Abstract
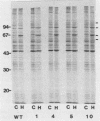
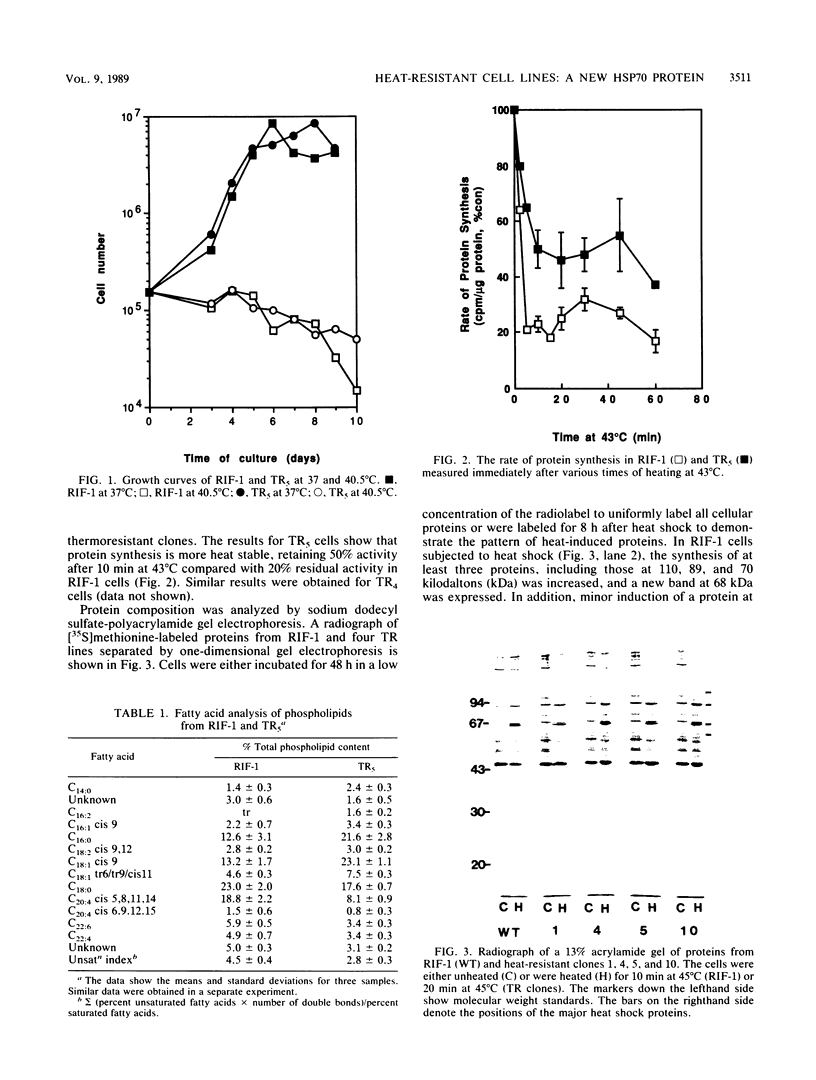
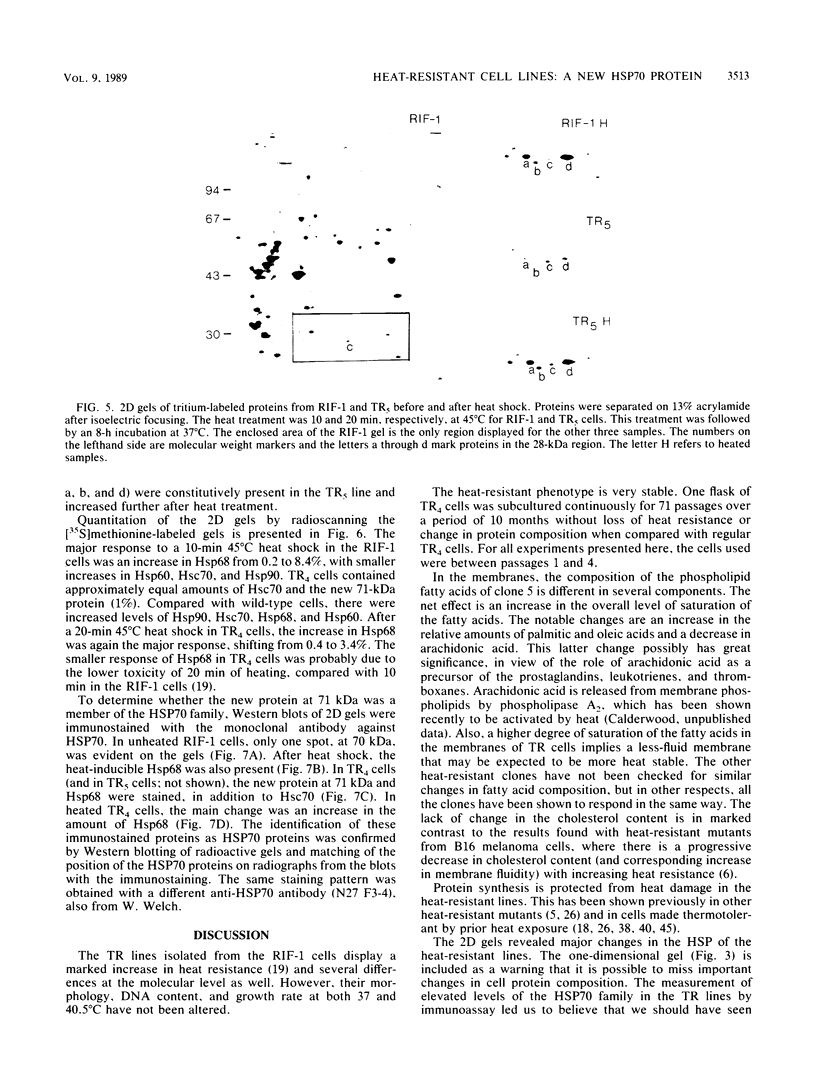
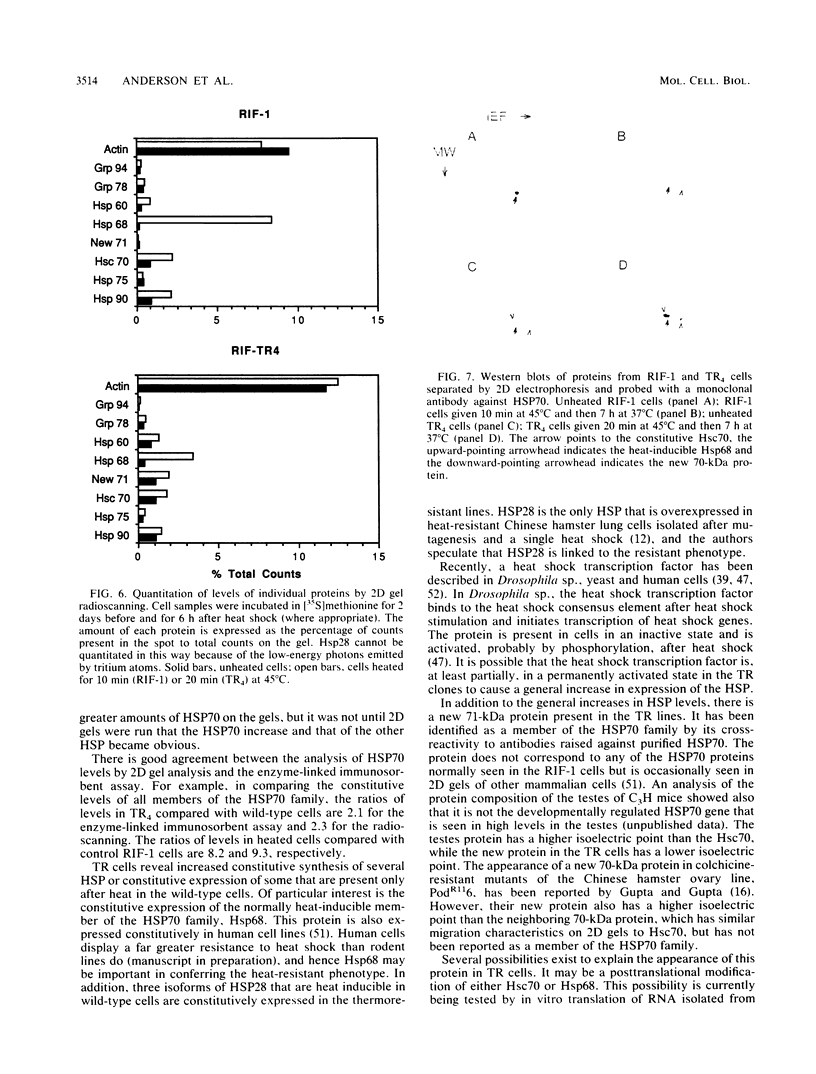
A series of heat-resistant mutants selected from a murine tumor cell line, RIF-1, display a markedly increased and stable resistance to heat shock. The mutant cell lines were analyzed for differences that may explain their increased resistance. Membrane lipid analysis showed no change in cholesterol content but an increase in the proportion of saturated fatty acids in the phospholipid fraction. Two-dimensional gel analysis revealed a generally increased constitutive synthesis of several major heat shock proteins (HSP), including HSP90, 68, 60, and 28. In addition, a new protein in the 70-kilodalton region is present in the resistant lines. The new protein has a lower isoelectric point than the constitutive HSP70 does, is only weakly induced by heat shock, and is immunologically cross-reactive with other members of the HSP70 family. After heat shock, the mutants display increases in HSP similar to those seen in the wild-type cells and they develop further transient tolerance to heat. Analysis of these mutants may help in understanding the function of HSP, both in normal growth and after heat shock.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Hahn G. M. Differential effects of hyperthermia on the Na+,K+-ATPase of Chinese hamster ovary cells. Radiat Res. 1985 Jun;102(3):314–323. [PubMed] [Google Scholar]
- Anderson R. L., Parker R. Analysis of membrane lipid composition of mammalian cells during the development of thermotolerance. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Jul;42(1):57–69. doi: 10.1080/09553008214550911. [DOI] [PubMed] [Google Scholar]
- Anderson R. L., Shiu E., Fisher G. A., Hahn G. M. DNA damage does not appear to be a trigger for thermotolerance in mammalian cells. Int J Radiat Biol. 1988 Aug;54(2):285–298. doi: 10.1080/09553008814551711. [DOI] [PubMed] [Google Scholar]
- Anderson R. L., Tao T. W., Betten D. A., Hahn G. M. Heat shock protein levels are not elevated in heat-resistant B16 melanoma cells. Radiat Res. 1986 Feb;105(2):240–246. [PubMed] [Google Scholar]
