Abstract
AIM: To evaluate clinical and laboratory parameters for prediction of bleeding from esophageal varices (EV) in children with portal hypertension.
METHODS: Retrospective study of 103 children (mean age: 10.1 ± 7.7 years), 95.1% with intrahepatic portal hypertension. All patients had no history of bleeding and underwent esophagogastroduodenoscopy for EV screening. We recorded variceal size (F1, F2 and F3), red-color signs and portal gastropathy, according to the Japanese Research Society for Portal Hypertension classification. Patients were classified into two groups: with and without EV. Seven noninvasive markers were evaluated as potential predictors of EV: (1) platelet count; (2) spleen size z score, expressed as a standard deviation score relative to normal values for age; (3) platelet count to spleen size z score ratio; (4) platelets count to spleen size (cm) ratio; (5) the clinical prediction rule (CPR); (6) the aspartate aminotransferase to platelet ratio index (APRI); and (7) the risk score.
RESULTS: Seventy-one children had EV on first endoscopy. On univariate analysis, spleen size, platelets, CPR, risk score, APRI, and platelet count to spleen size z score ratio showed significant associations. The best noninvasive predictors of EV were platelet count [area under the receiver operating characteristic curve (AUROC) 0.82; 95%CI: 0.73-0.91], platelet: spleen size z score (AUROC 0.78; 95%CI: 0.67-0.88), CPR (AUROC 0.77; 95%CI: 0.64-0.89), and risk score (AUROC 0.77; 95%CI: 0.66-0.88). A logistic regression model was applied with EV as the dependent variable and corrected by albumin, bilirubin and spleen size z score. Children with a CPR < 114 were 20.7-fold more likely to have EV compared to children with CPR > 114. A risk score > -1.2 increased the likelihood of EV (odds ratio 7.47; 95%CI: 2.06-26.99).
CONCLUSION: Children with portal hypertension with a CPR below 114 and a risk score greater than -1.2 are more likely to have present EV. Therefore, these two tests can be helpful in selecting children for endoscopy.
Keywords: Portal hypertension, Clinical predictors, Pediatric patients, Esophageal varices
Core tip: Children with portal hypertension (PH) are at risk for variceal bleeding. The standard test for screening varices is endoscopy, an invasive method. We evaluated non-invasive markers for diagnosing esophageal varices (EV) in 103 children (95% intrahepatic PH). All patients had no bleeding history and underwent endoscopy for EV screening. Platelet count (< 115 000), clinical prediction rule (< 114) and risk score (cutoff > -1.2) were the best predictors of EV. Limitations are the retrospective design and the small number of pre-hepatic PH patients. The strength is the paucity of pediatric studies related to this issue and the assessment of risk score in children.
INTRODUCTION
Portal hypertension is the underlying pathophysiological process that leads to the formation of portosystemic collaterals and heralds the onset of a severe complication: variceal hemorrhage. It is estimated that approximately 50% of pediatric patients with chronic liver disease and 90% of those with extrahepatic portal vein obstruction (EHPVO) will experience gastrointestinal bleeding[1,2]. Esophagogastroduodenoscopy (EGD) is considered the primary modality for detection and surveillance of esophageal varices (EV) and to determine the risk of bleeding.
Guidelines for adult cirrhotic patients recommend universal EV screening by EGD at the time of the diagnosis of cirrhosis[3-7].
Many studies have sought to determine clinical, laboratory, or other noninvasive methods that could predict the presence of EV. Preliminary data suggests that laboratory tests such as platelet count, albumin and ultrasonographic parameters such as presence of splenomegaly, spleen size z score and platelet count to spleen size ratio and the clinical prediction rule (CPR; calculated from platelet count, spleen size z-score, and albumin concentration) developed by Gana may be useful as first-line tools for identification of adults and pediatric patients at risk of variceal development and thus reduce the number of unnecessary EGDs[8-22].
The aim of this study was to analyze the following non-invasive methods for predicting EV in pediatric patients with portal hypertension submitted to EGD: platelet count, spleen size z score, platelet count to spleen size (cm) ratio, platelet count to spleen size z score ratio, CPR, risk score and the aspartate aminotransferase to platelet ratio index (APRI).
