Abstract
Studies in people and animal models suggest that depression is influenced by natural, fluctuations in the levels of 17β-oestradiol (E2), as well as administration of E2-based therapies, such as selective oestrogen receptor modulators (SERMs). Elucidating the effects and mechanisms of E2 is important to improve future E2-based therapeutics. An important question is whether effects of E2 or SERMs for mood regulation act at the α or β isoform of the oestrogen receptor (ER) because some of the unwanted trophic effects of E2-based therapies may involve actions at ERα, rather than ERβ. In the present study, whether there are sex differences in depression-like behaviour of adult mice (experiment 1), and the effects of natural fluctuations in E2 (experiment 2), or administration of E2 or a SERM that has higher affinity for ERβ than for ERα (diarylpropionitrile; DPN) to ovariectomised (experiment 3) wildtype and ERβ knockout (βERKO) mice were investigated. Results of this study supported our hypotheses that: there would be sex differences favouring males for depression-like behaviour and endogenous increases in, or exogenous administration of, E2 or administration of an ERβ SERM would decrease depression-like behaviour in wildtype, but not βERKO, mice. In experiment 1, adult male mice spent less time immobile in the forced swim test (i.e., showed less depression-like behaviour) compared with female mice. In experiment 2, pro-oestrous (higher circulating E2 levels), compared with dioestrous (lower circulating E2 levels), mice had reduced immobility in the forced swim test; this effect was not observed in βERKO mice. In experiment 3, administration of E2 or DPN to ovariectomised wildtype, but not βERKO, mice decreased immobility compared with vehicle administration, these data suggest that ERβ may be required for some of the anti–depressant-like effects of E2.
Keywords: affect, oestrogen, oestrous cycle, SERMs, sex differences
Introduction
Sex differences in incidence and severity of depression disorders suggest that women may be more vulnerable than men. Women are twice as likely to be diagnosed with major depression disorders, such as unipolar depression, compared with men (Earls, 1987; Kessler, et al., 1993; Nolen-Hoeksema, 1987). There are longer and more frequent episodes of depression among women than among men who have been diagnosed with a depression disorder (Earls, 1987; Nolen-Hoeksema, 1987). There are similar sex differences reported in animal models of depression. Compared with male rats, female rats have increased immobility in the forced swim test animal model of depression-like behaviour (Frye and Wawrzycki, 2003). However, there are some inconsistencies reported in the pattern of sex differences in the forced swim test of rats and mice when differences related to oestrous cycle, stressor exposure, gene knockout, central lesions and/or treatment with compounds that may have anti-depressant actions, are taken into account (Akanmu, et at, 2007; Barros and Ferigolo, 1998; Bhatnagar, et al., 2004; Bielajew, et al., 2003; Brotto, et al., 2000; Consoli, et al., 2005; Contreras, et al., 1995; Marvan, et al., 1996). For example, gestational stress or chronic mild stress exposure increases immobility in the forced swim test or decreases sucrose consumption (which is considered one index of anhedonia in rodents) of female, but not, male rodents (Alonso, et al., 2000; Dalla, et al., 2005). In another animal model of depression–induction, the novel open space swim test, female rats expressed a depression-like phenotype more readily than males (Sun and Alkon, 2006). Although the timing of this sex difference in animal models is not clearly reported in the literature, among people, sex differences in depression occur post-puberty, suggesting a role of ovarian steroids, such as 17β-E2 (E2), in depression (Seeman, 1997; Young, 1998; Young and Altemus, 2004; Young and Korszun, 2002). At puberty, females begin secreting E2 from their ovaries in a cyclical pattern, whereas E2 levels among males is typically very low. As such, these data suggest that some of these post-pubertal sex differences in depression are not because of absolute levels of E2, but they may be related to responses to fluctuations in E2 as has been shown among some women (Schmidt, et al., 1998). Indeed, sex differences in depression-like behaviour of rodents are more evident when endogenous changes in E2 levels are considered.
Endogenous changes in E2 alter depression behaviour. Some of the most convincing evidence that fluctuations in E2 levels may underlie the increased propensity for some women to develop neuropsychiatric disorders with depressive symptoms is that there is an increase in these disorders coincident with substantial fluctuations in E2, such as that occuring in premenstrual, postpartum and peri- and post-menopausal women (Bebbington, et al., 1981; reviewed in Bloch, et al., 2003; Jenkins, 1987; reviewed in Rubinow and Schmidt, 1995; Weissman and Klerman, 1977). Furthermore, women with postpartum depression or premenstrual syndrome, compared with those who have not been diagnosed with these disorders, respond favourably to gonadotropin-releasing hormone agonists, which stabilise E2 levels (Bloch, et al., 2000; Schmidt, et al., 1998). Evident in animal models are the effects of endogenous increases in E2 levels to alter depression-like behaviour. Pro-oestrous or pregnant rats with acute and chronic elevations in E2, respectively, show less depressive behaviour in the forced swim test than rats with lower E2 levels, such as dioestrous, post-partum or male rats (Frye and Walf, 2002, 2004). Additionally, depressive behaviour in the differential reinforcement model of depression is increased among rats three days post-parturition, a time characterised with lower and/or fluctuating levels of E2 (Molina-Hernández, et al., 2000). There are also oestrous cycle phase–dependent effects of inescapable shock to enhance, and clomipramine to attenuate, depression behaviour in the forced swim task of female rats (Marvan, et al., 1996, 1997). Notably, cyclical changes in E2 throughout the lifespan of women and female rodents cannot be separated from the effects of co-varying progestins. Thus, it is important to investigate effects of natural fluctuations in E2 and effects of E2 replacement.
