Abstract
Background
Strong associations exist between tumor necrosis factor-α (TNF-α) and metabolic syndrome (MetS). While TNF-α is associated with Bipolar depression, its role in atypical antipsychotic (AAP) associated MetS in Bipolar Disorder (BD) is unclear. Here we investigate the potential intervening role TNF-α in the indirect relationship between AAP treatment and MetS in BD.
Materials and Methods
Using a cross-sectional design, 99 euthymic BD volunteers were stratified by presence/absence of MetS (NCEP-ATP-III). Serum TNF-α concentration, determined via chemiluminescent immunometric assays, was compared between groups (i.e. MetS or no MetS). We investigated the intervening effect of TNF-α on the relation between AAP treatment and MetS in BD using regression techniques.
Results
Treatment with antipsychotics believed associated with a higher risk for MetS (i.e. AAPs; olanzapine, quetiapine, risperidone, paliperidone, clozapine), was found to be associated with significantly greater TNF-α (F1,88=11.2, p=0.001, mean difference of 1.7 +/− 0.51) and a higher likelihood of MetS (F1,88=4.5, p=0.036) than in those not receiving treatment with an AAP. Additionally, TNF-α was greater (trending towards significance; T52=2.0, p=0.05) in BD volunteers with MetS and was found to have a statistically significant effect on the indirect relationship between AAP treatment and elevated waist circumference in these BD volunteers.
Discussion
These results identify TNF-α as a potential intervening variable of AAP associated MetS in BD, not previously identified in this population. Future prospective studies could assess the predictive potential of TNF-α in determining risk of AAP associated MetS in BD. Given previous evidence relating TNF-α and mood state in BD, this study increases the importance in understanding the role of TNF-α in “mind-body” interactions and renews discussions of the utility of research into the clinical efficacy of TNF-α antagonist treatment in mood disorders.
Keywords: cytokines, psychoneuroimmunology, bipolar disorder, metabolic syndrome, mediation, atypical antipsychotic, tumor necrosis factor
Background
Concurrent with an increased use of atypical antipsychotic medications (AAPs) as mood stabilizers in bipolar disorder has been a growth in incidence of metabolic syndrome [1,2,3]. While existing evidence implicates AAP treatment in the emergence of obesity and hyperglycemia (i.e. Metabolic syndrome parameters), these investigations are largely confined to samples with schizophrenia[4] contributing little to the mechanistic understanding of these associations, particularly in bipolar disorder. Investigating these relationships in samples with bipolar disorder is of great importance given the increased prescription of AAPs as mood stabilizing alternatives to traditional agents (i.e. valproate, lithium, carbamazepine, lamotrigine) in bipolar disorder. Clinical psychiatric practice would benefit from greater differentiation of the metabolic syndrome risks associated with these two broad classes of medications (i.e. AAPs vs. traditional mood stabilizers). Furthermore, enhanced mechanistic understanding of the mediators between AAP treatment and Metabolic syndrome parameters (i.e. obesity, hyperglycemia) will serve as a foundation for clinical advancement (i.e. biological risk stratification, novel patient tailored treatment approaches, etc.) permitting increased personalization of medication management in bipolar disorder.
Metabolic syndrome, an objective characterization of cardiovascular risk factors, is reaching epidemic proportion in America[5,6] affecting 20–24% of the general population[7]. Individuals with bipolar disorder are at greater risk of metabolic syndrome than the general population[1,2] with rates similar to those reported with schizophrenia[8]. The diagnostic criteria outlined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP-III) recommends metabolic syndrome be identified in an individual with 3 or more of the following risks factors: increased waist circumference, elevated triglycerides, reduced high density lipoprotein (HDL), elevated blood pressure, and elevated fasting glucose[5,6]. Consequent to these factors are the increased morbidity and mortality associated with cardiovascular illness and diabetes[5,6]. The morbidity and mortality associated with metabolic syndrome is further increased in the setting of co-morbid bipolar disorder with up to 70% of the treatment costs associated with bipolar disorder, being attributed to the management of cardiovascular morbidity[9].
Evidence implicating certain AAP medications for increasing the likelihood of developing metabolic syndrome[4,10,11] has resulted in an FDA issued AAP class warning in schizophrenia. However, in a pivotal study that has addressed these issues, certain AAPs (i.e. Olanzapine, Quetiapine, Risperidone, Clozapine) had a greater association with metabolic syndrome than others (i.e. Ziprazidone) [4]. However, research in this area (i.e. relation between AAP treatment and Metabolic syndrome) has disproportionately focused on AAP treatment of schizophrenia[4]. Concurrent with the increased clinical prescription of AAPs for their mood stabilizing potential is an increased prevalence of abdominal obesity and hyperglycemia in patients with bipolar disorder. While such research is only beginning to emerge in bipolar disorder populations[11,12,13,14,15,16], this clinically apparent association between increased AAP treatment and the evolving medical epidemic of metabolic syndrome in bipolar disorder, further underscores the need for more research in this area.
A number of biological variables, including pro-inflammatory cytokines, have been investigated for their role in the development of metabolic syndrome within the general population[17,18]. Tumor Necrosis Factor-α (TNF-α), a pro-inflammatory cytokine protein expressed by fat cells, is secreted in greater concentrations in patients with metabolic syndrome, inducing cellular changes (i.e. insulin resistance, hyperglycemia) upon binding with cell surface TNF-α receptors[19,20,21,22]. Existing research implicating TNF-α for its association with mood states in samples with bipolar disorder [23,24,25], and AAP associated metabolic syndrome in samples with schizophrenia[26], provides the basic rationale for investigating the mechanistic role of TNF-α in AAP associated Metabolic syndrome in a sample with bipolar disorder. [5,6]. Using a cross sectional design, we investigated the role of plasma TNF-α as an intervening variable of the indirect relation between AAPs and Metabolic syndrome in a sample with bipolar disorder. It was believed that TNF-α would play a mediating role in the relations between AAP treatment and Metabolic syndrome via its previously reported relationship with waist circumference, one of the main parameters of metabolic syndrome.