MATERIALS AND METHODS
We conducted a retrospective evaluation of patients aged < 18 years with a diagnosis of chronic liver disease or EHPVO who underwent EGD between the 2000 and 2011 at Hospital de Clinicas de Porto Alegre, a tertiary referral center in Southern Brazil. Portal hypertension was defined after the diagnosis of some conditions which natural progression occurs along with portal hypertension such as chronic liver disease and extra hepatic portal vein thrombosis. The following exclusion criteria were applied: active or previous variceal bleeding, prior variceal treatment (any type) or variceal bleeding prophylaxis (including nonselective β-blocker use, endoscopic variceal ligation or sclerotherapy, surgical portosystemic shunt or transjugular intrahepatic portosystemic shunt insertion), liver transplantation, and malignancy.
The presence of EV on endoscopy was the primary endpoint. Clinical and demographic data, diagnoses, medication use, physical examination findings, and severity of liver disease, as assessed by pediatric end-stage liver disease and model for end-stage liver disease (for children > 12 years old) and the Child-Pugh classification, were reviewed. The results of laboratory tests and ultrasound scans were considered for analysis if performed within 3 mo of EGD.
Endoscopy was carried out as part of routine clinical care. Four different endoscopists, recorded variceal size (F1, F2 and F3), red-color signs, and portal gastropathy, according to Japanese Research Society for Portal Hypertension classification[23], and gastric varices according to the Sarin classification[24].
Three thousand EGDs were reviewed and 127 patients with chronic liver disease or EHPVO were identified. Twenty-four patients were excluded: eleven due to previous variceal bleeding, four due to non-selective β-blocker therapy, four due to liver transplantation, two with laboratory test performed over than 3 mo of EGD, one due to surgical shunting, one due to no etiologic confirmation and one due to band ligation.
Seven non-invasive markers, previously described in adults and pediatric patients with portal hypertension, were evaluated as potential predictors of EV: (1) platelet count; (2) spleen size z score, expressed as a standard deviation score relative to normal values for age[25]; (3) platelet count to spleen size z score ratio; (4) platelet count to spleen size (cm) ratio; (5) the CPR, proposed by Gana et al[22] which is calculated according to the following formula: [(0.75 × platelets)/(spleen z score + 5)] + (2.5 × albumin); (6) the APRI test; and (7) a risk score, calculated as follows: [14.2 - 7.1 × log10 platelets (109/L)] + [4.2 × log10 bilirubin (mg/dL)][21].
For statistical analyses, patients were classified into two groups: patients with EV and patients without EV.
Statistical analysis
Data are expressed as mean and standard deviation, median and interquartile range, and proportions and 95%CI as appropriate. A P value of < 0.05 was considered statistically significant in all analyses. Continuous variables (such as laboratory data, spleen size z score, CPR, risk score) were compared using the Student t-test or the Mann-Whitney U test. Categorical variables (such as ascites, encephalopathy, and splenomegaly) were compared by the chi-square test or Fisher’s exact test.
To determinate test performance for prediction of EV, a receiver operator characteristic (ROC) curve was constructed and the area under the ROC curve (AUROC) was calculated. The cutoff value of the variables was determined at the point of highest sensitivity and specificity. Sensitivity, specificity, predictive values and likelihood ratios were calculated for these cutoff values. A logistic regression model was used to evaluate the variables that reached statistical significance on univariate analysis, with EV as the dependent variable. All statistical analyses were performed in the SPSS 18.0. This study was approved by the local Research Ethics Committee.
RESULTS
A hundred and three patients were included, with a mean age of 8.9 (± 4.7) years. Fifty-six (56/103; 54.3%) patients were female. Ninety-eight (98/103; 95%) patients had a diagnosis of chronic liver disease and five (5/103; 4.8%) had EHPVO. Seventy-one of the (71/103; 68.9%) patients had EV. Varices were classified as F2 and F3 in 35 patients, with red spots in 12 patients. Sixteen (16/71; 22.5%) patients presented both esophageal and gastric varices, and one had isolated gastric varices. Twenty (20/103; 19.4%) patients had portal hypertensive gastropathy.
Spleen size, platelet count, CPR, APRI, platelet count to spleen size ratio, platelet count to spleen size z score ratio, and the risk score were able to discriminate patients with and without varices (Table 1).
Table 1.
