Abstract
The normal heart responds to changes in its metabolic milieu by changing relative oxidation rates of energy providing substrates. We hypothesized that this flexibility is lost when genetically obese rats are fed a high caloric, high fat ‘Western’ diet (WD).
Male Zucker obese (ZO) and Zucker lean (ZL) rats were fed either control or WD composed of 10 kcal% and 45 kcal% fat respectively for 7 or 28 days. Cardiac triglycerides and mRNA transcript levels were measured in situ. Substrate oxidation rates and cardiac power were measured ex vivo. Hearts from ZO rats fed WD for 7 days showed decreased cardiac power and increased cardiac triglyceride content, but no change in oleate oxidation rates or mRNA transcript levels of pyruvate dehydrogenase kinase-4 (PDK-4), uncoupling protein-3 (UCP-3), mitochondrial (MTE-1) and cytosolic thioesterase 1(CTE-1). When fed WD for 28 days, ZO rats showed no further decrease in cardiac power and no further increase in intramyocardial triglyceride levels compared to ZO rats fed the same diet for 7 days only, but did show significantly increased oleate oxidation rates and transcript levels of CTE-1, MTE-1, PDK-4 and UCP-3. In contrast, hearts from ZL rats fed WD showed increased rates of oleate oxidation and increased transcript levels of the fatty acid responsive genes investigated, and no further deterioration of contractile function.
We conclude that exposing a genetic model of obesity to the nutrient stress of WD results in an early reversible loss of metabolic flexibility of the heart which is accompanied by contractile dysfunction.
Keywords: diet, rat model of obesity, cardiac dysfunction, fatty acid metabolism
Introduction
The normal mammalian heart prefers fatty acids as energy providing substrates. However, it is capable of changing the proportion of substrates it oxidizes in response to changes in its physiologic environment (1,2). We have proposed that prolonged exposure to an abnormal metabolic milieu in vivo results in a loss of this “metabolic flexibility”, which ultimately leads to contractile dysfunction (3). A case in point is the Zucker Diabetic Fatty rat, which exhibits cardiac dilatation, reduced contractility and intramyocardial lipid accumulation (4). We have observed in the same model that administration of a PPAR-γ agonist, which restores a normal metabolic milieu, also restores cardiac function (5).
We now propose that diet-induced obesity in a genetically predisposed model of insulin resistance results in a loss of metabolic flexibility. We examined the time course of metabolic adaptation and malaptation as well as contractile function of the heart. We found that in this model, metabolic dysregulation is accompanied by an early contractile dysfunction of the heart which is subsequently compensated by mechanisms of adaptation to the altered metabolic milieu or restoration of metabolic flexibility.
Methods
Animals
Six-week-old male Zucker lean (ZL) rats and male Zucker obese (ZO) fa/fa rats were purchased from Harlan (Indianapolis, Indiana). Animals were kept in the Animal Care Center of the University of Texas Medical School at Houston under controlled conditions (23 ± 1° C; 12:12-h light-dark cycle) and received standard laboratory chow and water ad libitum for 2 weeks. ZL and ZO rats were then randomly assigned to one of four feeding groups which varied both with respect to diet (control versus WD) and duration of feeding (0, 7 or 28 days). Animals were sacrificed 6 ± 1 hours after the onset of the dark phase. All protocols were approved by the Institutional Animal Care and Use Committee. Comparisons between the ZL and ZO with the same composition of diet and duration of diet (7 or 28 days) were performed to characterize the animal model. Comparisons within individual animal models exposed to different diets (e.g. ZL on control vs. WD) allowed us to characterize the effect of diet.
Control diet (CD, D12450B, Research Diet, New Brunswick, NJ) contained 10% kcal fat, 70% kcal carbohydrates, 20% kcal proteins with a caloric density of 3.85 kcal/g. High fat Western diet (WD, D12451, Research Diets, New Brunswick, NJ) had 45% kcal fat, 35% kcal carbohydrates, 20% kcal proteins with a caloric density of 4.73 kcal/g. We have used both diets previously to investigate the effects of diet-induced obesity on the heart of Wistar rats (6).
