Abstract
AIM: To compare effects of different resuscitation fluid on microcirculation, inflammation, intestinal barrier and clinical results in severe acute pancreatitis (SAP).
METHODS: One hundred and twenty patients with SAP were enrolled at the Pancreatic Disease Institute between January 2007 and March 2010. The patients were randomly treated with normal saline (NS group), combination of normal saline and hydroxyethyl starch (HES) (SH group), combination of normal saline, hydroxyethyl starch and glutamine (SHG group) in resuscitation. The ratio of normal saline to HES in the SH and SHG groups was 3:1. The glutamine (20% glutamine dipeptide, 100 mL/d) was supplemented into the resuscitation liquid in the SHG group. Complications and outcomes including respiratory and abdominal infection, sepsis, abdominal hemorrhage, intra-abdominal hypertension, abdominal compartment syndrome (ACS), renal failure, acute respiratory distress syndrome (ARDS), multiple organ dysfunction syndrome (MODS), operation intervention, length of intensive care unit stay, length of hospital stay, and mortality at 60 d were compared. Moreover, blood oxygen saturation (SpO2), gastric intramucosal pH value (pHi), intra-abdominal pressure (IAP), inflammation cytokines, urine lactulose/mannitol (L/M) ratio, and serum endotoxin were investigated to evaluate the inflammatory reaction and gut barrier.
RESULTS: Compared to the NS group, patients in the SH and SHG groups accessed the endpoint more quickly (3.9 ± 0.23 d and 4.1 ± 0.21 d vs 5.8 ± 0.25 d, P < 0.05) with less fluid volume (67.26 ± 28.53 mL/kg/d, 61.79 ± 27.61 mL/kg per day vs 85.23 ± 21.27 mL/kg per day, P < 0.05). Compared to the NS group, incidence of renal dysfunction, ARDS, MODS and ACS in the SH and SHG groups was obviously lower. Furthermore, incidence of respiratory and abdominal infection was significantly decreased in the SH and SHG groups, while no significant difference in sepsis was seen. Moreover, less operation time was needed in the SH and SHG group than the NS group, but the difference was not significant. The mortality did not differ significantly among these groups. Blood SpO2 and gastric mucosal pHi in the SH and SHG groups increased more quickly than in the NS group, while IAP was significantly decreased in the SH and SHG group. Moreover, the serum tumor necrosis factor-α, interleukin-8 and C-reactive protein levels in the SH and SHG groups were obviously lower than in the NS group at each time point. Furthermore, urine L/M ratio and serum endotoxin were significantly lower in the SH group and further decreased in the SHG group.
CONCLUSION: Results indicated that combination of normal saline, HES and glutamine are more efficient in resuscitation of SAP by relieving inflammation and sustaining the intestinal barrier.
Keywords: Microcirculation, Intestinal barrier, Inflammatory reaction, Intra-abdominal hypertension, Capillary leakage syndrome
INTRODUCTION
Severe acute pancreatitis (SAP) has a mortality rate about 30% and is characterized by pancreatic necrosis, cytokine activation, systemic inflammatory response sydrome (SIRS), and multiple organ dysfunction syndrome (MODS)[1,2]. Accumulative results have demonstrated that microcirculation perfusion and hypoxia have a significant impact on the early stages of disease and play an important role in the pathogenesis of necrosis[3]. Different from normal hypovolemia caused by trauma or bleeding, microcirculatory disorder of SAP is caused by special SIRS. Overexpressed inflammatory media such as tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 in SIRS will injury the microcirculation endothelium and then increase capillary permeability and fluid sequestration, leading to capillary leakage syndrome and MODS[4].
Moreover, the microcirculatory disorder of the intestinal will lead to intestinal ischemia reperfusion injury and damage the intestinal barrier, which could facilitate translocation of intestinal bacteria and enhance leukocyte activation and inflammatory cytokine release[5]. If the microcirculatory disorder cannot be blocked at the initial stage, it will be exaggerated and form positive feedback. Thus, the excess cytokine will further injury the distal organs and lead to irreversible multiorgan failure[6,7]. Moreover, bacterial translocation from the gut will cause severe infection[8]. Therefore, adequate prompt fluid resuscitation is crucial for prevention of systemic complications[9-11]. Thus, the purpose of effective fluid resuscitation of SAP is not only to supply the deficiency of blood volume, but also especially to stabilize the capillary permeability, modulate the inflammation reaction, and sustain the intestinal barrier function.
Two types of fluids frequently used today for active resuscitation are colloid fluids with large molecules (hetastarch, dextran 40, and albumin) that keep fluid intravascular, and crystalloid fluids with added electrolytes (normal saline, Ringer’s, and lactated Ringer’s). Both crystalloid and colloid solutions are considered effective for the resuscitation of a hypovolemic patient, because neither fluid provides a survival benefit that is superior to the other[12]. When trying to augment cardiac output and blood pressure, colloids have an advantage over crystalloid solutions, because a larger percentage enters the intravascular space and remains there for a longer period of time. This is because colloids provide the greatest effect on intravascular volume expansion and improve flow secondary to their low viscosity, which is equal to that of water[13].
In the septic shock model with concomitant capillary leakage in the presence of a marked albumin loss, the hydroxyethyl starch (HES) could preserve systemic oxygenation and hemodynamics[14]. Increased capillary permeability leading to fluid loss from the intravascular space and fluid sequestration into the third space are hallmarks of SAP[15]. Clinically, capillary leakage is reflected by intravascular fluid loss leading to hypovolemia [low central venous pressure (CVP)], hemoconcentration (high packed cell volume), and extravascular fluid sequestration in the retroperitoneal, lungs, pleural and abdominal cavities[16]. Although colloids such as HES are supposed more suitable than crystalloids for volume resuscitation of hypovolemia, prospective clinic studies have seldom involved comparing effects of crystalloids and HES in volume resuscitation in SAP[10,11,17].