Univariate analysis for esophageal varices
| Variables | Varices (n = 71) | No varices (n = 32) | P value |
| Age (yr) | 9.1 ± 4.9 | 8.5 ± 4.4 | 0.530 |
| AST (U/L) | 87 (51-158) | 68 (36-178) | 0.417 |
| ALT (U/L) | 78 (40-141) | 54 (22-160) | 0.197 |
| INR | 1.2 (1.1-1.4) | 1.1 (1.1-1.3) | 0.066 |
| Bilirubin (mg/dL) | 1.4 (0.8-2.4) | 0.6 (0.4-2.2) | 0.016 |
| Albumin (g/dL) | 3.8 ± 0.6 | 4.1 ± 0.7 | 0.077 |
| Creatinine (mg/dL) | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.686 |
| Splenomegaly | 64 (95.5%) | 22 (73.3%) | 0.001 |
| Spleen size (cm) | 14.6 ± 3.3 (n = 65) | 12.2 ± 2.7 (n = 24) | 0.001 |
| Spleen size z score | 6.3 ± 3.2 (n = 65) | 3.7 ± 2.6 (n = 24) | 0.000 |
| Platelets (103/μL) | 102 ± 50.8 | 195 ± 85.2 | 0.000 |
| Encephalopathy(1/2/3)1 | 31/0/0 | 68/3/0 | 0.245 |
| Ascites (1/2/3)2 | 31/0/1 | 59/9/3 | 0.100 |
| Model of end-stage liver disease | 6.6 ± 4.6 (n = 23) | 3.6 ± 7.1 (n = 8) | 0.290 |
| Pediatric end-stage liver disease | -1.3 ± 8.6 (n = 48) | -2.2 ± 10.2 (n = 24) | 0.708 |
| Child-Pugh A/B/C | 35/31/5 | 22/8/2 | 0.169 |
| Child score | 7.0 ± 1.4 | 6.6 ± 1.3 | 0.074 |
| Clinical prediction rule | 103.6 ± 17.5 (n = 65) | 121.1 ± 21.1 (n = 24) | 0.001 |
| Platelets/spleen size z score | 16.7 (7.9-31.1) | 47.1 (27.2-123.3) | 0.000 |
| APRI | 2.3 (1.0-3.7) | 1.0 (0.3-2.3) | 0.001 |
| Platelets/spleen size | 0.7 (0.4-1.1) (n = 65) | 1.3 (0.8-2.2) (n = 24) | 0.000 |
| Risk score | 1.2 ± 2.6 | -1.6 ± 2.9 | 0.000 |
Determined clinically or by electroencephalogram: 1 = none; 2 = grade 1 or 2; 3 = grade 3 or 4;
Determined clinically or by ultrasound: 1 = no ascites; 2 = controlled or mild; 3 = moderate or tense. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; INR: International normalized ratio; APRI: Aspartate aminotransferase to platelet ratio index.
On ROC curve analysis, the best predictors of EV were: platelet count; platelet count to spleen size z score ratio; CPR; risk score; platelet count to spleen size (cm) ratio; spleen size z score; and the APRI test (Figure 1). The cutoff points were established with the best relationship between sensitivity and specificity for each variable as follows: platelet count, 115 000; platelet count to spleen size z score ratio, 25; CPR, 114; risk score, -1.2; platelet count to spleen size ratio, 1.0; APRI test, 1.4.
Figure 1.
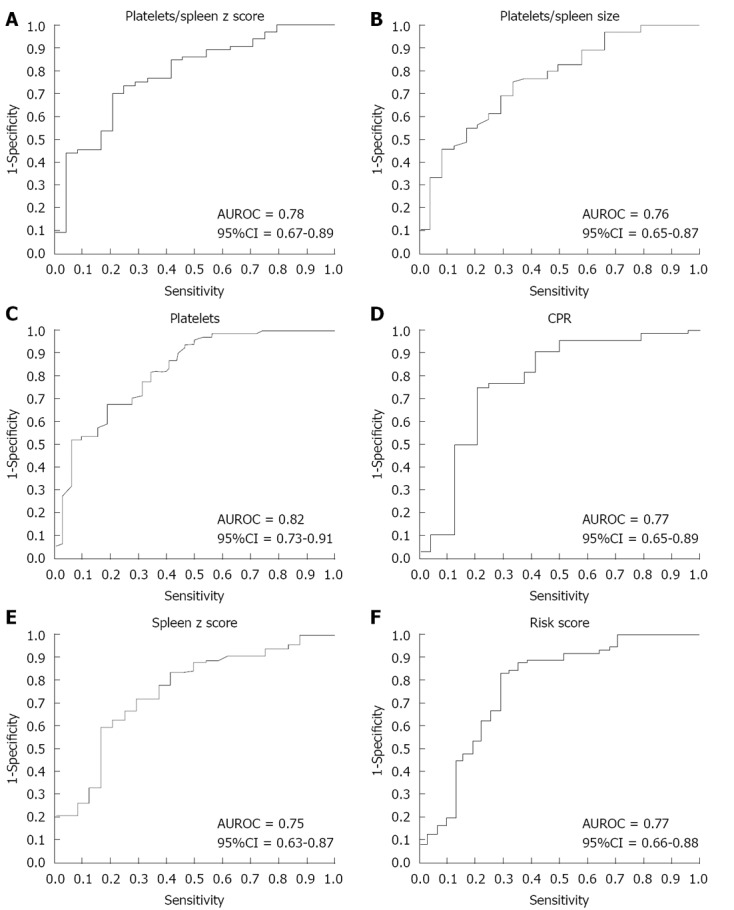
Receiver operator characteristic curves for presence of esophageal varices. AUROC: Area under the receiver operating characteristic curve.