Determination of non-esterified fatty acids in plasma
To determine non-esterified free fatty acid (NEFA) levels, spectrophotometric analysis was performed on EDTA-plasma using a commercially available kit (NEFA C, Wako Chemicals, Richmond, VA). Specimen blanks were prepared for all samples to correct for possible hemolysis.
Triglyceride extraction and quantification in heart
Tissue triglyceride concentrations were determined by digesting ~70-100mg of heart tissue in chloroform-methanol and using a triglyceride assay kit (Sigma, St. Louis, MO) as described previously (7).
Working heart perfusions
In order to investigate the time course of metabolic adaptation and maladaptation in the heart, we started our studies with hearts from animals that were six weeks old. The working heart preparation has been described previously (2). Animals were anaesthetized with chloral hydrate (60mg/100g body weight) i.p. and heparin was administered directly into the inferior vena cava. Hearts were removed rapidly and placed in ice cold Krebs-Henseleit buffer (KHB). Hearts were perfused in the working mode with recirculating KHB containing D-glucose (5 mM plus 20 μCi/L [U-14C] glucose), insulin (1ng/mL), sodium lactate (0.5 mM) and sodium oleate (0.4 mM plus 30 μCi/L [9,10-3H] oleate), which was bound to 1% BSA and dialyzed overnight. The buffer was equilibrated with O2:CO2 (95:5 % v/v). The preload was set to 15 cm H2O, the afterload was set to 100 cm H2O and hearts were perfused for forty minutes at 37° C. Cardiac power, glucose and oleate oxidation rates were measured at 5 minute intervals and averaged over the entire time of perfusions. At the end of the protocol, oxidation rates and cardiac power were normalized to dry weights of freeze-clamped hearts as described previously (5).
RNA extraction and quantitative RT-PCR
RNA extraction and quantitative RT-PCR was performed on non-perfused hearts as described previously (8). Primers and probes were designed across splice sites to prevent amplification of genomic DNA. The assays have been published previously (9). Data are presented as number of transcripts per nanogram of total RNA molecules.
Statistical analysis
Data are presented as mean ± standard error (SE). Statistically significant differences between groups were calculated using the analysis of variance (ANOVA), with repeated measures with post-hoc tests (Scheffe’s and Dunnett’s test) where appropriate.
Results
Plasma fatty acids and heart triglycerides
ZO animals fed WD showed no increase in plasma NEFA at 7 days of WD but a significant increase (70.6%) in plasma NEFA levels at 28 days compared to ZO rats fed CD for the same duration. Plasma NEFA levels were significantly increased in the ZL rats fed WD both at 7 (42.3%) and 28 days (47.2%) (Figure 1A) compared to ZL rats fed a CD for the same duration.
Figure 1.
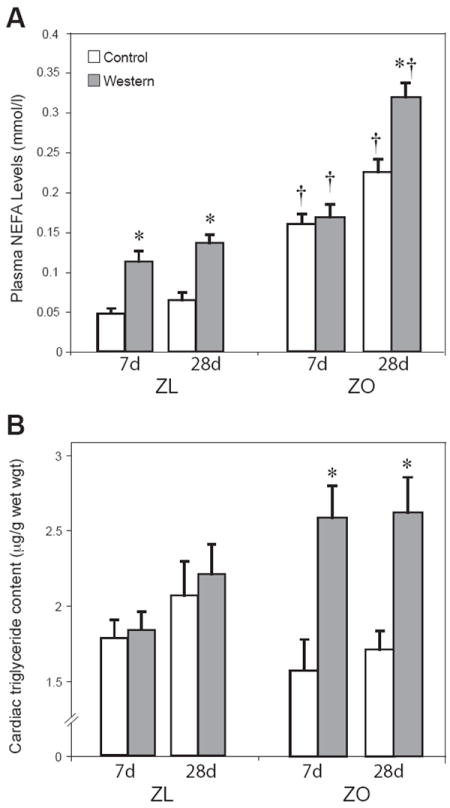
Plasma NEFA (A), and cardiac triglyceride content (B). Groups: ZL = Zucker lean; ZO = Zucker obese. WD = high fast diet; CD = control diet. Data are means ± SE for 8 independent observations. * p < 0.01 for WD vs. CD; † p < 0.05 for ZL vs. ZO.
