Abstract
The aim of this study was to compare zinc, copper, lead, cadmium, and mercury concentrations in the bones of long-living mammals—humans (Homo sapiens) and Canidae (dogs Canis familiaris and foxes Vulpes vulpes) from northwestern Poland and to determine the usefulness of Canidae as bioindicators of environmental exposure to metals in humans. Zinc concentrations in cartilage with adjacent compact bone and in spongy bone were highest in foxes (∼120 mg/kg dry weight (dw)) and lowest in dogs (80 mg/kg dw). Copper concentrations in cartilage with adjacent compact bone were greatest in foxes (1.17 mg/kg dw) and smallest in humans (∼0.8 mg/kg dw), while in spongy bone they were greatest in dogs (0.76 mg/kg dw) and lowest in foxes (0.45 mg/kg dw). Lead concentrations in both analyzed materials were highest in dogs (>3 mg/kg dw) and lowest in humans (>0.6 mg/kg dw). Cadmium concentration, also in both the analyzed materials, were highest in foxes (>0.15 mg/kg dw) and lowest in humans (>0.04 mg/kg dw). Mercury concentration in bones was low and did not exceed 0.004 mg/kg dw in all the examined species. The concentrations of essential metals in the bones of the examined long-living mammals were similar. The different concentrations of toxic metals were due to environmental factors. As bone tissues are used in the assessment of the long-term effects of environmental exposure to heavy metals on the human body, ecotoxicological studies on the bones of domesticated and wild long-living mammals, including Canidae, may constitute a significant supplement to this research.
Keywords: Human, Dog, Red fox, Bioindicator, Bone tissue, Trace elements
Introduction
Medium size and large wild mammals (mainly hunted species) and domesticated mammals are often used in comparative ecotoxicological studies [1–5]. Two important examples are the domestic dog (Canis familiaris) [6, 7] and the fox (Vulpes vulpes), commonly found in the entire Palearctic [8–10]. These species meet a number of criteria for bioindicators and show a measurable response to environmental pollutants, as documented in the works of various authors [3, 4, 7–10]. Researchers usually determine trace elements in their kidneys and liver, key organs in detoxification; bones are examined much less frequently [5, 7, 9, 11–13].
The concentrations of trace elements in bones indirectly indicate the extent of environmental pollution and the long-term impact, for example with regard to heavy metals [1, 14–16]. There are many signs that the concentrations of elements in bones are strongly linked to environmental conditions, diet, and health status of various populations of humans and mammals, but wider and more extensive research is necessary, including Central Europe. Although there are no reports comparing bone metal concentrations between humans and Canidae, several studies have used Canidae for the biomonitoring of human exposure to metals. For example, a study on pets and their owners living near a secondary lead (Pb) smelter in Illinois showed that when a dog or cat in the household had a high blood–Pb concentration, there was a significant increase in the likelihood of finding a person with a high blood Pb concentration in the same household [17, 18]. Canidae have a higher metabolic rate compared to Homo sapiens, but due to the longevity of these animals, it can be expected the concentrations of heavy metals in their bones may be similar to concentrations in humans. So far, most works have used rodents, including rats and mice, which live much shorter than humans and exhibit much lower bioaccumulation.
The aim of this study was to compare the concentrations of zinc (Zn), copper (Cu), Pb, cadmium (Cd), and mercury (Hg) in the bones of long-living mammals—humans (H. sapiens) and Canidae (dogs C. familiaris and foxes V. vulpes) from northwestern Poland and to determine the usefulness of Canidae as bioindicators of human exposure to metals.
Methods
Materials
We examined the femoral joint of humans (H. sapiens) and two Canidae species: the dog (C. familiaris) and the fox (V. vulpes). A total of 146 samples were collected of (1) cartilage with adjacent compact bone and (2) spongy bone, in the West Pomeranian voivodship, NW Poland, from November 2007 to December 2009.
Human bone material (n = 37) were sourced from residents of north-western Poland who had undergone femoral joint surgery. The study group comprised men between 53 to 78 years (62.6 ± 15.5) and women aged from 32 to 82 years (69.9 ± 10.76). The patients were mainly inhabitants of urban areas (approximately 90 %). Among the patients, 15 people were nonsmokers and 22 smokers. The majority of patients (approximately 90 %) consumed fish and seafood at least once a month. The exact characteristics of the study group are shown in a previous work on the same population [19].
The studies were approved by the Bioethics Committee of the Pomeranian Medical University in Szczecin (BN/001/111/08).
In addition, we examined 24 dogs (9 females and 15 males) and 12 foxes (four females and eight males). The animal study was performed only on adult individuals. The dogs were collected with the help of veterinarians performing euthanasia of dogs for various reasons (e.g., respiratory failure and cancer). The dogs were fed with commercial feed.
The foxes were from hunters in the voivodship, acting in compliance with the national law. Fox age categories were based on the examination of one single-root lower canine.
The exact characteristics of the study groups are presented in a previous work on the same population [20, 21].
Femoral heads were removed with a glass tool. Chemical analysis was performed on two materials: cartilage with directly adjacent compact bone and spongy bone. Bone material was dried to a constant weight at 55 then at 105 °C. This procedure was used to determine water content (gravimetric method). Dried samples were ground in an agate mortar.
Determination of Cu, Zn, Pb, Cd, and Hg
The samples were divided into doses weighing from 0.5 to 1.0 g. Bone material was mineralized by wet digestion using a Velp Scientifica mineralizer (Italy) [5]. Concentrations of Cu, Zn, Pb, and Cd were determined by ICP-AES in inductively coupled argon plasma, using a Perkin-Elmer Optima 2000 DV. The limits of detection for Cu, Zn, Pb, and Cd were 0.4, 0.2, 1.0, and 0.1 μg/l, respectively.