A logistic regression model was applied with EV as the dependent variable, corrected by albumin, bilirubin and spleen size z score. Patients with a CPR < 114 were 20.74-fold more likely to have EV compared to children with CPR > 114. Risk score > -1.2 increased the likelihood of varices (odds ratio 7.47; 95%CI: 2.06-26.99) (Table 2). Sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio and negative likelihood ratio values for CPR, platelet count, platelet count to spleen size z score ratio, platelet count to spleen size (cm) ratio, risk score and APRI as EV predictors are presented on Table 3.
Table 2.
Odds ratios for esophageal varices
| Variables | OR1 | 95%CI | P value |
| CPR < 115 | 7.99 | 1.45-43.82 | 0.017 |
| CPR < 114 | 20.74 | 2.66-161.50 | 0.004 |
| Platelets/spleen size z score < 25 | 4.27 | 0.90-20.26 | 0.067 |
| Platelets/spleen size < 1 | 2.20 | 0.65-7.43 | 0.203 |
| Platelets | 0.98 | 0.97-0.99 | 0.016 |
| Platelets < 115 | 3.10 | 0.97-9.88 | 0.056 |
| Risk score > -1.2 | 7.47 | 2.06-26.99 | 0.002 |
| APRI > 1.4 | 1.85 | 0.56-6.10 | 0.309 |
Odds ratio computed by multivariate logistic regression model. CPR: Clinical prediction rule; APRI: Aspartate aminotransferase to platelet ratio index.
Table 3.
Diagnostic performance of variables as esophageal varices predictors
| Variables | Sensitivity (95%CI) | Specificity (95%CI) | Positive predictive value (95%CI) | Negative predictive value (95%CI) | Positive likelihood ratio (95%CI) | Negative likelihood ratio (95%CI) |
| Clinical prediction rule < 115 | 76.6 (64.0-85.8) | 70.8 (48.7-86.5) | 87.5 (75.3-94.4) | 53.1 (35.0-70.4) | 2.63 (1.38-4.96) | 0.33 (0.20-0.53) |
| Clinical prediction rule < 114 | 75.0 (62.3-84.6) | 79.2 (57.3-92.0) | 90.5 (78.5-96.5) | 54.2 (36.8-70.8) | 3.60 (1.63-7.95) | 0.32 (0.20-0.49) |
| Platelets < 115 | 67.6 (55.3-77.9) | 81.3 (62.9-92.1) | 88.8 (76.6-95.4) | 53.0 (38.4-67.2) | 3.61 (1.72-7.54) | 0.40 (0.28-0.56) |
| Platelets/spleen size z score < 25 | 68.8 (55.8-79.4) | 79.2 (57.3-92.0) | 89.8 (76.9-96.2) | 48.7 (32.7-64.9) | 3.30 (1.48-7.32) | 0.39 (0.27-0.58) |
| Platelets/spleen size < 1 | 72.3 (59.6-82.3) | 66.7 (44.6-83.6) | 85.4 (72.8-93.0) | 47.0 (30.1-64.6) | 2.17 (1.20-3.89) | 0.42 (0.27-0.64) |
| Risk score > -1.2 | 80.3 (67.2-89.3) | 70.9 (51.7-85.1) | 86.3 (75.2-93.2) | 61.1 (43.5-76.4) | 2.77 (1.57-4.85) | 0.28 (0.17-0.45) |
| APRI > 1.4 | 63.4 (51.0-74.2) | 65.6 (46.7-80.8) | 80.3 (67.2-89.3) | 44.7 (30.4-59.8) | 1.84 (1.10-3.07) | 0.56 (0.39-0.78) |
APRI: Aspartate aminotransferase to platelet ratio index.