In the ZO rats fed WD, triglyceride content in the heart increased at both 7 and 28 days, by 165% and 153 % respectively, compared to ZO rats fed CD for the same duration Cardiac triglyceride content did not change in ZL rats fed WD or CD at 7 or 28 days. (Figure 1B). In both groups, cardiomyocytes increased in size when animals were fed WD for 28 days (Supplementary Fig 1).
Cardiac power and substrate oxidation
Cardiac power decreased by 25% in ZO rats fed WD for 7 days compared to ZO rats fed CD for 7 days. Furthermore, cardiac power significantly decreased in ZO rats fed either WD or CD for 28 days compared to ZO rats fed CD. The WD had no significant effects on cardiac power in ZL rats. Among all groups fed WD, cardiac power decreased in ZO rats compared to ZL rats for the same duration. However, ZO rats fed WD for 28 days showed no further deterioration in cardiac power compared to ZO rats fed WD for 7 days only, suggesting adaptation (Figure 2A).
Figure 2.
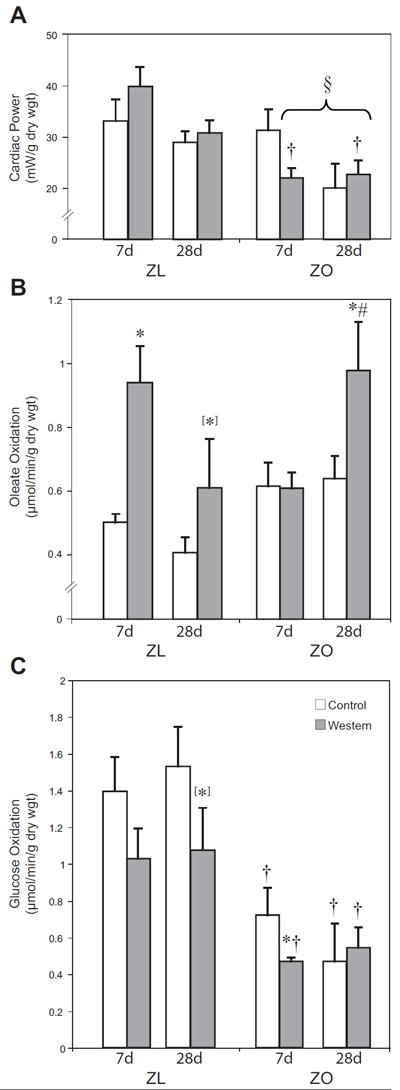
Cardiac power (A), oleate oxidation (B) and glucose oxidation (C) of perfused hearts from lean and obese Zucker rats. Groups are the same as in Figure 1. Data are means ± SE. * p < 0.05 for WD vs. CD; † p < 0.05 for ZL vs. ZO; [*] p < 0.1 for WD vs. CD; § p < 0.05 for ZO fed CD for 7 days vs. ZO fed WD for 7 days, ZO fed WD for 28 days and ZO fed CD for 28 days.
We also investigated rates of oxidative substrate metabolism to study changes in ZO and ZL hearts exposed to WD. Figures 2B and C depict rates of oleate oxidation and glucose oxidation. At 7 days, both ZO rats fed WD and those fed CD had similar rates of oleate oxidation. However, 28 days of WD led to a significant increase in oleate oxidation rates (161%) in ZO rats compared to ZO rats fed CD and compared to ZO rats fed WD for only 7 days (Figure 2B). Hearts from ZL rats fed WD for 7 days also exhibited increased rates of oleate oxidation. With 28 days of WD feeding, oleate oxidation rates only trended to increase compared to ZL fed CD for the same duration.
Rates of glucose oxidation significantly decreased in hearts of ZO rats on both diets at both 7 and 28 days relative to ZL rats. The decrease in rates of glucose oxidation by hearts of ZL rats fed WD did not reach significance (Figure 2C).