Some research has shown that HES reduces intestinal permeability by modulating inflammatory response and has a promising effect on survival, together with antibiotics under septic conditions[18]. Although glutamine has been proved to protect the intestinal barrier as a nutrient supplement in nutritional support of SAP, the effects of glutamine delivered as a supplement in resuscitation fluid in the early stage of SAP have not been defined[19-21].
In the present study, we compared the clinical results of different resuscitation fluids including normal saline, combination of normal saline and HES, and combination of normal saline, HES and glutamine in resuscitation of SAP. Moreover, the effects on microcirculation, inflammation reaction and intestinal barrier were investigated.
MATERIALS AND METHODS
Patients
One hundred and twenty patients with SAP were enrolled at the Pancreatic Disease Institute, Union Hospital (Wuhan, China) between January 2007 and March 2010. All of the patients were diagnosed with SAP according to the Atlanta Classification System[22], and were aged 18-60 years. Exclusion criteria were heart disease, severe renal and hepatic dysfunction, coagulation disturbances, and allergy to HES or glutamine. Those patients with manifestation more than 48 h or who received resuscitation from the other hospital were also excluded. Informed consent was obtained from the patients and approval was obtained from the Ethics Committee of Union Hospital, Huazhong University of Science and Technology. The demographic information of the patients is shown in Table 1. If the patient could not successfully achieve a balance of output and input within 7 d, we considered them to have failed resuscitation. However, these cases were not removed from the study and all the results were still included.
Table 1.
Demographic information of patients with severe acute pancreatitis treated by different resuscitation fluid (mean ± SD)
| NS group (n = 40) | SH group (n = 40) | SHG group (n = 40) | |
| Age (yr) | 41.86 ± 13.85 | 44.50 ± 9.77 | 45.11 ± 11.57 |
| Sex n (%) | |||
| Male | 20 (50) | 22 (55) | 21 (52.5) |
| Female | 20 (50) | 18 (45) | 19 (47.5) |
| Height (cm) | 165.86 ± 6.04 | 165.00 ± 9.03 | 169.25 ± 6.67 |
| Weight (kg) | 66.5 ± 8.63 | 69.00 ± 9.68 | 72.38 ± 8.43 |
| APACHE II score | 11.2 ± 0.7 | 10.9 ± 0.6 | 11.3 ± 0.4 |
| MAP (mmHg) | 62.3 ± 9.3 | 64.8 ± 9.2 | 63.6 ± 8.9 |
| BUN/Cr ratio | 23.9 ± 3.6 | 24.2 ± 3.2 | 23.7 ± 3.7 |
No significant differences were observed among the study groups regarding demographic data. APACHE: Acute physiology and chronic health evaluation; MAP: Mean arterial pressure; BUN/Cr: Blood urea nitrogen/creatinine; NS group: Normal saline; SH group: Combination of normal saline and hydroxyethyl starch; SHG group: Combination of normal saline, hydroxyethyl starch and glutamine.
Management of resuscitation
The patients were randomly divided into three groups that were resuscitated with normal saline (NS group), combination of normal saline and hydroxyethyl starch (SH group; 130 kD, Sino-Swed Pharmaceutical Corp. Ltd.), or combination of normal saline, hydroxyethyl starch and glutamine (SHG group; glutamine dipeptide, Sino-Swed Pharmaceutical Corp. Ltd.). The ratio of normal saline to HES in the SH and SHG groups was 3:1. Glutamine (20% glutamine dipeptide, 100 mL/d) was supplemented into the resuscitation liquid in the SHG group.
All of the patients with suspected SAP were initially transferred to the pancreatic intensive care unit (PICU). The vital signs, oxygen saturation (SpO2), gastric intramucosal PH value (pHi), arterial blood-gas analysis, mean arterial pressure (MAP) and intra-abdominal pressure (IAP) were monitored to guide the treatment. All the patients received a central venous catheter capable of measuring central venous oxygen saturation (ScvO2) and CVP.
Meanwhile, the fluid resuscitation was promptly conducted as early as possible. Generally, we infused 1 L normal saline in the NS group or combination of 500 mL normal saline and 500 mL HES in the HS and HSG groups in the first 2 h (500 mL/h) to achieve a CVP of 8-12 mmHg. If the MAP was < 65 mmHg, vasopressors were given to maintain an MAP of at least 65 mmHg. If the MAP was > 90 mmHg, vasodilators were given until it was ≤ 90 mmHg[23]. If the urine output was < 0.5 mL/kg/h after the CVP and MAP were stabilized, 20 mg dihydrochlorothiazide bolus was infused and followed with 1-2 mL/h continuous infusion via syringe pump to maintain the urine output at > 0.5 mL/kg per hour. After that, resuscitation fluid was continually infused at a speed of 150-300 mL/h (approximately 2-3 mL/kg per hour, ratio of normal saline to HES is 3:1) and modulated depending on the reaction of early resuscitation and parameters in the later course, which maintained the urine at 0.5-1 mL/kg per hour and prevented excess resuscitation.
Moreover, if the oliguria continued for > 2 d and the ratio of blood urea nitrogen/creatinine (BUN/Cr) significantly increased, continuous venovenous hemofiltration (CVVH) with ultrafiltration modulation was performed to extra the liquid sequestration in the “3rd space” and overexcited inflammatory mediators. Most of the patients were accompanied with respiratory dysfunction, and for those patients with ScvO2 < 70% after stabilization of CVP and MAP, we did not perform blood transfusion but intensified oxygen supplementation through pressure oxygen mask or tracheal intubation with artificial respirator. Except for the CVP, MAP, urine and ScvO2 adopted as early resuscitation parameters, IAP was also used to assess the microcirculation dysfunction. Even without significant abnormality in the other parameters, the infusion was carefully controlled and the CVVH applied to balance the output and input in those patients with significantly increased IAP. The input of resuscitating liquid depended on the output including ultrafiltration volume of CVVH, urine and non-dominant water loss. The ultimate endpoint of resuscitation was defined as the balance of input and output. Those patients who approached the endpoint in 7 d were considered to have successful resuscitation, otherwise it had failed.