DISCUSSION
We evaluated seven non-invasive markers, two of which had never been tested in children, the platelet count to spleen size (cm) ratio and the risk score. We found that platelets, the platelet count to spleen size z score ratio, CPR, and the risk score were able to predict EV. The prevalence of EV observed in our sample was similar to those reported elsewhere[22,26,27].
Thrombocytopenia is a common complication of chronic liver disease, affecting 76% of cirrhotic patients[28]. Unlike in adults[13], isolated platelet count has been described as a predictor of EV in four out of four studies of pediatric patients[22,26,27,29]. Nevertheless, there is still no consensus as to the best cutoff points, ranging from 100 000 to 130 000[22,26]. Gana et al[27] demonstrated that platelet count (cutoff point = 115 000) was the best predictor of EV, with an area under the AUROC curve of 0.79 (95%CI: 0.69-0.90). In the present study, the cutoff of point was similar to that observed by Gana et al[22] with an area under the ROC curve = 0.82 (95%CI: 0.73-0.91).
Splenomegaly is an important clinical sign of portal hypertension, especially in patients with chronic liver disease[30]. It has been used as such in several studies, both as an isolated parameter and as a component of scores or mathematical algorithms[22,26,27,29]. In cirrhotic children studied by Fagundes et al[26] patients with splenomegaly were almost 15-fold more likely to have EV compared with those without splenomegaly. In our study, 83.5% of patients had splenomegaly on physical examination, and, as observed by others, this variable discriminated patients with and without EV (P = 0.001).
Based on the premise that both thrombocytopenia and splenomegaly may depend on several factors related to chronic liver disease per se, Giannini et al[8] proposed studying the platelet count to spleen diameter ratio as a noninvasive rule predicting EV. According to the authors, a ratio less than 909 was independently associated with the presence of EV, the negative predictive value found was reproducible even in patients with compensated disease, and it was cost effective[8].
In our study, a platelet count to spleen size (cm) ratio < 1.0 was able to discriminate patients with and without EV (P < 0.000), but did not reach statistical significance on logistic regression (OR = 2.2; 95%CI: 0.65-7.43; P = 0.203). This could be explained by age and gender differences in spleen size. We tried to minimize the impact of this factor by using the spleen size z score, but this parameter was also not able to reach statistical significance on logistic regression (OR = 4.7; 95%CI: 0.90-20.26; P = 0.067). A disadvantage of considering platelet count and spleen size is that this evaluation needs to be synchronously due the great variability of both.
More recently, a systematic review and meta-analysis sought to determine the evidence on the diagnostic accuracy of platelet count to spleen diameter ratio < 909 as a noninvasive predictor of EV and concluded that the quality of evidence of these studies was low, raising questions about the reliability of the platelet count to spleen diameter ratio as a good predictor of EV. We agree with Chawla et al[20] that the heterogeneity of patients studied may limit the value of this ratio as a noninvasive predictor of EV. The etiological diversity of patients in our sample may have played a role in our findings.
An interesting CPR was developed and validated by Gana et al[22] in a retrospective study, using platelet count, spleen size z score and albumin as variables[22]. In a prospective, multicenter clinical trial, a CPR ≤ 116 had a sensitivity of 81%, a specificity of 73% and an AUROC of 0.84. The authors suggested that CPR under 115 could screen patients for endoscopy[27].
Apart from Gana et al[22], we are the first group to test the CPR in children. To do so, we used two different cutoff points: 115 and 114. The best specificity was observed with a cutoff of 114 (79%). Others predictors identified by Gana et al[22] were platelet count under 115 000 and serum albumin level. On multivariate analysis, CPR (OR 0.62; 95%CI: 0.45-0.84; P = 0.002) and albumin (OR 3.1; 95%CI: 1.5-6.7; P = 0.004) were independent predictors[27]. In our study, logistic regression, adjusted for albumin, bilirubin and spleen size z score, had an OR of 7.79 (95%CI: 1.45-43.82) with a CPR cutoff of 115 and an OR of 20.74 (95%CI: 2.66-161.5; P = 0.004) with a CPR cutoff of 114. This mathematical algorithm is simple, composed by available and noninvasive variables, and our results suggest that it is reproducible.