Total mercury concentrations were determined by atomic absorption spectroscopy from samples dried at 55 °C. The assays were run in an AMA 254 Hg analyzer (Altach Ltd., Czech Republic) based on flameless atomic absorption. For the analysis, from 100 to 300 mg of each sample was collected and placed in the analyzer’s nickel nacelle where it was automatically weighed and dried. The sample was thermally decomposed in a stream of oxygen to obtain a gaseous form, and its degradation products were transferred to the amalgamator for the selective off take of Hg. After the determination of the parameters of measurement, Hg vapor was released from the amalgamator by a brief heating. The amount of released Hg was measured by atomic absorption (detector in the AMA 254 analyzer is a silicon UV diode) at a wavelength of 254 nm in an arrangement of two measuring cells. The limit of detection for this method is 0.01 ng of Hg in the sample. For each sample, two or three repetitions were performed, and statistical analysis used the average of the data, expressed in milligrams per kilogram dry weight (dw).
The reliability of the analytical procedure was controlled by the determination of elements in two reference materials with known concentrations: National Institute of Standards and Technology (NIST) SRM 1486 bone meal and International Atomic Energy Agency (IAEA)-407 trace elements and methylmercury in fish (Table 1). Concentrations of metals in the reference materials were provided by the product manufacturers.
Table 1.
Concentrations of selected elements in the certified reference materials in milligrams per kilogram dry weight
| Metal | Bone meal SRM NIST 1486 | OD/RV (%) | Fish tissue IAEA-407 | OD/RV (%) | ||
|---|---|---|---|---|---|---|
| RV | OD (n = 7) | RV | OD (n = 8) | |||
| Zn | 147.0 ± 16.0 | 132.4 ± 4.1 | 90.0 | 67.1 | 65.8 ± 3.8 | 98.1 |
| Cu | 0.80a | 0.74 ± 0.01 | 92.5 | 3.28 | 3.12 ± 0.28 | 95.1 |
| Pb | 1.335 ± 0.014 | 1.190 ± 0.306 | 89.1 | 0.12 | 0.11 ± 0.03 | 91.7 |
| Cd | 0.003a | 0.0020 ± 0.0002 | 66.7 | 0.189 | 0.176 ± 0.010 | 93.1 |
| Hg | – | – | – | 0.222 | 0.237 ± 0.002 | 106.8 |
RV reference value, OD own determination
aEstimated value
Statistical Analysis
Analysis used Statistica 9.0 StatSoft software. In order to determine compliance with the expected normal distribution of results, a Kolmogorov–Smirnov test with Lillefors correction (p < 0.05) was used. Interspecies comparisons were made with regard to the corresponding bone material using a Kruskal–Wallis test. In the case of statistically significant differences, a Mann–Whitney U test was used (p < 0.05). Two samples of cartilage with compact bone obtained from dogs had Cu concentrations many times higher than the maximum value for other samples (3.1 mg/kg dw), 42.2 and 51.4 mg/kg dw, respectively, so were excluded from the statistical analysis.
Results
In Table 2 presents the date for the concentration of heavy metals in the (1) cartilage with compact bone and (2) spongy bone in humans and in two species of Canidae (dog and fox). Nonparametric Kruskal–Wallis tests showed the presence of statistically significant differences between the mean values of all studied metals in the cartilage with adjacent compact bone, and showed significant differences in the spongy bone concentrations of Zn and Cd between three compared species (Table 3). Further comparisons of the concentrations of metals in samples between the examined mammals were based on the Mann–Whitney U test.
Table 2.
Comparison of metal concentrations (in milligrams per kilogram dry weight) between bones from humans, dogs, and foxes
| Metal | Parameter | Human (n = 37) | Dog (n = 24) | Red fox (n = 12) |
|---|---|---|---|---|
| Cartilage with adjacent compact bone | ||||
| Zn | AM ± SD | 88.3 ± 22.5 | 80.5 ± 30.3 | 119.6 ± 42.8 |
| Median | 85.7 | 77.3 | 121.5 | |
| Min–max | 54.3–163.8 | 15.9–165.5 | 38.9–199.7 | |
| CV | 25.5 | 37.3 | 42.8 | |
| Cu | AM ± SD | 0.79 ± 0.40 | 0.89 ± 0.737 | 1.17 ± 1.15 |
| Median | 0.74 | 0.76 | 0.75 | |
| Min–max | 0.20–1.78 | 0.10–3.11 | 0.20–3.71 | |
| CV | 50.6 | 82.7 | 99.0 | |
| Pb | AM ± SD | 0.527 ± 0.204 | 2.829 ± 3.490 | 1.65 ± 1.88 |
| Median | 0.496 | 1.158 | 0.927 | |
| Min–max | 0.285–1.440 | 0.017–12.68 | 0.07–6.15 | |
| CV | 38.7 | 110.3 | 113.8 | |
| Cd | AM ± SD | 0.031 ± 0.204 | 0.105 ± 0.067 | 0.113 ± 0.049 |
| Median | 0.021 | 0.118 | 0.107 | |
| Min–max | 0.001–0.151 | 0.026–0.295 | 0.003–0.184 | |
| CV | 100.5 | 63.6 | 43.0 | |
| Hg | AM ± SD | 0.0032 ± 0.0021 | 0.0020 ± 0.0013 | 0.0028 ± 0.0025 |
| Median | 0.0027 | 0.0015 | 0.0020 | |
| Min–max | 0.0010–0.0123 | 0.0009–0.0650 | 0.0013–0.0105 | |
| CV | 68.1 | 65.0 | 91.1 | |
| Spongy bone | ||||
| Zn | AM ± SD | 83.1 ± 21.5 | 81.1 ± 41.1 | 99.9 ± 50.7 |
| Median | 79.4 | 94.5 | 105.0 | |
| Min–max | 44.2–160.5 | 5.9–159.8 | 11.2–157.1 | |
| CV | 25.9 | 50.6 | 50.7 | |
| n = 22a | ||||
| Cu | AM ± SD | 0.67 ± 0.36 | 0.76 ± 0.51 | 0.45 ± 0.34 |
| Median | 0.58 | 0.62 | 0.45 | |
| Min–max | 0.18–1.89 | 0.01–1.59 | 0.08–1.12 | |
| CV | 53.0 | 66.8 | 74.1 | |
| Pb | AM ± SD | 0.500 ± 0.142 | 1.55 ± 1.71 | 0.980 ± 1.0 |
| Median | 0.500 | 0.694 | 0.447 | |
| Min–max | 0.287–0.789 | 0.034–6.70 | 0.150–2.83 | |
| CV | 28.4 | 110.3 | 108.3 | |
| Cd | AM ± SD | 0.028 ± 0.040 | 0.096 ± 0.074 | 0.131 ± 0.07 |
| Median | 0.023 | 0.058 | 0.132 | |
| Min–max | 0.001–0.269 | 0.013–0.210 | 0.034–0.260 | |
| CV | 155.5 | 77.3 | 53.2 | |
| Hg | AM ± SD | 0.0022 ± 0.0013 | 0.0027 ± 0.0022 | 0.0043 ± 0.0033 |
| Median | 0.0018 | 0.0021 | 0.0030 | |
| Min–max | 0.0002–0.0060 | 0.0009–0.0113 | 0.0012–0.0104 | |
| CV | 61.6 | 81.9 | 76.2 | |
AM arithmetic mean, SD standard deviation, CV coefficient of variation in percent, K-W Kruskal–Wallis test, p level of significance, NS statistically nonsignificant
aAnalysis after removing samples with exceptionally high Cu concentrations
Table 3.