Transcript levels of PPARα-regulated genes
To examine whether changes in metabolism correlate to changes in gene expression, we used real-time quantitative RT-PCR to analyze transcript levels of genes involved in fatty acid metabolism. Transcript levels of PPAR-α were the same in hearts isolated from ZL and ZO animals fed either WD or CD (Figure 3A) at all time points.
Figure 3.
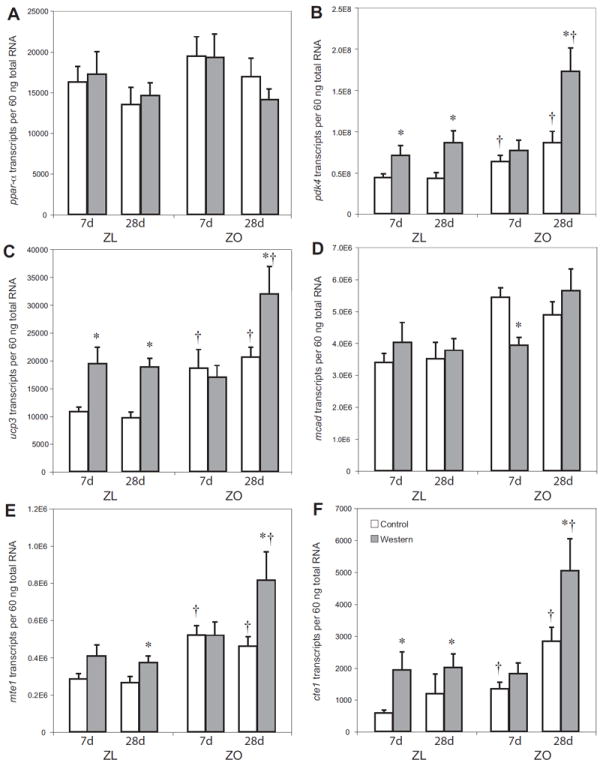
Expression of PPAR-α and fatty acid response gene-regulated genes in lean and obese Zucker rat hearts. Transcripts for PPAR-α (A), PDK-4 (B), UCP-3 (C), MCAD (D). MTE-1 (E) and CTE-1 (F). Groups are the same as in Figure 1. Data are means ± SE for seven to eight independent observations. * p < 0.05 for WD vs. CD; † p < 0.05 for ZL vs. ZO.
After 7 days of WD, hearts from ZO rats showed no changes in transcript levels of pyruvate dehydrogenase kinase 4 (PDK-4), uncoupling protein 3 (UCP-3), medium chain acyl-CoA dehydrogenase (MCAD), mitochondrial thioesterase-1 (MTE-1) and cytosolic thioesterase-1 (CTE-1) compared to CD. However, in ZO rats fed WD for 28 days there were strikingly increased transcript levels of CTE-1(+178%), MTE-1(+176%), PDK-4 (+200%), and UCP-3 (+276%), all p<0.001 compared to ZO rats fed CD for 28 days (Figure 3B-F) and compared to ZO rats fed WD for 7 days only. ZL rats fed WD showed a significant increase in the expression of fatty acid responsive genes compared to ZL fed CD both at 7 and 28 days. Only MCAD did not change (Figure 3B-F). Transcript levels of MCAD were significantly decreased in ZO rats fed WD for 7 days compared to ZO rats fed CD for the same time (Figure 3D).
Transcript levels of genes of the lipid biosynthetic pathway
To see if changes in myocardial triglyceride content correlate to changes in gene expression, we analyzed transcript levels of glycerol-3-phosphate O-acyltransferase (GPAT), which is an important flux generating step in myocardial glycerolipid synthesis. We also investigated transcript levels of steroyl-CoA desaturase-1 (SCD-1) were investigated, which is a key enzyme in myocardial lipogenesis. Transcript levels of GPAT increased in hearts of ZO rats fed WD for both 7 and 28 days. In hearts of ZL rats fed WD, GPAT transcript levels remained at baseline at both 7 and 28 days (Figure 4A). Hearts of ZO rats showed no change in SCD-1 transcript levels in response to diet whereas transcript levels of SCD-1 were significantly decreased in hearts of ZL rats fed WD compared to those fed CD for 7 days (Figure 4B).
Figure 4.
