Abstract
Background
The feeling of hunger and feeding, a wake–state-dependent behavior, is regulated by specific centers within the hypothalamus. While paraventricular nucleus (PVN), arcuate nucleus (ARC), and dorso- and ventromedial hypothalamus (DMH/VMH) regulate feeding, the lateral hypothalamus (LH) is associated both with feeding and wake/REM sleep regulation. In order to examine the effects of sleep and wakefulness on food intake and body weight, we also measured hypothalamic ATP concentrations, which are known to be involved in feeding behavior and sleep–wake regulation.
Methods
In rats, food intake and body weight was measured during a 24-h light–dark cycle and during 6 h of sleep deprivation (SD) performed by gentle handling. Tissue samples from the PVN, ARC/DMH/VMH, and LH were collected after 6 h of SD and from time-matched diurnal controls. ATP was measured by luciferin-luciferase bioluminescence assay.
Results
Across the 24-h light–dark period, rats consumed approximately 28.13±4.48 g of food and gained 5.22±1.65 g with a positive correlation between food intake and body weight. During SD, while food intake increased significantly +147.31±6.13%, they lost weight significantly (–93.29±13.64%) when compared to undisturbed controls. SD resulted in a significant decrease in ATP levels only in LH (–44.60±21.13%) with no change in PVN, ARC/DMH/VMH region when compared with undisturbed controls.
Conclusion
The results indicate a strong overall correlation between ATP concentrations in the LH and individual food intake and suggest a sleep–wake dependent neuronal control of food intake and body weight.
Keywords: ATP, Food intake, Body weight, Sleep deprivation, Hypothalamus
There is convincing evidence that sleep is important for overall health and performance. The fact that reduced or disrupted sleep impairs a variety of physiological processes, including the immune system and neurocognitive behavior, supports its indispensable role in general emotional and physical health [1, 2, 3, 4]. Also, recent studies suggest that sleep plays an important role in the regulation of the whole body's energy balance. Disrupted non-rapid eye movement (NREM) sleep, which is the most restorative sleep, affects glucose metabolism and insulin sensitivity and elevated risks for metabolic disorders such as obesity and type 2 diabetes [5]. However, the direct relationship between sleep and the regulation of food intake and body weight is still unknown. Body weight reflects the balance between energy intake and utilization. On the basis of bidirectional signaling between cerebral structures and peripheral organs, the brain controls not only the consumption of calories but also whole body's energy balance. Appetite and satiety that control feeding behavior are regulated by processes within hypothalamic centers of the brain [6, 7] and disruptions in hypothalamic nutrition sensing have been linked to increased body weight and obesity [8, 9].
Previous studies have shown that hypothalamic energy metabolism, in particular, the concentrations of adenosine tri-phosphate (ATP) and adenosine mono-phosphate (AMP)-activated protein kinase (AMPK), are important in the regulation of food intake and body weight regulation [6, 10, 11]. In a recent study, we showed that ATP and AMPK concentrations in the frontal cortex, basal forebrain, cingulated cortex, lateral hypothalamus, and hippocampus change between sleep and wakefulness and correlate positively with the amount of slow-wave sleep (0.5–4.5 Hz) [12].
These findings prompted us to examine in the following study the relationship between sleep, the ATP concentrations in feeding centers within the hypothalamus, and associated food intake and body weight, since changes in hypothalamic energy metabolism during sleep and SD might have potential effects on food intake and whole body's energy balance.
Materials and methods
Animals
Male Sprague-Dawley rats (250–300 g) used in this study were housed individually in cages in a temperature-controlled and noise-shielded environment (23°C) with a 12 h light–dark cycle (lights on 7 a.m. to 7 p.m.). All rats had free access to food and water except when noted otherwise. Animals were treated in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care and Use Committee at Boston VA Healthcare system, Harvard University and U.S.National Institute of Health. Every effort was made to minimize animal suffering and to reduce the number of animals used. Daily food intake and body weights were measured. Food intake was controlled by supplying to each rat a pre-weighed, fixed amount (15 g) of pellets (standard rodent diet, Harlan Laboratories, South Easton, USA) in a bowl. To determine the amount of consumed food, all remaining pellets were weighed at the specific time points and calculated with the initial weight (7 a.m.). Body weight and food intake were measured at 7 a.m. and 1 p.m. For the 6-h food deprivation (FD) experiments, rats had no access to food. Water was provided as during control conditions. Between the different experiments, rats had 48 h to recover.
