Abstract
Lithium (Li) is a trace element that is essential in the human diet due to its importance for health and proper functioning of an organism. However, the biological activity of this metal in crop plants, which are the primary dietary sources of Li, is still poorly understood. The aim of the presented study was to comparatively analyse two Li chemical forms on the growth, as well as the l-ascorbic acid content, the Li accumulation and translocation in butterhead lettuce (Lactuca sativa L. var. capitata) cv. Justyna. The plants were grown in a nutrient solution enriched with Li in the form of LiCl or LiOH at the following concentrations: 0, 2.5, 20, 50 or 100 mg Li dm−3. The obtained results indicate that the presence of Li+ ions in the root environment reduced the yield of edible parts of the lettuce if the Li concentration in a nutrient solution had reached 20 mg Li dm−3. However, a yield reduction under these conditions was found to be significant only for LiOH. In plants exposed to 50 mg Li dm−3, both shoot and root fresh weights (FW) significantly decreased, regardless of the supplied Li chemical form. On the other hand, under the lowest LiOH dose, a significant increase in the root FW was noted, suggesting beneficial effects of Li on the growth of lettuce plants. However, applied Li concentrations and forms did not affect the l-ascorbic acid content in the lettuce leaves. Regardless of which Li form was used, Li accumulated mainly in the root tissues. An exception was the higher concentration of this metal in the shoots than in the roots of plants supplied with 100 mg Li dm−3 in LiCl, and there were almost the same Li concentrations in both examined organs of plants supplied with 100 mg Li dm−3 in LiOH. The effectiveness of Li translocation from roots to shoots rose with increasing Li concentrations in the growth medium, and this suggests a relatively ready translocation of this metal throughout the plant. Moreover, these results suggest that Li toxicity in lettuce plants is related to a high accumulation of this element in the root and shoot tissues, causing a drastic reduction in the yield, in the presence either of LiCl or LiOH, but not affecting the l-ascorbic acid accumulation in the leaves.
Keywords: Lithium accumulation, Lettuce, l-Ascorbic acid, Phytotoxicity
Introduction
Lithium (Li) is one of the alkali metals with a high chemical activity, and it is relatively widespread in the environment. Although Li has not been shown to serve as a required cofactor of any enzyme or enzymatic transport system, it is increasingly regarded as an essential trace element for animals and humans. The physiological role of Li in the human body is still not clearly recognised; however, deficiency of this element may disturb protein metabolism and reproductive ability. It was noted that elevated Li levels in drinking water had a beneficial influence on the circulatory system and can prevent cardiovascular diseases [1]. Furthermore, the latest study suggests a positive role in the prevention of Alzheimer’s disease [2]. As Li appears to alter neurotransmission at a synaptic level in the brain, carbonate and other Li salts have been used in psychiatry for over 50 years, mainly to moderate mood swings [1–4]. Deficiency of Li in humans is unlikely to reach a degree of severity as observed in experimental animals, but if any symptoms of Li insufficiency in humans had occurred, they would be expected to be mild and would manifest themselves by behavioural rather than physiological abnormalities [3].
Primary dietary sources of Li are grains and vegetables, which may contribute from 66 % to more than 90 % of the total Li intake [3]. This element is taken up by all plant species, and although Li appears not to be essential for their proper growth and development, the stimulation of plant growth under Li supplementation has been observed [3, 4]. The physiological function, toxicity and tolerance of crop plants to Li are relatively poorly recognised, despite many years of investigation. Various plant species differ greatly in their tolerance to Li [5]. Li-accumulating plants belong mainly to the Rosaceae and Solanaceae families and halophilic plants; these plants are known to have the highest tolerance to Li [1, 3]. Relatively high contents of this metal have been reported also for lettuce plants grown in Hungary [6]. However, it is known that the excess of Li in the soil environment can be toxic to some plants. The extremely strong sensitivity of citrus trees (avocado, orange) [1, 6] can be a good example. Morphological and physiological processes can be affected by Li+ ions. In the Li-rich soils, damage of root tips and chlorotic and necrotic spots on leaves have been observed in corn [1]. Moreover, an excess of Li can disturb normal pollen development, inducing symmetrical mitosis in microspores [7], and blocks its germination [8]. The toxic concentrations of this metal induce a hypersensitive-like response in tobacco plants [9]. Furthermore, results from several studies demonstrate that Li affects plant metabolism at a membrane level; however, its role seems to be non-specific as it substitutes other monovalent cations in plant cells [1].
