Abstract
AIM: To investigate the effect of surgery and chemotherapy for gastric cancer with multiple synchronous liver metastases (GCLM).
METHODS: A total of 114 patients were entered in this study, and 20 patients with multiple synchronous liver metastases were eligible. After screening with preoperative chemotherapy, 20 patients underwent curative gastrectomy and hepatectomy for GCLM; 14 underwent major hepatectomy, and the remaining six underwent minor hepatectomy. There were 94 patients without aggressive treatment, and they were in the non-operative group. Two regimens of perioperative chemotherapy were used: S-1 and cisplatin (SP) in 12 patients, and docetaxel, cisplatin and 5-fluorouracil (DCF) in eight patients. These GCLM patients were given preoperative chemotherapy consisting of two courses chemotherapy of SP or DCF regimens. After chemotherapy, gastrectomy and hepatectomy were preformed. Evaluation of patient survival was by follow-up contact using telephone and outpatient records. All patients were assessed every 3 mo during the first year and every 6 mo thereafter.
RESULTS: Twenty patients underwent gastrectomy and hepatectomy and completed their perioperative chemotherapy and hepatic arterial infusion before and after surgery. Ninety-four patients had no aggressive treatment of liver metastases because of technical difficulties with resection and severe cardiopulmonary dysfunction. In the surgery group, there was no toxicity greater than grade 3 during the course of chemotherapy. The response rate was 100% according to the response evaluation criteria in solid tumors criteria. For all 114 patients, the overall survival rate was 8.0%, 4.0%, 4.0% and 4.0% at 1, 2, 3 and 4 years, respectively, with a median survival time (MST) of 8.5 mo (range: 0.5-48 mo). For the 20 patients in the surgery group, MST was 22.3 mo (range: 4-48 mo). In the 94 patients without aggressive treatment, MST was 5.5 mo (range: 0.5-21 mo). There was a significant difference between the surgery and unresectable patients (P = 0.000). Three patients in surgery group were still alive at the end of the cut-off date.
CONCLUSION: Perioperative weekly DCF and SP achieved a good response, and combined with surgery, they could improve prognosis of GCLM.
Keywords: Gastric cancer, Liver metastases, Surgery, Chemotherapy, Pilot study
Core tip: We investigated the effect of surgery and chemotherapy for gastric cancer with multiple synchronous liver metastases (GCLM). Perioperative weekly docetaxel, cisplatin and 5-fluorouracil and S-1 and cisplatin achieved a good response, and combined with surgery, they could improve prognosis of GCLM.
INTRODUCTION
Surgery for gastric cancer with multiple synchronous liver metastases (GCLM) is a major challenge to every surgeon; not only because of coexisting factors, but each GCLM patient has his/her own clinicopathological features. It is difficult to determine the suitable candidates for treatment. At present, the justification for surgical resection is still controversial[1], and the prognosis is dismal. In contrast, for patients with colorectal carcinoma with liver metastases, a second liver resection is safe and feasible. Hepatic resection has been widely accepted as a potentially curative approach in patients with liver metastases of colorectal carcinoma[2].
One study demonstrated that patients with GCLM limited to one lobe, who underwent radical gastrectomy with D2 lymphadenectomy, had the most favorable outcomes following hepatic surgical treatment[3]. A further study found that the number of metastases was no longer considered to be an important predictor of long-term survival[4]. Some positive effect of liver resection in these patients seemed to imply that hepatic surgical treatment should be recommended for appropriate GCLM candidates[5-7]. The United Kingdom myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial of perioperative chemotherapy in gastric cancer found that perioperative systemic chemotherapy improved 5-year survival from 23% to 36%[8], compared with surgery alone. What is the optimal dosing appropriate for Chinese patients, and how do we schedule perioperative chemotherapy that could improve tolerability while maintaining efficacy? In our previous pilot study, we found that liver resection combined with a weekly docetaxel-based regimen (docetaxel, cisplatin and 5-fluorouracil, DCF) were well tolerated, with a good response. In the present study, we assessed more GCLM patients who underwent aggressive treatment, in comparison with non-surgical treatment.