The degree of fibrosis can determine significant changes in the hepatic venous pressure gradient, and seems related to complications such as the development of EV[7]. Non-invasive markers for fibrosis have been tested in children with biliary atresia[29,31]. There was good correlation between APRI and Metavir scores in patients studied by Kim et al[31], suggesting that the APRI test could predict the appearance of fibrosis in those patients (a cutoff of 1.42 was correlated with grade 4 fibrosis).
The APRI was studied by Colecchia et al[29] as a noninvasive marker of EV in pediatric patients with chronic liver disease. A cutoff > 0.96 had a total accuracy of 83%. These results were not confirmed on multivariate analysis[29]. APRI, with a cutoff of > 1.4, was also used as a variable in this study, and we did not find this parameter to be statistically significant for prediction of EV on logistic regression. We did not test other cutoffs. In fact, the exact thresholds of APRI for prediction of fibrosis constitute the main issue related to its diagnostic accuracy[32].
The risk score was another clinical model tested for predicting EV in adults with advanced fibrosis and portal hypertension. The AUROC of the risk score for prediction of EV was 0.82. The -1.0 cutoff had a sensitivity of 82% and a specificity of 76%. The authors suggested that this score should be validated as a noninvasive test to detect the presence of EV[29]. This was the first pediatric study to use the risk score. The cutoff of -1.2 had a sensitivity of 80.3%, a specificity of 70.9%, an AUROC of 0.77 (95%CI: 0.66-0.88), and an OR of 7.47 (95%CI: 2.06-26.99; P = 0.002). This method is also composed by simple and available variables that proved to be good noninvasive parameters for EV detection in our patients. Furthermore, this method avoids the frequent use of ultrasound. It is worth noting that this method has not been tested in patients with pre-hepatic portal hypertension, and may not be an effective method in such patients, whose bilirubin levels are usually normal.
We tried to apply all known non-invasive clinical methods for prediction of EV to the study population. According to other pediatric studies, we also found platelet count to be a good predictor of EV, with a cutoff of 115 000. Children with a CPR under 114, in a logistic regression model, were 20.7-fold more likely to have EV compared to children with CPR > 114. More studies of this rule are required to find the optimal cutoff value.
The risk score, previously studied in adults, was a good and inexpensive predictor of EV in our patients. We believe it should be tested as a tool that can potentially limit the number of endoscopies in pediatric patients.
This study has some limitations. The retrospective design precludes blinding of the endoscopists or controlling for interobserver variability in ultrasound tests. The small number of patients with pre-hepatic portal hypertension could not be compared with those with intrahepatic portal hypertension.
In conclusion, the results of this study suggest that platelet count, the CPR and risk score could be used to screen children with portal hypertension for endoscopy. Further studies with a prospective design are necessary to confirm these suggestions.
ACKNOWLEDGMENTS
The authors would like acknowledge Professor Renato Borges Fagundes and Dr. Alexandre Araújo for suggestions.
COMMENTS
Background
Esophageal varices (EV) bleeding is a severe complication of portal hypertension. The standard diagnostic screening tool for EV is endoscopy, which is considered an invasive procedure in pediatric patients. Evaluate clinical and laboratory parameters for prediction of EV is very important to avoid unnecessary endoscopy, especially in children. Some studies have reported that platelet count and spleen size could be used to predict EV, but there is no agreement in the cut-off point.
Research frontiers
The development of mathematical models, such as clinical prediction rule (CPR) and risk score, that involves variables associated with intrahepatic portal hypertension seems to be promising. The research hotspot is to evaluate the parameters that could predict EV in children and reduce the indication of endoscopy.
Innovations and breakthroughs
Few previous studies in pediatric patients evaluated platelet count, CPR splenomegaly isolated in different population. The risk score was studied only in adults and aspartate aminotransferase to platelet ratio index was not used to predict EV in children. The risk score, that use platelet count and bilirubin as variables, should be used as a tool that can limit endoscopies indications in pediatric patients with the advantage of not using spleen size. The predictive value was similar to CPR and better than platelet count isolated.
Applications
The study suggests that both CPR and risk score could be used to screen children with portal hypertension for endoscopy.
Terminology
CPR is a clinical prediction rule, proposed by Gana et al that use as independent variables platelet count, spleen size z score (based on age and gender) and albumin. Risk score is a score proposed by Park et al to be used in patients with advanced fibrosis to determine clinically significant portal hypertension and was used to predict EV, using platelet count and bilirubin.
Peer review
This is a very interesting manuscript that further delineates clinical variables that are readily available and which can be used to increase the yield of endoscopy for identifying EV in children with portal hypertension. Though the variables studied have all been reported previously, the validation of these variables in children is an important extension of this work.