Administration of E2 to females with low endogenous E2 levels decreases depression behaviour, but this effect may depend upon many factors, such as individual differences in sensitivity to E2, E2 regimen utilised and/or individual history. In support, in two double-blind, placebo-controlled studies of E2 replacement to perimenopausal women, baseline E2 levels or those produced by E2 therapy, did not predict a favourable response to E2 for mood scores (Schmidt, et al., 2000; Soares, et al., 2001). Furthermore, oral-conjugated E2 therapy, compared with placebo, improved ratings on the Hamilton Scale of Depression of severely depressed pre- and post-menopausal women; however, this favourable response to E2 varied as a function of depression duration (i.e., was greater among women with a shorter history of depression; Klaiber, et al., 1979). Reports from the literature also support the notion that E2 regimen utilised contributes to its efficacy (Gregoire, et al., 1996; Saletu, et al., 1995). Indeed, negligible findings for E2 to improve mood of older women in the Women’s Health Initiative studies (who were many years post-menopausal) further support the idea that response to E2 may be sensitive to all of these factors (Brunner, et al., 2005; Hays, et al., 2003; Smoller, et al., 2003). Despite these findings, some studies show a beneficial effect of E2 for mood in perimenopausal, and to a much lesser extent postmenopausal, women (Cohen, et al., 2003; Ditkoff, et al., 1991; Heinrich and Wolf, 2005; Morrison, et al., 2004; Rausch and Parry, 1993; Schmidt, et al., 2000; Sherwin, 1991; Sherwin and Gelfand, 1985; Soares, et al., 2001; Zweifel and O’Brien, 1997). Indeed, the most consistent positive effects of E2 to improve mood are observed in perimenopausal, depressed women who were administered transdermal E2. Given some of these limitations in clinical studies, evidence of the effects of E2 from animal models may be particularly informative. Among rodents, ovariectomy (ovx), the surgical removal of the primary endogenous source of E2, can be used as an animal model of menopause in which individual differences related to age, life history, length of ovarian cessation and E2 dosing/regimen are experimentally controlled. Ovx mice or rats have increased depressive-like behaviour compared with hormone-replete counterparts (Bekku, et al., 2006; Galea, et al., 2001; Stoffel and Craft, 2004), and these effects are sustained throughout the period of endogenous hormone deprivation (Bekku and Yoshimura, 2005). Furthermore, the effects of fluoxetine to reduce depressive behaviour in the forced swim test are attenuated by ovx (Estrada-Camarena, et al., 2004, 2006). In a genetic model of depression, the Flinders Sensitive Line, a lower expression of 5-HT2A was reversed by E2 administration (Osterlund, et al., 1999). Administration of E2 to ovx rodents can produce anti–depressant-like effects (Bernardi, et al., 1989; Estrada-Camarena, et al., 2003; Frye and Wawrzycki, 2003; Hilakivi-Clarke, 1996; Okada, et al., 1997; Rachman, et al., 1998; Walf and Frye, 2005; Walf, et al., 2004). This effect is dose-responsive, such that anti–depressant-like effects are observed following administration of E2 that produces pro-oestrous-like, and not higher or lower, E2 levels (Walf and Frye, 2005). Despite this indication that E2 can have clear anti–depressant-like effects, another important issue that is related to efficacy of E2-based therapies is E2’s targets and mechanisms of action.
A likely target for E2’s anti–depressant-like effects is oestrogen receptors (ERs), of which there are two identified types (i.e., ERα and ERβ). In support, intra-hippocampal administration of an ER antagonist attenuates anti–depressive-like behaviour of pro-oestrous rats (Walf and Frye, 2006). More recent investigations suggest that this effect may be related to ERβ activity. For instance, rendering ERβ inactive via intra-cerebroventricular administration of antisense oligonucleotides decrease ERβ expression in the hippocampus and obviates the anti–depressive-like effects of E2 administration to ovx rats (Walf, et al., 2008). Activating ERβ with administration of ERβ agonists (i.e., selective oestrogen receptor modulators – SERMs), such as diarylpropionitrile (DPN) or coumestrol, produces similar effects as E2 (which has equal affinity for ERα and ERβ) to decrease depressive-like behaviour of ovx rats (Walf, et al., 2004). A similar effect is observed when E2, DPN or coumestrol are administered directly to the hippocampus, a limbic brain region that predominantly expresses ERβ (Shughrue, et al., 1997, 1998; Walf and Frye, 2007). Indeed, differential distribution of these receptor types, as well as determining whether E2 has actions via ERα and ERβ, is important and relevant because of the potential for negative side-effects associated with E2 therapies, such as increased risk for certain cancers. E2’s proliferative effects are likely via its binding to ERα, which is predominant in mammary and uterine tissue (as reviewed in Gustafsson, 2003). ERβ is more widely distributed in the limbic regions of the brain and in bone, which may underlie some of the beneficial effects related to mood and/or osteoporosis treatment associated with this receptor subtype (Gustafsson, 2003; Shughrue, et al., 1997, 1998). Furthermore, it has been suggested that increases in ERβ expression may be associated with increased survival in breast cancer patients (Gruvberger-Saal, et al., 2007; Lin, el al., 2007). As such, it is critical to elucidate the mechanisms of E2 for its favourable effects.