- Anderson R. L., Tao T. W., Hahn G. M. Membrane lipids of B16 melanoma cells and heat-resistant variants. Int J Radiat Biol. 1988 Nov;54(5):813–823. doi: 10.1080/09553008814552241. [DOI] [PubMed] [Google Scholar]
- Bowler K. Cellular heat injury: are membranes involved? Symp Soc Exp Biol. 1987;41:157–185. [PubMed] [Google Scholar]
- Burdon R. H., Cutmore C. M. Human heat shock gene expression and the modulation of plasma membrane Na+, K+-ATPase activity. FEBS Lett. 1982 Apr 5;140(1):45–48. doi: 10.1016/0014-5793(82)80517-6. [DOI] [PubMed] [Google Scholar]
- Calderwood S. K., Hahn G. M. Thermal sensitivity and resistance of insulin-receptor binding. Biochim Biophys Acta. 1983 Mar 15;756(1):1–8. doi: 10.1016/0304-4165(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Campbell S. D., Kruuv J., Lepock J. R. Characterization and radiation response of a heat-resistant variant of V79 cells. Radiat Res. 1983 Jan;93(1):51–61. [PubMed] [Google Scholar]
- Carper S. W., Duffy J. J., Gerner E. W. Heat shock proteins in thermotolerance and other cellular processes. Cancer Res. 1987 Oct 15;47(20):5249–5255. [PubMed] [Google Scholar]
- Chrétien P., Landry J. Enhanced constitutive expression of the 27-kDa heat shock proteins in heat-resistant variants from Chinese hamster cells. J Cell Physiol. 1988 Oct;137(1):157–166. doi: 10.1002/jcp.1041370119. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Tobey R. A. Cell-cycle analysis in 20 minutes. Science. 1974 Jun 21;184(4143):1297–1298. doi: 10.1126/science.184.4143.1297. [DOI] [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Evaluation of isoelectric focusing running conditions during two-dimensional isoelectric focusing/sodium dodecyl sulfate-polyacrylamide gel electrophoresis: variation of gel patterns with changing conditions and optimized isoelectric focusing conditions. Anal Biochem. 1984 Apr;138(1):144–155. doi: 10.1016/0003-2697(84)90783-8. [DOI] [PubMed] [Google Scholar]
- Fuhr J. E. Effect of hyperthermia on protein biosynthesis in L5178Y murine leukemic lymphoblasts. J Cell Physiol. 1974 Dec;84(3):365–372. doi: 10.1002/jcp.1040840305. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Gupta R. Mutants of chinese hamster ovary cells affected in two different microtubule-associated proteins. Genetic and biochemical studies. J Biol Chem. 1984 Feb 10;259(3):1882–1890. [PubMed] [Google Scholar]