MATERIALS AND METHODS
From July 2007 to October 2012, 1821 patients with gastric cancer were treated in Beijing Cancer Hospital of Beijing University and Qingdao Municipal Hospital. Only patients with adenocarcinoma were enrolled in this study. Among these patients, 114 developed multiple liver metastases. The inclusion and exclusion criteria are described in our previous study[9]. Patients had adequate physical condition and received two course of preoperative chemotherapy. After effective screening with preoperative chemotherapy, 20 patients underwent curative gastrectomy and hepatectomy for GCLM. Two regimens of perioperative chemotherapy were used. Twelve patients received the S-1 and cisplatin (SP) regimen: 40 mg S-1 orally, twice daily for 3 consecutive weeks, and 60 mg/m2 cisplatin intravenously on day 8, followed by a 2-wk rest period, within a 5-wk cycle[10]. Eight patients received the DCF regimen: 20 mg/m2 cisplatin over 1 h; 20 mg/m2 docetaxel, over 30 min; and 350 mg/m2 5-fluorouracil over 15 min on day 1. This was administered weekly for 6 wk, followed by a 2-wk break[9].
According to the Japanese Research Society for Gastric Cancer guidelines, our surgical procedure was total or subtotal gastrectomy, at a minimum of 5 cm clearance. Hepatic resection with D2 lymphadenectomy was performed[11].
After surgery, two courses of chemotherapy (SP or DCF regimen) were administered. After completion of chemotherapy, patients without other distant disease, except for hepatic metastasis, underwent hepatic arterial infusion (HAI). If liver lesions progressed in the course of postoperative chemotherapy, HAI was commenced immediately. Safety evaluation was standardized by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (May 28, 2009). Evaluations were classified by the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines[12]. The study was approved by the medical ethics committees of Qingdao Municipal Hospital and Beijing Cancer Hospital. Written informed consent was obtained according to the principles of the institution.
Evaluation of patient survival was by follow-up contact using telephone and outpatient records. All patients were assessed every 3 mo during the first year and every 6 mo thereafter. Patient follow-up lasted until death or the cut-off date of October 1, 2012. Three patients (2.6%) were lost to follow-up, and survival information was censored at their last visit. Four (3.5%) patients were still alive and were censored at the cut-off date. The median follow-up period for the 114 patients was 10 mo (range: 2-53 mo).
Statistical analysis
Statistical analysis was performed with SPSS version 13.0 (SPSS, Chicago, Illinois, United States). For univariate analysis, binomial and categorical data were evaluated by cross-linked tables and the Fisher’s exact test. Results were regarded as being statistically significant when P < 0.05. For survival analysis, the Kaplan-Meier method was used.
RESULTS
Patient characteristics
The mean age of the 114 patients was 56.7 years (range: 33-75 years), and the male to female ratio was 3.1:1. Twenty patients underwent gastrectomy and hepatectomy. These 20 patients completed their perioperative chemotherapy and HAI before and after surgery. The other 94 patients were not considered for aggressive treatment of liver metastases. In most cases (n = 91), the reason for deciding against aggressive treatment was patient refusal; the remaining three were not eligible for surgery due to severe cardiopulmonary dysfunction. There was no perioperative mortality. There were no obviously different clinicopathological characteristics between patients with and without hepatectomy.
Surgery
There were 12 male and eight female patients in the surgery group. The median age of this group was 54 years (range: 31-74 years). Seventeen patients had a lymph-node-positive stage of the primary tumor, and only three had no lymph node involvement. There were 13 patients with distal gastric cancer, seven had proximal gastric cancer, and nine had bilobar metastases. The clinicopathological characteristics of the patients who underwent hepatectomy are listed in Table 1.
Table 1.