To begin to investigate the role and mechanisms of E2 for depression-like behaviour, the following experimental series was conducted using the forced swim test animal model of depression. In experiment 1, evidence for sex differences in the forced swim test performance of mice was determined by comparing duration of immobility of intact, adult male and female mice. In experiment 2, the requirement of ERβ for the effects of endogenous fluctuations in E2 for duration of immobility was determined by comparing pro-oestrous and dioestrous mice that were wildtype (WT) or ERβ knockouts (βERKO). In experiment 3, the requirement of ERβ for the effects of SERMs (E2 or DPN) to ovx WT and βERKO mice to alter immobility in the forced swim test was compared with administration of vehicle to WT and βERKO mice. We hypothesized that if there are sex differences in depression-like behaviour, and if these are due to effects of E2 and its actions involving ERβ, then, in general: 1) Female mice would have increased immobility compared with male mice. 2) When oestrous cycle is taken into account, pro-oestrous mice would have decreased depression-like behaviour compared with dioestrous mice, and this effect would only be evident in WT, but not βERKO, mice. 3) E2 or DPN administration to ovx WT, but not βERKO, mice would produce anti–depressant-like effects.
Materials and methods
All methods used were pre-approved by the Institutional Animal Care and Use Committee at University at Albany – State University of New York, Albany, New York, USA.
Animals and housing
Subjects (N = 151) were adult (8 to 10-week old) female (n = 141) or male (n = 10) mice. Mice were group-housed by sex (4–5/cage) in polycarbonate cages (26 × 16 × 12.5 cm3), containing woodchip shavings for bedding, in a temperature-controlled room (21 ± 1 °C) in the Laboratory Animal Care Facility. Mice were maintained on a 12/12-h reversed light cycle (lights off at 8:00 a.m.) with Purina Mice Chow (Purina, St. Louis, MO, USA) and tap water in their home cages ad libitum.
Mouse strain and genotyping
All experimental mice were bred in our colony in the Laboratory Animal Care Facility (original breeder pairs on a C57BL/6 background were from Jackson Laboratories, Bar Harbor, Maine, USA). There are no phenotypic characteristics that can be used to determine whether experimental mice were WT or heterozygous or homozygous for the ERβ gene knockout. Genotypes of experimental mice were determined with polymerase chain reaction (PCR). PCR was conducted using tails collected from experimental mice, isolating genomic DNA from these tails and running a typical PCR analyses as per the Jackson Laboratory protocol for this strain. In brief, DNA was denatured at 94 °C for 3 min, followed by 35 cycles of amplification: 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s and a final primer extension step at 72 °C for 2 min. Specific primers (obtained from Integrated DNA Technologies; Coralville, Illinois, USA) were used: ESR2-1, which lies upstream of insertion site in exon 2 (5′-GTTGTGCCAGCCCTGTTACT-3′), ESR2-1, which lies downstream of the insertion site in exon 2 (5′-TCACAGGACCAGACACCGTA-3′) and ESR2-3, a neo gene-specific primer (5′-GCAGCCTCTGTTCCACATACAC-3′). Bands of approximately 106 and 160 bp were amplified for WT (n = 80) and homozygous βERKO (n = 71) mice, respectively. PCR was done in the laboratory of Dr Anne Messer at The Wadsworth Center (Albany, New York, USA), the Molecular Core Facility at the University of Albany or in our laboratory at the University of Albany, Albany, New York, USA.
Determination of oestrous cycle
In experiment 2, mice were removed from their housing room and transported on a cart in their home cages to the adjacent core behavioural testing facility between 7:00 and 9:00 a.m. daily. Vaginal epithelium of experimental mice was obtained by lavage and examined under a light microscope daily. After 2 weeks of regular, 4–5 day cycles, mice were tested when in pro-oestrus or diestrus. Mice were considered to be in pro-oestrus when their vaginal smears had characteristic nucleated cells, 4–5 days following the previous smear of this type. Mice in diestrus had a heterogeneous cell type in their vaginal smears for two consecutive days, and nucleated cells were not evident.
Ovariectomy
In experiment 3, mice were ovx under sodium pentobarbital anaesthesia (80 mg/kg; i.p.). In brief, an incision was first made in the skin and then in the abdominal wall. The ovary, oviduct and top of the fallopian tube were ligated and removed. The abdominal wall was sutured. The skin was closed with surgical adhesive and/or wound clips. After full recovery from anaesthesia, mice were returned to group-housing in their home cages.
Hormone administration
In experiment 3, ovx mice were administered vegetable oil vehicle (0.2 cm3), E2 (0.1 mg/kg; Steraloids, Newport, Rhode Island, USA) or DPN (0.1 mg/kg; Tocris, Ellisville, Missouri, USA). Compounds were dissolved in vegetable oil and administered via subcutaneous injections in the scruff of the neck 44 h before behavioural testing.
Screening/handling procedure
Before behavioural testing, mice were evaluated for their general health and normative responses to stimuli (as modified from Crawley, et al., 2007; and described in Frye and Walf, 2008). Briefly, health status of mice (based upon appearance of clean fur, whiskers, posture, gait, muscle tone and normative behaviour, such as presence of fur grooming, building nests with Nestlet squares provided in home cage, huddling with cage mates, ability to cage climb, paw withdrawal) was determined. Additionally, whether there were normative sensory responses (i.e., a blink or twitch reflex to a cotton swab placed close to their eyes or ears, respectively). In this study, mice did not differ based upon genotype for health status or reflex measure.