The statistically significant differences (using a Kruskal–Wallis test) between the concentrations of metals in examined long-living mammals bones materials
| Species | Zn | Cu | Pb | Cd | Hg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CACB | SB | CACB | SB | CACB | SB | CACB | SB | CACB | SB | ||
| Human vs. dog vs. red fox | |||||||||||
| Human (n = 37) vs. dog (n = 24) | p | NS | NS | NS | NS | NS | NS | 0.0001 | 0.0001 | 0.02 | NS |
| Human (n = 37) vs. red fox (n = 12) | p | NS | NS | NS | NS | NS | NS | 0.0001 | 0.0001 | NS | 0.04 |
| Dog (n = 24) vs. red fox (n = 12) | p | 0.02 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
CACB cartilage with adjacent compact bone, SB spongy bone, p level of significance, NS statistically nonsignificant
Differences Between Man and Dog
Zn and Cu concentrations did not differ significantly between humans and dogs, either in cartilage with adjacent compact bone or in spongy bone. Statistically confirmed differences were found for Pb (U test = 385.0; p < 0.03) and Cd (U test = 16.0; p < 0.0001) which were higher in dogs. Pb concentrations were higher in dog cartilage with adjacent compact bone and higher in spongy bone about five and three times, respectively (Table 2). Similar regularity was observed for Cd, about 70 % higher in both types of materials.
Differences Between Man and Fox
In the cartilage with adjacent compact bone, significant differences were found for Zn (U test = 259.0; p < 0.0002) and Cu (U test = 362.0; p < 0.02), both about 30 % higher in foxes. There were also significant differences in highly toxic elements: Pb (U test = 385.0; p < 0.03), Cd (U test = 16.0; p < 0.0001) and Hg (U test = 325.0; p < 0.01). Pb was three times higher in foxes than in humans. Cd was about four times higher than in humans furthermore Hg concentration between this two species were similar.
In the spongy bone, there were two statistically confirmed differences: Zn (U test = 273.0; p < 0.0001) and Cd (U test = 47.0; p < 0.0001). Concentrations of both metals were higher in the fox, about 20 (Zn) and 98 % (Cd) greater than in humans.
Most Frequent Metal Concentration Ranges in Man, Dog and Fox
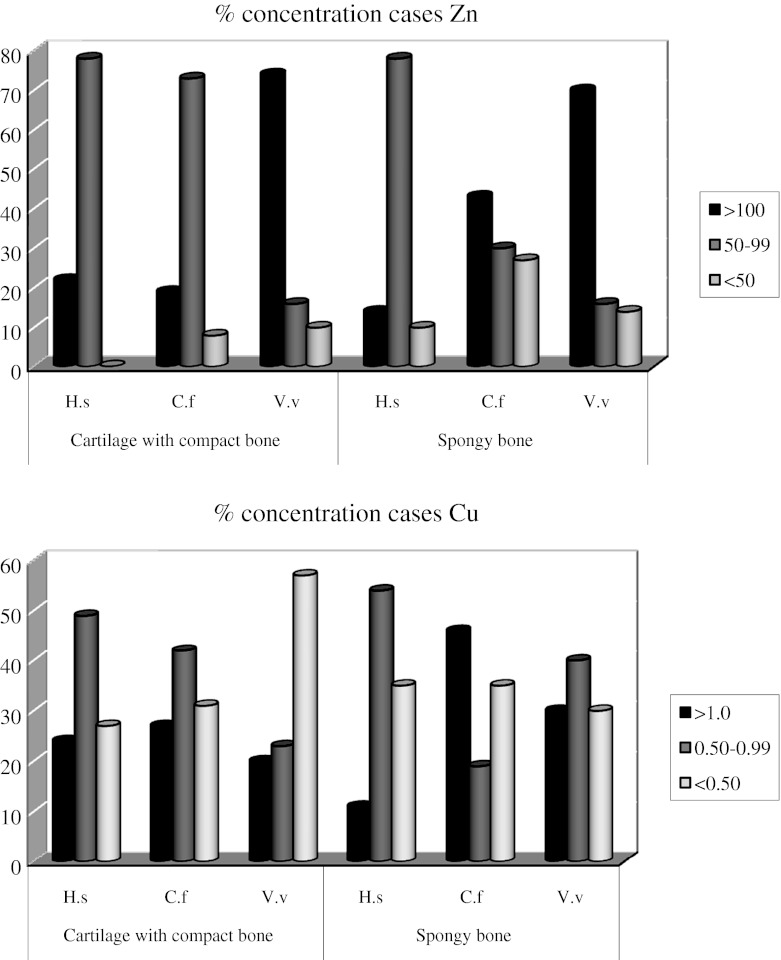
In humans and dogs, Zn concentrations were most frequently (70 %) within the range 50–100 mg/kg dry weight while in foxes it exceeded 100 mg/kg dry weight (Fig. 1).