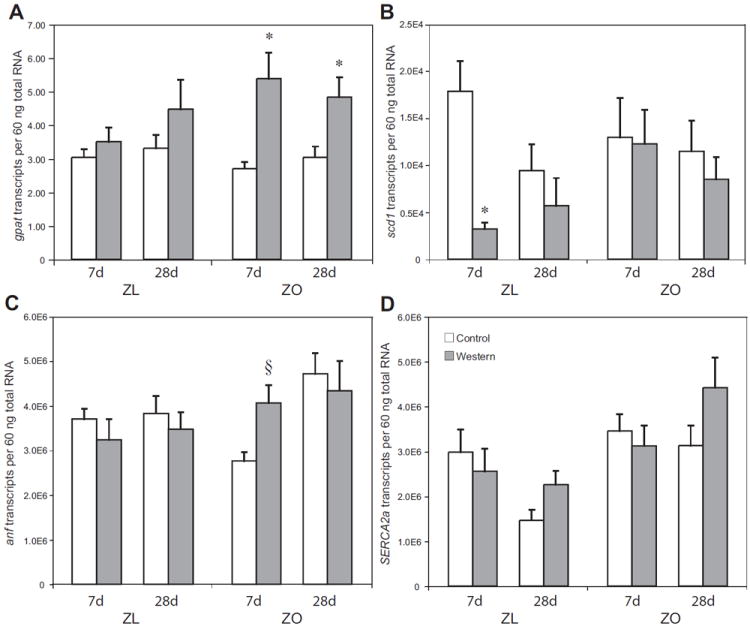
Expression of enzymes involved in fat synthesis and potential modulators of contractile function in lean and obese Zucker rat hearts. Groups are the same as in Figure 1. Transcripts coding for GPAT (A), SCD-1 (B), ANF (C), and SERCA2a (D). Data are means ± SE for seven to eight independent observations. * p < 0.05 for WD vs. CD; † p < 0.05 for ZL vs. ZO; § p < 0.05 for ZO fed CD for 7 days vs. ZO fed WD for 7 days, ZO fed WD for 28 days and ZO fed CD for 28 days.
Transcript levels of mediators and markers of contractile function
To further elucidate changes we observed in cardiac function, we also measured transcript levels of atrial naturitic factor (ANF) and sarcoendoplasmic reticulum Ca 2+-ATPase 2a (SERCA2a). ANF gene expression was significantly increased in the ZO rats on WD at 7 days and in ZO rats fed either diet for 28 days compared to ZO rats fed CD for 7 days only whereas ZL animals showed no change in ANF transcript levels with either CD or WD at either timepoint (Figure 4C). This data supports the functional data, as increases in ANF transcript levels mirror decreases in cardiac function. There were no changes in the gene expression of SERCA2a in the ZO or ZL rats fed either diet at all timepoints of the study (Figure 4D).
Discussion
We investigated the effects of WD on cardiac metabolism and function in a genetically obese rat model with a missense mutation in the leptin receptor (10,11). In this model of insulin resistance and obesity, the nutrient stress of WD leads to early cardiac dysfunction, which is followed by metabolic adaptation, increased rates of long-chain fatty acid oxidation, and decreased cardiac efficiency (Supplementary Figure 2). In other words, when a nutrient stress meets a genetically predisposed animal, the heart decompensates, but a further decline in function is halted by subsequent metabolic compensatory responses, resulting in restoration of metabolic flexibility.
Alteration in substrate use and fatty acid responsive gene expression
Previous work from our laboratory has suggested that prolonged exposure to an abnormal metabolic milieu of increased fatty acids leads to a reversible loss of “metabolic flexibility” (3). For example, we have shown that administering a PPAR-γ agonist to the “metabolically inflexible” Zucker Diabetic Fatty rat improves cardiac output, reduces cardiac triglyceride content, restores suppressed glucose oxidation, and allows a tighter coupling of oxidative metabolism and contraction in Zucker diabetic fatty rats (5). We have also shown that a loss of synchrony between fatty acid delivery and oxidation rates in the “metabolically inflexible” failing human heart is associated with decreased fatty acid responsive gene expression and intramyocardial triglyceride accumulation (12). Taken together, our previous work has suggested that a chronic exposure to a high fat environment results in metabolic maladaptation due to a breakdown in the feed forward system of fatty acid oxidation, potentially resulting in loss of metabolic flexibility and lipotoxicity (13).