l-Ascorbic acid is one of the most important compounds in vegetables having anti-oxidative and immunomodulatory effects in an organism. It is commonly known as vitamin C—a water-soluble vitamin important for humans and animals that is involved in the biosynthesis of neurotransmitters, collagen and carnitine [10]. l-Ascorbic acid along with glutathione belongs to the non-enzymatic antioxidant defence system [11]. To our knowledge, the effect of applied Li compounds on l-ascorbic acid concentrations in plant tissues has not been studied earlier. Therefore, one of the aims of this study was to learn more about the accumulation of l-ascorbic acid in edible parts of the lettuce under Li supplementation, depending on the chemical form of this metal. Moreover, we have determined how these two Li forms (LiCl and LiOH) affect the growth and Li accumulation and translocation in lettuce plants grown under hydroponic conditions.
Materials and Methods
Plant Material and Growth Conditions
Seeds of butterhead lettuce (Lactuca sativa var. capitata) cv. Justyna were germinated in wet quartz sand for about 19–21 days. The healthiest, best-developed seedlings were transferred to 1-dm3 glass jars (three per jar) filled with Hoagland’s II nutrient solution supplemented with micronutrients as ferric citrate and A-Z solution [12]. The pH of the medium was adjusted to 6.2 (±0.05). Plant vegetation was carried out in a phytotron room, with a 14-h photoperiod at a PPFD of 270 μmol m−2 s−1, a day/night temperature of 23 °C/18 °C, and 60–70 % relative humidity. After 3 days of plant adaptation to a pure nutrient solution, the medium was enriched with Li in the form of LiCl or LiOH at final concentrations of 0 (control), 2.5, 20, 50 or 100 mg Li dm−3. Li was added to the growth medium in two divided doses (2 × 0.5 of final concentration) given at 3 days interval. The plants were exposed to Li for the next 3 weeks from the addition of a second Li dose. During the growth period, the nutrient solution was replenished with a fresh solution periodically. The plants were then harvested, and the fresh weight (FW), index of tolerance (IT) of roots, and l-ascorbic acid contents were determined as will be described in the next sections. The samples were then dried in order to determine the percentage of water content, and then they were put through a grinder to determine the Li content.
Plant Analysis
For FW determination, all aboveground vegetative plant parts (shoot FW) and all belowground parts (root FW) were harvested and weighed. The dry biomasses of the shoots and roots were determined after heating the samples in a drying oven at 105 °C to a constant DW. The percentage of water content was calculated using the following equation: Percentage of H2O = ((FW − DW) / DW) × 100. The IT of roots was calculated by comparing the mean length of the longest root of plants grown in the presence of Li+ ions with the mean length of the longest root from the control plants according to Mc Neilly [13].
The l-ascorbic acid contents in the leaves were estimated by Tillman’s titration method, which was modified by Pijanowski [14]. In short, the leaf material (10 g FW) was homogenised with 30 cm3 of 2 % oxalic acid (v/v) and filtrated. The filtrate was filled with 1 % oxalic acid (v/v) to a total volume of 100 cm3, then 10 cm3 of the obtained extract was transferred to an Erlenmeyer flask and then 40 cm3 of 1 % oxalic acid (v/v) was added. The solution was quickly titrated using 2,6–dichlorophenolindophenol until the pink colour held for 30 s. The concentrations of the total vitamin C, as the sum of the contents of l-ascorbic acid and dehydroascorbic acid, were expressed as milligrams per 100 g FW.
Li concentrations in the plant tissues were determined by inductively coupled plasma–optical emission spectroscopy (ICP–OES) technique. For sample mineralisation, 6 cm3 of concentrated nitric acid was added to the reaction dish containing 0.25 g of ground plant material. After 1 h, 2 cm3 of 30 % hydrogen peroxide (v/v) was added and left until the violent reaction stopped (up to 12 h). Mineralisation was carried out in a CEM MARS 5 microwave oven according to the producer’s procedure. After cooling, the dish contents were quantitatively transferred to 25-cm3 volumetric flasks, and the concentrations of Li were estimated in a VARIAN emission spectrophotometer ICP–OES VISTA MPX at 670 and 783 nm. The calibration curve was established using standard Li solutions of 5, 10, 50 and 100 mg Li dm−3, and the Li concentrations were expressed as milligrams per kilogram DW.