To habituate mice to steroid injections, handling by the investigator, and behavioural testing, mice were first subjected to a habituation/handling protocol. The protocol used was one modified from published methods (Frye, et al., 2006). Mice were picked up from their home cage, handled for 15 s and returned to their home cage (Day 1). Mice were then transferred from their home cage to a novel cage (Day 2) and weighed and then returned to their home cage (Day 3). On Day 4, mice were transferred to another room via a cart. On Day 5, they were transferred to another room via a cart, injected with 0.2 cm3 vegetable oil subcutaneously and placed in novel environment for 5 min.
Experimental procedure
In this study, mice were tested on one occasion in the forced swim test. Mice that were to be tested on a given day were then individually housed immediately before testing and returned to their home cages following testing. Behavioural data were collected by trained observers and simultaneously video-recorded with a video-tracking system (Any-maze- Stoelting, Inc., Wood Dale, Illinois, USA). In experiment 1, adult, gonadally-intact male (n = 10) and female (n = 10) mice were tested in the forced swim task. In experiment 2, mice were cycled daily and tested in pro-oestrus (WT: 8; βERKO: 12) or diestrus (WT: 9; βERKO: 11). In experiment 3, mice were randomly assigned to be administered vegetable oil vehicle (WT: 17; βERKO: 18), E2 (WT: 12; βERKO: 13) or DPN (WT: 14; βERKO: 17).
Forced swim test
The forced swim test is a commonly used bioassay to investigate depressive behaviour of mice and a screening tool for anti-depressants (Barros and Ferigolo, 1998; Petit-Demouliere, et al., 2005). In the forced swim test, the dependent measure of depression-like behaviour is time spent immobile. Immobility is quantifiable and readily identifiable as the absence of active behaviours (i.e., swimming, jumping or diving) following placement in the testing chamber (Porsolt, et al., 1977). The protocol in our lab for the forced swim test was used as per previous descriptions (Frye, et al., 2004). Briefly, mice were placed in a glass cylinder, which was 20.5 cm in diameter and 21.5 cm in depth, and was filled with 18 cm of 25 °C water. The time spent immobile was simultaneously recorded by the video-tracking system and an experimenter, who was blind to the treatment of mice and/or the hypothesized outcome of the study.
Statistical analyses
In experiment 1, a one-way analyses of variance test (ANOVA) was used to determine effects of sex for time spent immobile in the forced swim test. In experiment 2 and 3, two-way ANOVAs were used to determine effects of hormone condition and genotype (WT vs βERKO) for time spent immobile. As appropriate, group differences were determined by Fisher’s post-hoc tests. In the case of significant interactions, main effects for these measures are not described. A P value of ≤0.05 was considered significant.
Results
Male mice have decreased depressive behaviour compared with female mice (Figure 1)
Figure 1.
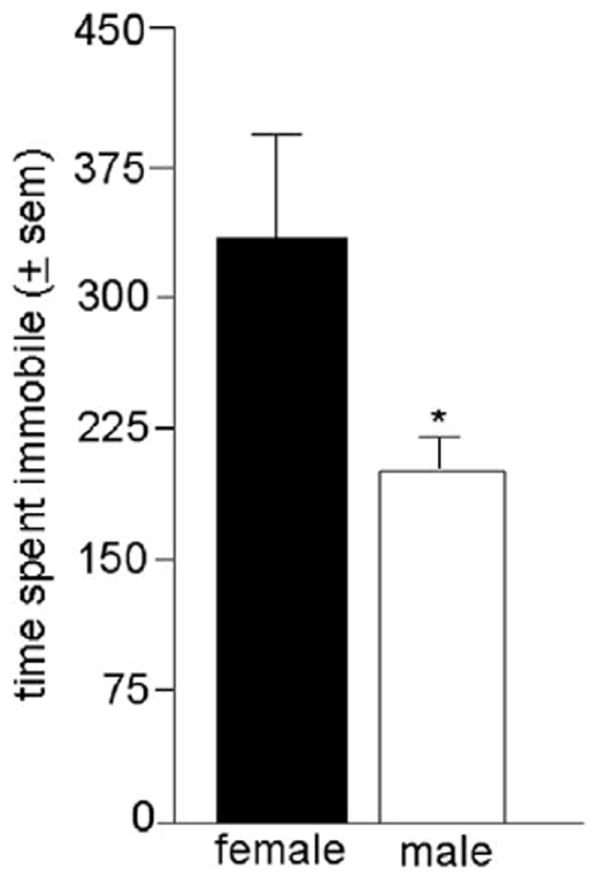
Mean (±SEM) time spent immobile by adult female or male mice. *above bar indicates a significant decrease (P ≤ 0.05).
There was a significant effect of sex for time spent immobile in the forced swim test [F(1,18) = 4.86, P < 0.04]. Female mice spent more time immobile than did male mice.
Pro-oestrous WT, but not βERKO, mice have decreased depressive behaviour compared with dioestrous mice (Figure 2)
Figure 2.