In this study, there is a disconnect between cardiac triglycerides and fatty acid oxidation. With 7 days of WD feeding, ZO rats display an increase in cardiac triglyceride content by 165% but no increase in oleate oxidation and no corresponding changes in transcript levels of fatty acid responsive genes investigated (except MCAD, which decreased) compared to ZO rats on CD for the same time (Figure 1B). Though contrary to data from ZDF and failing human hearts, this observation is in line with our previously published study investigating the effects of acute fasting in this model, which also noted an increase in myocardial triglyceride levels and a decrease in cardiac power without corresponding increases in fatty acid responsive genes or oleate oxidation rates (13).
In addition, we observed decreased transcript levels of MCAD in ZO rats fed WD for 7 days compared to ZO rats on CD for the same time. Decreased MCAD transcript levels are associated with a reduction in MCAD activity and a reduction in rates of fatty acid oxidation in canine models of pacing-induced cardiac failure (14,15). Accordingly, the decreased cardiac power noted in ZO rats at 7 days on WD may reflect a delay in the ability to increase myocardial mitochondrial fatty acid oxidation and may be responsible for the cardiac dysfunction seen at that time (Supplementary Figure 3). In contrast, ZL rats fed WD for 7 days increase oleate oxidation rates and transcript levels of fatty acid responsive genes and maintain cardiac power. This suggests that obese rats have lost their capacity to adapt to a WD and show no change in gene expression or exogenous substrate oxidation at 7 days. It is reasonable to assume that this inability of the ZO hearts to increase oleate oxidation rates and to adapt to WD after 7 days may be the cause for a mismatch between fatty acid uptake and oxidation in the cardiomyocytes, resulting in the massive myocardial triglyceride levels seen in our model (13). After 28 days of WD, ZO rats display a long-term metabolic adaptation and show no further decrease in cardiac power. Exogenous oleate oxidation rates increase, which is associated with increased transcript levels of fatty acid responsive genes and there is no further increase in intramyocardial triglyceride levels. Taken together, ZO rats exposed to WD show contractile dysfunction at 7 days and a later adaptation to the abnormal metabolic milieu by 28 days, which prevents a further decrease in cardiac power.
Lipotoxicity
In addition to reduced fatty acid oxidation levels, increased de novo lipogenesis also likely contributes to the rapid accumulation of intramyocardial lipid in ZO rat hearts by 7 days. Increased expression of lipogenic enzymes such a GPAT, an enzyme of fatty acid esterification, at both 7 and 28 days also likely contributes to the rapid accumulation of triglyceride in cardiac tissue of these rat hearts during these timepoints. ZO rats on both diets were also unable to decrease expression levels of stearoyl Co-A desaturase (SCD-1), which regulates fatty acid synthesis, unlike ZL rats which displayed a significant decrease in SCD-1 gene expression with exposure to WD both at 7 days. These observations suggest that ZO rats are unable to downregulate fatty acid synthesis to the same extent as the ZL rats, which may be due to the effects of defective leptin signaling on SCD-1 and AMP-activated protein kinase (AMPK) activity in our model (16,17).
Although the exact mechanism remains elusive, the phenomenon of cardiac “lipotoxicity” is characterized by the accumulation of ceremide, diacyl glycerol, and triglycerides (18). Cardiac overexpression of fatty acyl-CoA synthetase (FACS) or human lipoprotein lipase has also been shown to result in lipid accumulation, cardiac hypertrophy and gradual development of contractile dysfunction (19). An interesting new aspect of the metabolic changes in lipotoxicity is the possible role of AMPK.
