.35.429. [DOI] [PubMed] [Google Scholar]
- Itin A., Keshet E. Diverse long terminal repeats are associated with murine retroviruslike (VL30) elements. Mol Cell Biol. 1986 Apr;6(4):1276–1282. doi: 10.1128/mcb.6.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaghad M., Maillet L., Brûlet P. Retroviral characteristics of the long terminal repeat of murine E.Tn sequences. EMBO J. 1985 Nov;4(11):2911–2915. doi: 10.1002/j.1460-2075.1985.tb04022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser N., Bourne H. R., Insel P. A., Coffino P. Regulation of phosphodiesterase and ornithine decarboxylase by cAMP is cell cycle independent. J Cell Physiol. 1979 Dec;101(3):369–374. doi: 10.1002/jcp.1041010304. [DOI] [PubMed] [Google Scholar]
- Kaiser N., Edelman I. S. Calcium dependence of glucocorticoid-induced lymphocytolysis. Proc Natl Acad Sci U S A. 1977 Feb;74(2):638–642. doi: 10.1073/pnas.74.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Itin A. Patterns of genomic distribution and sequence heterogeneity of a murine "retrovirus-like" multigene family. J Virol. 1982 Jul;43(1):50–58. doi: 10.1128/jvi.43.1.50-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Shaul Y., Kaminchik J., Aviv H. Heterogeneity of "virus-like" genes encoding retrovirus-associated 30S RNA and their organization within the mouse genome. Cell. 1980 Jun;20(2):431–439. doi: 10.1016/0092-8674(80)90629-7. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Cohen D. I. Preserving primary cDNA libraries. Anal Biochem. 1987 Feb 15;161(1):85–88. doi: 10.1016/0003-2697(87)90655-5. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D., Ho P. L. Induction of chondroitin sulfate proteoglycan synthesis and secretion in lymphocytes and monocytes. J Cell Biol. 1983 Aug;97(2):351–358. doi: 10.1083/jcb.97.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D., Olmstead L. B-cell stimulation by T-cell-secreted proteoglycan. Cell Immunol. 1987 Dec;110(2):425–430. doi: 10.1016/0008-8749(87)90134-1. [DOI] [PubMed] [Google Scholar]
- MacDonald R. G., Martin T. P., Cidlowski J. A. Glucocorticoids stimulate protein degradation in lymphocytes: a possible mechanism of steroid-induced cell death. Endocrinology. 1980 Nov;107(5):1512–1524. doi: 10.1210/endo-107-5-1512. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Duddy S. K., Håkansson H., Orrenius S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Science. 1988 Oct 14;242(4876):256–259. doi: 10.1126/science.3262923. [DOI] [PubMed] [Google Scholar]
- McDonald A. R., Goldfine I. D. Glucocorticoid regulation of insulin receptor gene transcription in IM-9 cultured lymphocytes. J Clin Invest. 1988 Feb;81(2):499–504. doi: 10.1172/JCI113347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A. Metabolic site and time course of cortisol action on glucose uptake, lactic acid output, and glucose 6-phosphate levels of rat thymus cells in vitro. J Biol Chem. 1968 Mar 10;243(5):1039–1042. [PubMed] [Google Scholar]
- Nakamura T., Niimi S., Nawa K., Noda C., Ichihara A., Takagi Y., Anai M., Sakaki Y. Multihormonal regulation of transcription of the tryptophan 2,3-dioxygenase gene in primary cultures of adult rat hepatocytes with special reference to the presence of a transcriptional protein mediating the action of glucocorticoids. J Biol Chem. 1987 Jan 15;262(2):727–733. [PubMed] [Google Scholar]
- Nawa K., Nakamura T., Kumatori A., Noda C., Ichihara A. Glucocorticoid-dependent expression of the albumin gene in adult rat hepatocytes. J Biol Chem. 1986 Dec 25;261(36):16883–16888. [PubMed] [Google Scholar]
- Nebes V. L., Morris S. M., Jr Regulation of messenger ribonucleic acid levels for five urea cycle enzymes in cultured rat hepatocytes. Requirements for cyclic adenosine monophosphate, glucocorticoids, and ongoing protein synthesis. Mol Endocrinol. 1988 May;2(5):444–451. doi: 10.1210/mend-2-5-444. [DOI] [PubMed] [Google Scholar]
- Russo A. F., Nelson C., Roos B. A., Rosenfeld M. G. Differential regulation of the coexpressed calcitonin/alpha-CGRP and beta-CGRP neuroendocrine genes. J Biol Chem. 1988 Jan 5;263(1):5–8. [PubMed] [Google Scholar]
- Scollay R., Shortman K. Thymocyte subpopulations: an experimental review, including flow cytometric cross-correlations between the major murine thymocyte markers. Thymus. 1983 Sep;5(5-6):245–295. [PubMed] [Google Scholar]
- Shapiro D. J., Blume J. E., Nielsen D. A. Regulation of messenger RNA stability in eukaryotic cells. Bioessays. 1987 May;6(5):221–226. doi: 10.1002/bies.950060507. [DOI] [PubMed] [Google Scholar]
- Vedeckis W. V., Bradshaw H. D., Jr DNA fragmentation in S49 lymphoma cells killed with glucocorticoids and other agents. Mol Cell Endocrinol. 1983 May;30(2):215–227. doi: 10.1016/0303-7207(83)90049-7. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wynshaw-Boris A., Short J. M., Hanson R. W. Regulation of gene transcription by multiple hormones: organization of regulatory elements. Prog Nucleic Acid Res Mol Biol. 1987;34:59–87. doi: 10.1016/s0079-6603(08)60493-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Sabol S. L. Glucocorticoids and cyclic AMP synergistically regulate the abundance of preproenkephalin messenger RNA in neuroblastoma-glioma hybrid cells. Biochem Biophys Res Commun. 1986 Aug 29;139(1):1–10. doi: 10.1016/s0006-291x(86)80071-7. [DOI] [PubMed] [Google Scholar]
- Yuh Y. S., Thompson E. B. Complementation between glucocorticoid receptor and lymphocytolysis in somatic cell hybrids of two glucocorticoid-resistant human leukemic clonal cell lines. Somat Cell Mol Genet. 1987 Jan;13(1):33–45. doi: 10.1007/BF02422297. [DOI] [PubMed] [Google Scholar]