Fig. 1.
The percentage of concentrations of essential elements (Zn, Cu) in bone material of humans (H.s. Homo sapiens) and Canidae: dog (C.f. Canis lupus familiaris), fox (V.v. Vulpes vulpes)
In contrast Cu in the cartilage with adjacent compact bones of foxes was generally low, in more than 55 % of cases not exceeding 0.50 mg/kg dw, while most humans and dogs had usually higher Cu, with ranges of >1 and 0.50–0.99 mg/kg found in 65–70 % of the samples. Interestingly, Cu concentration in spongy bone of >1 mg/kg was observed in 45 % of dog samples and 30 % of fox samples, while in humans it was 10 % (Fig. 1).
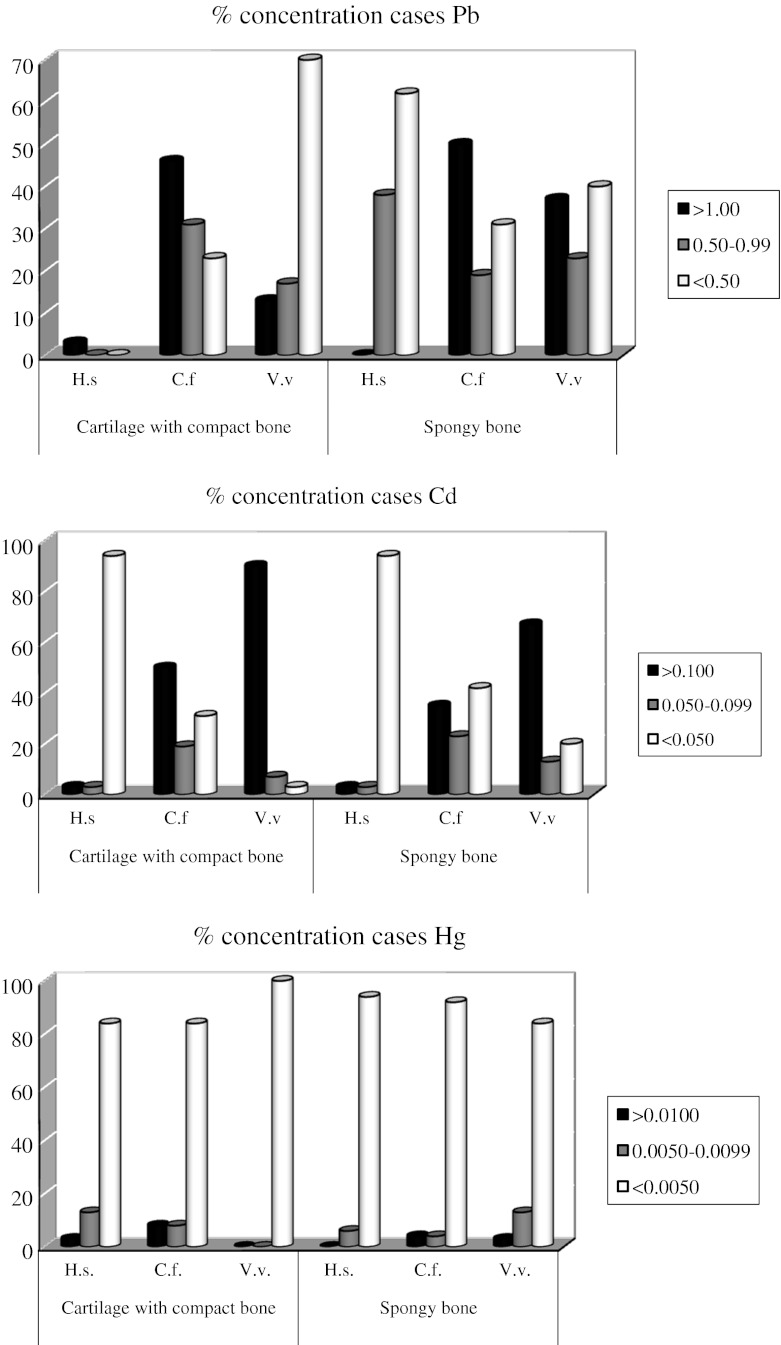
Pb concentration in human bone material was generally low, only occasionally exceeding 1 mg/kg dw in both materials. This limit was exceeded in more than 45 % of dog samples (Fig. 2). In fox samples, Pb of >1 mg/kg dw was found in less than 35 % of spongy bone samples and around 10 % of cartilage with adjacent compact bone.
Fig. 2.
The percentage of concentrations of toxic metals (Pb, Cd and Hg) in bone material of (H.s. Homo sapiens) and Canidae: dog (C.f. Canis lupus familiaris), fox (V.v. Vulpes vulpes)
Cd in the bone material of all three examined mammalian species was significantly lower than Pb. The samples were divided into three groups regarding Cd concentration, with the highest range of >0.1 mg/kg dw. The highest percentage of samples in this group was found in the fox, 85 % samples of cartilage with adjacent compact bone and 65 % of spongy bone samples. In humans, Cd concentration was usually very low <0.05 mg/kg dw in more than 90 % samples.
In all three species, Hg concentrations in 80–90 % of the samples did not exceed 0.005 mg/kg dw in both materials and can be regarded as very low (Fig. 2).
Discussion
This research, using Canidae bones to indirectly assess environmental pollution and human exposure to metals, is a pioneering ecotoxicological biomonitoring study, not only in Poland but also in Europe. We have demonstrated similar concentrations of essential metals and considerably varied toxic metal concentrations in the bones of long-living mammals in the same area, due to environmental conditions. Differences in metal levels in tissues between the observed species reflected different feeding selection and behavior of animals, and the utilization of urban and suburban habitat locations. The diet of the dogs is either similar to the human diet or based solely on commercial feeds, while foxes feed on a variety of animals, mostly rodents (e.g., mice) and other small animals (amphibians, birds and bird eggs, reptiles, and fish) and carrion.