Sleep deprivation (SD)
SD was done by “gentle handling” which, according to standard protocols [13] involved presentation of new objects into the cage or gentle touching by a brush. SD for 6 h began at 7 a.m. and was ended at 1 p.m. During SD, the rats continued to have access to food and water ad libitum.
During this period, rats consumed twice the usual amount of food compared to undisturbed controls.
Tissue collection and measurement of ATP content
The rats were killed by decapitation and brains removed. Coronal slices (2 mm thick) were carefully placed on a dry ice (–78.5°C) containing covered Petri dish for rapid freezing and subsequent dissection. Three brain regions were dissected: frontal cortex (tissue vol. ~2 mm × 2 mm × 1 mm, Bregma 4.2 to 2.2), lateral hypothalamus (~1 mm × 1.0 mm × 1.0 mm, Bregma –1.2 to –2.2) and the ARC/DMH/VMH (~1.0 mm × 1.0 mm × 1.0 mm, Bregma –2.2 to –3.2). Tissue samples were collected at 1 p.m. after 6-h SD or 6-h undisrupted control conditions. Extreme precaution was exercised to complete this process, with an average time of 80±9 s for tissue collection for all the animals. The dissected regions were kept frozen on dry ice and stored at –80°C until used for biochemical measurements.
Determination of ATP was performed by a luciferin-luciferase based assay [14, 15] using a commercial ATP assay system with bioluminescence detection kit (Enliten, Promega). The assay principle is that, in the presence of ATP and oxygen, luciferase from Photinus pyralis catalyses D-luciferin to oxyluciferin, Pi, AMP, carbon dioxide, and light. The light intensity is measured by luminometry. This technique has been widely used for ATP measurement in cell cultures, slices and also to measure in vivo changes using frozen dissected tissue including brain tissue [16, 17, 18, 19]. ATP was measured according to the manufacturer's protocol. Briefly, weighed tissue samples were homogenized in 5% trichloroacetic acid (TCA) and transferred to 1.5 ml Eppendorf tubes. The samples were centrifuged at 5,000 rpm in cold for 5 min and the supernatant were transferred to a fresh tube. Samples (10 μl) were neutralized with tris-acetate buffer (490 μl) adjusted to a pH value of 7.75. The luciferase reagent was added immediately before measurement in the luminometer (Flexstation III, Molecular Devices, Sunnyvale, CA), as described by the supplier. A new standard curve was made daily before each measurement using known standards and ATP-free water. The absolute concentration was calculated per mg wet tissue weight by using known ATP standards which were provided in the commercial kit. ATP concentrations are expressed as 10-8 M/mg tissue. This method gave highly reproducible results. The mean coefficient of variation (standard deviation/mean) at the same time of day 10 p.m. in the four regions was 0.13.
Data analysis
Reported values are means ± standard error (SE) from at least five rats per group. Data were analyzed by one-way analysis of variance (ANOVA) or student t-test for group interactions, and Pearson's product-moment correlation analysis for data correlation analysis, using SigmaPlot 11.0 (Systat Software, Chicago, IL, USA) for MS Windows. All statistical tests were considered significant if p<0.05.