Complications and clinical outcomes
Complications and outcomes were recorded throughout the whole course, including respiratory infection, abdominal infection, sepsis, abdominal hemorrhage, intra-abdominal hypertension (IAH), abdominal compartment syndrome (ACS), renal failure, acute respiratory distress syndrome (ARDS), MODS, operation intervention, length of intensive care unit stay, length of hospital stay and mortality at 60 d.
All the clinical parameters were record from day 1 to day 7 after administration. Resuscitation fluid input, abdominal drainage fluid and urine output were recorded every day. Oxygen supply and microcirculation perfusion was evaluated by pulse SpO2 and pHi, which were detected by bedside monitor (Life Scope A, Nihon Kohden, Irvine, CA, Untied States) and gastric mucosal pH monitor (Tonocap, Datex-Ohmeda, Finland) respectively[24]. Harvested heparinized blood was centrifuged and the plasma was removed and stored at -80 °C for later examination.
Measurements of IAP
IAP was measured according to the method of Oda et al[25]. In brief, an 18-gauge catheter was inserted into the culture aspiration port of the urine catheter, and a line filled with saline was connected to the pressure transducer. After urine had been completely drained from the bladder, the urine catheter line was clamped, and then 100 mL of saline was instilled in the bladder and the pressure was measured at the end of expiration. IAP was measured first on admission to the PICU, and twice daily thereafter in the morning and evening, in principle. According to the consensus guidelines, IAH was defined as IAP ≥ 12 mmHg and ACS as IAP > 20 mmHg with evidence of organ dysfunction.
Plasma cytokines assay
Serum TNF-α, IL-8 and C-reactive protein (CRP) from day 1 to day 7 were evaluated by enzyme-linked immuno sorbent assay (R-D Systems, Minneapolis, MN, United States) according to the manufacturer’s instructions.
Urine lactulose/mannitol ratio assay
Urine lactulose/mannitol (L/M) ratio during 7 d was analyzed to evaluate intestinal permeability as described before[21]. All the patients fasted for at least 6 h and their bladders were emptied before the test. The test solution consisted of 10 g lactulose and 5 g mannitol in a total volume of 50 mL through nasojejunal tube. The urine volume was collected for the subsequent 6 h. The urine volume was recorded, and 10 mL was frozen and stored at -80 °C. L/M ratio in urine was analyzed by Hi-Crush Partners LP.
Endotoxin assay
Serum concentration of endotoxin at 7 d was detected by quantitative chromogenic limulus amebocyte lysate assay (QCL-1000; Whittaker MA Bioproducts, Walkersville, MD, United States) according to the manufacturer’s instructions. Blood was drawn aseptically into lipopolysaccharide-free tubes. All samples were processed in a laminar flow hood. To minimize nonspecific plasma inhibitors, samples were diluted with pyrogen-free water and heat inactivated at 100 °C for 10 min. Escherichia coli 055:B5 reference endotoxin (1 endotoxin unit = 0.6 ng/mL) was used for the standard curve (Whittaker MA Bioproducts).
Statistical analysis
Statistical analyses were performed with SPSS version 12.0.2. Data are presented as mean ± SD. χ2 analysis and one-way repeated-measures analysis of variance were used for the analysis of differences. P < 0.05 was considered significant.
RESULTS
Demographic information
As shown in Table 1, no significant differences were observed between the study groups regarding demographic data including age, agenda, height, weight, Acute physiology and chronic health evaluation II score, MAP and BUN/Cr ratio at the initial time.
Fluid resuscitation and fluid balance
All patients in the three groups received resuscitation at an early stage after manifestation of SAP (12.8 ± 6.7 h, 13.1 ± 5.4 h, and 12.2 ± 6.3 h). The balance of input and output was considered as the ultimate endpoint of resuscitation. Compared to the NS group, it took a significantly shorter time to approach the resuscitation endpoint in the SH and SHG groups (5.8 ± 0.25 d vs 3.9 ± 0.23 d and 4.1 ± 0.21 d, P < 0.05). Nevertheless, the volumes of fluid administered in the SH and SHG groups was obviously lower than that in the NS group (67.26 ± 28.53 and 61.79 ± 27.61 vs 85.23 ± 21.27 mL/kg per day, P < 0.05), while the abdominal drainage fluid in the SH and SHG groups was significantly lower than that in the NS group (Table 2).
Table 2.
Window of manifestation to resuscitation, time of resuscitation endpoint, volume of resuscitation fluid, urine output, and abdominal drainage fluid in different groups
| NS group | SH group | SHG group | |
| Window to resuscitation (h) | 12.8 ± 6.7 | 13.1 ± 5.4 | 12.2 ± 6.3 |
| Time to endpoint (d) | 5.8 ± 0.25 | 3.9 ± 0.231 | 4.1 ± 0.211 |
| Resuscitation fluid (mL/kg per day) | 61.79 ± 7.61 | 46.93 ± 12.381 | 44.75 ± 8.531 |
| Urine output/d (mL/kg per day) | 31.3 ± 5.47 | 28.71 ± 11.62 | 27.94 ± 10.62 |
| Abdominal drainage fluid (mL/kg per day) | 11.32 ± 2.13 | 6.28 ± 3.261 | 6.35 ± 1.421 |
P < 0.05 vs normal saline (NS group). Statistic measure of variation is standard error. SH group: Combination of normal saline and hydroxyethyl starch; SHG group: Combination of normal saline, hydroxyethyl starch and glutamine.