- Hahn G. M., Li G. C., Shiu E. Interaction of amphotericin B and 43 degrees hyperthermia. Cancer Res. 1977 Mar;37(3):761–764. [PubMed] [Google Scholar]
- Hahn G. M., Shiu E. C. Protein synthesis, thermotolerance and step down heating. Int J Radiat Oncol Biol Phys. 1985 Jan;11(1):159–164. doi: 10.1016/0360-3016(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Hahn G. M., van Kersen I. Isolation and initial characterization of thermoresistant RIF tumor cell strains. Cancer Res. 1988 Apr 1;48(7):1803–1807. [PubMed] [Google Scholar]
- Hallberg R. L. No heat shock protein synthesis is required for induced thermostabilization of translational machinery. Mol Cell Biol. 1986 Jun;6(6):2267–2270. doi: 10.1128/mcb.6.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. Stable heat-resistant variants in populations of Chinese hamster cells. J Natl Cancer Inst. 1980 Jun;64(6):1495–1501. doi: 10.1093/jnci/64.6.1495. [DOI] [PubMed] [Google Scholar]
- Harris M. Temperature-resistant variants in clonal populations of pig kidney cells. Exp Cell Res. 1967 May;46(2):301–314. doi: 10.1016/0014-4827(67)90068-7. [DOI] [PubMed] [Google Scholar]
- Henle K. J., Leeper D. B. Effects of hyperthermia (45 degrees) on macromolecular synthesis in Chinese hamster ovary cells. Cancer Res. 1979 Jul;39(7 Pt 1):2665–2674. [PubMed] [Google Scholar]
- Kwock L., Lin P. S., Hefter K. A comparison of the effects of hyperthermia on cell growth in human T and B lymphoid cells: relationship to alterations in plasma membrane transport properties. Radiat Res. 1985 Jan;101(1):197–206. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landry J., Bernier D., Chrétien P., Nicole L. M., Tanguay R. M., Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982 Jun;42(6):2457–2461. [PubMed] [Google Scholar]