Results
Correlation between food intake and body weight during sleep–wake cycle
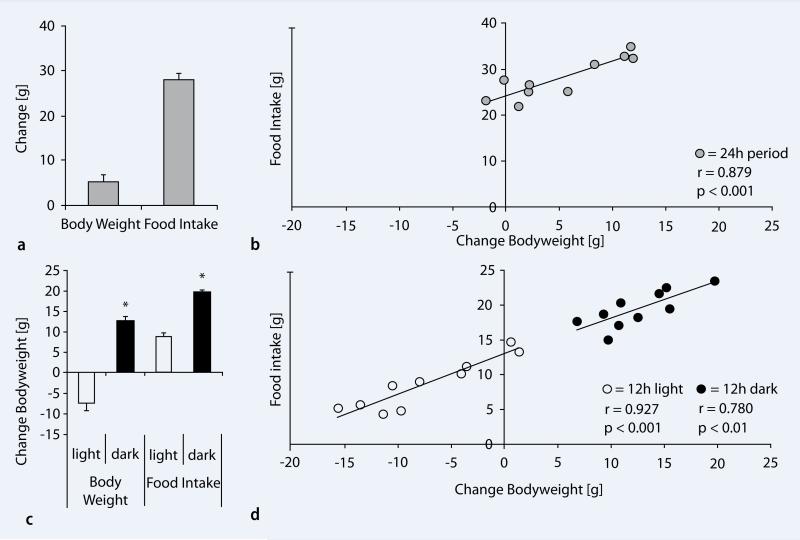
Across the 24-h light–dark period, rats consumed approximately 28.13±4.48 g of food and gained 5.22±1.65 g ( . Fig. 1a). There was a strong positive correlation (n=10; r=0.879, p<0.001) between food intake and body weight over the 24-h period (
. Fig. 1a). There was a strong positive correlation (n=10; r=0.879, p<0.001) between food intake and body weight over the 24-h period ( Fig. 1b). Next, we examined the pattern of food intake and body weight selectively during the light and dark period of rats. During the light period, when rats slept most of the time, 8.69±1.16 g of food was consumed, but they lost 7.36±1.83 g of body weight. In contrast, during the dark (active) period, food intake was significantly higher (19.44±0.82 g, p<0.001), so was the body weight gain (12.58±1.19 g, p<0.001) when compared to the light (inactive) period (
Fig. 1b). Next, we examined the pattern of food intake and body weight selectively during the light and dark period of rats. During the light period, when rats slept most of the time, 8.69±1.16 g of food was consumed, but they lost 7.36±1.83 g of body weight. In contrast, during the dark (active) period, food intake was significantly higher (19.44±0.82 g, p<0.001), so was the body weight gain (12.58±1.19 g, p<0.001) when compared to the light (inactive) period ( Fig. 1c). There was a strong positive correlation between food intake and body weight during the light period (n=10; r=0.927, p<0.001) and during the dark period (n=10; r=0.780, p<0.01) (
Fig. 1c). There was a strong positive correlation between food intake and body weight during the light period (n=10; r=0.927, p<0.001) and during the dark period (n=10; r=0.780, p<0.01) ( Fig. 1d).
Fig. 1d).
Fig. 1.
Correlation between body weight and food intake during the light–dark cycle. a Across a 24-h period experimental rats consumed approximately 28.13±4.48 g food and increased body weight. b Positive correlation between the average food intake and body weight across a 24-h light–dark cycle. c Body weight and food intake during the light and dark period. During the light (sleep) period rats lost body weight compared to the dark (wake) period. Food intake was significantly higher during the wake period of the rats. d Strong positive correlation between food intake and body weight. Note the stronger (r=0.927) positive correlation during the light (sleep) period when compared to the dark (wake) period (r=0.780)
Reciprocal relationship between food intake and body weight during 6-h SD
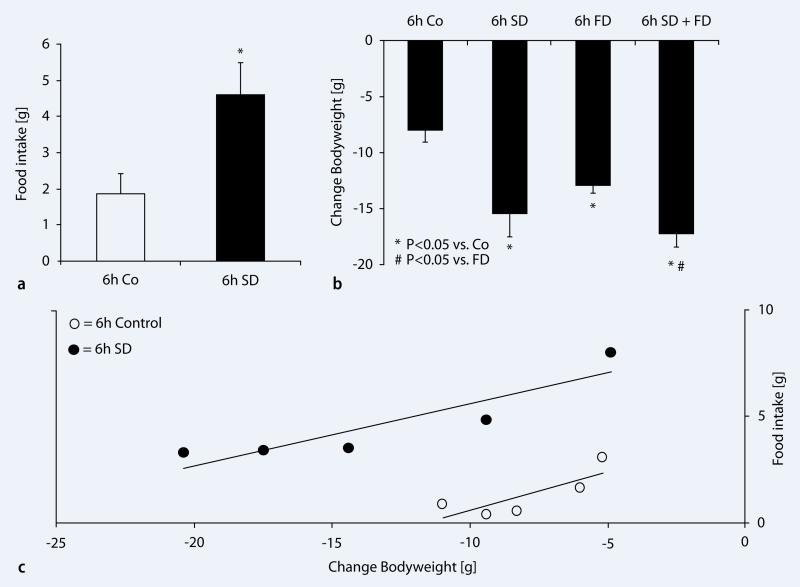