Methods and Materials
Participants included 99 volunteers with a prior diagnosis of Bipolar Disorder type I, recruited via advertisements in mental health clinics and via pamphlets distributed in an academic medical setting. Subjects met the following inclusion criteria: 1) DSM-IV diagnosis of bipolar disorder type I (having had at least one prior primary [i.e. not attributable to other causes] manic and/or mixed episode) and 2) Age 18–90 years. Exclusion criteria included 1) unwillingness to participate, 2) unable to give informed consent (assessed by our study personnel using a short questionnaire asking key questions about the study), 3) medical records documenting type II diabetes before antipsychotic use, or 4) active substance use disorder. In an effort to provide a “real world” estimate of medication use, participants were not restricted to specific psychiatric medication use. Clozapine is prescribed by clinicians “off-label” in the “real world” treatment of Bipolar Disorder, particularly when multiple other treatment trials have failed to yield significant benefit. While we do not endorse its use in the treatment of Bipolar Disorder, we believe that inclusion of clozapine treated patients in our study could potentially lead to more generalizable study findings. A thorough medication use history was obtained concurrent with metabolic assessments and blood sampling. This study was approved by the University of Michigan Institutional Review Board and carried out in accordance with the Declaration of Helsinki as revised in 1989. Informed consent was obtained from all study participants. Participants completed the Beck Depression Inventory (BDI)[27] and the Hamilton Depression Rating Scale (HAM-D)[28] at entry into the study, concurrent with time of blood sampling.
As part of the study enrollment process, participants were assessed by medical research personnel of the University of Michigan Clinical Research Unit (MCRU) obtaining various physical measures (i.e. height, weight, waist circumference, hip circumference, heart rate, blood pressure, physical activity (Total Activity Measure 2; TAM2)[29], medical co-morbidities, total number and duration of most recent psychiatric hospitalizations, number of suicide attempts, as well as both current and prior medications). Blood was drawn from patients for assessment of multiple fasting laboratory evaluations (i.e. glucose, high density lipoprotein (HDL), triglycerides, total cholesterol, low density lipoprotein (LDL), TNF-α). Time of blood draws were consistent across the study sample, occurring within 3 hours of the participants’ normal awakening time. Following the NCEP-ATP-III recommendations, the presence of metabolic syndrome was identified in an individual with 3 or more of the following risks factors: increased waist circumference, elevated triglycerides, reduced HDL, elevated blood pressure, and/or elevated fasting glucose[5,6]. The number of criteria for metabolic syndrome that were met by each individual study subject was recorded.
Blood samples were collected from participants by the MCRU staff and centrifuged for 15 minutes at 4750 rpm. Serum was extracted, aliquotted, and the resulting serum samples frozen at −80° C until the time of assaying. Samples are analyzed on an Immulite Analyzer (Siemens Healthcare Diagnostics Inc., SHD) using a solid-phase chemiluminescent immunometric assay (SHD). Sample is added to a test unit containing a bead coated with murine monoclonal anti-TNF-α antibody. Reagent containing alkaline phosphatase conjugated to rabbit polyclonal anti-TNF-α is added to the test unit. Following incubation, a chemiluminescent substrate is added and the fluorescence measured. Fluorescence is directly proportional to the TNF-α concentration of the sample. Calibration range is 4–1000 pg/ml. Intra-assay precision is 3% at 96 pg/ml and 2% at 548 pg/ml. External quality control samples are completed with all of the assays run through the MDRTC. This work utilized the Chemistry Core of the Michigan Diabetes Research and Training Center (MDRTC) funded by DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases. Other medical lab results (i.e. HDL, LDL, triglycerides, glucose) were obtained from blood samples (obtained by MCRU staff concurrent with above samples) provided to MDRTC.
The distribution of TNF-alpha was in keeping with that of a “normal” or “Gaussian” distribution and as such, normalization procedures were not employed. Raw TNF-alpha data was used in all analyses. Raw TNF-α results were used in the study analyses. Using PASW Statistics software version 18.0 (SPSS Inc., Chicago, IL), these values were used to plot the data, rule out the presence of outliers and perform additional statistical analyses. Data are expressed as mean ± S.D. in text and tables, and mean ± S.E.M. in Figures. Gender was not constrained, but given the evidence of sex differences in inflammatory processes[30], was used as a covariate in post-hoc analyses (see below).
Included in the group defined as atypical antipsychotics (AAPs) were those atypical antipsychotic medications previously shown to present the greatest risk of metabolic syndrome in Schizophrenia illness including olanzapine, quetiapine, risperidone, and clozapine[4,10,11,31]. Doses equivalent to 100 mg of clorpromazine (CPZ) per day were calculated for each of the AAPs with results as follows: 5 mg/day for olanzapine, 75 mg/day for quetiapine, and 2 mg/day for risperidone[32,33]. However, clozapine was left out of this portion of the analyses due to the paucity of clozapine treated participants (n=3).
Metabolic syndrome variables of interest (i.e. TNF-α, waist circumference, hip/waist ratio, cholesterol, triglycerides, and glucose) were identified based on a priori hypotheses and used in subsequent analyses. Planned analyses included multivariate analyses of variance (MANOVA) to examine relationships between TNF-α values across metabolic syndrome diagnostic and treatment groups. Statistical significance was calculated using a statistical threshold that controls for a Type-I error rate at p = 0.05. Results of MANOVA (and post-hoc) testing shown are corrected for multiple comparisons using automated Bonferroni techniques provided within the MANOVA function of the PASW Statistics software (version 18.0, SPSS Inc., Chicago, IL). PASW Statistics software was used to complete the statistical analyses. Mediation models were completed using previously published algorythms provided by Preacher and Hayes as scripts for use within PASW Statistics software [34,35,38,39].
Exploratory, medication specific effects are uncorrected and are reported for reader interest. Simple mediation models[34] and a mediation model with two serial intervening variables [35] were employed to test more detailed a priori hypotheses as to the indirect effects of TNF-α and waist circumference (two serial intervening variables) on the relationship between medication treatment, and glucose. We assume that multiple unknown variables intervene (either positively or negatively) in the indirect relationship between AAP and either waist circumference and/or glucose. Whether the summation of the indirect effects of all of these individual intervening variables is negligible or not, will determine whether the relationship between the independent and dependent variables is significant. Indeed, recent evidence justifies the investigation of indirect effects of an intervening variable in situations when no association is found between the dependent and independent variables[38,39]. Statistical significance was calculated using a statistical threshold that controls for a Type-I error rate at p = 0.05. Note that we acknowledge the potential limitations in testing for indirect effects of an intervening variable in a cross sectional design. While we believe the rationale for testing such effects in a cross-sectional study are valid, we do not infer that the results will necessarily be equivalent to those obtained from a longitudinal design.