Complications and outcomes
When infection was suspected, ultrasound-guided percutaneous aspiration of pancreatic tissue, sputum and blood was performed with Gram’s stain and culture. If ultrasound-guided percutaneous aspiration confirmed the infection, antibiotics were administered following the results of culture and antibiotic sensitivity. If the episode is not effectively relieved, the patient should undergo debridement. Compared to the NS group, the incidence of renal dysfunction, ARDS, MODS and ACS in the SH and SHG groups was obviously lower. Furthermore, the incidence of respiratory and abdominal infection was significantly decreased in the SH and SHG groups, while no significant difference in sepsis was seen. Moreover, less operation time was needed in the SH and SHG groups than in the NS group, but the difference was not significant (Table 3). IAP in the NS group was significantly higher than in the SH and SHG groups at each time point (Figure 1). Patients in the SH and SHG groups had a notably shorter length of stay in the intensive care unit (ICU) and hospital than the NS group had. Although mortality in the NS group was higher than in the SH and SHG groups, no significant difference was observed. No significant difference in any of the parameters was noted between the SH and SHG groups.
Table 3.
Complications and outcomes of patients treated by different resuscitation fluid
| NS group | SH group | SHG group | |
| Renal dysfunction | 11 | 31 | 41 |
| ARDS | 16 | 61 | 71 |
| MODS | 10 | 31 | 31 |
| ACS | 6 | 11 | 11 |
| Lung infection | 13 | 51 | 51 |
| Abdominal infection | 11 | 31 | 21 |
| Sepsis | 3 | 2 | 3 |
| Operation | 6 | 2 | 2 |
| LOIS | 14 ± 8.2 | 10 ± 9.41 | 11 ± 6.31 |
| LOHS | 31 ± 22.8 | 22 ± 18.91 | 21 ± 23.71 |
| Mortality | 5 | 2 | 3 |
P < 0.05 vs normal saline (NS group). SH group: Combination of normal saline and hydroxyethyl starch; SHG group: Combination of normal saline, hydroxyethyl starch and glutamine; ARDS: Acute respiratory distress syndrome; MODS: Multiple organ dysfunction syndrome; ACS: Abdominal compartment syndrome; LOIS: Length of intensive care unit stay; LOHS: Length of hospital stay.
Figure 1.
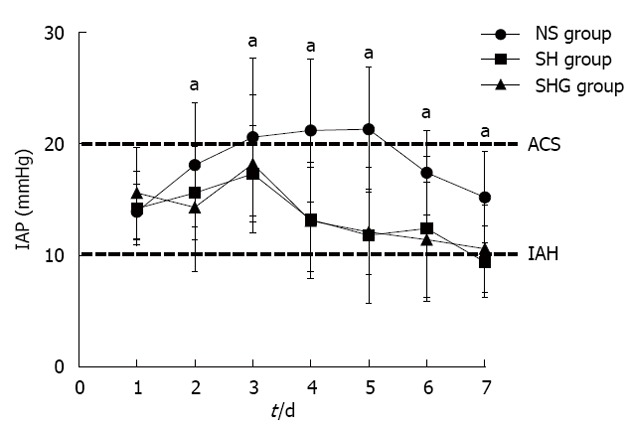
Effect of different resuscitation fluids on changes in intra-abdominal pressure in severe acute pancreatitis. Intra-abdominal pressure (IAP) was indirectly measured via a bladder catheter during 7 d and performed twice daily. All of the patients had intra-abdominal hypertension on d 1 (IAP > 10 mmHg). aP < 0.05 vs normal saline (NS group). SH group: Combination of normal saline and hydroxyethyl starch; SHG group: Combination of normal saline, hydroxyethyl starch and glutamine; ACS: Abdominal compartment syndrome; IAH: Intra-abdominal hypertension.
SpO2 and microcirculation perfusion
The SpO2 of the SH and SHG groups increased more quickly than in the NS group and was significantly higher at day 4 and day 5. Similarly, pHi in the SH and SHG groups also increased faster than in the NS group from day 2 to day 4 (Figure 2).
Figure 2.
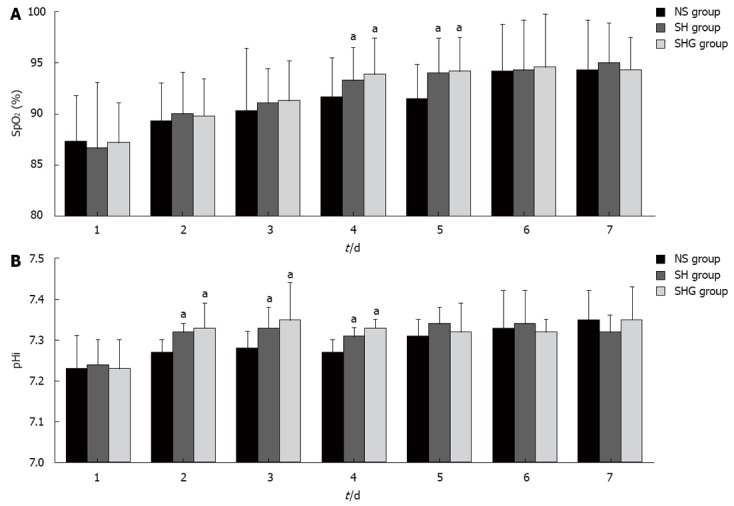
Effect of different resuscitation fluids on circulation oxygen supply and microcirculation perfusion. A: Effect of different fluids on circulation oxygen supply was evaluated with pulse oxygen saturation (SpO2) by automatic monitoring; B: Microcirculation perfusion was assessed with gastric intramucosal pH value (pHi) by Tonocap monitor. aP < 0.05 vs normal saline (NS group). SH group: Combination of normal saline and hydroxyethyl starch; SHG group: Combination of normal saline, hydroxyethyl starch and glutamine.