There is a lack of information in literature regarding to the usefulness of Canidae as bioindicators of human exposure to metals and also no study has been carried out to correlate the hard tissue levels of heavy metals in dog, fox and human exposure. Some studies do suggest that dogs which for years shared the same habitat with humans may be an appropriate animal model to assess human exposure to metals [17, 22, 23]. However, in the available scientific literature there are no comparative works on concentrations of trace elements in the bones of long-living mammals and interspecies relationships between man, dog, and fox.
Zn and Cu
In a human biomonitoring study, Zaichick and Zaichick [24–27] established physiological levels of Zn in the femoral head, iliac crest and rib among inhabitants of Central Europe: 58.1, 60.8, and 92.4 mg/kg dw, respectively while the physiological levels of Cu in the bone were deemed to be 5–6 mg/kg dw. The average concentration of Cu in samples of ribs from Russians ranged from 0.84 to 1.0 mg/kg dw [24, 26]. In this study, the concentration of Zn in femur samples obtained from patients with osteoporosis in NW Poland was approximately 50 % higher than the physiological concentration. Zn and Cu concentrations in the spongy bone of the femoral head in patients from NW Poland and vicinity differed from results obtained from the most contaminated region of Poland, Upper Silesian, recorded by Brodziak-Dopierala et al. [28]. The concentration of Zn in the bones of these patients was more than two times lower and amounted to 35.7 mg/kg dw, and the concentration of Cu was more than five times higher and reached a value of 2.95 mg/kg dw. In addition, in the residents of Taiwan and China, Zn concentrations in the femur head were found in the range of 109 to 115 mg/kg dw, Cu 1.4 to 3.1 mg/kg dw [29, 30]. In patients from USA, Brazil, and Japan, Zn and Cu concentrations in ribs ranged from 114 to 149 and from 0.2 to 1.4 mg/kg dw, respectively [31–34].
Pb and Cd
More than 90% of Pb accumulates in bones of the human, of which 70 % accumulates in the cortical bone (the major part of the human skeleton). A reference level of Pb in the femoral head is approximately 1 mg/kg dw, while in highly exposed children, it is 40 mg/kg dw [35].
Comparisons of our results with data in literature on Pb content in cartilage with adjacent compact bone show dozens of times higher concentrations of Pb in bones of Upper Silesians than in the residents of north-western Poland [24, 28, 29, 34, 36]. In the Upper Silesian Industry Area, where the main factors of exposure to heavy metals include numerous heavy metal smelters, and high Pb and Cd contamination of soil, water, and air, concentration of Pb in bones ranged from 0.87 to 10.6 mg/kg dw [28, 36].
Cd affinity to bone is much lower than Pb and therefore Cd is determined less frequently. Its concentration is two orders of magnitude lower than Pb. In our study, the cartilage with adjacent compact bone in the inhabitants of the NW Poland had a low concentration of Cd (0.03 mg/kg dw). Similar values were detected in individuals not occupationally exposed to this xenobiotic in other parts of Europe, including Spain, the Czech Republic, Great Britain, and various regions of Poland [28, 36–38], with Cd concentrations ranging from 0.03 to 0.07 mg/kg dw. People not exposed occupationally to Pb and Cd, living in various Asian countries including South Korea and Taiwan, had much higher concentrations of these xenobiotics in comparison to north-western Poland [39]. The concentration of Pb ranged from 1.9 to 7.1 mg/kg dw, and Cd concentrations from 0.11 and 1.2 mg/kg dw.
Lanocha et al. [11] determined Pb and Cd in the cartilage of the femoral bone in dogs and foxes. The concentration of Pb in the cartilage of dogs was ∼2.3 mg/kg, about 15 % larger than in the fox, although the difference was not confirmed statistically. However, the concentration of Cd in the cartilage was significantly lower (p < 0.05) in the dog than in the fox (0.13 and 0.16 mg/kg dw, respectively). Similar to the cited work, in this study, Cd in the cartilage with adjacent compact bone differed statistically significantly between the analyzed adult dogs and foxes (p < 0.0001). In foxes from NW Poland, the average values of Pb fluctuated around 1.6 mg/kg dw. Perhaps the accumulation of Pb in their bodies was not only due to air pollution, but also to their specific diet, with a considerable share of carcasses and/or birds and small wild hunted animals with Pb pellets in their bodies [40].
Differences in diet are probably the reason for the differences in Cd concentrations between the examined Canidae in north-western Poland. The fox is a predator that prefers food of animal origin, especially rodents, but also feeds on small invertebrates (beetles, grubs, and earthworms) which accumulate large amounts of Cd. The dog’s diet is similar to humans and generally contains only small amounts of this toxic metal. Similarly, Lopez-Alonso et al. [10] suggest that differences in the concentrations of highly toxic metals, including Pb and Cd, in the liver and kidneys of Canidae may be due to the different food preferences of these animals. Moreover, it should also be noted that sometimes Cd concentrations vary significantly in different parts of the long bones of the fox. For example, in the spongy bone concentrations ranged from 0.034–0.026 mg/kg dw.
In our study, the observed dog bone Pb concentrations in West Pomerania were quite high (from 0.017 to 12.68 mg/kg dw). This may be due to environmental pollution with Pb, mainly from car fumes, as unleaded petrol had only begun to be introduced in Poland since the mid-1990s. A total ban on the use of Pb antiknock agents in fuel was introduced as late as 2005 [41, 42]. It appears that Pb from gasoline could have been intensely accumulated in bones when Pb was used as a fuel additive.
In Uruguay (slum settlements in La Teja), which until 2004 used tetraethyl Pb in gasoline and Pb pipes for drinking water supply in older buildings, Pb concentration in the soil exceeded 3,000 ppm [43]. Children living in that area were found to have blood Pb levels (BLL) higher than 20 μg/dL. Similarly, dogs in the area had higher BLL than children when exposed to the same polluted environment and developed symptoms of Pb intoxication earlier and at lower BLL than did the children [43]. Furthermore, after the withdrawal of Pb in gasoline in Uruguay, there was a significant decrease in blood Pb levels in children, similar to the USA and Sweden.