Statistical Analysis
The experimental design was randomised with nine treatments and five replications per treatment. Each experimental series included 15 plants, and the experiment was repeated three times under the same conditions. A two-way ANOVA test was used to compare the obtained results followed by post hoc multiple comparisons of means using Tukey’s test. All statistical calculations were performed using a statistical package (StatSoft Statistica 6.0), and differences were considered significant at p < 0.05.
Results
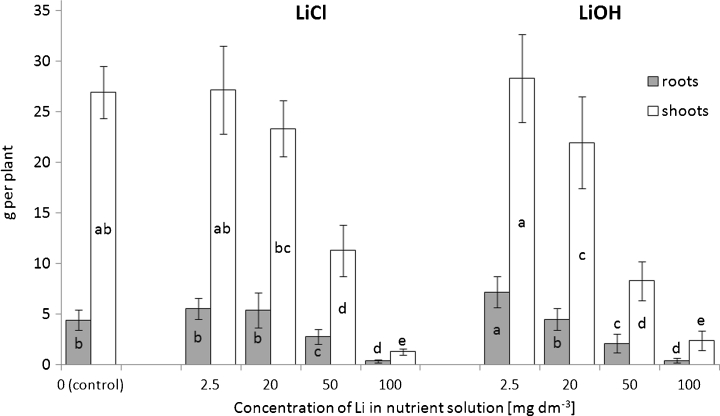
In lettuce plants, increased Li concentration in a nutrient solution strongly correlated with a reduction in plant growth after treating it with ≥20 mg Li dm−3 (Fig. 1). In plants grown in a nutrient solution containing 20 mg Li dm−3 ,, the yields of the aboveground parts were also reduced by 13 and 19 % in the presence of LiCl and LiOH, respectively. However, only the FW decrease after LiOH exposure was statistically significant. Above this concentration, a large decline in the FW of these plants’ shoots and roots was found as compared with the control plants. At a 50-mg Li dm−3 concentration, a severe reduction in the shoot biomass occurred, in up to 58 and 69 % in LiCl- and LiOH-exposed plants, respectively. An increase of Li concentration in a nutrient solution of 100 mg dm−3 almost completely inhibited lettuce growth, as the plant’s FW only managed to reach 5–9 % of the control plants’ FW. Furthermore, the formation of necrotic spots of various sizes in older leaves and the oldest leaves withering, regardless of Li chemical form (Fig. 2a, b), was observed in plants supplied with 50 and 100 mg Li dm−3.
Fig. 1.
Effects of increasing concentrations of Li in nutrient solution on the biomass of lettuce plants exposed to LiCl or LiOH. The mean values for each organ marked with the same letters are not significantly different at p < 0.05
Fig. 2.
The morphology of lettuce plants under different Li forms and concentrations (a) and necrotic spots on the older leaves of lettuce in the presence of 100 mg Li dm−3 (b)
However, in lettuce supplied with 2.5 mg Li dm−3 in LiOH, a significant increase (by 62 %) in the root system FW was found. Under these conditions, a slight increase (about 5 %) in the yield of edible parts was also observed, but these differences did not reach a statistical significance, just as an increase in the root FW after an application of 2.5 and 20 mg Li dm−3 in LiCl (Fig. 1). Li addition to the nutrient solution in the form of LiCl at concentrations of 2.5 and 20 mg Li dm−3 exerted stimulatory effects on the elongation of the root system (Table 1). Nevertheless, if the Li dose rose to 100 mg Li dm−3, the IT values of roots are significantly reduced by about 36 and 21 % under the presence of LiCl and LiOH, respectively, when compared with those of control plants. In comparing changes in the root length and their biomass, it is evident that, at a lower concentration of Li, a significant reduction of the root FW appeared rather than the inhibition of root elongation (Fig. 1; Table 1).
Table 1.