Footnotes
Supported by FIPE-HCPA (Research and Events Support Fund at Hospital de Clínicas de Porto Alegre)
P- Reviewers Skill NJ, Schuchert MJ, Ge D, Koulaouzidis A S- Editor Huang XZ L- Editor A E- Editor Xiong L
References
- 1.Ling SC, Walters T, McKiernan PJ, Schwarz KB, Garcia-Tsao G, Shneider BL. Primary prophylaxis of variceal hemorrhage in children with portal hypertension: a framework for future research. J Pediatr Gastroenterol Nutr. 2011;52:254–261. doi: 10.1097/MPG.0b013e318205993a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poddar U, Thapa BR, Rao KL, Singh K. Etiological spectrum of esophageal varices due to portal hypertension in Indian children: is it different from the West? J Gastroenterol Hepatol. 2008;23:1354–1357. doi: 10.1111/j.1440-1746.2007.05102.x. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Lam S, Rapuano CJ, Krachmer JH, Lam BL. Lamellar corneal autograft for corneal perforation. Ophthalmic Surg. 1991;22:716–717. [PubMed] [Google Scholar]
- 5.Shneider B, Emre S, Groszmann R, Karani J, McKiernan P, Sarin S, Shashidhar H, Squires R, Superina R, de Ville de Goyet J, et al. Expert pediatric opinion on the Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. Pediatr Transplant. 2006;10:893–907. doi: 10.1111/j.1399-3046.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 6.Jensen DM. Endoscopic screening for varices in cirrhosis: findings, implications, and outcomes. Gastroenterology. 2002;122:1620–1630. doi: 10.1053/gast.2002.33419. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445–1449. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V, et al. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200–1205. doi: 10.1136/gut.52.8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zein CO, Lindor KD, Angulo P. Prevalence and predictors of esophageal varices in patients with primary sclerosing cholangitis. Hepatology. 2004;39:204–210. doi: 10.1002/hep.20029. [DOI] [PubMed] [Google Scholar]
- 10.Giannini EG, Botta F, Borro P, Dulbecco P, Testa E, Mansi C, Savarino V, Testa R. Application of the platelet count/spleen diameter ratio to rule out the presence of oesophageal varices in patients with cirrhosis: a validation study based on follow-up. Dig Liver Dis. 2005;37:779–785. doi: 10.1016/j.dld.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Giannini EG, Zaman A, Kreil A, Floreani A, Dulbecco P, Testa E, Sohaey R, Verhey P, Peck-Radosavljevic M, Mansi C, et al. Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation study. Am J Gastroenterol. 2006;101:2511–2519. doi: 10.1111/j.1572-0241.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 12.Sharma SK, Aggarwal R. Prediction of large esophageal varices in patients with cirrhosis of the liver using clinical, laboratory and imaging parameters. J Gastroenterol Hepatol. 2007;22:1909–1915. doi: 10.1111/j.1440-1746.2006.04501.x. [DOI] [PubMed] [Google Scholar]
- 13.Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Maurer R, Planas R, Escorsell A, Garcia-Pagan JC, et al. Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis. Hepatology. 2008;47:153–159. doi: 10.1002/hep.21941. [DOI] [PubMed] [Google Scholar]
- 14.Barrera F, Riquelme A, Soza A, Contreras A, Barrios G, Padilla O, Viviani P, Pérez-Ayuso RM. Platelet count/spleen diameter ratio for non-invasive prediction of high risk esophageal varices in cirrhotic patients. Ann Hepatol. 2009;8:325–330. [PubMed] [Google Scholar]
- 15.Treeprasertsuk S, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, et al. The predictors of the presence of varices in patients with primary sclerosing cholangitis. Hepatology. 2010;51:1302–1310. doi: 10.1002/hep.23432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarzenberger E, Meyer T, Golla V, Sahdala NP, Min AD. Utilization of platelet count spleen diameter ratio in predicting the presence of esophageal varices in patients with cirrhosis. J Clin Gastroenterol. 2010;44:146–150. doi: 10.1097/MCG.0b013e3181a745ff. [DOI] [PubMed] [Google Scholar]