Clinicopathological characteristics of patients with and without hepatectomy
| Clinicopathological characteristics | With hepatectomy | Without hepatectomy |
| Sex | ||
| Male | 12 | 59 |
| Female | 8 | 35 |
| Primary gastric tumors | ||
| Median diameter of primary gastric tumors (cm) | 4.3 (2.4-8.8) | 4.5 (2.1-9.3) |
| Tumor location | ||
| Upper | 7 | 27 |
| Lower | 13 | 67 |
| Pathological T-stage of the primary1 | ||
| pT1 | 2 | 9 |
| pT2 | 4 | 21 |
| pT3 | 12 | 51 |
| pT4 | 2 | 13 |
| N stage of the primary tumor | ||
| N0 | 3 | 18 |
| N1 | 9 | 48 |
| N2 | 5 | 21 |
| N3 | 3 | 7 |
| Differentiation of the primary tumor | ||
| Well | 2 | 9 |
| Moderate | 14 | 64 |
| Poor | 4 | 21 |
| Liver metastases | ||
| Median diameter of liver metastases (cm) | 4.1 (1.7-16) | 4.5 (1.5-18) |
| No. of metastases | ||
| Solitary | 8 | 43 |
| ≥ 2 | 12 | 51 |
| Vascular invasion of metastases | ||
| Present | 3 | 26 |
| Absent | 17 | 68 |
| Site of metastases | ||
| Left lobe | 4 | 18 |
| Right lobe | 7 | 31 |
| Bilobar | 9 | 45 |
| Interruption of hepatic hilum | ||
| Present | 5 | 28 |
| Absent | 15 | 66 |
According to tumor-nodes-metastasis-classification.
The patients in the surgery group finished two courses of SP or DCF chemotherapy before the operation. In the two courses of chemotherapy with different regimens, no patients had toxicity greater than grade 3. The most common adverse effects in the two regimens were diarrhea, nausea, leukopenia, neutropenia and thrombocytopenia, at grade 1 or 2 intensity. Most adverse effects could be modified by premedication, such as dexamethasone and antiemetics. Granulocyte colony-stimulating factor support was given to 12 patients. Response to treatment was assessed by monthly magnetic resonance imaging or computed tomography. All patients achieved a partial response according to the RECIST[12] criteria (Table 2). The response rate was 100% according to the RECIST (Figures 1 and 2). There was no treatment-related mortality.
Table 2.
Response evaluation after first two courses of preoperative chemotherapy
| No. of cases |
Diameter of metastases (mm3) |
Evaluation of response (according to RECIST) | Adverse events grade | |
| Pre-chem | Post-chem | |||
| 1 | 128 | 0-10 | CR | 2 |
| 2 | 135 | 56 | PR | 3 |
| 3 | 188 | 65 | PR | 1 |
| 4 | 64 | 22 | PR | 2 |
| 5 | 48 | 24 | PR | 2 |
| 6 | 148 | 38 | PR | 1 |
| 7 | 205 | 83 | PR | 1 |
| 8 | 162 | 65 | PR | 2 |
| 9 | 78 | 30 | PR | 2 |
| 10 | 228 | 94 | PR | 3 |
| 11 | 206 | 108 | PR | 2 |
| 12 | 144 | 56 | PR | 1 |
| 13 | 67 | 41 | PR | 1 |
| 14 | 104 | 67 | PR | 3 |
| 15 | 163 | 103 | PR | 2 |
| 16 | 134 | 92 | PR | 3 |
| 17 | 88 | 61 | PR | 3 |
| 18 | 225 | 134 | PR | 2 |
| 19 | 143 | 96 | PR | 2 |
| 20 | 78 | 43 | PR | 3 |
When the number of liver lesions was > 5, the diameters of the five largest lesions were summed. RECIST: Response evaluation criteria in solid tumors; CR: Complete response; PR: Partial response; Pre-chem: Pre-chemotherapy; Post-chem: Post-chemotherapy.
Figure 1.