Next, we addressed whether SD has an effect on food intake and body weight in rats. Rats were sleep deprived for 6 h during the light period (7:00 a.m.–1:00 p.m.) by gentle handling. Food intake and body weight were measured as described previously. Food intake was significantly increased by +147.31±6.13% (n=5/group; p<0.008) in sleep-deprived rats (1.86±0.56 g vs. 4.60±0.89 g/6 h) when compared to undisturbed controls ( Fig. 2a). To further test if the SD-induced decrease in body weight is related to increased energy use during SD, we performed additional experiments in the same rats using four different conditions. (1) food provided ad libitum and allowed to sleep, (2) food provided ad libitum but deprived of sleep for 6 h (7:00 a.m.–1:00 p.m.), (3) food deprived (water was given ad libitum) for 6 h but allowed to sleep, (4) food deprived and sleep deprived for 6 h. The rats that were deprived of food but were not deprived of sleep showed significant decrease in (–61.65±5.55%; p<0.001; n= 5/group) body weight in comparison with undisturbed controls with access to food ad libitum, but not significantly different when compared to 6-h sleep-deprived rats with ad libitum food (
Fig. 2a). To further test if the SD-induced decrease in body weight is related to increased energy use during SD, we performed additional experiments in the same rats using four different conditions. (1) food provided ad libitum and allowed to sleep, (2) food provided ad libitum but deprived of sleep for 6 h (7:00 a.m.–1:00 p.m.), (3) food deprived (water was given ad libitum) for 6 h but allowed to sleep, (4) food deprived and sleep deprived for 6 h. The rats that were deprived of food but were not deprived of sleep showed significant decrease in (–61.65±5.55%; p<0.001; n= 5/group) body weight in comparison with undisturbed controls with access to food ad libitum, but not significantly different when compared to 6-h sleep-deprived rats with ad libitum food ( Fig. 2b). Rats which underwent SD concomitant with food deprivation showed a significant reduction in body weight compared to 6-h control rats (–116.29±15.06%) and 6-h food-deprived rats (–33.79±9.32%;p=0.014), but no change when compared to 6-h SD rats (p=0.293) (
Fig. 2b). Rats which underwent SD concomitant with food deprivation showed a significant reduction in body weight compared to 6-h control rats (–116.29±15.06%) and 6-h food-deprived rats (–33.79±9.32%;p=0.014), but no change when compared to 6-h SD rats (p=0.293) ( Fig. 2b). Significant correlation between the food intake and body weight was observed in both groups of rats, with and without sleep, but were allowed access to food (with sleep (r=0.883, p<0.05) 6-h SD (r=0.902, p<0.05)).
Fig. 2b). Significant correlation between the food intake and body weight was observed in both groups of rats, with and without sleep, but were allowed access to food (with sleep (r=0.883, p<0.05) 6-h SD (r=0.902, p<0.05)).
Fig. 2.