Pb levels in sediments in the USA closely followed the rise and fall of leaded gasoline, demonstrating that leaded gasoline was the major contributor to atmospheric and environmental Pb pollution in the twentieth century [44].
Gamberg and Braune [45] found that in the wolf preying on medium and large mammals (often shot by hunters) Pb concentration in bone amounted to 2.12 mg/kg ww (∼2.6 mg/kg dw). This concentration was similar to that observed in dog bones and about 40 % larger than in the fox from north-western Poland. The results concerning Pb in predatory mammals indicate interspecies differences related to the degree of environmental pollution and the risk of Pb intoxication from organs of the animals shot by hunters.
Mercury
Data on Hg in bone and skeletal system are even scarcer than for Pb and Cd, albeit in laboratory studies pregnant rats and mice that inhaled Hg chloride were reported to experience changes in bone and decreased ossification in the offspring [46]. In Eastern Europe (Russia), biomonitoring studies on the concentration of Hg in the iliac crest and the ribs of humans were carried out by Zaichick and Zaichick [25, 26]. Hg concentration in the iliac crest ranged from 0.004 to 0.008 mg/kg dw and in ribs from 0.008 to 0.018 mg/kg dw. Average Hg concentration in the ribs of the Russian population was 0.006 mg/kg dw where they were found to be a typical value for those not occupationally exposed to this xenobiotic [25, 26]. In our study, in samples of cartilage with compact bone, average Hg concentrations ranged from 0.001 to 0.0123 mg/kg dw. The highest concentrations of Hg were twice as high compared with typical concentrations of Hg given by Zaichick and Zaichick [25, 26].
In research by Yoo et al. [39] in South Korea very high concentrations of Hg were found in the bones of the inhabitants of Seoul. Hg concentration was as high as 2.2 mg/kg dw (2.75 mg/kg dw). These results differ from concentrations specified in the bones of inhabitants of the north-western Poland and Russia—they were about 3–4 orders of magnitude larger. Probably the concentration of >2 mg/kg dw in bones of Koreans were associated with air pollution in Asia and a diet rich in fish and seafood.
An interesting ecotoxicological study was carried out in the south of Spain (Donana Park), which a few years earlier had been heavily contaminated with mine water containing significant amounts of heavy metals [15]. The researchers examined the concentration of Zn, Cu, Pb, and Hg in the bones of wild mammals. Probably the transfer to food chains resulted in a significant increase in the concentration of heavy metals, especially Cu. These studies used a number of predatory mammals, including the fox, Iberian lynx (Lynx pardinus), common genet (Genetta genetta), Egyptian mongoose (Herpestes ichneumon), and badger (Meles meles). The highest concentrations of Zn (>170 mg/kg dw) were detected in the common genet, feeding mainly on insect, small vertebrates and fish. Lesser concentrations (∼130 mg/kg dw) of the metal were found in the fox and badger. The concentration of Zn in the spongy bone of the Western Pomerania fox was ~105 mg/kg and was about 20 % lower than those observed in the fox in Spain. However, the concentration of Cu in the bones of the fox analyzed by Millan et al. [15] was about nine and five times higher in the spongy bone of the fox and dog from West Pomerania. Cd concentration was not examined in the bones of these species but the concentrations of Pb and Hg were characterized by large interspecies variability. These animals have different diets which could be a key cause for the considerable variation in the concentration of Pb in their bones [15]. The highest Pb concentrations (>2 mg/kg dw) were detected in the Iberian lynx mainly feeding on rabbits which may have been shot with Pb pellets by hunters, probably the main source of Pb intoxication of this predator.
A much lower Pb concentration was found in the bones of the fox (∼0.4 mg/kg dw) and the lowest was observed in common genet and Egyptian mongoose (<0.15 mg/kg dw). Compared with the foxes from the Iberian Peninsula, the dogs and foxes coming from NW Poland had twice and four times higher concentrations of Pb in the spongy bone, which could have resulted from greater Pb pollution in Poland. However, it should be emphasized that Pb accumulates in cartilage of warm-blooded vertebrates more intensely than in bones, as documented by many researchers [5, 28, 47]. Furthermore, in Canidae from north-western Poland, Hg concentrations were an order of magnitude lower than those detected by Millan et al. [15]. The highest concentration of Hg was observed in the common genet (0.023 mg/kg dw) and the smallest in the Egyptian mongoose (0.011 mg/kg dw). The concentration of Hg in the fox from Spain was three and four times higher compared with spongy bone of the fox and dog from NW Poland.
Based on the results and data from the scientific literature, the bones of Canidae living near humans (especially dogs) can be considered as a convenient alternative reference material in relation to the species H. sapiens in research on the long-term effects of heavy metals on the skeletal system. They show a measurable response to environmental pollution with trace elements and meet the requirements for good bioindicators.