- 17.Sarangapani A, Shanmugam C, Kalyanasundaram M, Rangachari B, Thangavelu P, Subbarayan JK. Noninvasive prediction of large esophageal varices in chronic liver disease patients. Saudi J Gastroenterol. 2010;16:38–42. doi: 10.4103/1319-3767.58767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tafarel JR, Tolentino LH, Correa LM, Bonilha DR, Piauilino P, Martins FP, Rodrigues RA, Nakao FS, Libera ED, Ferrari AP, et al. Prediction of esophageal varices in hepatic cirrhosis by noninvasive markers. Eur J Gastroenterol Hepatol. 2011;23:754–758. doi: 10.1097/MEG.0b013e3283488a88. [DOI] [PubMed] [Google Scholar]
- 19.Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Ripoll C, Maurer R, Planas R, Escorsell A, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7:689–695. doi: 10.1016/j.cgh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chawla S, Katz A, Attar BM, Gupta A, Sandhu DS, Agarwal R. Platelet count/spleen diameter ratio to predict the presence of esophageal varices in patients with cirrhosis: a systematic review. Eur J Gastroenterol Hepatol. 2012;24:431–436. doi: 10.1097/MEG.0b013e3283505015. [DOI] [PubMed] [Google Scholar]
- 21.Park SH, Park TE, Kim YM, Kim SJ, Baik GH, Kim JB, Kim DJ. Non-invasive model predicting clinically-significant portal hypertension in patients with advanced fibrosis. J Gastroenterol Hepatol. 2009;24:1289–1293. doi: 10.1111/j.1440-1746.2009.05904.x. [DOI] [PubMed] [Google Scholar]
- 22.Gana JC, Turner D, Roberts EA, Ling SC. Derivation of a clinical prediction rule for the noninvasive diagnosis of varices in children. J Pediatr Gastroenterol Nutr. 2010;50:188–193. doi: 10.1097/MPG.0b013e3181b64437. [DOI] [PubMed] [Google Scholar]
- 23.Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, Kokudo N, Kokubu S, Sakaida I, Sata M, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition) Dig Endosc. 2010;22:1–9. doi: 10.1111/j.1443-1661.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- 24.Sarin SK, Sundaram KR, Ahuja RK. Predictors of variceal bleeding: an analysis of clinical, endoscopic, and haemodynamic variables, with special reference to intravariceal pressure. Gut. 1989;30:1757–1764. doi: 10.1136/gut.30.12.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Megremis SD, Vlachonikolis IG, Tsilimigaki AM. Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology. 2004;231:129–134. doi: 10.1148/radiol.2311020963. [DOI] [PubMed] [Google Scholar]
- 26.Fagundes ED, Ferreira AR, Roquete ML, Penna FJ, Goulart EM, Figueiredo Filho PP, Bittencourt PF, Carvalho SD, Albuquerque W. Clinical and laboratory predictors of esophageal varices in children and adolescents with portal hypertension syndrome. J Pediatr Gastroenterol Nutr. 2008;46:178–183. doi: 10.1097/MPG.0b013e318156ff07. [DOI] [PubMed] [Google Scholar]
- 27.Gana JC, Turner D, Mieli-Vergani G, Davenport M, Miloh T, Avitzur Y, Yap J, Morinville V, Brill H, Ling SC. A clinical prediction rule and platelet count predict esophageal varices in children. Gastroenterology. 2011;141:2009–2016. doi: 10.1053/j.gastro.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 28.Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–1007. doi: 10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Colecchia A, Di Biase AR, Scaioli E, Predieri B, Iughetti L, Reggiani ML, Montrone L, Ceccarelli PL, Vestito A, Viola L, et al. Non-invasive methods can predict oesophageal varices in patients with biliary atresia after a Kasai procedure. Dig Liver Dis. 2011;43:659–663. doi: 10.1016/j.dld.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Orlando R, Lirussi F, Basso SM, Lumachi F. Splenomegaly as risk factor of liver cirrhosis. A retrospective cohort study of 2,525 patients who underwent laparoscopy. In Vivo. 2011;25:1009–1012. [PubMed] [Google Scholar]
- 31.Kim SY, Seok JY, Han SJ, Koh H. Assessment of liver fibrosis and cirrhosis by aspartate aminotransferase-to-platelet ratio index in children with biliary atresia. J Pediatr Gastroenterol Nutr. 2010;51:198–202. doi: 10.1097/MPG.0b013e3181da1d98. [DOI] [PubMed] [Google Scholar]
- 32.Leroy V. Other non-invasive markers of liver fibrosis. Gastroenterol Clin Biol. 2008;32:52–57. doi: 10.1016/S0399-8320(08)73993-9. [DOI] [PubMed] [Google Scholar]