Effects of sleep deprivation on food intake and body weight in rats. a Average food intake during 6h spontaneous sleep–wake (control, white bar) and during 6 h of sleep deprivation (6h SD, black bar). Food intake was significantly (p=0.008, n=10) higher during the sleep deprivation period when compared to control rats. b Changes in body weight during 6 h of sleep deprivation, food deprivation, and respective controls. During spontaneous sleep–wake, rats showed a decrease in body weight (–7.89±1.06 g). Sleep-deprived rats showed significant higher decreases in body weight when compared to controls. 6h of food deprivation also significantly reduced body weight, but to a lesser extend than 6h SD. When the rats were food- and sleep-deprived at the same time, body weight decreased significantly more, when compared to food deprivation alone. c Correlation between food intake and body weight during 6h SD and respective controls. The rats showed a strong and significant correlation between the amount of food intake and body weight (6h SD (r=0.902, p<0.05), Control (r=0.883, p<0.05))
ATP concentrations in the LH decrease with 6-h SD
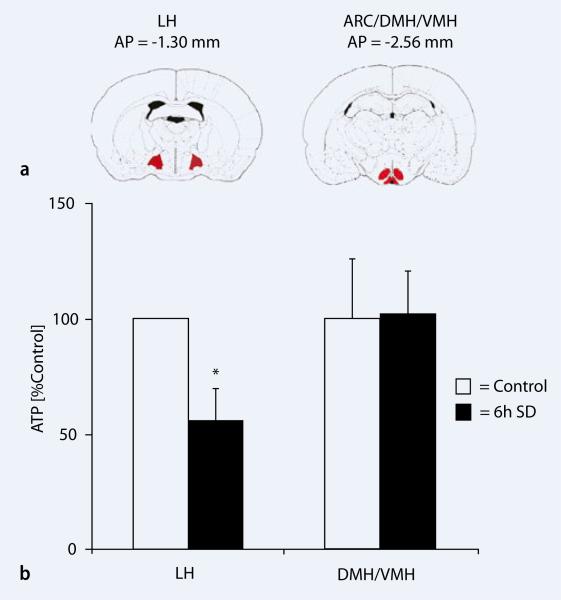
The hypothalamic energy content has been considered as a primary neural control center for regulation of appetite and body weight [20, 21, 22]. Therefore, we tested the hypothesis if SD affects the ATP levels in the LH, a region involved in wake and REM sleep regulation as well as in feeding and in ARC/DMH/VMH regions involved in satiety but not in sleep–wakefulness ( Fig. 3a). SD resulted in a significant decrease in ATP concentrations (–44.60±21.13%; p=0.041) in LH, whereas no significant change was observed in the ARC/DMH/VMH region (p=0.953) when compared with undisturbed time matched undisturbed controls (
Fig. 3a). SD resulted in a significant decrease in ATP concentrations (–44.60±21.13%; p=0.041) in LH, whereas no significant change was observed in the ARC/DMH/VMH region (p=0.953) when compared with undisturbed time matched undisturbed controls ( Fig. 3b). These results suggest that SD reduces hypothalamic (LH) ATP concentrations despite a higher food intake during sleep deprivation.
Fig. 3b). These results suggest that SD reduces hypothalamic (LH) ATP concentrations despite a higher food intake during sleep deprivation.
Fig. 3.
Brain ATP concentrations during sleep and sleep deprivation. a Coronal sections of the brain showing bregma coordinates of dissected brain regions: LH and ARC/DMH/VMH. b Rats (N5/group), sleep deprived for 6 h (7:00 a.m.–1:00 p.m.; black bar) showed significant decrease in ATP in the LH (p=0.041), whereas no significant change was observed in the ARC/DMH/VMH region (p=0.953) when compared with undisturbed time matched controls (white bars). Error bars indicate SEM
Correlation between hypothalamic regional ATP concentrations and food intake
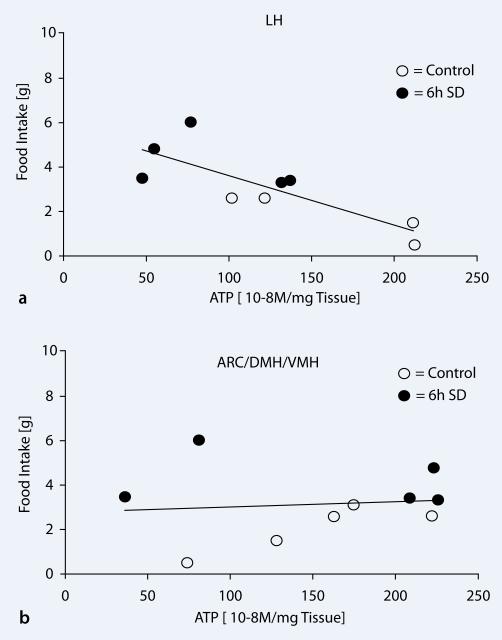
To test the relationship between hypothalamic ATP concentrations and food intake in rats, we correlated the data for measured ATP and food intake by Pearson's correlation analysis. Sleep-deprived rats showed reduced levels of ATP concentrations in LH with higher food intake leading to a strong negative correlation (r=–0.807, p=0.008). No correlation between food intake and brain ATP was observed in the ARC/DMH/VMH region (r=0.105, p=0.772).