Results
Subject Demographics
Of the 99 volunteers included in the analyses, 70.7 % were female, the mean age=43.7, standard deviation (SD) =12.1 years; and mean age at diagnosis=31.0, SD = 11.4 years. Participants were hospitalized a mean of 4.2 +/− 4.8 number of times, for a mean duration of 0.5 +/− 1.0 months, 55% reporting a history of one or more prior suicide attempt. Overall, 26 % of study participants met criteria for metabolic syndrome. Included in Table 1 are the average values of the individual metabolic syndrome parameters (identified as variables of interest in our analyses) among all study participants, those with metabolic syndrome, and those without metabolic syndrome. In some participants, criteria for 1 or 2 risk factors may have been met, but the participants failed to meet criteria for a third risk factor, thus were not identified as having metabolic syndrome. While 64 % of study subjects were identified as having a waist circumference within the range noted to be at risk for metabolic syndrome, 33 % evidenced glucose, triglyceride, and/or blood pressure risk criteria for metabolic syndrome. Only 18 % of study subjects met HDL risk criteria for metabolic syndrome.
Table 1. Average Values of Study Measures within the Study Population.
Table 1 outlines the average values of the various measures investigated in this study. Five broad columns are represented in this table. The first broad column lists the particular measures identified in the study. The second, third and fourth broad columns include data on mean, standard deviation, and number of subjects for each of the variables of interest within the respective group. The second broad column lists the value for the particular measure averaged across the study participants who have not met the overall criteria for metabolic syndrome (Metabolic syndrome). The third broad column lists the value for the particular measure averaged across the study participants who have met the overall criteria for metabolic syndrome (Metabolic syndrome). The fourth broad column lists the value for the particular measure averaged across the entire study population. The fifth broad column provides results from statistical comparisons (Mann Whitney U testing for all variables with the exception of TNF-α where the Student’s T-Test was used) between those participants receiving AAP treatment and those not receiving AAP treatment. Significance reported for p < 0.05. Trend towards significance reported for 1.0 > p > 0.05.
| Study Measures | No Metabolic Syndrome | Metabolic Syndrome | Total Study Population | Statistical Comparison (Metabolic Syndrome vs. No Metabolic Syndrome) |
|---|---|---|---|---|
| Mean +/− SD | Mean+/− SD | Mean+/− SD | ||
| Age (years) | 42.5 +/− 12.3 (n= 73) | 46.8 +/− 11.2 (n= 26) | 43.7 +/− 12.1 (n= 99) | p > 0.10 |
| Age at Diagnosis (years) | 30.4 +/− 11.3 (n= 73) | 33.0 +/− 11.6 (n= 26) | 31.0 +/− 11.4 (n= 99) | p > 0.10 |
| Number of Hospitalizations | 3.9 +/− 5.0 (n= 73) | 4.8 +/− 4.1 (n= 26) | 4.2 +/− 4.8 (n= 99) | P > 0.10 |
| Average Duration of Hospitalization (months) | 0.4 +/− 0.8 (n= 73) | 0.8 +/− 1.4 (n= 26) | 0.5 +/− 1.0 (n= 99) | Test Statistic = 1.8, p = 0.066 (trend towards significance) |
| TNF-α (pg/ml) | 8.9 +/− 2.4 (n= 70) | 9.9 +/− 2.0 (n= 26) | 9.2 +/− 2.3 (n= 96) | T94 = 2.0, p = 0.05 (trend towards significance) |
| Systolic Blood Pressure (mm Hg) | 119.5 +/− 15.4 (n= 72) | 134.0 +/− 20.0 (n= 26) | 123.0 +/− 18.0 (n= 98) | Test Statistic = 3.7, p < 0.001 (significant) |
| Diastolic Blood Pressure (mm Hg) | 72.6 +/− 11.7 (n= 72) | 76.0 +/− 10.1 (n= 26) | 73.0 +/− 11.0 (n= 98) | p > 0.10 |
| Waist Circumference (cm) | 99.0 +/− 18.0 (n= 70) | 112.0 +/− 9.8 (n= 26) | 102.5 +/− 17.2 (n= 96) | Test Statistic = 3.5, p = 0.001 (significant) |
| HDL (mg/dl) | 62.1 +/− 15.7 (n= 70) | 48.0 +/− 10.0 (n= 26) | 58.3 +/− 15.7 (n= 96) | Test Statistic = −4.4, p < 0.001 (significant) |
| Triglycerides (mg/dl) | 111.0 +/− 89.3 (n= 70) | 234.3 +/− 102.9 (n= 26) | 144.4 +/− 107.7 (n= 96) | Test Statistic = 5.6, p < 0.001 (significant) |
| Total Cholesterol (mg/dl) | 181.6 +/− 35.3 (n= 70) | 216.5 +/− 62.6 (n= 26) | 191.0 +/− 46.7 (n= 96) | Test Statistic = 2.5, p = 0.01 (significant) |
| Fasting Glucose (mg/dl) | 95.5 +/− 16.5 (n= 69) | 114.7 +/− 36.4 (n= 25) | 100.6 +/− 24.8 (n= 94) | Test Statistic = 3.7, p < 0.001 (significant) |
Description of Study Subjects’ Medications
Mood stabilizing treatments varied across participants with 72 % of participants being treated with classical mood stabilizing treatments (i.e. valproate, lithium, carbamazepine, lamotrigine) and 68 % being treated with AAPs. Combined treatment with both a mood stabilizer and an AAP medication was found in 46% of participants. Six% of participants were receiving neither antipsychotic nor mood stabilizing treatments. Roughly half (49%) of all study participants were receiving monotherapy for the mood stabilization of their bipolar disorder; the largest portion within the monotherapy group being treated with AAPs (46%). Within the monotherapy group, AAPs were the chosen treatments in 29% of participants. Mood stabilizers were the chosen treatments in 54% of participants on a monotherapy regimen. Treatment with a mood stabilizer did not appear to be associated with metabolic syndrome when looking at either the entire study population (Pearson Chi Square = 0.7, p = 0.404) or only those participants receiving monotherapy (Pearson Chi Square = 1.8, p = 0.177). However, a trend was identified whereby AAP treatment was related to the presence of metabolic syndrome when looking at both the entire study population (Pearson Chi Square = 3.2, p = 0.073) and those participants on monotherapy approaches (i.e. AAP monotherapy vs. mood stabilizer mood stabilizer monotherapy) (Pearson Chi Square = 4.4, p = 0.035).
