Abstract
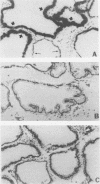
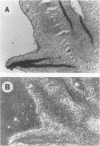
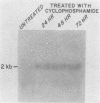
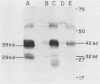
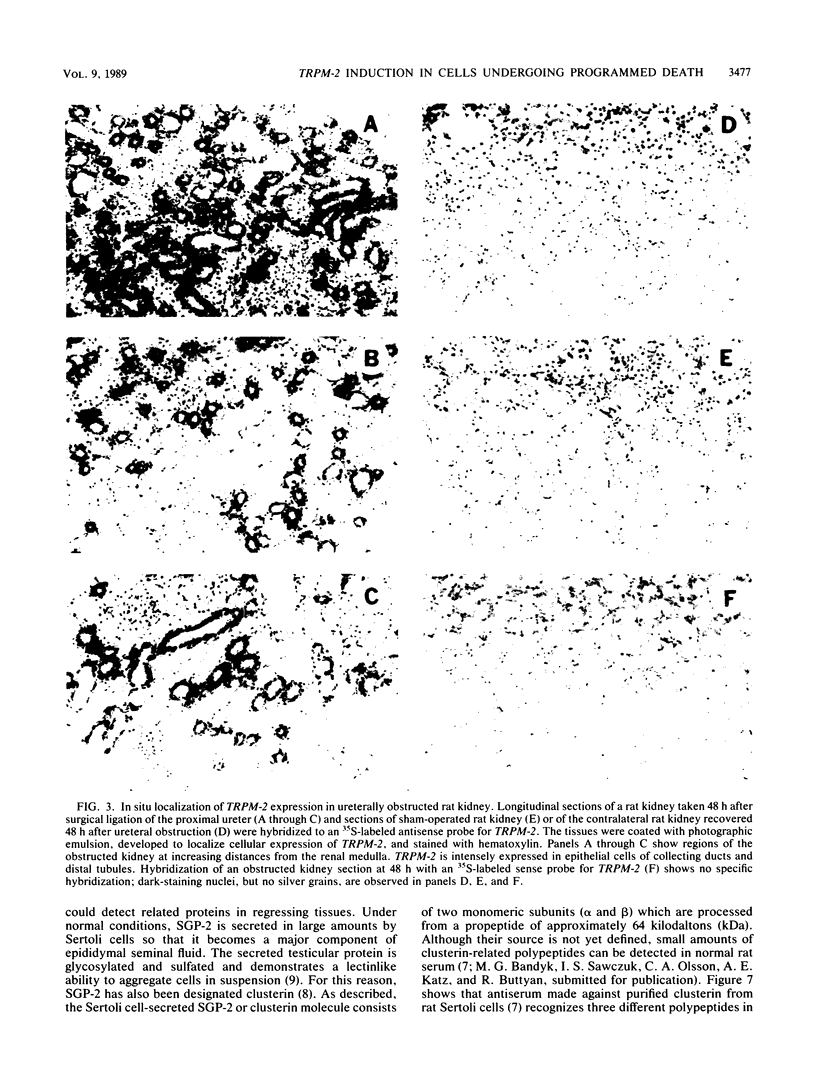
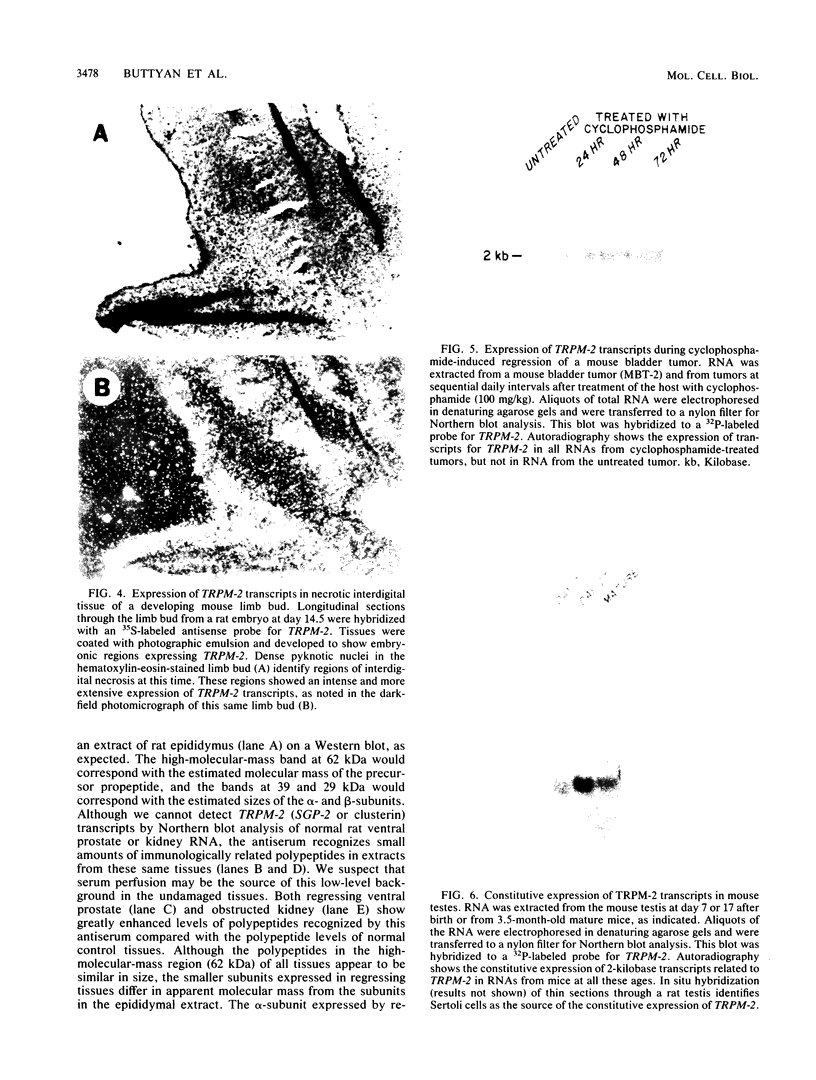
RNA and protein products encoded by the testosterone-repressed prostate message-2 gene (TRPM-2) are induced to high levels, coordinate with the onset of cell death, in numerous rodent models of inducible tissue damage. These models include cell death initiated by hormonal stimuli (prostate regression), pressure insult (renal atrophy after ureteral obstruction), developmental stimuli (necrosis of interdigital tissue), and cytotoxic injury (chemotherapeutic regression of a tumor). Sequence analysis of cDNA encoding TRPM-2 revealed its close homology with a product referred to as SGP-2 or clusterin expressed constitutively by Sertoli cells; however, the immunologically related polypeptides expressed in regressing tissues differ in molecular mass from the forms secreted by the testis. Although the function(s) of the products encoded by the TRPM-2 gene remains unclear, their presence provides a remarkable and early indicator of programmed cell death in many types of mammalian cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettuzzi S., Hiipakka R. A., Gilna P., Liao S. T. Identification of an androgen-repressed mRNA in rat ventral prostate as coding for sulphated glycoprotein 2 by cDNA cloning and sequence analysis. Biochem J. 1989 Jan 1;257(1):293–296. doi: 10.1042/bj2570293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Lesser B., Van Doorn E., Craven S. Hormonal effects on cell proliferation in rat prostate. Vitam Horm. 1975;33:61–102. doi: 10.1016/s0083-6729(08)60951-6. [DOI] [PubMed] [Google Scholar]
- Buttyan R., Zakeri Z., Lockshin R., Wolgemuth D. Cascade induction of c-fos, c-myc, and heat shock 70K transcripts during regression of the rat ventral prostate gland. Mol Endocrinol. 1988 Jul;2(7):650–657. doi: 10.1210/mend-2-7-650. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Bardin C. W. Identification of two testosterone-responsive testicular proteins in Sertoli cell-enriched culture medium whose secretion is suppressed by cells of the intact seminiferous tubule. J Biol Chem. 1987 Sep 15;262(26):12768–12779. [PubMed] [Google Scholar]
- Cheng C. Y., Chen C. L., Feng Z. M., Marshall A., Bardin C. W. Rat clusterin isolated from primary Sertoli cell-enriched culture medium is sulfated glycoprotein-2 (SGP-2). Biochem Biophys Res Commun. 1988 Aug 30;155(1):398–404. [PubMed] [Google Scholar]