Discussion
Our data provide evidence for a differential regulation of ATP in different areas within hypothalamus involved in feeding. ATP levels are stringently maintained in the medial hypothalamic areas of dorso- and ventromedial hypothalamus and arcuate nucleus (DMH/VMH/AC), irrespective of the amount of food intake or sleep–wake conditions. On the other hand, in the lateral hypothalamus, an area involved in feeding as well as wake and REM sleep regulation, the ATP levels decline with SD as was also observed earlier in other wake areas in the forebrain [12].
The present data indicate that feeding and body weight changes are impacted by spontaneous sleep and wake. On a daily basis rats feed less during the 12 h of light period as they sleep most of the time. Although cerebral energy levels are observed to be restored [12, 23, 24], the body weight shows a decrease during the resting period of rats. These findings support the assumption that anabolic processes within the brain occur predominantly during sleep [25], whereas peripheral anabolism is likely to occur during wakefulness and is associated with food consumption [26]. The strong positive correlation between the average food intake and body weight across the spontaneous sleep and wake period supports the general assumption between calorie intake and body weight regulation.
Contrary to the spontaneous waking-associated feeding and weight gain, 6 h of forced waking (sleep deprivation) though caused hyperphagia, resulted in body weight loss. In humans, sustained sleep restriction has been shown to increase food intake [27]. But contrary to humans, in rats we observed that despite of increased food intake during SD the rats lose body weight. This observation is in agreement with the previous report suggesting that although SD led to an increase in food intake, it resulted in a significant loss of weight [28]. This observation is contradictory to previous observations in humans showing that reduced sleep increases body weight and the risk for obesity [2]. Numerous cross-sectional and longitudinal epidemiological studies have revealed associations between chronic sleep restriction and increased body mass index in children, adolescents, and adults [29, 30]. A robust hypothesis in this field is that chronic sleep restriction leads to increased or altered dietary intake. Reduced caloric expenditure due to feelings of fatigue is also believed to contribute to weight gain in humans. Chronic sleep deprivation or sleep restriction affects glucose homeostasis and metabolic function in animals and humans [30, 31, 32] and has long-term effects on body weight and energy metabolism, whereas acute effects of SD led to an increase in food intake and physical activity [33]. The major difference between the animals and humans is the availability of variety of food to humans with a potential for unhealthy choices that may contribute to increased body weight and obesity as observed in humans.
Cerebral energy metabolism has been suggested to have an important function in body weight regulation [7, 34, 35]. On the basis of bidirectional signaling between cerebral structures and peripheral organs, the brain controls not only food intake but also the whole body's energy homeostasis [7, 35]. Sensations of appetite and satiety that regulate food intake are processed in hypothalamic centers within the brain [36, 37]. Detailed lesioning experiments identified the LH as “hunger center”, while the ventromedial hypothalamic nucleus that includes ARC/VLH/ DMH, was named as the “satiety center” [50, 51, 52]. Accordingly, disruptions in hypothalamic nutrition sensing have been shown to induce increased food intake, overweight, and obesity [38, 39]. Thus, alterations in hypothalamic energy content might have significant effects on food intake and associated body weight. While, a hunger peptide, ghrelin, expressed by the neurons in the arcuate nucleus [40] have been shown to be associated with diurnal rhythm of sleep–wakefulenss and disturbed by SD [41], we have not investigated changes in ghrelin in our current study.