When examining the entire study population, regardless of treatment regimen, participants treated with AAPs did not differ in their age, age of bipolar disorder diagnosis, number of hospitalizations, average duration of hospitalization, or history of suicide attempts from those not treated with AAPs (p > 0.05). Similarly, of those treated with AAPs, participants who met criteria for metabolic syndrome did not differ significantly in these variables from those who did not meet metabolic syndrome criteria (p > 0.05). However, in an attempt to remove the confound of polypharmacy, we investigated whether those participants receiving monotherapy AAP treatment differed with respect to these parameters from those receiving a polypharmacy treatment approach. Participants receiving AAP monotherapy had a history of fewer suicide attempts as compared to those treated with both AAPs and mood stabilizers (Mann Whitney U - Test Statistic= −2.1; p = 0.036), but no significant difference in likelihood of metabolic syndrome (p > 0.05) or in the other variables of interest (age, age of Bipolar diagnosis, number of hospitalizations, or average duration of hospitalization) (p > 0.05).
Relationship between TNF-α and metabolic syndrome
The average TNF-α concentration in the population studied was calculated to be 9.2 +/− 2.3 (pg/ml) with the average TNF-α for those participants with metabolic syndrome calculated to be 9.9 +/− 2.0 (pg/ml) and those without metabolic syndrome calculated to be 8.9 +/− 2.4 (pg/ml). Using a Student’s T-Test, we identified a trend whereby participants with metabolic syndrome had higher levels of TNF-α than those without metabolic syndrome (T52 = 2.0, p = 0.05) (see Figure 1).
Figure 1. Distribution of TNF-α by Diagnosis of Metabolic Syndrome.
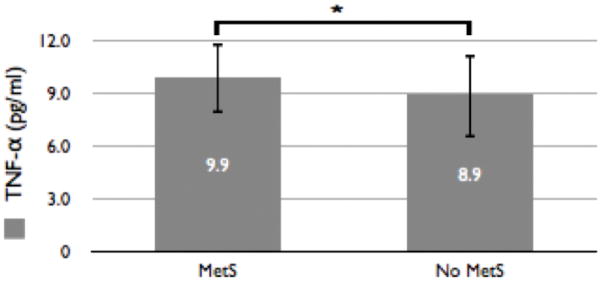
This figure outlines the differences in average TNF-α concentrations between those participants meeting criteria for metabolic syndrome and those without. The average TNF-α concentration in participants meeting criteria for metabolic syndrome (n = 26; 10.2 +/− 2.0 pg/ml) is higher (with results trending towards significance; T52=2.0, p=0.05) than in participants without metabolic syndrome (n = 70; 8.9 +/− 2.4 pg/ml).
Atypical Antipsychotics
In the entire study population, we tested (using the MANOVA test) whether treatment with antipsychotics believed to be associated with a higher risk for metabolic syndrome (i.e. AAPs; olanzapine, quetiapine, risperidone, paliperidone, clozapine), was associated with our dependent variables of interest (TNF-α, Metabolic Syndrome diagnosis, waist circumference, hip/waist ratio, HDL, triglycerides, glucose. Our model included one independent factors (AAP treatment). AAP treatment (without accounting for the particular AAP medication) was found to be associated with significantly greater TNF-α (F1,84 = 7.9, p = 0.006; mean difference of 1.4 +/− 0.5) (see figure 2) and a higher likelihood of metabolic syndrome (F1,84 = 5.6, p = 0.020; mean difference of 0.2 +/− 0.1) but not wither either waist circumference (or hip/waist ratio), HDL, triglycerides, or glucose (p > 0.05) as compared to those participants not receiving treatment with an AAP.
Figure 2. Distribution of TNF-α by Presence or Absence of AAP Treatment.
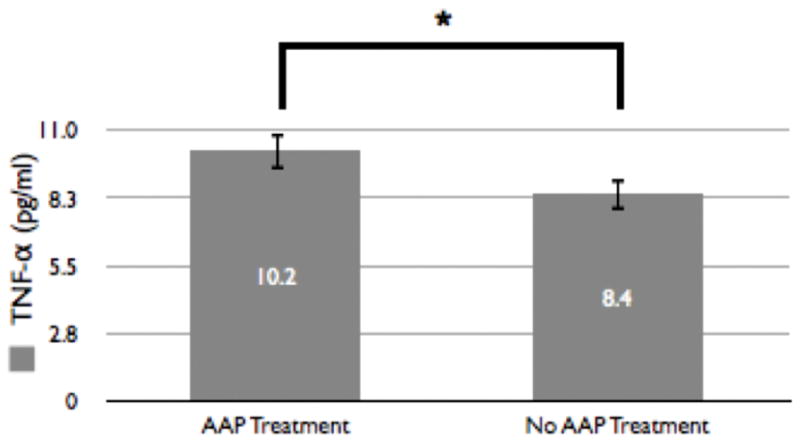
This figure outlines the differences in average TNF-α concentrations between those participants receiving AAP treatment (n = 44) and those not receiving AAP treatment (n = 52). The average TNF-α concentration in participants receiving AAP treatment (9.9 +/− 0.6 pg/ml) is significantly (denoted by *, p<0.05; T94 = 2.8, p = 0.005) higher than in participants not receiving AAP treatment (8.6 +/− 0.6 pg/ml).
However, given that these results likely suffered from the confounding issues of polypharmacy, we attempted to reduce the confounder of polypharmacy by investigating whether monotherapy AAP treatment was associated with these variables of interest. AAP monotherapy treatment was found to be associated with higher TNF-α (F1,84 = 7.3, p = 0.008; mean difference 1.3 +/− 0.5), a trend towards a greater likelihood of a diagnosis of metabolic syndrome (F1,84 = 4.5, p = 0.036; mean difference 0.2 +/− 0.1), and higher triglycerides (F1,84 = 4.6, p = 0.035; mean difference 46.9 +/− 21.9), but not associated with HDL, hip/waist ratio, waist circumference or glucose (p > 0.05). Results of MANOVA testing shown above are corrected for multiple comparisons using automated Bonferroni techniques provided within the MANOVA function of the PASW Statistics software (version 18.0, SPSS Inc., Chicago, IL). Results are designated as being significant (p<0.05) or marginally significant (p<0.10) based on the resulting p values. We did not find significant associations between CPZ equivalents and the metabolic syndrome variables of interest (p > 0.05).