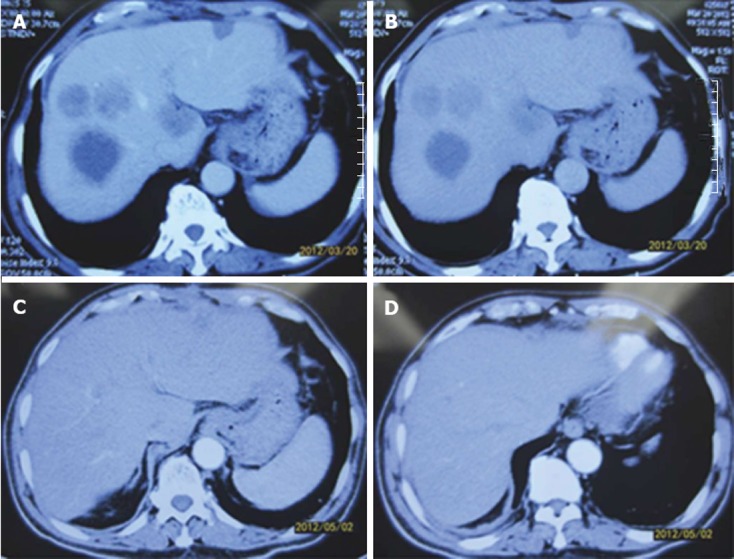
Patients with complete response. A, B: Abdominal computed tomography (CT) in patients with gastric cancer with multiple synchronous liver metastases (GCLM) after preoperative chemotherapy; C, D: Abdominal CT in patients with GCLM after neoadjuvant chemotherapy.
Figure 2.
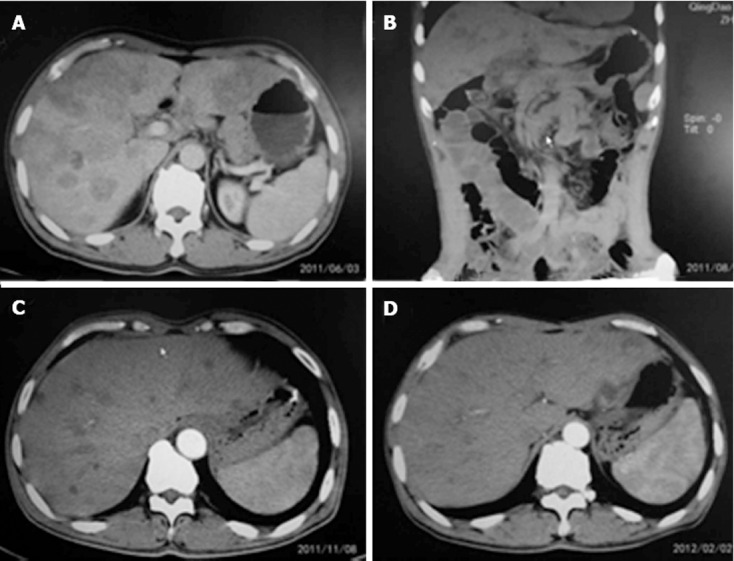
Patients with partial response. A: Abdominal computed tomography (CT) in gastric cancer with multiple synchronous liver metastases (GCLM) patient treated with preoperative chemotherapy (June 3, 2011); B: Abdominal CT in patient with GCLM after neoadjuvant chemotherapy (August 29, 2011); C: Abdominal CT in patient with GCLM after neoadjuvant chemotherapy (November 8, 2011); D: Abdominal CT in patient with GCLM after neoadjuvant chemotherapy (February 2, 2012).
We performed gastric and liver resection only in cases that were potentially curative. The common complications in the perioperative course were impaired wound healing (surgical therapy in two patients), and pleural effusion in four. Fourteen patients underwent major hepatectomy (hepatic resection of more than three segments: hemihepatectomy in 12 and trisectionectomy in 2); and the remaining six patients underwent minor hepatectomy (sectionectomy in 2 and limited resection in 4). The types of hepatectomy were classified according to the Brisbane 2000 terminology[13].
Survival rate in surgery and nonoperative groups
For all 114 patients, the overall survival rate was 8.0%, 4.0%, 4.0% and 4.0% at 1, 2, 3 and 4 years, respectively, with an median survival time (MST) of 8.5 mo (range: 0.5-48 mo). For the 20 patients in the surgery group, MST was 22.3 mo (range: 4-48 mo). In the 94 patients without aggressive treatment, MST was 5.5 mo (range: 0.5-21 mo). A significant difference was observed between the surgery and nonoperative patients (P = 0.000, Figure 3). Three patients in the surgery group were still alive at the end of the cut-off date.
Figure 3.