Acknowledgment
The study was financed as research project No. NN 404 507738 by the Polish Ministry of Education from the resources for the years 2010–2011
References
- 1.Lazarus M, Orct T, Blanusa M, Vickovic I, Sostaric B. Toxic and essential metal concentrations in four tissues of red deer (Cervus elaphus) from Baranja, Croatia. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25:270–283. doi: 10.1080/02652030701364923. [DOI] [PubMed] [Google Scholar]
- 2.Kalisinska E, Budis H, Lanocha N, Podlasinska J, Jedrzejewska E, Kosik-Bogacka DI. Comparison of hepatic and nephric total mercury concentrations between feral and ranch American mink (Neovison vison) from northwestern Poland. Bull Environ Contam Toxicol. 2012;88:802–806. doi: 10.1007/s00128-012-0555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalisinska E, Lisowski P, Kosik-Bogacka DI. Red fox Vulpes vulpes (L., 1758) as a bioindicator of mercury contamination in terrestrial ecosystems of north-western Poland. Biol Trace Elem Res. 2012;145:172–180. doi: 10.1007/s12011-011-9181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalisinska E, Lisowski P, Salicki W, Kucharska T, Kavetska K. Mercury wild terrestrial carnivorous mammals from north-western Poland and unusual fish diet of red fox. Act Theriol. 2009;54:345–356. doi: 10.4098/j.at.0001-7051.032.2008. [DOI] [Google Scholar]
- 5.Kalisinska E, Salicki W, Kavetska KM, Ligocki M. Trace metal concentrations are higher in cartilage than in bones of scaup and pochard wintering in Poland. Sci Total Environ. 2007;388:90–103. doi: 10.1016/j.scitotenv.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 6.Macpherson CNL, Meslin FX, Wandeler AI. Dogs, zoonoses and public health. Oxon: CABI; 2001. [Google Scholar]
- 7.Lanocha N, Kalisinska E, Kosik-Bogacka DI, Budis H. Evaluation of dog bones in the indirect assessment of environmental contamination with trace elements. Biol Trace Elem Res. 2012;147:103–112. doi: 10.1007/s12011-011-9315-3. [DOI] [PubMed] [Google Scholar]
- 8.Dip R, Stieger C, Deplazes P, Hegglin D, Muller U, Dafflon O, Koch H, Naegeli H. Comparison of heavy metal concentrations in tissues of red foxes from adjacent urban, suburban, and rural areas. Arch Environ Contam Toxicol. 2001;40:551–556. doi: 10.1007/s002440010209. [DOI] [PubMed] [Google Scholar]
- 9.Lanocha N, Kalisinska E, Kosik-Bogacka DI, Budis H, Noga-Deren K. Trace metals and micronutrients in bone tissues of the red fox Vulpes vulpes (L., 1758) Acta Theriol. 2012;57:233–244. doi: 10.1007/s13364-012-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Alonso M, Miranda M, García-Partida P, Cantero F, Hernandez J, Benedito JL. Use of dogs as indicators of metal exposure in rural and urban habitats in NW Spain. Sci Total Environ. 2007;372:668–675. doi: 10.1016/j.scitotenv.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Lanocha N, Kalisinska E, Budis H (2009) Cadmium and lead in femur cartilage of canids from north-western Poland. Ecotoxicology in the real world. Krakow 16–19 September 2009, p. 111
- 12.Brodziak-Dopierala B, Kwapulinski J, Rzepka J, Nogaj E, Bogunia M, Ahnert B. Influence of smoking tobacco on the occurrence metals in some parts and profiles of femur head. Przegl Lek. 2007;64:720–722. [PubMed] [Google Scholar]
- 13.Kwapulinski J, Miroslawski J, Wiechula D, Jurkiewicz A, Tokarowski A. The femur capitulum as a biomarker of contamination due to indicating lead content in the air by participation of the other metals. Sci Total Environ. 1995;175:57–64. doi: 10.1016/0048-9697(95)04844-8. [DOI] [PubMed] [Google Scholar]
- 14.Bjora R, Falch JA, Staaland H, Nordsletten L, Gjengedal E. Osteoporosis in the Norwegian moose. Bone. 2001;29:70–73. doi: 10.1016/S8756-3282(01)00469-0. [DOI] [PubMed] [Google Scholar]
- 15.Millan J, Mateo R, Taggart MA, Lopez-Bao JV, Viota M, Monsalve L, Camarero PR, Blazquez E, Jimenez B. Levels of heavy metals and metalloids in critically endangered Iberian lynx and other wild carnivores from Southern Spain. Sci Total Environ. 2008;399:193–201. doi: 10.1016/j.scitotenv.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Reglero MM, Taggart MA, Monsalve-Gonzalez L, Mateo R. Heavy metal exposure in large game from a lead mining area: effects on oxidative stress and fatty acid composition in liver. Environ Pollut. 2009;157:1388–1395. doi: 10.1016/j.envpol.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt PL. Companion animals as sentinels for public health. Vet Clin North Am Small Anim Pract. 2009;39:241–250. doi: 10.1016/j.cvsm.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Serpe FP, Russo R, De Simone A, Florio S, Esposito M, Severino L. Levels of heavy metals in liver and kidney of dogs from urban environment. Open Vet J. 2012;2:15–18. [PMC free article] [PubMed] [Google Scholar]
- 19.Lanocha N, Kalisinska E, Kosik-Bogacka DI, Budis H, Sokolowski S, Bohatyrewicz A. Concentrations of trace elements in bones of the hip joint from patients after hip replacement surgery. J Trace Elem Med Biol. 2012;26:20–25. doi: 10.1016/j.jtemb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Lanocha N, Kalisinska E, Kosik-Bogacka DI, Budis H. Evaluation of dog bones in the indirect assessment of environmental contamination with trace elements. Biol Trace Elem Res. 2012;147:103–112. doi: 10.1007/s12011-011-9315-3. [DOI] [PubMed] [Google Scholar]
- 21.Lanocha N, Kalisinska E, Kosik-Bogacka DI, Budis H, Noga-Deren K. Trace metals and micronutrients in bone tissues of the red fox Vulpes vulpes (L., 1758) Acta Theriol (Warsz) 2012;57:233–244. doi: 10.1007/s13364-012-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backer LC, Grindem CB, Corbett WT, Cullins L, Hunter JL. Pet dogs as sentinels for environmental contamination. Sci Total Environ. 2001;274:161–169. doi: 10.1016/S0048-9697(01)00740-9. [DOI] [PubMed] [Google Scholar]