Serum cytokine and CRP concentration
Serum TNF-α, IL-8 and CRP were detected daily from day 1 to day 7. Although the TNF-α concentration in all three groups increased to a peak at d 3 and then gradually decreased, it was significantly higher in the NS group at days 3-5 (Figure 3A). Unlike the highest IL-8 concentration in the NS group at day 4, the SH and SHG groups had the highest IL-8 concentration at day 3 and then it significantly decreased. Furthermore, the IL-8 concentration in the SHG group was obviously lower than in the SH group at days 4-7 (Figure 3B). The CRP concentration in the NS group continually increased until day 5, while it was significantly decreased in the SH and SHG groups after day 3. Moreover, the CRP concentration in the SHG group was significantly lower than that in the SH group after day 7 (Figure 3C).
Figure 3.
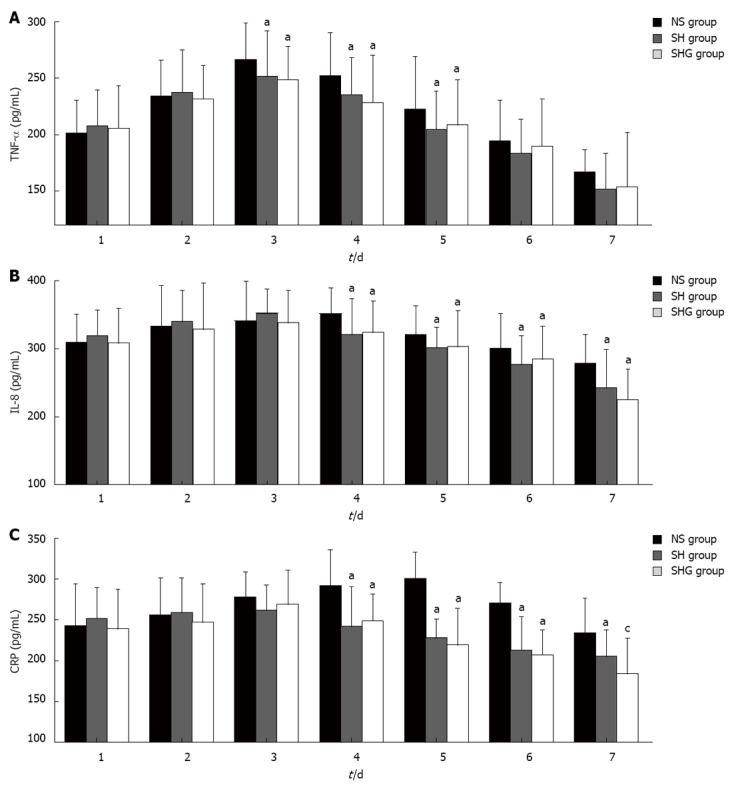
Effects of different resuscitation fluids on serum cytokine and C-reactive protein. Serum tumor necrosis factor (TNF)-α (A), interleukin (IL)-8 (B) and C-reactive protein (CRP) (C) concentration was evaluated by enzyme-linked immuno sorbent assay. aP < 0.05 vs normal saline (NS) group; cP < 0.05 vs combination of normal saline and hydroxyethyl starch (SH group). SHG group: Combination of normal saline, hydroxyethyl starch and glutamine.
Intestinal permeability
Intestinal permeability was assessed by urine L/M ratio and serum endotoxin. Urine L/M ratio in all three groups increased after administration of SAP. Compared to the NS group, urine L/M ratio in the SH and SHG groups was significantly lower after day 4. Moreover, compared to the SH group, it further decreased in the SHG group after day 5. Parallel to urine L/M ratio, serum endotoxin in the NS group was also significantly higher than that in the SH and SHG groups. Nevertheless, serum endotoxin in the SHG group was markedly lower than that in the SH group after day 6 (Figure 4).
Figure 4.
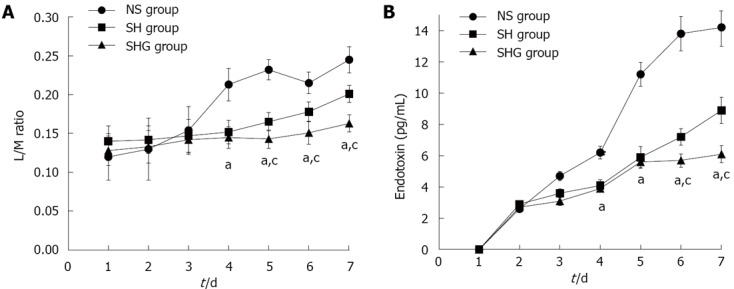
Effect of different fluids on intestinal mucosa barrier function. A: Lactulose/mannitol (L/M) ratio of urine in normal saline (NS group), combination of normal saline and hydroxyethyl starch (SH group) and combination of normal saline, hydroxyethyl starch and glutamine (SHG group) was measured by Hi-Crush Partners LP; B: Serum endotoxin in different groups was detected by quantitative chromogenic limulus amebocyte lysate assay reagent. aP < 0.05 vs NS group; cP < 0.05 vs SH group.
DISCUSSION
Many studies have indicated that colloids such as HES are more suitable than crystalline solutions for volume resuscitation in hypovolemia of sepsis, of which microcirculation arrangement are same as SAP[26-28]. Our present study demonstrated that less time and volume were needed to achieve the resuscitation endpoint in the SH and SHG groups. Moreover, higher urine output and lower abdominal drainage were seen in the SH and SHG groups. Furthermore, the incidence of MODS and ACS were significantly decreased in the SH and SHG groups.
This indicated that combination of HES and crystalloids might effectively attenuate the capillary leakage by maintaining colloid osmotic pressure and then decreasing extravascular fluid sequestration, such as pleural effusion and abdominal ascites. As an important clinical clue for extensive sequestration, the IAP and IAH in the SH and SHG groups were decreased significantly more than those in the NS group.