- Cheng C. Y., Mathur P. P., Grima J. Structural analysis of clusterin and its subunits in ram rete testis fluid. Biochemistry. 1988 May 31;27(11):4079–4088. doi: 10.1021/bi00411a026. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Collard M. W., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987 Jun 16;26(12):3297–3303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- Compton M. M., Cidlowski J. A. Rapid in vivo effects of glucocorticoids on the integrity of rat lymphocyte genomic deoxyribonucleic acid. Endocrinology. 1986 Jan;118(1):38–45. doi: 10.1210/endo-118-1-38. [DOI] [PubMed] [Google Scholar]
- Connor J., Sawczuk I. S., Benson M. C., Tomashefsky P., O'Toole K. M., Olsson C. A., Buttyan R. Calcium channel antagonists delay regression of androgen-dependent tissues and suppress gene activity associated with cell death. Prostate. 1988;13(2):119–130. doi: 10.1002/pros.2990130204. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. M., Horvitz H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986 Mar 28;44(6):817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- English H. F., Drago J. R., Santen R. J. Cellular response to androgen depletion and repletion in the rat ventral prostate: autoradiography and morphometric analysis. Prostate. 1985;7(1):41–51. doi: 10.1002/pros.2990070106. [DOI] [PubMed] [Google Scholar]
- Gobe G. C., Axelsen R. A. Genesis of renal tubular atrophy in experimental hydronephrosis in the rat. Role of apoptosis. Lab Invest. 1987 Mar;56(3):273–281. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- HAMBURGER J., RICHET G. Enseignements tirés de la pratique du rein artificiel pour l'interprétation des désordres électrolytiques de l'urémie aiguë. Rev Fr Etud Clin Biol. 1956 Jan;1(1):39–55. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Isaacs J. T. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5(5):545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988 Feb;122(2):552–562. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C., Tsai Y., Harrison H. H., Sensibar J. Proteins of the rat prostate: I. Preliminary characterization by two-dimensional electrophoresis. Prostate. 1985;7(2):171–182. doi: 10.1002/pros.2990070207. [DOI] [PubMed] [Google Scholar]
- Léger J. G., Montpetit M. L., Tenniswood M. P. Characterization and cloning of androgen-repressed mRNAs from rat ventral prostate. Biochem Biophys Res Commun. 1987 Aug 31;147(1):196–203. doi: 10.1016/s0006-291x(87)80106-7. [DOI] [PubMed] [Google Scholar]
- Montpetit M. L., Lawless K. R., Tenniswood M. Androgen-repressed messages in the rat ventral prostate. Prostate. 1986;8(1):25–36. doi: 10.1002/pros.2990080105. [DOI] [PubMed] [Google Scholar]