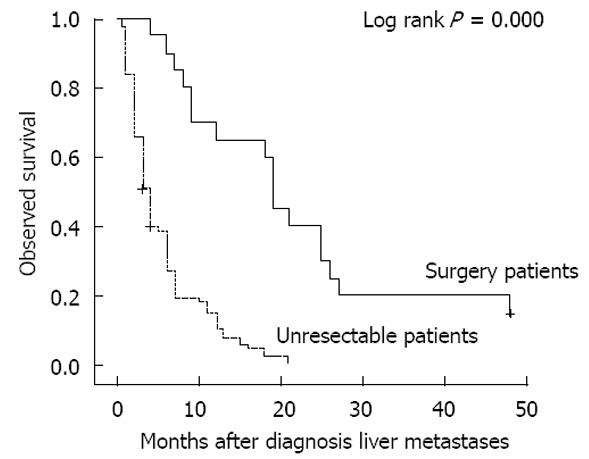
Overall survival of patients with hepatic metastases from gastric cancer.
DISCUSSION
We reviewed retrospectively 20 macroscopically complete liver resections for patients with GCLM at two institutions. After hepatectomy, their MST was 22.3 mo. These results compare favorably with patients without surgery, whose MST was only 5.5 mo. The survival time in patients with hepatectomy was longer than in those without hepatectomy. However, our MSTs were shorter than the 34 mo reported by Takemura et al[14]. The discrepancy may have been caused by the different operating procedures. In the Takemura et al[14] study, 14/64 (21.9%) patients underwent major hepatectomy and the remaining 50 (78.1%) minor hepatectomy. In our study, 70% patients had major hepatectomy and 30% had minor hepatectomy. Both studies indicate that hepatectomy is beneficial for some patients with GCLM despite the remaining controversy surrounding surgical resection.
Liver metastases is reported to develop in 5%-9% of patients with gastric cancer[15]. One study has shown that only a limited number of GCLM patients are eligible for surgical treatment[4]. After the promising results of the MAGIC trial, in Europe, current practice for treatment of GCLM patients has become surgery with perioperative chemotherapy[10,16]. However, the optimal surgical strategy for GCLM remains a matter of debate. Only some patients with GCLM are ideal candidates for hepatectomy, therefore, many patients are unsuitable for surgical resection, either due to other distant metastases, extensive lymph node metastases, multiple bilateral metastases, or comorbidity.
In recent decades, multimodality approaches using chemotherapy, radiotherapy, or both have been evaluated in an attempt to improve outcomes following gastric cancer surgery. Some benefit has been seen in adjuvant chemotherapy after gastric cancer resection. One recent trial conducted in East Asia, ACTS-GC30, evaluated S-1 chemotherapy and found significant 10% improvement in 3-year overall survival with adjuvant chemotherapy after surgery[17]. A more compelling study of perioperative chemotherapy was the phase 3 United Kingdom MAGIC trial. This trial demonstrated that perioperative chemotherapy could significantly improve overall survival and progression-free survival in 503 patients with resectable adenocarcinoma. However, this trial also highlighted the challenges involved in delivering postoperative treatment; only 50% of patients were able to receive postoperative chemotherapy, compared with nearly 91% who received preoperative chemotherapy.
In the late stage of gastric cancer, with high rates of toxicity in perioperative chemotherapy, adoption of the perioperative approach could be useful for a large proportion of GCLM patients. Our results also showed that weekly SP and low-dose DCF in perioperative chemotherapy had a positive effect in GCLM. In our study, two patients with initially unresectable multiple liver metastases were converted to resectable after preoperative chemotherapy. Our results also showed that D2 resection provides better locoregional control and significantly better survival compared with unresectable patients. We recommend more personally tailored multimodality treatment approaches (surgery + chemotherapy ± radiation) in patients with GCLM.
Some researchers have reported that even a generous surgical margin may not be essential for curative hepatic resection of liver metastases, because recurrence is strongly associated with systemic spread rather than local invasion[6]. This conclusion highlights the essentiality of perioperative chemotherapy. GCLM recurrence after surgery is most likely due to occult metastatic disease in the tumor bed and at distant sites, so locoregional resection alone is not a complete 100% successful procedure. Therefore, multimodality approaches using systemic chemotherapy or radiation, or a combination of both have been used in an attempt to improve outcomes following surgery, especially in patients with multiple metastases.