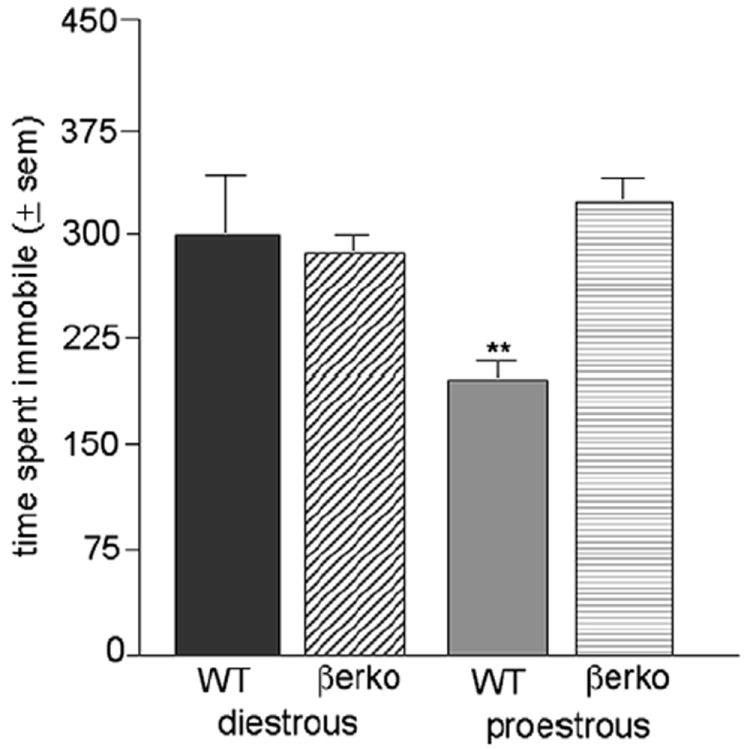
Mean (±SEM) time spent immobile by cycling WT or βERKO mice in pro-oestrous or dioestrous. ** indicates a significant (P ≤ 0.05) interaction between genotype and oestrous cycle phase condition.
There was a significant interaction between oestrous cycle phase and genotype [F(1,36) = 4.07, P < 0.05]. This was due to pro-oestrous WT, but not βERKO mice, spending less time immobile in the forced swim test compared with dioestrous mice.
SERMs decrease depressive-like behaviour of WT, but not βERKO, mice (Figure 3)
Figure 3.
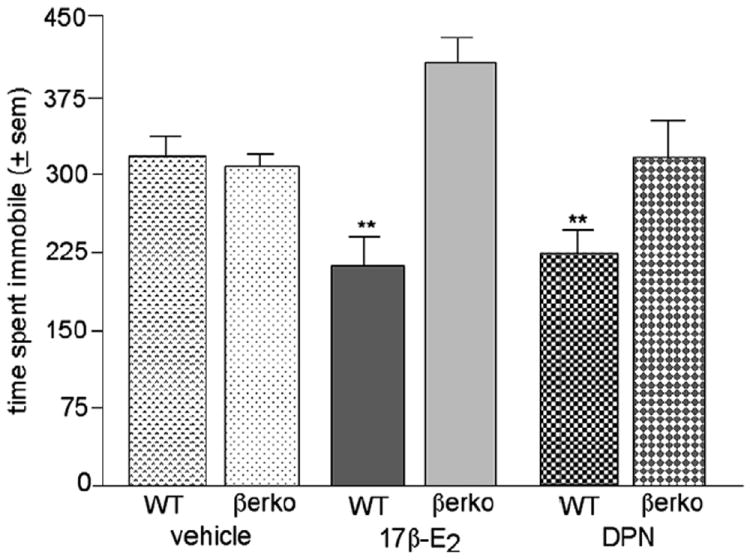
Mean (±SEM) time spent time spent immobile by ovx WT or βERKO mice in administered vehicle, E2 or DPN. * *indicates a significant (P ≤ 0.05) interaction between genotype and SERM condition.
There was a significant interaction between genotype and SERM condition [F(2,85) = 6.70, P < 0.01] in ovx mice. This was due to E2 or DPN, compared with vehicle, administration to ovx WT, but not βERKO, mice decreasing time spent immobile.
Discussion
The results of the present study supported our hypothesis that sex differences in depression-like behaviour may be due to effects of E2 and its actions involving ERβ. First, gonadally intact female mice showed more depressive-like behaviour than did male mice in the forced swim test. Second, mice in pro-oestrus had decreased immobility in the forced swim test compared with dioestrous mice; these effects were obviated in βERKO mice. Third, ovx WT mice that were administered E2 or DPN spent less time immobile than mice that were administered vehicle; E2 or DPN did not decrease immobility, compared with vehicle, when administered to ovx βERKO mice. Together, these data suggest that ERβ is required for E2’s anti–depressant-like effects.
The present data confirm and extend the literature supporting the existence of sex differences for depression behaviour in rodents. Although not all studies show a clear sex difference in depression-like behaviour in animal models, and few studies have investigated this question in mice, the present study confirmed published findings that female rats show more depression-like behaviour than males (Frye and Wawrzycki, 2003). Indeed, some differences may be due to stressor exposure. In our forced swim test paradigm, mice are exposed to the chamber once, eliminating any effects of habituation to this task, which likely produces a greater response given its novelty. Indeed, in the studies that report clear sex differences favouring males, stressor exposure was a typical method to induce depression-like behaviour (Alonso, et al., 2000; Dalla, et al., 2005; Sun and Alkon, 2006). Depression is considered a neuropsychiatric disorder that is mediated, in part, by the hypothalamic–pituitary–adrenal axis (HPA) and likely involves modulation by steroids of the hypothalamic–pituitary–gonadal axis (HPG; Young, 1998; Young and Korszun, 2002; Young, et al., 2000, 2007). For example, females may be particularly sensitive to the effects of stress. As one example, gestational stress produces greater loss in hippocampal cells among females compared with their male rat counterparts (Schmitz, et al., 2002). Another consideration to make is that, in addition to the role of the HPA, HPG steroids produce robust effects on affective behaviour of rodents, which may mask the role of sex differences for these behaviours. In Experiment 1, we did not take into consideration what oestrous cycle phase female mice were in when they were tested. However, this was investigated in Experiment 2. We found that, as has been reported in rats, pro-oestrous mice show less depressive behaviour in the forced swim test than do dioestrous mice (Frye and Walf, 2002). As an extension to these data, we found that this oestrous cycle effect only occurred in WT mice with functional ERβ, and not in their βERKO counterparts. These data suggest that ERβ may be an important target for the pro-oestrous-associated decreases in depression-like behaviour.