Although the mortality was not significantly decreased, the incidence of respiratory infection and abdominal infection was significantly reduced in the SH and SHG groups. This indicated that the deceased liquid sequestration in the abdomen and lungs significantly reduced the infection risk. Furthermore, less operation time was needed and a lower level of organ dysfunction occurred in the SH and SHG groups. Thus, shorter length of ICU and hospital stay was seen in the SH and SHG groups. These results further suggest that combination of HES and normal saline might be effective in resuscitation and effectively decrease infective complications and organ failure in SAP. Although the NS group had a higher incidence of respiratory and abdominal infection, incidence of sepsis and mortality in the NS did not differ significantly from that in the SH and SHG groups. This might because the treatment of SAP is multimodal including resuscitation, organ support, nutrition support, operative intervention, and antibiotics and these different single resuscitation managements were not sufficient to affect mortality.
The target of effective resuscitation is not only maintaining the blood volume, but more importantly, to improve tissue oxygenation and microcirculation perfusion. In our research, arterial SpO2 and pHi were detected to evaluate the oxygenation and microcirculation perfusion. We showed that SpO2 and pHi in the SH and SHG groups recovered more quickly than in the NS group. We showed that combination of HES and normal saline was effective in expanding the blood volume and improving the microcirculation and tissue oxygenation.
Our research demonstrated that inflammatory factors including TNF-α, IL-8 and CRP, in the SH and SHG groups were significantly lower and decreased earlier than those in the NS group, which indicates that HES might modulate the inflammatory reaction. Other research has also discovered that HES attenuates capillary leakage through modulation of the inflammation reaction in sepsis and abdominal surgery. It is speculated that HES may inhibit nuclear factor-κB activation and neutrophil adhesion and migration[29-31], but the exact mechanism has not been defined. Other research implies that HES prevents the inflammatory reaction through relieving ischemia-reperfusion injury in the intestine[18,32]. The present study demonstrated that intestinal permeability significantly decreased in the SH and SHG groups, which also implied that the inflammation reaction was modulated by improvement of intestinal ischemia-reperfusion injury.
Flint et al[33] have shown that the severity of SAP can be exacerbated by intestinal ischemia-reperfusion injury. When the intestinal barrier is disrupted, the luminal content invades the portal venous and lymphatic systems. This translocation activates immune cells downstream from the intestinal mucosa to release inflammatory mediators that drive the onset of SIRS and MODS in SAP[34-36]. Our present study demonstrated that urine L/M ratio and endotoxin were significantly decreased after treatment with HES and normal saline. It indicated that sustaining the intestinal barrier by HES is one of the important reasons for decreased mortality from infection and MODS.
Many studies have shown that nutrition support supplemented with specific immunonutrients such as glutamine may protect the intestinal barrier and modulate the acute phase responses, thereby potentially improving outcome in SAP[19-21]. We speculated that supplementation of glutamine in resuscitation could further sustain intestinal function and improve the clinical outcomes of SAP. Our results showed that the IL-8, CRP, urine L/M ratio and serum endotoxin were further decreased after supplementation with glutamine, while the clinical outcomes and complications had no significant change. This implies that ischemia-reperfusion injury is a major reason for intestinal dysfunction in the early stage of SAP.
In summary, the present study showed that combination of HES and normal saline was more efficient in fluid resuscitation of SAP by modulating inflammation and the intestinal barrier, which resulted in a lower level of infection and organ failure. Nevertheless, although supplementation with glutamine could further modulate inflammatory reaction and intestinal, while no significant changes were seen in clinical results.
COMMENTS
Background
Accumulative results demonstrated that microcirculation perfusion and hypoxia leading to capillary leakage syndrome (CLS) and multiple organ dysfunction syndrome (MODS) in severe acute pancreatitis (SAP). Although the colloid such as hydroxyethyl starch (HES) are supposed more suitable than crystalloid in volume resuscitation in hypovolemia, seldom prospective clinic researches have involved in SAP. Given to protect intestinal barrier as nutrient supplement in SAP, the effects of glutamine delivered as supplement of resuscitation fluid in early stage of SAP were not defined. Thus, the present in present set to compare clinical results of different resuscitation fluid including normal saline, combination of normal saline and HES as well as combination of normal saline, HES and glutamine in resuscitation of SAP, respectively.
Research frontiers
Overexpressed inflammatory cytokine of SAP such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-8 will injury the capillary permeability and lead to CLS, which further damage the intestinal barrier and induce translocation of intestinal bacterial. Thus, the effective fluid resuscitation of SAP should be not only to sustain body volume but also improve capillary permeability and intestinal barrier function. Although the colloid such as HES are supposed more suitable than crystalloid in volume resuscitation in hypovolemia, seldom prospective clinic researches have involved in comparing effects of crystalloid and HES in volume resuscitation in SAP.
Innovations and breakthroughs
Many researches indicated that colloid such as HES are supposed more suitable than crystalline in volume resuscitation in hypovolemia of sepsis, but it is unknown whether the HES is also more efficient in SAP which microcirculation arrangement is similar to septic. The present results firstly demonstrated that less time and volume were needed to get resuscitation endpoint in combination of normal saline and hydroxyethyl starch (HS) group and HES and glutamine (SHG group). Moreover, higher urine output and lower abdominal drainage were showed in SH and SHG groups. Furthermore, the incidence of MODS and abdominal compartment syndrome (ACS), were significantly decreased in SH and SHG groups. The results displayed that oxygen saturation and intramucosal pH value of combination of normal saline and hydroxyethyl starch (SH group) and SHG group recovered more quickly than normal saline (NS) group which implied that combination of HES and normal saline is not only effective expanding blood volume but also improving the microcirculation and tissue oxygenation. This research demonstrated that the inflammatory factor including TNF-α, IL-8 and C-reactive protein of SH and SHG group were significantly lower and decreased earlier than those of NS group. Moreover, our present study demonstrated that urine lactulose/mannitol ration and endotoxin were significantly decreased after treated with HES and normal saline.