- Mutter G. L., Wolgemuth D. J. Distinct developmental patterns of c-mos protooncogene expression in female and male mouse germ cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5301–5305. doi: 10.1073/pnas.84.15.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie P. S., Bruchovsky N., Buttyan R., Benson M., Cheng H. Gene expression during the early phases of regression of the androgen-dependent Shionogi mouse mammary carcinoma. Cancer Res. 1988 Nov 15;48(22):6309–6312. [PubMed] [Google Scholar]
- Sandford N. L., Searle J. W., Kerr J. F. Successive waves of apoptosis in the rat prostate after repeated withdrawal of testosterone stimulation. Pathology. 1984 Oct;16(4):406–410. doi: 10.3109/00313028409084731. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. W., Jr Death in embryonic systems. Science. 1966 Nov 4;154(3749):604–612. doi: 10.1126/science.154.3749.604. [DOI] [PubMed] [Google Scholar]
- Stanisic T., Sadlowski R., Lee C., Grayhack J. T. Partial inhibition of castration induced ventral prostate regression with actinomycin D and cycloheximide. Invest Urol. 1978 Jul;16(1):19–22. [PubMed] [Google Scholar]
- Sugimura Y., Cunha G. R., Donjacour A. A. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod. 1986 Jun;34(5):973–983. doi: 10.1095/biolreprod34.5.973. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Requirement for RNA and protein synthesis for induced regression of the tadpole tail in organ culture. Dev Biol. 1966 Feb;13(1):77–94. doi: 10.1016/0012-1606(66)90050-9. [DOI] [PubMed] [Google Scholar]
- Ucker D. S. Cytotoxic T lymphocytes and glucocorticoids activate an endogenous suicide process in target cells. Nature. 1987 May 7;327(6117):62–64. doi: 10.1038/327062a0. [DOI] [PubMed] [Google Scholar]
- Umansky S. R. The genetic program of cell death. Hypothesis and some applications: transformation, carcinogenesis, ageing. J Theor Biol. 1982 Aug 21;97(4):591–602. doi: 10.1016/0022-5193(82)90360-5. [DOI] [PubMed] [Google Scholar]
- Wadewitz A. G., Lockshin R. A. Programmed cell death: dying cells synthesize a co-ordinated, unique set of proteins in two different episodes of cell death. FEBS Lett. 1988 Dec 5;241(1-2):19–23. doi: 10.1016/0014-5793(88)81022-6. [DOI] [PubMed] [Google Scholar]
- Walsh W. G., Tomashefsky P., Olsson C. A., deVere White R. Keyhole-limpet haemocyanin (KLH) immunotherapy of murine transitional cell carcinoma. Urol Res. 1983;11(6):263–265. doi: 10.1007/BF00256343. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Zelman S. J., Zenser T. V., Davis B. B. Renal growth in response to unilateral ureteral obstruction. Kidney Int. 1983 Apr;23(4):594–598. doi: 10.1038/ki.1983.63. [DOI] [PubMed] [Google Scholar]