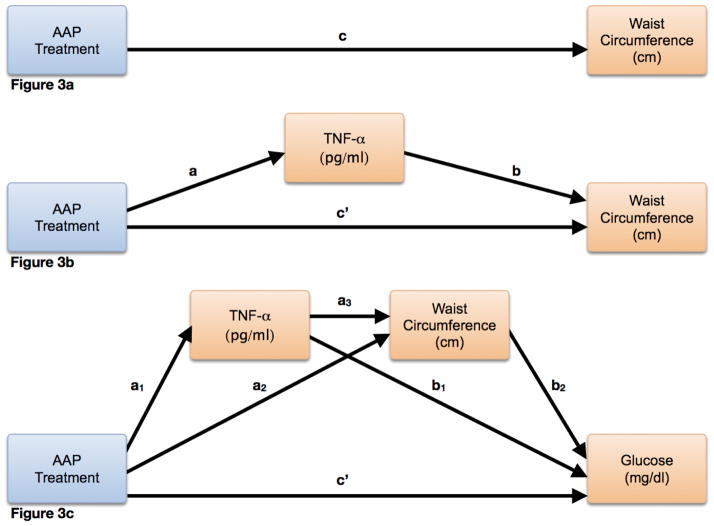
Using a simple mediation model[34] with AAP use as the independent variable, TNF-α as the intervening variable, and waist circumference as the dependent variable (Figure 3b), TNF-α was found to intervene significantly in the indirect relationship between AAP and waist circumference (effect size = 3.5, Z94 = 2.2, p = 0.03). In an attempt to better understand the mechanism by which TNF-α accounted for the indirect effect of AAP associated metabolic syndrome, we tested the indirect effect of two serial intervening variables (often referred to as mediators)[35] (m1 = TNF-α, m2 = waist circumference, bootstrapping set to 10000), using AAP as the independent variable, and glucose as the dependent variable (Figure 3c). Indeed, AAP monotherapy treatment conferred an increased likelihood of elevated glucose, doing so indirectly via both TNF-α and waist circumference (effect = 3.4, LL95%CI = 0.6, UL95%CI = 6.6)(note that significance of this multiple serial mediation analysis is reported as upper and lower limits of 95% confidence intervals per Hayes et al.)[35].
Figure 3. Schematic Representation of Mediation Analyses.
These schematic diagrams outline the mediation models used to test the corresponding hypotheses in the manuscript. This was adapted from prior work by Preacher, Hayes, and colleagues[34,35]. In Figure 3a, the general hypothesis that AAP treatment may have an impact on waist circumference is modeled. The overall (i.e. direct) effect is represented by c. Figure 3b builds on the model in figure 3a, modeling the simple hypothesis that the TNF-α variable plays an intervening role in the indirect relationship between AAP treatment and waist circumference. In this model, the direct effect is represented by c′ and the indirect effect is represented by both a and b. This model is further evolved in Figure 3c, using a serial mediation approach to test whether an indirect effect on the relationship between AAP treatment and serum glucose concentration exists and is explained by 2 serial intervening variables, TNF-α and waist circumference. The direct effect is again represented by c′, and the indirect effect is represented by a1, a3, and b2. Other partial effects are represented by a2 and b1.
Mood Stabilizers
Mood stabilizers utilized by our study participants included the following: lamotrigine (29% of subjects), valproate (26%), lithium (21%), and carbamazepine (6%). Within the monotherapy group, lamotrigine was the most frequently used mood stabilizer (25%). Within our study population, 72% of participants were being treated with any combination of these mood stabilizer medications either with or without concurrent antipsychotic medications and 26% were being treated with a mood stabilizer in the absence of an antipsychotic medication.
Using the MANOVA function, with the same dependent variables as above and mood stabilizer treatment as the independent variable, we tested whether mood stabilizer treatment was associated with our dependent variables of interest. In general, participants receiving mood stabilizers had significantly higher HDL (F1,84 = 4.5, p = 0.038; mean difference 8.4 +/− 4.0), lower LDL (F1,84 = 4.3, p = 0.041; mean difference −52.0 +/− 25.0), and higher hip/waist ratio (F1,84 = 7.2, p = 0.009; mean difference 0.1 +/− 0.0), but no significant difference in waist circumference, TNF-α, diagnosis of Metabolic Syndrome, number of metabolic syndrome criteria, or glucose (p > 0.05) as compared to those who were not receiving mood stabilizer treatment. However, of the study participants being treated with a mood stabilizer, 45% (n = 32) were concurrently being treated with an AAP and 55% (n = 39) without an AAP medication. Again, using the MANOVA test (this time with mood stabilizer treatment in the absence of AAP treatment as the independent variable, we found that treatment with mood stabilizers (and no AAP treatment) was not associated with a diagnosis of metabolic syndrome, waist circumference, hip/waist ratio, glucose, TNF-α, HDL, triglycerides, or total cholesterol (p > 0.05).
Results of MANOVA testing shown above are corrected for multiple comparisons using automated Bonferroni techniques provided within the MANOVA function of the PASW Statistics software (version 18.0, SPSS Inc., Chicago, IL).
Discussion
For this investigation we report relationships seen between bipolar disorder pharmacotherapy, TNF-α and the occurrence of metabolic syndrome in a sample with bipolar disorder. These data provide more evidence as to potential mediating relationships involved in AAP associated metabolic complications in samples with bipolar disorder. These particular investigations examined a frequently studied inflammatory cytokine as it relates to hyperglycemia seen in this population. It was hypothesized that this inflammatory cytokine may be a mediating factor between AAP treatment and its metabolic consequences (i.e. increased waist circumference). While existing evidence supports the relationships between this inflammatory cytokine and mood symptoms in samples with bipolar disorder [23,24,25], this study is novel in that it focuses on the metabolic consequences of AAPs in a sample with bipolar disorder and in particular, the role of TNF-α in these processes.