In low energy states, the enzyme AMPK plays a distinct role in the regulation of malonyl CoA levels and increases both glucose uptake and fatty acid oxidation in the heart in response to decreased intracellular ATP/AMP ratio (20). The induction of fatty acid oxidation by leptin has already been shown to be mediated by AMPK in skeletal muscle (21) and in isolated cardiomyocytes (22). AMPK activation is also associated with increased β-oxidation, reduction in fatty acid CoA, palmitoyl-CoA and ceremide in oxidative skeletal muscle from ob/ob mice (17,23). In this study, ZO rats show no change in p-AMPK or p-ACC levels after 7 days of WD but show significant increases in p-AMPK and p-ACC after 28 days of WD (Supplementary Figure 4B and 4C). AMPK activation may be responsible for increased oleate oxidation rates and preservation of glucose oxidation rates seen at 28 days in the ZO rats fed a WD. Additionally, AMPK activation may serve to increase the cells capacity to utilize both glucose and oleate to produce ATP in the face of impaired insulin signaling and explain the maintenance of contractile function in ZO hearts fed WD for 28 days.
Insulin resistance
Insulin suppresses myocardial fatty acid oxidation. As such, increased reliance on fatty acids could be coupled to insulin resistance. Mice with heart-specific overexpression of PPAR-α develop a cardiomyopathy with increased sensitivity to ischemic insult, increased rates of lipid oxidation and reduced glucose metabolism, all of which are characteristic features of the diabetic heart (24). Here we report that ZO rats show a significant decrease in AKT phosphorylation at 28 days irrespective of diet (Supplementary Figure 4A). The data indicate that the development of insulin resistance with age in the ZO rats is associated with cardiac dysfunction after 28 days on both diets. Whether insulin resistance is a marker or a mediator of contractile dysfunction (or altered fatty acid oxidation) remains unclear.
Limitations
The effects of a WD on cardiac function and metabolism in a genetic model of obesity have never been investigated using a longitudinal approach. The present study has determined substrate utilization in cardiac muscle in the ZO rat and provided a framework for explaining cardiac dysfunction after short and long term feeding of WD in animals that are genetically predisposed to growing obese. While we continue to investigate the causality, in this study we display a striking association between early contractile dysfunction and delayed metabolic adaptation in the ZO hearts faced with the nutrient stress of a WD when compared to the hearts of ZL rats, which exhibit normal leptin signaling. The exact mechanism by which aberrant leptin signaling leads to a loss of metabolic flexibility is still unknown. Studies utilizing the same isolated working rat heart perfusion apparatus have suggested that stimulation of cardiac fatty acid oxidation by leptin is mediated by p38 MAPK, which is activated by AMPK (25,26). However, further studies are required to identify the targets of p30 MAPK which mediate this effect. Manipulating fatty acid oxidation in a controlled manner may be able to address these important questions.
Conclusions
We conclude that exposing a genetic model of obesity to WD results in an early reversible loss of metabolic flexibility of the heart which is accompanied by contractile dysfunction. The exact mechanisms for this delayed metabolic await elucidation. Nonetheless, it is tempting to speculate that the genetic make up of an organism predisposes an organ like the heart to an abnormal metabolic stress including lipotoxicity, insulin resistance, and aberrant leptin signaling.
Supplementary Material
Supplementary Figure 1
Myocardial cell size in Zucker lean and obese rats fed WD and CD. See text for further details.
Supplementary Figure 2
Myocardial oxygen consumption and cardiac efficiency in Zucker lean and obese rats fed WD (WD) and control diet (CD). See text for further details.
Supplementary Figure 3
The effect of nutrient stress (WD) on hearts of ZO and ZL hearts. See text for further details.
Supplementary Figure 4
Insulin signaling and AMPK activation in obese Zucker rat hearts. See text for further details.
Acknowledgments
The work was supported in part by a grant from the US Public Health Service (R01 HL73162) to HT and by a scholarship from the Studienstiftung des Deutschen Volkes to MB. We thank Roxy A. Tate for editorial assistance and Patrick H. Guthrie and Rebecca Salazar for technical help.
Footnotes
Disclosures
None of the authors have any financial conflicts of interests to disclose related to this work.