The present results that administration of E2 or the ERβ agonist, DPN, decreased depression-like behaviour confirm and extend previous findings showing the anti–depressant-like effects of E2 or SERMs. In support, subcutaneous administration of E2 or raloxifene, a SERM that is typically prescribed to menopausal women for treatment of osteoporosis, similarly decrease immobility in the forced swim test when administered to aged female mice (Walf and Frye, 2006). Subcutaneous administration of E2 or SERMs that have greater affinity for ERβ than ERα similarly decrease immobility in the forced swim test of young adult ovx rats (Walf, et al., 2004). In the present study, E2 and DPN similarly decreased immobility when administered to WT mice; however, the anti–depressant-like effects of these compounds were not observed among mice lacking ERβ. In fact, βERKO mice had increased immobility (i.e., greater depressive-like behaviour) compared with their WT counterparts, which is consistent with previous reports using the forced swim test (Rocha, et al., 2005). Furthermore, temporary knockdown of ERβ, which was relegated to duration of E2 priming, of ovx rats increased immobility in the forced swim test (Walf, et al., 2008). Indeed, the results from the present study provide some evidence that the effects of E2 or SERMs in mice for affective behaviour are not solely because of the gross motor changes that E2 can promote (Becker, et al., 1987; Joyce and Van Hartesveldt, 1984; Morgan and Pfaff, 2001, 2002). It is notable that the beneficial effects of E2 and SERMs in this animal model reflect a decrease in motor behaviour, rather than an increase in motor behaviour, which is typical in anxiety measures, such as the elevated plus maze or open field. Together, these data confirm that beneficial effects of E2 can be observed in young and aged mice and that ERβ may be required for these effects.
In summary, the results of the present study supported the notion that ERβ is an important target for the effects of E2 for depression behaviour. Sex differences were observed, favouring males, in the forced swim test. Mice in pro-oestrus had decreased immobility in the forced swim test, compared with dioestrous mice, and these oestrous cycle effects did not occur in βERKO mice. Lastly, ovx WT, but not βERKO, mice that were administered E2 or the ERβ SERM, DPN, spent less time immobile than mice that were administered vehicle. These data suggest that sex- and hormone-dependent effects on depression-like behaviour in the forced swim test can be measured reliably in mice and that ERβ may be required for E2’s anti–depressant-like effects. This study has clinical relevance given that, although there is some evidence for favourable effects of E2 on mood (and other symptoms associated with cessation in E2 secretion during menopause, such as osteoporosis, hot flashes, vaginal dryness, cognitive deficits) among women, there are controversies regarding the magnitude of this effect. In addition, whether these beneficial effects can occur independently of the proliferative effects E2 can have in E2-sensitive organs, which can increase risk of cancer in breast and/or uterine tissue is also of great concern. Indeed, although the present data suggest a role of E2 via ERβ for anti–depressant-like effects, investigating the targets of E2 for its functional effects needs further investigation.
Acknowledgments
This research was supported in part by grants from the Department of Defense (BC051001), National Science Foundation (1BN03-16083) and National Institute of Mental Health (MH0676980). Assistance, provided by Theresa Blakesley, Judy Horan and Allicia Ryan, is greatly appreciated.
Contributor Information
AA Walf, Department of Psychology, The University at Albany – State University of New York, Albany, NY, USA.
CJ Koonce, Department of Psychology, The University at Albany – State University of New York, Albany, NY, USA.
CA Frye, Department of Psychology, The University at Albany – State University of New York, Albany, NY, USA; Department of Biological Sciences, The University at Albany – State University of New York, Albany, NY, USA; The Center for Neuroscience, The University at Albany – State University of New York, Albany, NY, USA; The Center for Life Sciences, The University at Albany – State University of New York, Albany, NY, USA.