Within the population studied, treatment with AAPs was found to be associated with increased likelihood of metabolic syndrome and elevated TNF-α, itself shown to be related to the particular parameters of the metabolic syndrome. Indeed, development of metabolic syndrome in conjunction with AAP treatment in bipolar disorder is believed precipitated by multiple factors (i.e. mediators and moderators). The current study provides evidence of TNF-α as an intervening variable in some of these indirect relationships, further characterizing a niche for TNF-α as a potential biomarker associated with particular metabolic risks (i.e. obesity, hyperglycemia) in bipolar disorders treated with AAPs. This is of particular interest in bipolar disorder given existing evidence of associations between TNF-α and variations in mood, particularly mood episodes in bipolar disorder[25,36,37]. What remains unclear is whether the association between TNF-α and risks for metabolic syndrome (i.e. waist circumference) are related to cause-effect or epi-phenomena. Future prospective studies focusing on AAP pre- and post-treatment factors (i.e. TNF-α, waist circumference, glucose) and their impact on the development of AAP associated metabolic syndrome in samples with bipolar disorder would be informative and permit more extensive statistical testing (i.e. longitudinal mediation). Indeed, further understanding in this area would prove critical in the translation of this work toward combining metabolic syndrome preventative and bipolar disorder treatment strategies with TNF-α blocking agents such as etanercept.
Limitations of the current study include the cross-sectional nature and future designs could readily overcome this by studying subjects before and after a substantial time period of AAP treatment, enhancing cause-effect interpretability of the results. However, interpretation of data from such designs is commonly confounded by the dynamic nature of multiple factors (i.e. number of medications, doses, etc.). Further, TNF-α is but one inflammatory cytokine and does not provide a comprehensive measure of overall inflammatory state. Expanding the inflammatory profile via additional cytokine assays would potentially enhance understanding of the broader inflammatory system involvement in AAP associated metabolic syndrome in bipolar disorder. While treatment with AAP medications has been shown to be associated with risk criteria for metabolic syndrome, these effects likely vary across different AAP medications. An increased sample size will facilitate future investigations related to metabolic associations of specific AAP medications. Expanding the sample size will allow for the increased power essential to investigating various polypharmacy (and other) issues that complicate the interpretation of these data. The data presented would benefit from additional information pertaining to individual blood levels of the various AAPs. The lack of drug concentration data in the current study limits investigation of more extensive medication specific inflammatory and metabolic effects. Future studies, which are planned to incorporate larger samples, will also include these additional measures pertaining to drug concentrations. Expanding recruitment across centers (i.e. multi-site studies) would substantially improve the quality of future work. Such a design would 1) increase the generalizability of the data across the broader American bipolar disorder population, 2) facilitate implementation of various data validation tools, and 3) increase the study subject number so as to permit enhanced statistical flexibility. Additionally, the measured variance in TNF-α concentrations detected can differ from study to study, thereby affecting the overall generalizability of the results presented. Such differences in variance in TNF-α appear likely to be attributed to many factors including (but not limited to): Different assay procedures and/or kits employed, usage of multiplex ELISA kits (increasingly being used in studies) with potentially less sensitivity and reliability, variation (either big of small) in lab technique within (from one time to another) and between labs, the extent of this variation, and the presence of heterophilic antibodies in the sample may interfere with the assay causing erroneous results. Additionally, the relatively small group sizes (n = 26 within the MetS group) potentially limit the interpretation of effect size. Expansion of the study numbers will hopefully yield more accurate and interpretable results as compared to the results obtained from this pilot dataset.
The intervening role of TNF-α in AAP associated metabolic syndrome in a sample with bipolar disorder outlined above is further clinically substantiated with previously identified relationships between TNF-α and mood state in samples with bipolar disorder. Interestingly, on the surface, it would appear impossible to identify an indirect effect of AAP treatment on metabolic syndrome through a third variable, TNF-α, given that association between AAP treatment and metabolic syndrome was identified to be non-significant in our data. However, recent evidence justifies the investigation of indirect effects of an intervening variable in situations when no association is found between the dependent and independent variables[38,39]. Indeed, we would hypothesize that multiple indirect effects exist between the AAP treatment and metabolic syndrome, but testing for multiple intervening variables is outside the scope of this data set. However, future investigations with larger data sets will indeed test for indirect effects (both positive and negative) of multiple intervening variables, some of which we hypothesize will yield indirect effects opposing that of TNF-α, explaining why the relationship between AAP treatment and metabolic syndrome was negligible in our data. Additionally, future designs would benefit from addressing not only metabolic factors, but extensive clinical factors pertaining to mood and characterize these “mind-body” interactions that are readily appreciated in clinical settings.
Conclusions
The results presented renew discussions on research on the utility of TNF-α antagonists in mood disorders, not only as treatment of the dysregulated mood itself, but of the metabolic consequences of effective mood stabilizing treatments. Such innovative approaches to the management of mood disorders are in need of investigation and indeed may potentially facilitate development of more personalized treatment of bipolar disorder in an effort to improve clinical practice and reduce the cardiovascular burden associated with this illness. Further investigation of TNF-α as a potential biomarker of risk for AAP associated metabolic effects would is warranted within mental illness in general and Bipolar Disorder in particular.
Table 2. Average Values of Study Measures in those Participants Treated with AAPs & those Participants not Treated with AAPs.
Table 2 outlines the values of the various study measures investigated. Five broad columns are represented in this table. The first broad column lists the particular measures identified in the study. The second broad column lists the value for the particular measure averaged across the study participants who have not been treated with AAPs. The third broad column (with results of measures pertaining to those participants treated with AAPs) is further divided into six smaller columns. The first of these columns presents results of measures averaged across all participants treated with any AAP. The remaining five columns (one column for each specific AAP) each presents results from those participants receiving treatment with each respective AAP. The fourth broad column lists the value for the particular measure averaged across the entire study population. The fifth broad column provides results from statistical comparisons (Mann Whitney U testing for all variables with the exception of TNF-α where the Student’s T-Test was used) between those participants receiving AAP treatment and those not receiving AAP treatment. Significance reported for p < 0.05. Trend towards significance reported for 1.0 > p > 0.05.