Our data indicate that the local energy status in the LH decreased during 6h SD, whereas no significant change was observed in the VLH. Furthermore, we found a strong correlation between LH ATP concentrations and food intake. The LH plays a crucial role in feeding behavior and energy homeostasis and contains a variety of functionally distinct neuronal populations involved in these processes. One of these cell groups, the orexin (also named hypocretin) neurons regulate both feeding and sleep–wakefulness [42, 43]. Previous studies supported the physiological relevance of orexin in the control of feeding by showing that intracerebroventricular (ICV) administration of an antiorexin antibody or an orexin 1 receptor-selective antagonist reduced food intake [44, 45], whereas ICV injections of orexin induce a rapid increase in food intake [46]. The orexinergic system also demonstrates a molecular link between the neuroendocrine control of appetite and sleep–wake regulation. Orexinergic neurons are active during wakefulness and quiescent during sleep and are overactive, when sleep deprivation is behaviorally enforced [47, 48]. That SD resulted in a decrease in the ATP level in LH is potentially due to the increased activity-dependent consumption of neuronal ATP.
The overall energy supply within the brain has been assumed to be an important factor in body weight regulation [23]. Recent studies showed that cerebral high-energy phosphate content in healthy humans correlates with subject's body mass index supporting the close relationship between energy supply of the brain and body weight regulation [49]. Our findings suggest that sleep loss, either behavioral or disease-related might have the potential to promote the development of metabolic disorders such obesity and diabetes by affecting feeding behavior and whole body metabolism. Thus, restorative sleep is not only essential for general well being and cognitive processes, but also for processes related to general energy metabolism and calory intake.
Conclusion
Our findings indicate that the strong overall correlation between ATP concentrations in the “neuronal hunger center” and individual food intake suggests a sleep–wake dependent neuronal control of food intake and body weight and implicates that disruptions in normal sleep– wake behavior can affect these processes significantly.
Fig. 4.
Correlation between brain ATP concentrations and food intake. a There was a strong negative correlation between the ATP concentrations in the LH and food intake (r=–0.807, p=0.008). Sleep-deprived rats (black circles) showed lower LH ATP concentrations and higher food intake when compared to undisturbed controls (white circles). b No correlation between food intake and brain ATP was observed in the ARC/DMH/VMH region (r=0.105, p=0.772)
Acknowledgment
We gratefully acknowledge Farzana Pervin Nipa for technical assistance, and Diane Ghera and Dewayne Williams for help with animal care. This work was supported by a Deutsche Forschungsgemeinschaft fellowship (DW66/1–1) to MD; awards of the Department of Veterans Affairs Medical Research Service Award to RB, and of the National Institute of Mental Health (NIMH39683) to RWM.
Footnotes
Conflict of interest. The corresponding author states that there are no conflicts of interest.
References
- 1.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 2.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119(1–3):56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Irwin MR, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64(6):538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minokoshi Y, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428(6982):569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MW, Woods SC, Porte D, Jr, et al. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 8.Morton GJ, Cummings DE, Baskin DG, et al. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 9.Fryer LG, Carling D. AMP-activated protein kinase and the metabolic syndrome. Biochem Soc Trans. 2005;33(Pt 2):362–366. doi: 10.1042/BST0330362. [DOI] [PubMed] [Google Scholar]
- 10.Minokoshi Y, Shiuchi T, Lee S, et al. Role of hypothalamic AMP-kinase in food intake regulation. Nutrition. 2008;24(9):786–790. doi: 10.1016/j.nut.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 11.MacLean DB, Luo LG. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004;1020(1–2):1–11. doi: 10.1016/j.brainres.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 12.Dworak M, McCarley RW, Kim T, et al. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30(26):9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261(1 Pt 2):R198–208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 14.McElroy WD, DeLuca MA. Firefly and bacterial luminescence: basic science and applications. J Appl Biochem. 1983;5(3):197–209. [PubMed] [Google Scholar]
- 15.Lundin A, Thore A. Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal Biochem. 1975;66(1):47–63. doi: 10.1016/0003-2697(75)90723-x. [DOI] [PubMed] [Google Scholar]