Effects of increasing concentrations of Li in nutrient solution on l-ascorbic acid contents, percentage of water and IT of lettuce roots exposed to LiCl or LiOH
| Li in nutrient solution | l-Ascorbic acid (mg 100 g−1 FW) | Water content (%) | IT of roots (%) | ||
|---|---|---|---|---|---|
| Form | Concentration (mg dm−3) | Shoots | Roots | ||
| Control | 0 | 26.71 ± 1.74 (ns) | 94.1 ± 0.58 a | 95.5 ± 1.86 a | 100.0 ± 0.00 c |
| LiCl | 2.5 | 28.11 ± 1.55 | 93.1 ± 1.07 ab | 95.2 ± 1.74 ab | 113.8 ± 7.30 a |
| 20 | 28.79 ± 1.16 | 91.8 ± 0.73 b | 95.7 ± 1.45 a | 111.4 ± 8.96 ab | |
| 50 | 26.98 ± 1.07 | 88.3 ± 2.22 c | 92.9 ± 2.98 bc | 98.4 ± 5.20 c | |
| 100 | 24.14 ± 0.49 | 80.4 ± 1.58 d | 92.5 ± 2.36 cd | 63.9 ± 4.67 e | |
| LiOH | 2.5 | 26.15 ± 3.94 | 92.1 ± 0.94 ab | 94.6 ± 1.26 abc | 105.5 ± 7.68 abc |
| 20 | 25.75 ± 2.73 | 91.8 ± 1.37 ab | 95.1 ± 1.47 ab | 107.9 ± 11.52 abc | |
| 50 | 30.25 ± 3.02 | 86.2 ± 2.07 c | 94.3 ± 2.08 abc | 102.8 ± 7.25 bc | |
| 100 | 23.25 ± 0.04 | 80.9 ± 4.46 d | 90.0 ± 3.44 d | 78.6 ± 2.62 d | |
The mean values in each column marked with the same letters are not significantly different at p < 0.05, ns not significant
The percentage of water content under Li treatments depended on the Li concentrations in the growth medium rather than on its chemical form (Table 1). Hydration of the aboveground parts showed almost no changes up to the Li concentration in the nutrient solution that reached a toxicity threshold (50 mg Li dm−3). There was an exception when there was a decreased percentage of H2O in the presence of 20 mg Li dm−3 in LiCl. After exposure of plants to 100 mg Li dm−3, the water content in shoots was about 14 % lower than in control plants, regardless of the Li chemical form. A similar effect was observed in the case of the roots; however, a decline in water content was lower in this case than that in the shoot tissues.
In lettuce leaves, Li supplementation caused slight fluctuations in the l-ascorbic acid contents, regardless of the chemical form of this element (Table 1). In general, in Li-exposed plants, the quantity of l-ascorbic acid remained at the control level. However, there was a tendency for a decrease in l-ascorbic acid contents (by 10–13 %) under the highest used metal concentration (100 mg Li dm−3), but this reduction was not statistically significant.
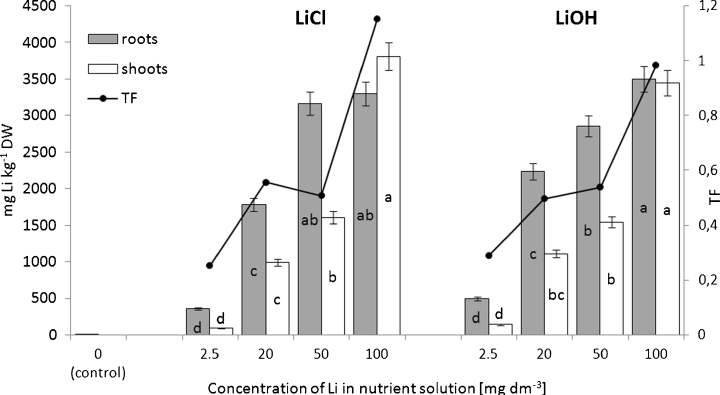
The analysis of Li concentrations in the shoots and roots of lettuce plants (Fig. 3) showed that Li accumulation directly correlated with concentrations of this element in a nutrient solution rather than with its chemical form. The Li contents, which were low in control plants, significantly increased after Li supplementation; both forms presented their maximum values after a plant exposure to 100 mg Li dm−3. Regardless of the chemical form of Li, its ions accumulated mainly in the root tissues of plants treated with 5–50 mg Li dm−3. However, in the presence of the highest dose of Li, the concentrations of this metal were higher in the shoots than in the roots of LiCl-supplied lettuce and were almost the same in the shoots and roots of LiOH-supplied plants. The differences between Li accumulation depending on its chemical form were dose-dependent. Under lower Li doses (2.5 or 20 mg dm−3), the higher concentrations of this metal were observed in the LiOH-exposed plants than in the LiCl-exposed plants, whereas the opposite tendency was noted for higher Li doses (50 and 100 mg dm−3). However, these differences did not attain any statistical significance.
Fig. 3.