| Study Measures | Not Treated with AAP | Treated with AAP | Total Population | Statistical Comparison (AAP Treatment vs. No AAP Treatment) | |||||
|---|---|---|---|---|---|---|---|---|---|
| All AAPs Included | Clozapine | Olanzepine | Quetiapine | Risperidone | Paliperidone | ||||
| Mean +/− SD (n = 53) | Mean +/− SD (n = 46) | Mean +/− SD (n = 3) | Mean +/− SD (n = 6) | Mean +/− SD (n = 27) | Mean +/− SD (n = 8) | Mean +/− SD (n = 4) | Mean +/− SD (n = 99) | ||
| Age (years) | 43.1 +/− 11.9 | 44.3 +/− 12.4 | 42.3 +/− 20.4 | 43.8 +/− 16.4 | 47.0 +/− 10.1 | 43.1 +/− 14.4 | 31.8 +/− 6.8 | 43.7 +/− 12.1 | P > 0.10 |
| TNF-α (pg/ml) | 8.6 +/− 2.4 | 9.9 +/− 2.2 | 10.1 +/− | 10.6 +/− 2.0 | 10.1 +/− 2.2 | 9.4 +/− 2.1 | 8.5 +/− 2.4 | 9.2 +/− 2.3 | T94=2.8, p = 0.005 (significant) |
| Mean Duration of Hospitalizations (months) | 0.6 +/− 1.3 | 0.5 +/− 0.7 | 0.4 +/− 0.3 | 0.3 +/− 0.2 | 0.5 +/− 0.8 | 0.6 +/− 0.7 | 0.2 +/− 0.1 | 0.5 +/− 1.0 | P > 0.10 |
| Age of Bipolar Diagnosis (years) | 30.1 +/− 11.3 | 32.2 +/− 11.5 | 36.0 +/− 18.7 | 27.7 +/− 8.7 | 35.2 +/− 11.3 | 29.3 +/− 12.5 | 25 +/− 8.6 | 31.1 +/− 11.4 | P > 0.10 |
| Number of Hospitalizations | 3.9 +/− 3.6 | 4.5 +/− 5.9 | 4.7 +/− 3.1 | 4.5 +/− 3.5 | 3.2 +/− 4.1 | 8.0 +/− 11.4 | 3.9 +/− 2.9 | 4.2 +/− 4.8 | P > 0.10 |
| Systolic BP (mm Hg) | 119.7 +/− 15.0 | 127.7 +/− 20.0 | 118.0 +/− 6.9 | 132.5 +/− 3.1 | 127.0 +/− 19.0 | 134.9 +/− 22.7 | 117.0 +/− 20.7 | 123.4 +/− 17.9 | Test Statistic = 2.0, P = 0.04 (significant) |
| Diastolic BP (mm Hg) | 70.0 +/− 10.2 | 77.5 +/− 11.4 | 71.0 +/− 10.4 | 76.5 +/− 7.1 | 78.3 +/− 12.6 | 86.1 +/− 59.4 | 70.3 +/− 12.4 | 73.5 +/− 11.4 | Test Statistic = 3.1, P = 0.002 (significant) |
| Waist Circumference (cm) | 102.4 +/− 17.2 | 102.7 +/− 17.2 | 90.7 +/− 4.7 | 105.0 +/− 17.9 | 104.1 +/− 17.2 | 108.5 +/− 16.6 | 90.0 +/− 16.8 | 102.5 +/− 17.2 | P > 0.10 |
| HDL (mg/dl) | 59.3 +/− 14.6 | 57.1 +/− 16.9 | 58.7 +/− 33.1 | 65.2 +/− 21.8 | 53.7 +/− 14.4 | 59.4 +/− 16.3 | 56.2 +/− 15.4 | 58.3 +/− 15.7 | P > 0.10 |
| Triglycerides (mg/dl) | 132.2 +/− 96.9 | 158.8 +/− 118.8 | 85.7 +/− 27.6 | 193.7 +/− 84.4 | 168.8 +/− 138.7 | 150.8 +/− 90.4 | 80.0 +/− 60.2 | 144.4 +/− 107.7 | P > 0.10 |
| Total Cholesterol (mg/dl) | 188.3 +/− 40.9 | 194.3 +/− 53.1 | 166.3 +/− 46.5 | 235.3 +/− 54.8 | 190.0 +/− 53.4 | 195.8 +/− 47.9 | 164.8 +/− 35.7 | 191.0 +/− 46.7 | P > 0.10 |
| Glucose (mg/dl) | 102.4 +/− 30.6 | 98.6 +/− 15.8 | 104.3 +/− 6.2 | 101.1 +/− 12.1 | 101.6 +/− 18.1 | 88.8 +/− 9.2 | 96.5 +/− 13.1 | 100.6 +/− 24.8 | P > 0.10 |
Table 3. Percentage of Participants Meeting Metabolic syndrome Criteria as Reported by Particular Treatment.
Table 3 outlines under each column heading, the number (and percentage) of participants receiving the various treatments as listed in column 1. Five columns are represented in this table. The first column lists the various treatment measures investigated in the study. The second, third and fourth columns list the number of participants receiving each respective treatment that 1) don’t meet criteria for metabolic syndrome, do meet criteria for metabolic syndrome, and 3) are receiving the particular treatment. The fifth column lists the percentage of participants receiving the particular treatment who meet criteria for metabolic syndrome. This is calculated by a ratio of column three/column four.
| Psychopharmacological Treatment | No Metabolic syndrome (N=73) | Metabolic syndrome (N=26) | Total Study Subjects (N=99) | % of Subjects who Meet Metabolic syndrome Criteria |
|---|---|---|---|---|
| AAP | 30 | 16 | 46 | 35 % |
| Olanzepine | 2 | 4 | 6 | 67 % |
| Quetiapine | 19 | 8 | 27 | 30 % |
| Risperidone | 5 | 3 | 8 | 38 % |
| Paliperidone | 2 | 2 | 4 | 50 % |
| Clozapine | 3 | 0 | 3 | 0 % |
| Monotherapy (i.e. one medication) | 37 | 11 | 48 | 23 % |
| Antipsychotics and Mood Stabilizers | 32 | 13 | 45 | 29 % |
| Mood Stabilizer | 54 | 17 | 71 | 24 % |
Footnotes
Presented in part at the annual meeting of the Psychoneuroimmunology Research Society (2010, Dublin, Ireland) (ARP).