Applications
The present study showed that combination of HES and normal saline was more efficient in fluid resuscitation of SAP by modulate inflammation and intestinal barrier, which resulted in lower modality of infection and organ failure. Moreover, the supplementary with glutamine could further modulate inflammatory reaction and intestinal. It implied that combination of normal saline, HES and glutamine might be an efficient resuscitation fluid in SAP which deserves to be investigated in further clinical study.
Terminology
CLS is a rare medical condition characterized by self-reversing episodes during which the endothelial cells which line the capillaries are thought to separate for a few days, allowing for a leakage of fluid from the circulatory system to the interstitial space, resulting in a dangerous hypotension (low blood pressure), hemoconcentration, and hypoalbuminemia. It is a life-threatening illness because each episode has the potential to cause damage to, or the failure of, vital organs due to limited perfusion. ACS occurs when the abdomen becomes subject to increased pressure. Specific cause of ACS is not known, although some causes can be sepsis and severe abdominal trauma. Increasing pressure reduces blood flow to abdominal organs and impairs pulmonary, cardiovascular, renal, and gastro-intestinal function, causing MODS and death.
Peer review
This is an interesting paper with potentially clinical use.
Footnotes
Supported by National Science Foundation Committee of China, No. 30600594 and 30972900; the Fundamental Research Funds for the Central Universities of China, No. 2011JC046
P- Reviewer Jeng JH S- Editor Song XX L- Editor A E- Editor Xiong L
References
- 1.Mitchell RM, Byrne MF, Baillie J. Pancreatitis. Lancet. 2003;361:1447–1455. doi: 10.1016/s0140-6736(03)13139-x. [DOI] [PubMed] [Google Scholar]
- 2.Perez A, Whang EE, Brooks DC, Moore FD, Hughes MD, Sica GT, Zinner MJ, Ashley SW, Banks PA. Is severity of necrotizing pancreatitis increased in extended necrosis and infected necrosis? Pancreas. 2002;25:229–233. doi: 10.1097/00006676-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kinnala PJ, Kuttila KT, Grönroos JM, Havia TV, Nevalainen TJ, Niinikoski JH. Pancreatic tissue perfusion in experimental acute pancreatitis. Eur J Surg. 2001;167:689–694. doi: 10.1080/11024150152619345. [DOI] [PubMed] [Google Scholar]
- 4.Bassi D, Kollias N, Fernandez-del Castillo C, Foitzik T, Warshaw AL, Rattner DW. Impairment of pancreatic microcirculation correlates with the severity of acute experimental pancreatitis. J Am Coll Surg. 1994;179:257–263. [PubMed] [Google Scholar]
- 5.Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130–135. doi: 10.1007/s002689900204. [DOI] [PubMed] [Google Scholar]
- 6.Ryan CM, Schmidt J, Lewandrowski K, Compton CC, Rattner DW, Warshaw AL, Tompkins RG. Gut macromolecular permeability in pancreatitis correlates with severity of disease in rats. Gastroenterology. 1993;104:890–895. doi: 10.1016/0016-5085(93)91027-f. [DOI] [PubMed] [Google Scholar]
- 7.Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252–262. doi: 10.1016/s1091-255x(99)80067-5. [DOI] [PubMed] [Google Scholar]
- 8.Cicalese L, Sahai A, Sileri P, Rastellini C, Subbotin V, Ford H, Lee K. Acute pancreatitis and bacterial translocation. Dig Dis Sci. 2001;46:1127–1132. doi: 10.1023/a:1010786701289. [DOI] [PubMed] [Google Scholar]
- 9.Tenner S. Initial management of acute pancreatitis: critical issues during the first 72 hours. Am J Gastroenterol. 2004;99:2489–2494. doi: 10.1111/j.1572-0241.2004.40329.x. [DOI] [PubMed] [Google Scholar]
- 10.Eckerwall G, Olin H, Andersson B, Andersson R. Fluid resuscitation and nutritional support during severe acute pancreatitis in the past: what have we learned and how can we do better? Clin Nutr. 2006;25:497–504. doi: 10.1016/j.clnu.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Kerner T, Vollmar B, Menger MD, Waldner H, Messmer K. Determinants of pancreatic microcirculation in acute pancreatitis in rats. J Surg Res. 1996;62:165–171. doi: 10.1006/jsre.1996.0190. [DOI] [PubMed] [Google Scholar]
- 12.Boldt J. Volume therapy in the intensive care patient--we are still confused, but. Intensive Care Med. 2000;26:1181–1192. doi: 10.1007/s001340000625. [DOI] [PubMed] [Google Scholar]
- 13.Lang K, Boldt J, Suttner S, Haisch G. Colloids versus crystalloids and tissue oxygen tension in patients undergoing major abdominal surgery. Anesth Analg. 2001;93:405–409 , 3rd contents page. doi: 10.1097/00000539-200108000-00034. [DOI] [PubMed] [Google Scholar]
- 14.Marx G, Pedder S, Smith L, Swaraj S, Grime S, Stockdale H, Leuwer M. Resuscitation from septic shock with capillary leakage: hydroxyethyl starch (130 kd), but not Ringer’s solution maintains plasma volume and systemic oxygenation. Shock. 2004;21:336–341. doi: 10.1097/00024382-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Eibl G, Hotz HG, Faulhaber J, Kirchengast M, Buhr HJ, Foitzik T. Effect of endothelin and endothelin receptor blockade on capillary permeability in experimental pancreatitis. Gut. 2000;46:390–394. doi: 10.1136/gut.46.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marx G. Fluid therapy in sepsis with capillary leakage. Eur J Anaesthesiol. 2003;20:429–442. doi: 10.1017/s0265021503000681. [DOI] [PubMed] [Google Scholar]