- Laszlo A., Li G. C. Heat-resistant variants of Chinese hamster fibroblasts altered in expression of heat shock protein. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8029–8033. doi: 10.1073/pnas.82.23.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo A. The relationship of heat-shock proteins, thermotolerance, and protein synthesis. Exp Cell Res. 1988 Oct;178(2):401–414. doi: 10.1016/0014-4827(88)90409-0. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Chapman D. The effects of temperature on biological membranes and their models. Symp Soc Exp Biol. 1987;41:35–52. [PubMed] [Google Scholar]
- Li G. C., Mak J. Y. Induction of heat shock protein synthesis in murine tumors during the development of thermotolerance. Cancer Res. 1985 Aug;45(8):3816–3824. [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Moran L. A. Molecular cloning and analysis of DNA complementary to three mouse Mr = 68,000 heat shock protein mRNAs. J Biol Chem. 1986 Feb 15;261(5):2102–2112. [PubMed] [Google Scholar]
- Magun B. E., Fennie C. W. Effects of hyperthermia on binding, internalization, and degradation of epidermal growth factor. Radiat Res. 1981 Apr;86(1):133–146. [PubMed] [Google Scholar]
- McCormick W., Penman S. Regulation of protein synthesis in HeLa cells: translation at elevated temperatures. J Mol Biol. 1969 Jan;39(2):315–333. doi: 10.1016/0022-2836(69)90320-9. [DOI] [PubMed] [Google Scholar]
- Minton K. W., Karmin P., Hahn G. M., Minton A. P. Nonspecific stabilization of stress-susceptible proteins by stress-resistant proteins: a model for the biological role of heat shock proteins. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7107–7111. doi: 10.1073/pnas.79.23.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzen L. A., Welch W. J. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol. 1988 Apr;106(4):1105–1116. doi: 10.1083/jcb.106.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Petersen N. S., Mitchell H. K. Recovery of protein synthesis after heat shock: prior heat treatment affects the ability of cells to translate mRNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1708–1711. doi: 10.1073/pnas.78.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay N. A mutant in a major heat shock protein of Escherichia coli continues to show inducible thermotolerance. Mol Gen Genet. 1988 Feb;211(2):332–334. doi: 10.1007/BF00330612. [DOI] [PubMed] [Google Scholar]