However, adequate chemotherapy can lead to intolerability and morbidity and mortality. In the present study, we wanted to explore some safe and effective regimens available to Chinese patients with GCLM. We investigated the safety and efficacy of liver resection combined with perioperative S1 regimen in patients with GCLM. We performed a retrospective analysis based on recent prospectively collected data. S-1 is an orally active combination of tegafur (5-fluorouracil prodrug), gimeracil (an inhibitor of dihydropyrimidine dehydrogenase, which degrades fluorouracil), and oteracil (which inhibits phosphorylation of 5-fluorouracil in the gastrointestinal tract) in a molar ratio of 1:0.4:1. S-1 has been the standard regimen for adjuvant chemotherapy for advanced primary gastric cancer[18], and its mild side effect profile and ease of administration make it a preferred choice. The DCF regimen has major myelotoxicity[19-25]. However, weekly DCF in our study was well tolerated, and both the regimens were well tolerated and achieved a good response. All GCLM patients with adequate physical condition obtained a benefit from preoperative chemotherapy, which assisted with their subsequent surgical procedure. Appropriately modified chemotherapy is necessary for the improvement of the GCLM resection rate and complete elimination of micrometastases[26-31]. In our initial results, weekly DCF yielded an unexpected high response as preoperative chemotherapy for GCLM[9]. We found that S-1 combined with cisplatin also yielded a high response and had better applicability. These modifications of altering the dose and frequency of the cytotoxic agents are an individualized approach for treatment of GCLM. Our aim is to improve the generally poor prognosis of this aggressive disease and further phase II and III trials are warranted to confirm the feasibility and efficacy of preoperative chemotherapy for GCLM.
COMMENTS
Background
Liver metastasis is a fatal event in gastric cancer patients, and remains a major cause of cancer-related death. Surgery for multiple liver metastases from gastric cancer (GCLM) has favorable outcomes. However, the efficacy and safety of perioperative chemotherapy is still a matter of debate.
Research frontiers
The glycosylated and myristoylated smaller surface antigen trial was conducted to compare gastrectomy with metastasectomy plus systemic therapy versus systemic therapy alone. The results of this trial showed that aggressive surgical resection in combination with systemic chemotherapy may improve the outcomes of the patients with metastatic gastric cancer.
Innovations and breakthroughs
In a previous pilot study, the authors found that liver resection combined with weekly docetaxel-based chemotherapy were well tolerated and had a good response. In the present study, the authors found that perioperative weekly docetaxel, cisplatin and 5-fluorouracil (DCF), and S-1 and cisplatin (SP) in patients with GCLM who underwent aggressive surgical treatment could improve prognosis and overall survival.
Applications
The study results suggest that perioperative weekly DCF and SP could be used to treat GCLM patients who underwent aggressive surgical treatment.
Terminology
Synchronous liver metastases are detected before or during surgery, or occur within 1 year after gastrectomy. Metachronous liver metastases are usually detected within a 2-year period following initial gastrectomy.
Peer review
This is a good study in which the authors evaluated the effect of perioperative weekly DCF and SP in GCLM patients who underwent aggressive surgical treatment. The results are interesting and suggest that perioperative weekly DCF and SP combined with resection could be applied in patients with GCLM.