- 16.Chang EJ, et al. Brain-type creatine kinase has a crucial role in osteoclast-mediated bone resorption. Nat Med. 2008;14(9):966–972. doi: 10.1038/nm.1860. [DOI] [PubMed] [Google Scholar]
- 17.Christian SL, et al. Arctic ground squirrel (Spermophilus parryii) hippocampal neurons tolerate prolonged oxygen-glucose deprivation and maintain baseline ERK1/2 and JNK activation despite drastic ATP loss. J Cereb Blood Flow Metab. 2008;28(7):1307–1319. doi: 10.1038/jcbfm.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao L, Avshalumov MV, Rice ME. Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion. J Neurosci. 2005;25(43):10029–10040. doi: 10.1523/JNEUROSCI.2652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441(7097):1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 20.Kalra SP, et al. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20(1):68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104(4):531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 22.Williams G, Harrold JA, Cutler DJ. The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc Nutr Soc. 2000;59(3):385–396. doi: 10.1017/s0029665100000434. [DOI] [PubMed] [Google Scholar]
- 23.Karnovsky ML, Reich P, Anchors JM, Burrows BL. Changes in brain glycogen during slow-wave sleep in the rat. J Neurochem. 1983;41(5):1498–1501. doi: 10.1111/j.1471-4159.1983.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 24.Reich P, Geyer SJ, Karnovsky ML. Metabolism of brain during sleep and wakefulness. J Neurochem. 1972;19(2):487–497. doi: 10.1111/j.1471-4159.1972.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 25.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41(1):35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132(10):3219S–3224S. doi: 10.1093/jn/131.10.3219S. [DOI] [PubMed] [Google Scholar]
- 27.Nedeltcheva AV, et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89(1):126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25(1):18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- 29.Magee CA, Iverson DC, Huang XF, Caputi P. A link between chronic sleep restriction and obesity: methodological considerations. Public Health. 2008;122(12):1373–1381. doi: 10.1016/j.puhe.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Magee CA, Huang XF, Iverson DC, Caputi P. Examining the pathways linking chronic sleep restriction to obesity. J Obes. 2010 doi: 10.1155/2010/821710. Epub 2010 Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barf RP, Meerlo P, Scheurink AJ. Chronic sleep disturbance impairs glucose homeostasis in rats. Int J Endocrinol. 2010:819414. doi: 10.1155/2010/819414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brondel L, Romer MA, Nougues PM, et al. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91(6):1550–1559. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 34.Peters A, et al. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004;28(2):143–180. doi: 10.1016/j.neubiorev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307(5708):375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 36.Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8(5):561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- 37.Gao Q, Horvath TL. Neuronal control of energy homeostasis. FEBS Lett. 2008;582(1):132–141. doi: 10.1016/j.febslet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 39.Elmquist JK, Coppari R, Balthasar N, et al. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493(1):63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 40.Cowley MA, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 41.Bodosi B, et al. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1071–1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 42.Lecea L de, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5):1 page following 696. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- 44.Haynes AC, et al. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96(1–2):45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 45.Yamada H, Okumura T, Motomura W, et al. Inhibition of food intake by central injection of anti-orexin antibody in fasted rats. Biochem Biophys Res Commun. 2000;267(2):527–531. doi: 10.1006/bbrc.1999.1998. [DOI] [PubMed] [Google Scholar]
- 46.Edwards CM, et al. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160(3):R7–R12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- 47.Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29(1):70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 49.Schmoller A, et al. Evidence for a relationship between body mass and energy metabolism in the human brain. J Cereb Blood Flow Metab. 2010;30(7):1403–1410. doi: 10.1038/jcbfm.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anand BK, Brobeck JR. Localization of a feeding center in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77:323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 51.Hetherinton AW, Ranson SW. Hypothalamic lesions and adipocity in the rat. Anat Rec. 1940;78:149. [Google Scholar]
- 52.Bray GA, Fisler J, York DA. Neuroendocrine control of the development of obesity: understaniding gained from studies of experimental animal models. Front Neuroendocrinil. 1990;11:128–181. [Google Scholar]