- Rice G. C., Fisher K. A., Fisher G. A., Hahn G. M. Correlation of mammalian cell killing by heat shock to intramembranous particle aggregation and lateral phase separation using fluorescence-activated cell sorting. Radiat Res. 1987 Nov;112(2):351–364. [PubMed] [Google Scholar]
- Rice G. C., Hahn G. M. Modulation of adriamycin transport by hyperthermia as measured by fluorescence-activated cell sorting. Cancer Chemother Pharmacol. 1987;20(3):183–187. doi: 10.1007/BF00570481. [DOI] [PubMed] [Google Scholar]
- Rowley R., Joyner D. E., Stewart J. R. In vitro response to hyperthermia or X-irradiation of diploid and tetraploid RIF-1 cells separated by centrifugal elutriation. Int J Hyperthermia. 1987 May-Jun;3(3):235–244. doi: 10.3109/02656738709140390. [DOI] [PubMed] [Google Scholar]
- SELAWRY O. S., GOLDSTEIN M. N., McCORMICK T. Hyperthermia in tissue-cultured cells of malignant origin. Cancer Res. 1957 Sep;17(8):785–791. [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R. Heat shock proteins and protection of proliferation and translation in mammalian cells. Cancer Res. 1984 Nov;44(11):5188–5194. [PubMed] [Google Scholar]
- Sorger P. K., Lewis M. J., Pelham H. R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- Subjeck J. R., Sciandra J. J., Johnson R. J. Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol. 1982 Aug;55(656):579–584. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- VanBogelen R. A., Acton M. A., Neidhardt F. C. Induction of the heat shock regulon does not produce thermotolerance in Escherichia coli. Genes Dev. 1987 Aug;1(6):525–531. doi: 10.1101/gad.1.6.525. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Mizzen L. A. Characterization of the thermotolerant cell. II. Effects on the intracellular distribution of heat-shock protein 70, intermediate filaments, and small nuclear ribonucleoprotein complexes. J Cell Biol. 1988 Apr;106(4):1117–1130. doi: 10.1083/jcb.106.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimarino V., Wu C. Induction of sequence-specific binding of Drosophila heat shock activator protein without protein synthesis. 1987 Jun 25-Jul 1Nature. 327(6124):727–730. doi: 10.1038/327727a0. [DOI] [PubMed] [Google Scholar]