Effects of increasing concentrations of Li in nutrient solution on the accumulation and translocation of Li in lettuce plants exposed to LiCl or LiOH. The mean values for each organ marked with the same letters are not significantly different at p < 0.05
The effectiveness of Li translocation from the roots to the shoots (expressed as translocation factor (TF)) increased with concentrations of this metal in a nutrient solution (Fig. 3). In plants supplied with the lowest Li dose, the concentrations of Li in the tissues of roots were about 3.5–4-fold greater than those of the shoots. With increasing concentrations of Li, the TF tended to rise, and under highest metal concentrations, the content of Li achieved a similar level in both plant parts.
Discussion
A better understanding of the effects of Li on plant growth and accumulation of this metal remains vital for the improvement of the knowledge about biological activity of Li compounds in crop plants. In our study, the enrichment of a nutrient solution with LiCl or LiOH greatly influenced Li concentrations in both the above-ground and belowground parts of the lettuce. The Li content is considered to be higher in dicotyledonous plants than in monocotyledonous [15], and it is believed that among dicotyledonous plants, the lettuce can contain relatively high levels of this metal [6]. Our previous research has demonstrated that the dicot sunflower accumulated considerably greater amounts of Li than the monocot maize, when plants are grown in a nutrient solution under increasing LiCl concentrations [16]. Comparing the contents of this metal between the two species mentioned above, as well as examining in the present study lettuce, it is evident that under the presence of 50 mg Li dm−3 in a nutrient solution, the lettuce plants are able to accumulate higher concentrations of Li in the shoots than the maize, though less than the sunflower. In the above-ground parts, the maize plants accumulated 695 mg Li kg−1 DW; sunflower, 3,292 mg Li kg−1 DW [16]; lettuce, 1,607 and 1,544 mg Li kg−1 DW after exposure to 50 mg Li dm−3 LiCl or LiOH, respectively. However, in lettuce plants supplied with 20 mg Li dm−3 , the Li concentrations were twofold higher than those in sunflower and maize supplied with 25 mg Li dm−3 [16]. This suggests some limiting dose-dependent steps in the processes involved in Li uptake, translocation and accumulation by different plant species. In the experiments performed by Magalhães et al. [17], the lettuce contained above twofold less Li in their leaves than the radish and watercress grown under hydroponic conditions; it suggests that lettuce is able to accumulate less Li among the studied dicotyledonous plants. The observed differences in Li accumulation by different species of plants may be also partially related to the influence of some experimental conditions, including different ages of plants when Li compounds were added to the nutrient solution and various times of exposure to the metal.
In the Li-supplied lettuce, the metal was accumulated mainly in the roots, regardless of the used chemical form (LiCl or LiOH). The differences between Li content in the individual plant parts were form- and dose-dependent. Accumulation of this metal in plants was greater when LiOH rather than LiCl was introduced to the nutrient solution but only at lower-used Li concentrations (2.5 and 20 mg Li dm−3). At higher Li concentrations (50 and 100 mg Li dm−3), the LiCl-supplied plants contained more Li. However, the differences in Li accumulation dependent on its chemical form were not statistically significant, indicating that Li is equally easily available to plants from both chemical compounds. Furthermore, the effectiveness of Li translocation from the roots to the shoots increased with the concentration of this metal in the growth medium, indicating a relatively easy translocation of Li+ ions throughout the plant. The results obtained in this study agree with those obtained in other works, which reported that increased concentrations of Li in the root zone (induced by soil fertilisation) can cause a significant increase of Li content in the above-ground parts of agricultural crops [18–20]. Since Li shares the potassium transport carrier, therefore, it is easily and effectively transported from roots to above-ground parts of plants and accumulates mainly in the leaf tissues [1]. However, in our study, the contents of Li in the shoot tissues generally were lower than those in the roots, particularly under lower Li concentrations in a nutrient solution (2.5–50 mg Li dm−3). The reason may be that plants cultivated under hydroponic conditions can easily uptake this metal, and the pattern of Li distribution between belowground and above-ground parts of plants can be different from those of plants grown in the soil.