References
- 1.Fagiolini A, Frank E, Scott JA, Turkin S, Kupfer DJ. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;7:424–430. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 2.Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, Miller DD, Haynes WG. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20:131–137. doi: 10.1080/10401230802177722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vuksan-Cusa B, Jakovljevic M, Sagud M, Mihaljevic Peles A, Marcinko D, et al. Metabolic syndrome and serum homocysteine in patients with bipolar disorder and schizophrenia treated with SGA. Psychiatry Res. 2011 doi: 10.1016/j.psychres.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Meyer JM, Davis VG, Goff DC, McEvoy JP, Nasrallah HA, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008;101:273–286. doi: 10.1016/j.schres.2007.12.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ATP-III. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 8.Correll CU, Frederickson AM, Kane JM, Manu P. Equally increased risk for metabolic syndrome in patients with bipolar disorder and schizophrenia treated with second-generation antipsychotics. Bipolar Disord. 2008;10:788–797. doi: 10.1111/j.1399-5618.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 9.Guo JJ, Keck PE, Li H, Patel NC. Treatment costs related to bipolar disorder and comorbid conditions among Medicaid patients with bipolar disorder. Psychiatr Serv. 2007;58:1073–1078. doi: 10.1176/ps.2007.58.8.1073. [DOI] [PubMed] [Google Scholar]
- 10.Kemp DE, Calabrese JR, Tran QV, Pikalov A, Eudicone JM, et al. Metabolic syndrome in patients enrolled in a clinical trial of aripiprazole in the maintenance treatment of bipolar I disorder: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:1138–1144. doi: 10.4088/JCP.09m05159gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yumru M, Savas HA, Kurt E, Kaya MC, Selek S, et al. Atypical antipsychotics related metabolic syndrome in bipolar patients. J Affect Disord. 2007;98:247–252. doi: 10.1016/j.jad.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Cardenas J, Frye MA, Marusak SL, Levander EM, Chirichigno JW, et al. Modal subcomponents of metabolic syndrome in patients with bipolar disorder. J Affect Disord. 2008;106:91–97. doi: 10.1016/j.jad.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Fagiolini A, Chengappa KN, Soreca I, Chang J. Bipolar disorder and the metabolic syndrome: causal factors, psychiatric outcomes and economic burden. CNS Drugs. 2008;22:655–669. doi: 10.2165/00023210-200822080-00004. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Portilla MP, Saiz PA, Benabarre A, Sierra P, Perez J, et al. The prevalence of metabolic syndrome in patients with bipolar disorder. J Affect Disord. 2008;106:197–201. doi: 10.1016/j.jad.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Taylor V, MacQueen G. Associations between bipolar disorder and metabolic syndrome: A review. J Clin Psychiatry. 2006;67:1034–1041. doi: 10.4088/jcp.v67n0704. [DOI] [PubMed] [Google Scholar]
- 16.van Winkel R, De Hert M, Van Eyck D, Hanssens L, Wampers M, et al. Prevalence of diabetes and the metabolic syndrome in a sample of patients with bipolar disorder. Bipolar Disord. 2008;10:342–348. doi: 10.1111/j.1399-5618.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 17.Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J Am Coll Cardiol. 2005;46:1978–1985. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 18.Lee SC, Pu YB, Thomas GN, Lee ZS, Tomlinson B, et al. Tumor necrosis factor alpha gene G-308A polymorphism in the metabolic syndrome. Metabolism. 2000;49:1021–1024. doi: 10.1053/meta.2000.7704. [DOI] [PubMed] [Google Scholar]
- 19.Kern PA. Potential role of TNFalpha and lipoprotein lipase as candidate genes for obesity. J Nutr. 1997;127:1917S–1922S. doi: 10.1093/jn/127.9.1917S. [DOI] [PubMed] [Google Scholar]
- 20.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest. 1996;97:1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens JM, Carter BZ, Pekala PH, Malter JS. Tumor necrosis factor alpha-induced glucose transporter (GLUT-1) mRNA stabilization in 3T3-L1 preadipocytes. Regulation by the adenosine-uridine binding factor. J Biol Chem. 1992;267:8336–8341. [PubMed] [Google Scholar]
- 22.Stephens JM, Pekala PH. Transcriptional repression of the C/EBP-alpha and GLUT4 genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. Regulations is coordinate and independent of protein synthesis. J Biol Chem. 1992;267:13580–13584. [PubMed] [Google Scholar]
- 23.Brietzke E, Stertz L, Fernandes BS, Kauer-Sant’anna M, Mascarenhas M, et al. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord. 2009;116:214–217. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Elisa B, Beny L. Induction of manic switch by the tumour necrosis factor-alpha antagonist infliximab. Psychiatry Clin Neurosci. 2010;64:442–443. doi: 10.1111/j.1440-1819.2010.02096.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. 2007;104:91–95. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Basile VS, Masellis M, McIntyre RS, Meltzer HY, Lieberman JA, et al. Genetic dissection of atypical antipsychotic-induced weight gain: novel preliminary data on the pharmacogenetic puzzle. J Clin Psychiatry. 2001;62(Suppl 23):45–66. [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA, Beck JS, Newman CF. Hopelessness, depression, suicidal ideation, and clinical diagnosis of depression. Suicide Life Threat Behav. 1993;23:139–145. [PubMed] [Google Scholar]
- 28.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orrell A, Doherty P, Miles J, Lewin R. Development and validation of a very brief questionnaire measure of physical activity in adults with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2007;14:615–623. doi: 10.1097/HJR.0b013e3280ecfd56. [DOI] [PubMed] [Google Scholar]
- 30.Berkley KJ, Zalcman SS, Simon VR. Sex and gender differences in pain and inflammation: a rapidly maturing field. Am J Physiol Regul Integr Comp Physiol. 2006;291:R241–244. doi: 10.1152/ajpregu.00287.2006. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 32.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 34.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 35.Hayes AF, Preacher KJ, Myers TA. In: Mediation and the estimation of indirect effects in political communication research. Bucy EP, Holbert RL, editors. New York, NY: Routledge; 2010. p. 586. [Google Scholar]
- 36.Kim YK, Myint AM, Lee BH, Han CS, Lee SW, et al. T-helper types 1, 2, and 3 cytokine interactions in symptomatic manic patients. Psychiatry Res. 2004;129:267–272. doi: 10.1016/j.psychres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Kunz M, Cereser KM, Goi PD, Fries GR, Teixeira AL, et al. Serum levels of IL-6, IL-10 and TNF-alpha in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Rev Bras Psiquiatr. 2011 doi: 10.1590/s1516-44462011000300010. [DOI] [PubMed] [Google Scholar]
- 38.Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76:13. [Google Scholar]
- 39.Mathieu JE, Taylor SR. Clarifying conditions and decision points for mediational type inferences in Organizational Behavior. Journal of Personality and Social Psychology. 2006;89:12. [Google Scholar]