References
- Akanmu MA, Adeoson SO, Ilesanmi OR. Neuropharmacological effects of oleamide in male and female mice. Behav Brain Res. 2007;182:88–94. doi: 10.1016/j.bbr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Alonso SJ, Damas C, Navarro E. Behavioral despair in mice after prenatal stress. J Physiol Biochem. 2000;56:77–82. doi: 10.1007/BF03179902. [DOI] [PubMed] [Google Scholar]
- Barros HM, Ferigolo M. Ethopharmacology of imipramine in the forced-swimming test: gender differences. Neurosci Biobehav Rev. 1998;23:279–286. doi: 10.1016/s0149-7634(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Bebbington P, Hurry J, Tennant C, Sturt E, Wing JK. The epidemiology of mental disorders in Camberwell. Psychol Med. 1981;11:561–579. doi: 10.1017/s0033291700052879. [DOI] [PubMed] [Google Scholar]
- Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav. 1987;27:53–59. doi: 10.1016/0091-3057(87)90476-x. [DOI] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H. Animal model of menopausal depressive-like state in female mice: prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacology. 2005;183:300–307. doi: 10.1007/s00213-005-0179-0. [DOI] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H, Araki H. Factors producing a menopausal depressive-like state in mice following ovariectomy. Psychopharmacology. 2006;187:170–180. doi: 10.1007/s00213-006-0395-2. [DOI] [PubMed] [Google Scholar]
- Bernardi M, Vergoni AV, Sandrini M, Tagliovini SB. Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiol Behav. 1989;45:1067–1068. doi: 10.1016/0031-9384(89)90238-2. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Nowak N, Babich L, Bok L. Deletion of the 5-HT3 receptor differentially affects behavior of males and females in the Porsolt forced swim test and defensive withdrawal tests. Behav Brain Res. 2004;153:527–535. doi: 10.1016/j.bbr.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Bielajew C, Konkle AT, Kentner AC, Baker SL, Stewart A, Hutchins AA, et al. Strain and gender specific effects in the forced swim test: effects of previous stress exposure. Stress. 2003;6:269–280. doi: 10.1080/10253890310001602829. [DOI] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Brotto LA, Barr AM, Gorzalka BB. Sex differences in forced swim and open-field test behaviors after chronic administration of melatonin. Eur J Pharmacol. 2000;402:87–93. doi: 10.1016/s0014-2999(00)00491-x. [DOI] [PubMed] [Google Scholar]
- Brunner RL, Gass M, Aragaki A, Hays J, Granek I, Woods N, et al. Women’s Health Initiative Investigators. Effects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: results from the women’s health initiative randomized clinical trial. Arch Intern Med. 2005;165:1976–1986. doi: 10.1001/archinte.165.17.1976. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Poitras JR, Prouty J, Alexander AB, Shifren JL. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519–1522. doi: 10.1176/appi.ajp.160.8.1519. [DOI] [PubMed] [Google Scholar]
- Consoli D, Fedotova J, Micale V, Sapronov NS, Drago F. Stressors affect the response of male and female rats to clomipramine in a model of behavioral despair (forced swim test) Eur J Pharmacol. 2005;520:100–107. doi: 10.1016/j.ejphar.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Lara-Morales H, Molina-Hernandez M, Saavedra M, Arrellin-Rosas G. An early lesion of the lateal septal nuclei produces changes in the forced swim test depending on gender. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:1277–1284. doi: 10.1016/0278-5846(95)00266-9. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, et al. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, et al. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Ditkoff EC, Crary WG, Cristo M, Lobo RA. Estrogen improves psychological function in asymptomatic postmenopausal women. Obstet Gynecol. 1991;78:991–995. [PubMed] [Google Scholar]
- Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatr Dev. 1987;5:1–23. [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology. 2003;28:830–838. doi: 10.1038/sj.npp.1300097. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Interaction between estrogens and antidepressants in the forced swimming test in rats. Psychopharmacology. 2004;173:139–145. doi: 10.1007/s00213-003-1707-4. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Lopez-Rubalcava C, Fernandez-Guasti A. Facilitating antidepressant-like actions of estrogens are mediated by 5-HT1A and estrogen receptors in the rat forced swimming test. Psychoneuroendocrinology. 2006;31:905–914. doi: 10.1016/j.psyneuen.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O’Malley BW, et al. Progesterone’s effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology. 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004;78:531–540. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Effects of progesterone administration and APPswe+PSENlΔe9 mutation for cognitive performance of mid-aged mice. Neurobiol Learn Mem. 2008;89:17–26. doi: 10.1016/j.nlm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004;1004:116–124. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–326. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Gregoire AJ, Kumar R, Everitt B, Henderson AF, Studd JW. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347:930–933. doi: 10.1016/s0140-6736(96)91414-2. [DOI] [PubMed] [Google Scholar]
- Gruvberger-Saal SK, Bendahl PO, Saal LH, Laakso M, Hegardt C, Eden P, et al. Estrogen receptor beta expression is associated with tamoxifen response in ERα-negative breast carcinoma. Clin Cancer Res. 2007;13:1987–1994. doi: 10.1158/1078-0432.CCR-06-1823. [DOI] [PubMed] [Google Scholar]
- Gustafsson JA. What pharmacologists can learn from recent advances in estrogen signaling. Trends Pharmacol Sci. 2003;24:479–485. doi: 10.1016/S0165-6147(03)00229-3. [DOI] [PubMed] [Google Scholar]
- Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- Heinrich AB, Wolf OT. Investigating the effects of estradiol or estradiol/progesterone treatment on mood, depressive symptoms, menopausal symptoms and subjective sleep quality in older healthy hysterectomized women: a questionnaire study. Neuropsychobiology. 2005;52:17–23. doi: 10.1159/000086173. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L. Role of estradiol in alcohol intake and alcohol-related behaviors. J Stud Alcohol. 1996;57:162–170. doi: 10.15288/jsa.1996.57.162. [DOI] [PubMed] [Google Scholar]
- Jenkins R. Sex differences in depression. Br J Hosp Med. 1987;38:485–86. [PubMed] [Google Scholar]