The results of the present study indicated that the used Li compounds did not considerably differ in their phytotoxicity to lettuce and that a concentration of 50 mg Li dm−3 causes a major decrease in the FW of plants. The same Li concentrations in nutrient solution were also toxic for maize and sunflower plants [16]. Similar results regarding a decrease of yield in Li-exposed plants were also obtained for oat (25 mg Li kg−1), maize and spinach (40 mg Li kg−1) cultivated in the soil [20]. Moreover, our results indicate that lettuce can accumulate high contents of Li in their organs, but simultaneously, this species is relatively sensitive to high concentration of this metal in the medium culture, which causes a decrease in the yield. One of the symptoms of Li phytotoxicity was the formation of necrotic spots of various sizes on older lettuce leaves. Naranjo et al. [9] also observed the formation of necrotic lesions and leaf curling on older leaves of tobacco plants exposed to 50 mM LiCl, which resulted from preferential Li+ accumulation in those leaves. The occurrence of necrotic spots on the leaf area under toxic concentrations of Li seems to be a characteristic of the dicotyledonous species; in monocotyledonous maize plants, its occurrence was not found [16]. The adverse effects of Li on a plant’s growth may be because the Li+ ion readily crosses cell membranes and interferes with calcium metabolism [21]. Li et al. [22] showed that with an exposure of up to 30 mM LiCl (about 200 mg Li dm−3), Li has almost no effect on the germination and growth parameters of Brassica carrinata seedlings. However, above this concentration, Li toxicity rapidly increased, causing a reduction of growth; it decreased in the chlorophyll content as well as affected the lipid and phenolic compositions of seedlings.
On the other hand, a slight increase was found (but insignificant) in the yielding capacity of the edible parts of lettuce, and there was a significant increase in the root system FW (in the presence LiOH only) after the application of the lowest concentration of Li (2.5 mg Li dm−3). In maize, a significant growth-stimulating effect of this metal was noted at a concentration of 5 mg Li dm−3 [16]; in lettuce, in the presence of 1 and 2 mM LiNO3 (about 7 and 14 mg Li dm−3, respectively) [17]. In spinach and mustard plants, Li addition to the soil also increased plant biomasses but under conditions of reduced light levels only [23]. This may be due to the hormetic effect that has been described in the literature as a stimulation of plant growth from sublethal doses of potentially toxic agents [21] or some other unknown effects that Li+ ions can exert on a plant’s metabolism. A possible catalytic mechanism including a cofactor function of Li+ ions for any enzyme activity has not been detected so far; however, Li, like other alkali metals, may appear as a metabolism regulator due to its affinity to enzymes activated by calcium and/or magnesium [1]. Therefore, the beneficial effect of Li compounds on plant growth still needs further research.
l-Ascorbic acid plays multiple roles in crucial physiological processes, and the steady-state values in plant cells are tightly controlled at various levels, such as enzyme activity, gene expression, regeneration of the oxidised form, compartmentation and transport of this molecule in response to the environmental stimuli [24]. In the present study, concentrations of l-ascorbic acid in lettuce leaves remained unaffected by Li compounds, regardless of the chemical form used. Nevertheless, a tendency for a decrease in the content of l-ascorbic acid under the highest and greatly phytotoxic concentration of this metal (100 mg Li dm−3) was observed. In the study of Makus et al. [23], Li addition to the soil also had no influence on the level of the total ascorbate in spinach and mustard leaves. Moreover, there is no evidence confirming that fertilising with potassium, and other alkali metals, can affect the content of l-ascorbic acid in the tissues of pineapple [25]. Thus, it seems that Li toxicity in plants is not related to the disturbances in the accumulation of l-ascorbic acid in the leaf tissues.