Footnotes
P- Reviewers Tsujimoto H, Nardone G, Wang CC S- Editor Zhai HH L- Editor A E- Editor Xiong L
References
- 1.Romano F, Garancini M, Uggeri F, Degrate L, Nespoli L, Gianotti L, Nespoli A, Uggeri F. Surgical treatment of liver metastases of gastric cancer: state of the art. World J Surg Oncol. 2012;10:157. doi: 10.1186/1477-7819-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolff HC, Calatayud D, Larsen PN, Wettergren A. Good results after repeated resection for colorectal liver metastases. Dan Med J. 2012;59:A4373. [PubMed] [Google Scholar]
- 3.Liu J, Li JH, Zhai RJ, Wei B, Shao MZ, Chen L. Predictive factors improving survival after gastric and hepatic surgical treatment in gastric cancer patients with synchronous liver metastases. Chin Med J (Engl) 2012;125:165–171. [PubMed] [Google Scholar]
- 4.Yang XW, Li Z, Liu K, Fu XH, Yang JH, Wu MC. Correlation between the survival rate of the patients with synchronous hepatic metastases from gastric carcinoma after surgical resection and patient’s index. Chin Med J (Engl) 2012;125:747–751. [PubMed] [Google Scholar]
- 5.Tsujimoto H, Ichikura T, Ono S, Sugasawa H, Hiraki S, Sakamoto N, Yaguchi Y, Hatsuse K, Yamamoto J, Hase K. Outcomes for patients following hepatic resection of metastatic tumors from gastric cancer. Hepatol Int. 2010;4:406–413. doi: 10.1007/s12072-009-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, Usuki H, Maeta H. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg. 2002;235:86–91. doi: 10.1097/00000658-200201000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujii K, Fujioka S, Kato K, Machiki Y, Kutsuna Y, Ishikawa A, Takamizawa J, Ko K, Yoshida K, Nimura Y. Resection of liver metastasis from gastric adenocarcinoma. Hepatogastroenterology. 2001;48:368–371. [PubMed] [Google Scholar]
- 8.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 9.Li ZY, Tang L, Zhang LH, Bu ZD, Wu AW, Wu XJ, Zong XL, Wu Q, Shan F, Li SX, et al. Weekly docetaxel and cisplatin plus fluorouracil as a preoperative treatment for gastric cancer patients with synchronous multiple hepatic metastases: a pilot study. Med Oncol. 2010;27:1314–1318. doi: 10.1007/s12032-009-9381-y. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 11.Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981;11:127–139. doi: 10.1007/BF02468883. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 14.Takemura N, Saiura A, Koga R, Arita J, Yoshioka R, Ono Y, Hiki N, Sano T, Yamamoto J, Kokudo N, et al. Long-term outcomes after surgical resection for gastric cancer liver metastasis: an analysis of 64 macroscopically complete resections. Langenbecks Arch Surg. 2012;397:951–957. doi: 10.1007/s00423-012-0959-z. [DOI] [PubMed] [Google Scholar]
- 15.Dittmar Y, Altendorf-Hofmann A, Rauchfuss F, Götz M, Scheuerlein H, Jandt K, Settmacher U. Resection of liver metastases is beneficial in patients with gastric cancer: report on 15 cases and review of literature. Gastric Cancer. 2012;15:131–136. doi: 10.1007/s10120-011-0080-y. [DOI] [PubMed] [Google Scholar]
- 16.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 17.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita T, Nashimoto A, Yamamura Y, Okamura T, Sasako M, Sakamoto J, Kojima H, Hiratsuka M, Arai K, Sairenji M, et al. Feasibility study of adjuvant chemotherapy with S-1 (TS-1; tegafur, gimeracil, oteracil potassium) for gastric cancer. Gastric Cancer. 2004;7:104–109. doi: 10.1007/s10120-004-0278-3. [DOI] [PubMed] [Google Scholar]
- 19.Yokota T, Hatooka S, Ura T, Abe T, Takahari D, Shitara K, Nomura M, Kondo C, Mizota A, Yatabe Y, et al. Docetaxel plus 5-fluorouracil and cisplatin (DCF) induction chemotherapy for locally advanced borderline-resectable T4 esophageal cancer. Anticancer Res. 2011;31:3535–3541. [PubMed] [Google Scholar]