- 23.Ghisleni G, Spagnolo V, Roccabianca P, Scanziani E, Paltrinieri S, Lupo F, Ferretti E, Nageli F. Blood lead levels, clinico-pathological findings and erythrocyte metabolism in dogs from different habitats. Vet Hum Toxicol. 2004;46:57–61. [PubMed] [Google Scholar]
- 24.Zaichick S, Zaichick V, Karandashev VK, Moskvina IR. The effect of age and gender on 59 trace-element contents in human rib bone investigated by inductively coupled plasma mass spectrometry. Biol Trace Elem Res. 2011;143:41–57. doi: 10.1007/s12011-010-8837-4. [DOI] [PubMed] [Google Scholar]
- 25.Zaichick S, Zaichick V. The effect of age and gender on 38 chemical element contents in human iliac crest investigated by instrumental neutron activation analysis. J Trace Elem Med Biol. 2010;24:1–6. doi: 10.1016/j.jtemb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Zaichick V, Zaichick S. Instrumental neutron activation analysis of trace element contents in the rib bone of healthy men. J Radioanal Nucl Chem. 2009;281:47–52. doi: 10.1007/s10967-009-0084-9. [DOI] [Google Scholar]
- 27.Zaichick S, Zaichick V. The effect of age and gender on 38 chemical element contents in human femoral neck investigated by instrumental neutron activation analysis. Biol Trace Elem Res. 2010;137:1–12. doi: 10.1007/s12011-009-8554-z. [DOI] [PubMed] [Google Scholar]
- 28.Brodziak-Dopierala B, Kwapulinski J, Kusz D, Gajda Z, Sobczyk K. Interactions between concentrations of chemical elements in human femoral heads. Arch Environ Contam Toxicol. 2009;57:203–210. doi: 10.1007/s00244-008-9228-0. [DOI] [PubMed] [Google Scholar]
- 29.Kuo HW, Kuo SM, Chou CH, Lee TC. Determination of 14 elements in Taiwanese bones. Sci Total Environ. 2000;255:45–54. doi: 10.1016/S0048-9697(00)00448-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Cheng F, Li D, Wang Y, Zhang G, Liao W, Tang T, Huang Y, He W. Investigation of elemental content distribution in femoral head slice with osteoporosis by SRXRF microprobe. Biol Trace Elem Res. 2005;103:177–185. doi: 10.1385/BTER:103:2:177. [DOI] [PubMed] [Google Scholar]
- 31.Saiki M, Takata MK, Kramarski S, Borelli A. Instrumental neutron activation analysis of rib bone samples and of bone reference materials. Biol Trace Elem Res. 1999;71–72:41–46. doi: 10.1007/BF02784189. [DOI] [PubMed] [Google Scholar]
- 32.Samudralwar DL, Robertson JD. Determination of major and trace elements in bones by simultaneous PIXE/PIGE analysis. J Radional Nucl Chem. 1993;169:259–267. doi: 10.1007/BF02046801. [DOI] [Google Scholar]
- 33.Takata MK, Saiki M, Sumita NM, Saldiva PHN, Passqualucci CA. Trace element determinations in human cortical and trabecular bones. J Radioanal Nucl Chem. 2005;264:5–8. doi: 10.1007/s10967-005-0666-0. [DOI] [Google Scholar]
- 34.Yoshinaga J, Suzuki T, Morita M, Hayakawa M. Trace elements in ribs of elderly people and elemental variation in the presence of chronic diseases. Sci Total Environ. 1995;162:239–252. doi: 10.1016/0048-9697(95)04470-L. [DOI] [PubMed] [Google Scholar]
- 35.Miculescu F, Miculescu M, Ciocan LT, Ernuteanu A, Antoniac I, Pencea I, Matei E. Comparative studies regarding heavy elements concentration in human cortical bone. Digest J Nanomater Biostruct. 2011;6:1117–1127. [Google Scholar]
- 36.Brodziak-Dopierala B, Kwapulinski J, Sobczyk K, Kowol J. The occurrence of nickel and other elements in tissues of the hip joint. Ecotoxicol Environ Saf. 2011;74:630–635. doi: 10.1016/j.ecoenv.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Benes B, Jakubec K, Smid J, Spevackova V. Determination of thirty-two elements in human autopsy tissue. Biol Trace Elem Res. 2000;75:195–203. doi: 10.1385/BTER:75:1-3:195. [DOI] [PubMed] [Google Scholar]
- 38.Helliwell TR, Kelly SA, Walsh HP, Klenerman L, Haines J, Clark R, Roberts NB. Elemental analysis of femoral bone from patients with fractured neck of femur or osteoporosis. Bone. 1996;18:151–157. doi: 10.1016/8756-3282(95)00440-8. [DOI] [PubMed] [Google Scholar]
- 39.Yoo YC, Lee SK, Yang JY, Kim KW, Lee SY, Oh SM, Chung KH. Interrelationship between the concentration of toxic and essential elements in Korean tissues. J Health Sci. 2002;48:195–200. doi: 10.1248/jhs.48.195. [DOI] [Google Scholar]
- 40.Goszczynski J. Red fox. Monograph of nature and hunting. Warsaw: Publishing House Oikos; 1995. [Google Scholar]
- 41.Directive 2003/17/EC of the European Parliament and of the Council of 3 March 2003 amending Directive 98/70/EC relating to the quality of petrol and diesel fuels
- 42.Directive 98/70/EC of the European Parliament and of the Council of 13 March 1998 relating to the quality of petrol and diesel fuels Directive 93/12/EC
- 43.Manay N, Cousillas AZ, Alvarez C, Heller T. Lead contamination in Uruguay: the "La Teja" neighborhood case. Rev Environ Contam Toxicol. 2008;195:93–115. doi: 10.1007/978-0-387-77030-7_4. [DOI] [PubMed] [Google Scholar]
- 44.Callender E, Metre PC. Environmental policy analysis, peer reviewed: reservoir sediment cores show U.S. lead declines. Environ Sci Technol. 1997;31:424A–428A. doi: 10.1021/es972473k. [DOI] [Google Scholar]
- 45.Gamberg M, Braune BM. Contaminant residue levels in arctic wolves (Canis lupus) from the Yukon Territory, Canada. Sci Total Environ. 1999;243–244:329–338. doi: 10.1016/S0048-9697(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 46.Bartolome J, Whitmore WL, Slotkin TA. Effects of neonatal mercuric chloride administration on growth and biochemical development of neuronal and non-neuronal tissues in the rat: comparison with methylmercury. Toxicol Lett. 1984;22:101–111. doi: 10.1016/0378-4274(84)90052-3. [DOI] [PubMed] [Google Scholar]
- 47.Jurkiewicz A, Wiechula D, Nowak R, Loska K. Lead content in the femoral heads of inhabitants of Silesia (Poland) J Trace Elem Med Biol. 2005;19:165–170. doi: 10.1016/j.jtemb.2005.07.010. [DOI] [PubMed] [Google Scholar]