- Joyce JN, Van Hartesveldt C. Behaviors induced by intrastriatal dopamine vary independently across the estrous cycle. Pharmacol Biochem Behav. 1984;20:551–557. doi: 10.1016/0091-3057(84)90304-6. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Klaiber EL, Broverman DM, Vogel W, Kobayashi Y. Estrogen therapy for severe persistent depressions in women. Arch Gen Psychiatry. 1979;36:550–554. doi: 10.1001/archpsyc.1979.01780050060006. [DOI] [PubMed] [Google Scholar]
- Lin CY, Strom A, Li Kong S, Kietz S, Thomsen JS, Tee JB, et al. Inhibitory effects of estrogen receptor beta on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer. Breast Cancer Res. 2007;9:R25. doi: 10.1186/bcr1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvan ML, Chavez-Chavez L, Santana S. Clomipramine modifies fluctuations of forced swimming immobility in different phases of the rat estrous cycle. Arch Med Res. 1996;27:83–86. [PubMed] [Google Scholar]
- Marvan ML, Santana S, Chavez-Chavez L, Bertran M. Inescapable shocks accentuate fluctuations of forced swimming immobility in different phases of the rat estrous cycle. Arch Med Res. 1997;28:369–372. [PubMed] [Google Scholar]
- Molina-Hernandez M, Contreras CM, Tellez-Alcantara P. Antidepressant-like effects of pregnancy and progesterone in Wistar rats as measured in the differential reinforcement of the low-rate 72 s task. Psychopharmacology. 2000;151:306–311. doi: 10.1007/s002130000496. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behav Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Kallan MJ, Ten Have T, Katz I, Tweedy K, Battistini M. Lack of efficacy of estradiol for depression in postmenopausal women: a randomized, controlled trial. Biol Psychiatry. 2004;55:406–412. doi: 10.1016/j.biopsych.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Sex differences in unipolar depression: evidence and theory. Psychol Bull. 1987;101:259–282. [PubMed] [Google Scholar]
- Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Influences of ovariectomy and continuous replacement of 17β-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Jpn J Pharmacol. 1997;73:93–96. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Overstreet DH, Hurd YL. The flinders sensitive line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17beta-estradiol. Brain Res Mol Brain Res. 1999;74:158–166. doi: 10.1016/s0169-328x(99)00274-0. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci U S A. 1998;95:13941–13946. doi: 10.1073/pnas.95.23.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch JL, Parry BL. Treatment of premenstrual mood symptoms. Psychiatr Clin North Am. 1993;16:829–839. [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17β-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-β knockout (βERKO) mice. Psychopharmacology. 2005;179:637–643. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. The neuroendocrinology of menstrual cycle mood disorders. Ann N Y Acad Sci. 1995;771:648–659. doi: 10.1111/j.1749-6632.1995.tb44717.x. [DOI] [PubMed] [Google Scholar]
- Saletu B, Brandstatter N, Metka M, Stamenkovic M, Anderer P, Semlitsch HV, et al. Double-blind, placebo-controlled, hormonal, syndromal and EEG mapping studies with transdermal oestradiol therapy in menopausal depression. Psychopharmacology. 1995;122:321–329. doi: 10.1007/BF02246261. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, et al. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry. 2002;7:810–813. doi: 10.1038/sj.mp.4001118. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Psychopathology in women and men: focus on female hormones. Am J Psychiatry. 1997;154:1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The impact of different doses of estrogen and pro-gestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab. 1991;72:336–343. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM. Sex steroids and affect in the surgical menopause: a double-blind, cross-over study. Psychoneuroendocrinology. 1985;10:325–335. doi: 10.1016/0306-4530(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-β mRNA and estrogen receptor-α immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Pollack MH, Wassertheil-Smoller S, Barton B, Hendrix SL, Jackson RD, et al. Women’s Health Initiative Investigators. Prevalence and correlates of panic attacks in postmenopausal women: results from an ancillary study to the women’s health initiative. Arch Intern Med. 2003;163:2041–2050. doi: 10.1001/archinte.163.17.2041. [DOI] [PubMed] [Google Scholar]
- Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced ’depression’ in female rats. Physiol Behav. 2004;83:505–513. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Sun MK, Alkon DL. Differential gender-related vulnerability to depression induction and converging antidepressant responses in rats. J Pharmacol Exp Ther. 2006;316:926–932. doi: 10.1124/jpet.105.093948. [DOI] [PubMed] [Google Scholar]
- Walf AA, Ciriza I, Garcia-Segura LM, Frye CA. Antisense oligodeoxynucleotides for estrogen receptor-β and α attenuate estradiol’s modulation of affective and sexual behavior, respectively. Neuropsychopharmacology. 2008;33:431–440. doi: 10.1038/sj.npp.1301416. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol’s effects to reduce anxiety and depressive behavior may be mediated by estradiol dose and restraint stress. Neuropsychopharmacology. 2005;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Administration of estrogen receptor β-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007;86:407–114. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Anti-depressant effects of ERβ selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Arch Gen Psychiatry. 1977;34:98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]
- Young EA. Sex differences and the HPA axis: implications for psychiatric disease. J Gend Specif Med. 1998;1:21–27. [PubMed] [Google Scholar]
- Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- Young EA, Korszun A. The hypothalamic-pituitary-gonadal axis in mood disorders. Endocrinol Metab Clin North Am. 2002;31:63–78. doi: 10.1016/s0889-8529(01)00002-0. [DOI] [PubMed] [Google Scholar]
- Young EA, Midgley AR, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry. 2000;57:1157–1162. doi: 10.1001/archpsyc.57.12.1157. [DOI] [PubMed] [Google Scholar]
- Young EA, Ribeiro SC, Ye W. Sex differences in ACTH pulsatility following metyrapone blockade in patients with major depression. Psychoneuroendocrinology. 2007;32:503–507. doi: 10.1016/j.psyneuen.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel JE, O’Brien WH. A meta-analysis of the effect of hormone replacement therapy upon depressed mood. Psychoneuroendocrinology. 1997;22:189–212. doi: 10.1016/s0306-4530(96)00034-0. [DOI] [PubMed] [Google Scholar]