- 17.Freitag M, Standl TG, Kleinhans H, Gottschalk A, Mann O, Rempf C, Bachmann K, Gocht A, Petri S, Izbicki JR, et al. Improvement of impaired microcirculation and tissue oxygenation by hemodilution with hydroxyethyl starch plus cell-free hemoglobin in acute porcine pancreatitis. Pancreatology. 2006;6:232–239. doi: 10.1159/000091962. [DOI] [PubMed] [Google Scholar]
- 18.Feng X, Liu J, Yu M, Zhu S, Xu J. Protective roles of hydroxyethyl starch 130/0.4 in intestinal inflammatory response and survival in rats challenged with polymicrobial sepsis. Clin Chim Acta. 2007;376:60–67. doi: 10.1016/j.cca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Sahin H, Mercanligil SM, Inanç N, Ok E. Effects of glutamine-enriched total parenteral nutrition on acute pancreatitis. Eur J Clin Nutr. 2007;61:1429–1434. doi: 10.1038/sj.ejcn.1602664. [DOI] [PubMed] [Google Scholar]
- 20.Pearce CB, Sadek SA, Walters AM, Goggin PM, Somers SS, Toh SK, Johns T, Duncan HD. A double-blind, randomised, controlled trial to study the effects of an enteral feed supplemented with glutamine, arginine, and omega-3 fatty acid in predicted acute severe pancreatitis. JOP. 2006;7:361–371. [PubMed] [Google Scholar]
- 21.Zhao G, Wang CY, Wang F, Xiong JX. Clinical study on nutrition support in patients with severe acute pancreatitis. World J Gastroenterol. 2003;9:2105–2108. doi: 10.3748/wjg.v9.i9.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 23.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 24.Bonham MJ, Abu-Zidan FM, Simovic MO, Windsor JA. Gastric intramucosal pH predicts death in severe acute pancreatitis. Br J Surg. 1997;84:1670–1674. [PubMed] [Google Scholar]
- 25.Oda S, Hirasawa H, Shiga H, Matsuda K, Nakamura M, Watanabe E, Moriguchi T. Management of intra-abdominal hypertension in patients with severe acute pancreatitis with continuous hemodiafiltration using a polymethyl methacrylate membrane hemofilter. Ther Apher Dial. 2005;9:355–361. doi: 10.1111/j.1744-9987.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 26.Meyer P, Pernet P, Hejblum G, Baudel JL, Maury E, Offenstadt G, Guidet B. Haemodilution induced by hydroxyethyl starches 130/0.4 is similar in septic and non-septic patients. Acta Anaesthesiol Scand. 2008;52:229–235. doi: 10.1111/j.1399-6576.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 27.Marx G, Pedder S, Smith L, Swaraj S, Grime S, Stockdale H, Leuwer M. Attenuation of capillary leakage by hydroxyethyl starch (130/0.42) in a porcine model of septic shock. Crit Care Med. 2006;34:3005–3010. doi: 10.1097/01.CCM.0000242755.74063.ED. [DOI] [PubMed] [Google Scholar]
- 28.Lv R, Zhou W, Chu C, Xu J. Mechanism of the effect of hydroxyethyl starch on reducing pulmonary capillary permeability in a rat model of sepsis. Ann Clin Lab Sci. 2005;35:174. [PubMed] [Google Scholar]
- 29.Feng X, Yan W, Liu X, Duan M, Zhang X, Xu J. Effects of hydroxyethyl starch 130/0.4 on pulmonary capillary leakage and cytokines production and NF-kappaB activation in CLP-induced sepsis in rats. J Surg Res. 2006;135:129–136. doi: 10.1016/j.jss.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Handrigan MT, Burns AR, Donnachie EM, Bowden RA. Hydroxyethyl starch inhibits neutrophil adhesion and transendothelial migration. Shock. 2005;24:434–439. doi: 10.1097/01.shk.0000180625.53800.63. [DOI] [PubMed] [Google Scholar]
- 31.Lang K, Suttner S, Boldt J, Kumle B, Nagel D. Volume replacement with HES 130/0.4 may reduce the inflammatory response in patients undergoing major abdominal surgery. Can J Anaesth. 2003;50:1009–1016. doi: 10.1007/BF03018364. [DOI] [PubMed] [Google Scholar]
- 32.Schäper J, Ahmed R, Schäfer T, Elster A, Enigk F, Habazettl H, Mousa S, Schäfer M, Welte M. Volume therapy with colloid solutions preserves intestinal microvascular perfusion in endotoxaemia. Resuscitation. 2008;76:120–128. doi: 10.1016/j.resuscitation.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 33.Flint RS, Phillips AR, Power SE, Dunbar PR, Brown C, Delahunt B, Cooper GJ, Windsor JA. Acute pancreatitis severity is exacerbated by intestinal ischemia-reperfusion conditioned mesenteric lymph. Surgery. 2008;143:404–413. doi: 10.1016/j.surg.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Warndorf MG, Kurtzman JT, Bartel MJ, Cox M, Mackenzie T, Robinson S, Burchard PR, Gordon SR, Gardner TB. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:705–709. doi: 10.1016/j.cgh.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trikudanathan G, Navaneethan U, Vege SS. Current controversies in fluid resuscitation in acute pancreatitis: a systematic review. Pancreas. 2012;41:827–834. doi: 10.1097/MPA.0b013e31824c1598. [DOI] [PubMed] [Google Scholar]
- 36.Mole DJ, Hall A, McKeown D, Garden OJ, Parks RW. Detailed fluid resuscitation profiles in patients with severe acute pancreatitis. HPB (Oxford) 2011;13:51–58. doi: 10.1111/j.1477-2574.2010.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