Supplementary Data is available online at www.nature.com/obesity:
References
- 1.Bing RJ. The metabolism of the heart. Harvey Lect. 1954-1955;50:27–70. [PubMed] [Google Scholar]
- 2.Taegtmeyer H, Hems R, Krebs HA. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980;186:701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taegtmeyer H, Golfman L, Sharma S, Razeghi P, van Arsdall M. Linking gene expression to function: metabolic flexibiliy in the normal and diseased heart. Ann N Y Acad Sci. 2004;1015:202–213. doi: 10.1196/annals.1302.017. [DOI] [PubMed] [Google Scholar]
- 4.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golfman LS, Wilson CR, Sharma S, et al. Activation of PPARgamma enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol Endocrinol Metab. 2005;289:E328–336. doi: 10.1152/ajpendo.00055.2005. [DOI] [PubMed] [Google Scholar]
- 6.Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J. 2007;406:457–467. doi: 10.1042/BJ20070392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 8.Depre C, Shipley GL, Chen W, et al. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med. 1998;4:1269–1275. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 9.Stavinoha MA, RaySpellicy JW, Essop MF, et al. Diurnal Variations in the Responsiveness of Cardiac and Skeletal Muscle to Fatty Acids. Am J Physiol Endocrinol Metab. 2004;287:E888–895. doi: 10.1152/ajpendo.00189.2004. [DOI] [PubMed] [Google Scholar]
- 10.Chua SC, Jr, White DW, Wu-Peng XS, et al. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr) Diabetes. 1996;45:1141–1143. doi: 10.2337/diab.45.8.1141. [DOI] [PubMed] [Google Scholar]
- 11.Phillips MS, Liu Q, Hammond HA, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 13.Young ME, Guthrie PH, Razeghi P, et al. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–2595. doi: 10.2337/diabetes.51.8.2587. [DOI] [PubMed] [Google Scholar]
- 14.Lei B, Lionetti V, Young ME, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol. 2004;36:567–576. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Osorio JC, Stanley WC, Linke A, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 16.Cohen P, Miyazaki M, Socci ND, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 17.Dobrzyn A, Dobrzyn P, Lee SH, et al. Stearoyl-CoA desaturase-1 deficiency reduces ceramide synthesis by downregulating serine palmitoyltransferase and increasing beta-oxidation in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E599–607. doi: 10.1152/ajpendo.00439.2004. [DOI] [PubMed] [Google Scholar]
- 18.Unger RH. The physiology of cellular liporegulation. Annu Rev Physiol. 2003;65:333–347. doi: 10.1146/annurev.physiol.65.092101.142622. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Fillmore JJ, Chen Y, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 21.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 22.Palanivel R, Eguchi M, Shuralyova I, Coe I, Sweeney G. Distinct effects of short- and long-term leptin treatment on glucose and fatty acid uptake and metabolism in HL-1 cardiomyocytes. Metabolism. 2006;55:1067–1075. doi: 10.1016/j.metabol.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Brandt JM, Djouadi F, Kelly DP. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. J Biol Chem. 1998;273:23786–23792. doi: 10.1074/jbc.273.37.23786. [DOI] [PubMed] [Google Scholar]
- 24.Finck BN, Kelly DP. Peroxisome proliferator-activated receptor alpha (PPARalpha) signaling in the gene regulatory control of energy metabolism in the normal and diseased heart. J Mol Cell Cardiol. 2002;34:1249–1257. doi: 10.1006/jmcc.2002.2061. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR, 3rd, Young LH. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res. 2005;97:872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- 26.Sharma V, Mustafa S, Patel N, Wambolt R, Allard MF, McNeill JH. Stimulation of cardiac fatty acid oxidation by leptin is mediated by a nitric oxide-p38 MAPK-dependent mechanism. Eur J Pharmacol. 2009;617:113–117. doi: 10.1016/j.ejphar.2009.06.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Myocardial cell size in Zucker lean and obese rats fed WD and CD. See text for further details.
Supplementary Figure 2
Myocardial oxygen consumption and cardiac efficiency in Zucker lean and obese rats fed WD (WD) and control diet (CD). See text for further details.
Supplementary Figure 3
The effect of nutrient stress (WD) on hearts of ZO and ZL hearts. See text for further details.
Supplementary Figure 4
Insulin signaling and AMPK activation in obese Zucker rat hearts. See text for further details.