- 20.Higuchi K, Koizumi W, Tanabe S, Sasaki T, Katada C, Ishiyama H, Hayakawa K. A phase I trial of definitive chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) for advanced esophageal carcinoma: Kitasato digestive disease & amp; oncology group trial (KDOG 0501) Radiother Oncol. 2008;87:398–404. doi: 10.1016/j.radonc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Robak T, Korycka A, Kasznicki M, Wrzesien-Kus A, Smolewski P. Purine nucleoside analogues for the treatment of hematological malignancies: pharmacology and clinical applications. Curr Cancer Drug Targets. 2005;5:421–444. doi: 10.2174/1568009054863618. [DOI] [PubMed] [Google Scholar]
- 22.Pasini F, de Manzoni G, Zanoni A, Grandinetti A, Capirci C, Pavarana M, Tomezzoli A, Rubello D, Cordiano C. Neoadjuvant therapy with weekly docetaxel and cisplatin, 5-fluorouracil continuous infusion, and concurrent radiotherapy in patients with locally advanced esophageal cancer produced a high percentage of long-lasting pathological complete response: A phase 2 study. Cancer. 2013;119:939–945. doi: 10.1002/cncr.27822. [DOI] [PubMed] [Google Scholar]
- 23.Oertel K, Spiegel K, Schmalenberg H, Dietz A, Maschmeyer G, Kuhnt T, Sudhoff H, Wendt TG, Guntinas-Lichius O. Phase I trial of split-dose induction docetaxel, cisplatin, and 5-fluorouracil (TPF) chemotherapy followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer (TISOC-1) BMC Cancer. 2012;12:483. doi: 10.1186/1471-2407-12-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keil F, Selzer E, Berghold A, Reinisch S, Kapp KS, De Vries A, Greil R, Bachtiary B, Tinchon C, Anderhuber W, et al. Induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil followed by radiotherapy with cetuximab for locally advanced squamous cell carcinoma of the head and neck. Eur J Cancer. 2013;49:352–359. doi: 10.1016/j.ejca.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Inal A, Kaplan MA, Kucukoner M, Isikdogan A. Docetaxel and Cisplatin Plus Fluorouracil compared with Modified Docetaxel, Cisplatin, and 5-Fluorouracil as first-line therapy for advanced gastric cancer: a retrospective analysis of single institution. Neoplasma. 2012;59:233–236. doi: 10.4149/neo_2012_030. [DOI] [PubMed] [Google Scholar]
- 26.Yeh YS, Tsai HL, Ma CJ, Wu DC, Lu CY, Wu IC, Hou MF, Wang JY. A retrospective study of the safety and efficacy of a first-line treatment with modified FOLFOX-4 in unresectable advanced or recurrent gastric cancer patients. Chemotherapy. 2012;58:411–418. doi: 10.1159/000345742. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Kim HS, Han AR, Moh IH, Chung DC, Choi DR, Jang HJ, Kim JB, Yang DH, Lee SI, et al. Irinotecan, leucovorin and 5-fluorouracil (modified FOLFIRI) as salvage chemotherapy for frail or elderly patients with advanced gastric cancer. Oncol Lett. 2012;4:751–754. doi: 10.3892/ol.2012.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catalano V, Bisonni R, Graziano F, Giordani P, Alessandroni P, Baldelli AM, Casadei V, Rossi D, Fedeli SL, D‘Emidio S, et al. A phase II study of modified FOLFOX as first-line chemotherapy for metastatic gastric cancer in elderly patients with associated diseases. Gastric Cancer. 2012:Oct 11 [Epub ahead of print]. doi: 10.1007/s10120-012-0204-z. [DOI] [PubMed] [Google Scholar]
- 29.Keskin S, Yıldız I, Sen F, Aydogan F, Kilic L, Ekenel M, Saglam S, Sakar B, Disci R, Aykan F. Modified DCF (mDCF) regimen seems to be as effective as original DCF in advanced gastric cancer (AGC) Clin Transl Oncol. 2012:Oct 2 [Epub ahead of print]. doi: 10.1007/s12094-012-0942-8. [DOI] [PubMed] [Google Scholar]
- 30.Polyzos A, Felekouras E, Karatzas T, Griniatsos J, Dimitroulis D, Polyzos K, Kontzoglou K, Mantas D, Karavokyros J, Nikiteas N, et al. Modified docetaxel-cisplatin in combination with capecitabine as first-line treatment in metastatic gastric cancer. a phase II study. Anticancer Res. 2012;32:4151–4156. [PubMed] [Google Scholar]
- 31.Jeong JH, Lim SM, Yun JY, Rhee GW, Lim JY, Cho JY, Kim YR. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology. 2012;83:292–299. doi: 10.1159/000342376. [DOI] [PubMed] [Google Scholar]