Abbreviations
- DW
Dry weight
- FW
Fresh weight
- IT
Index of tolerance
- TF
Translocation factor
- PPFD
Photosynthetic photon flux density
- ICP–OES
Inductively coupled plasma–optical emission spectroscopy
References
- 1.Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer-Verlag, Berlin, pp 87–93
- 2.Young AH. More good news about the magic ion: lithium may prevent dementia. B J Psych. 2011;198:336–337. doi: 10.1192/bjp.bp.110.082875. [DOI] [PubMed] [Google Scholar]
- 3.Schrauzer GN. Lithium: occurrence, dietary intakes, nutritional essentiality. J Americ Coll Nutr. 2002;21:14–21. doi: 10.1080/07315724.2002.10719188. [DOI] [PubMed] [Google Scholar]
- 4.Aral H, Vecchio-Sadus A. Toxicity of lithium to humans and the environment—a literature review. Ecotoxicol Environ Saf. 2008;70:349–356. doi: 10.1016/j.ecoenv.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Jurkowska H, Rogóż A, Wojciechowicz T. Comparison of lithium toxic influence on some plant species. Acta Agraria et Silvestra/Agraria. 1997;35:45–50. [Google Scholar]
- 6.Schäfer U. Lithium. In: Merian E, Anke M, Ihnat M, Stoepler M, editors. Elements and their compounds in the environment. Weinheim: Wiley–VCH; 2004. pp. 901–930. [Google Scholar]
- 7.Zonia LE, Tupy J. Lithium treatment of Nicotiana tabacum microspores blocks polar nuclear migration, disrupts the partitioning of membrane-associated Ca2+, and induces symmetrical mitosis. Sex Plant Repr. 1995;8:152–160. [Google Scholar]
- 8.Zonia LE, Tupy J. Lithium-sensitive calcium activity in the germination of apple (Malus x domestica Borkh.), tobacco (Nicotiana tabacum L.), and potato (Solanum tuberosum L.) pollen. J Exp Bot. 1995;46:973–979. doi: 10.1093/jxb/46.8.973. [DOI] [Google Scholar]
- 9.Naranjo MA, Romero C, Belles JM, Montesinos C, Vicente O, Serrano R. Lithium treatment induces a hypersensitive-like response in tobacco. Planta. 2003;217:417–424. doi: 10.1007/s00425-003-1017-4. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal K, Khan A, Khan Khattak MMA. Biological significance of ascorbic acid (vitamin C) in human health—a review. Pak J Nutr. 2004;3:5–13. doi: 10.3923/pjn.2004.5.13. [DOI] [Google Scholar]
- 11.Eraslan F, Inal A, Savasturk O, Gunes A. Changes in antioxidative system and membrane damage of lettuce in response to salinity and boron toxicity. Sci Hort. 2007;114:5–10. doi: 10.1016/j.scienta.2007.05.002. [DOI] [Google Scholar]
- 12.Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Col Agric Exp Stn Circ. 1950;347:1–32. [Google Scholar]
- 13.Mc Neilly T. Metal toxicity. In: Yeo AR, Flowers TJ, editors. Soil mineral stresses: approaches to crop improvement. Berlin: Springer-Verlag; 1994. pp. 145–174. [Google Scholar]
- 14.Pijanowski E, Mrożewski S, Horubała A, Jarczyk A. Fruit and vegetables processing. Warsaw: PWRiL; 1973. pp. 127–134. [Google Scholar]
- 15.Jurkowska H, Rogóż A. Influence of high doses of Cu, Zn, Pb and Cd on lithium content in oat plants. Polish J Soil Sci. 1993;26:77–80. [Google Scholar]
- 16.Hawrylak-Nowak B, Kalinowska M, Szymańska M. A study on selected physiological parameters of plants grown under lithium supplementation. Biol Trace Elem Res. 2012;149:425–430. doi: 10.1007/s12011-012-9435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magalhães JR, Wilox GE, Rocha ANF, Silva FLIM. Research on lithium-phytological metabolism and recovery of hypo-lithium. Pesq Agropec Bras. 1990;25:1781–1787. [Google Scholar]
- 18.Jurkowska H, Rogóż A, Wojciechowicz T. The effect of sodium on lithium uptake by plants. Polish J Soil Sci. 1995;28:135–138. [Google Scholar]
- 19.Jurkowska H, Rogóż A. Uptake of lithium by plants as depending on soil moisture content. Polish J Soil Sci. 1991;24:93–97. [Google Scholar]
- 20.Jurkowska H, Rogóż A, Wojciechowicz T. Comparison of lithium toxic influence on some cultivars of oats, maize and spinach. Acta Agraria et Silvestria/Agraria. 1998;36:37–42. [Google Scholar]
- 21.Allender WJ, Cresswell GC, Kaldor J, Kennedy IR. Effect of lithium and lanthanum on herbicide induced hormesis in hydroponically-grown cotton and corn. J Plant Nutr. 1997;20:81–95. doi: 10.1080/01904169709365235. [DOI] [Google Scholar]
- 22.Li X, Gao P, Gjetvaj B, Westcott N, Gruber MY. Analysis of the metabolome and transcriptome of Brassica carinata seedlings after lithium chloride exposure. Plant Sci. 2009;177:68–80. doi: 10.1016/j.plantsci.2009.03.013. [DOI] [Google Scholar]
- 23.Makus DJ, Zibilske L, Lester G. Effect of light intensity, soil type, and lithium addition on spinach and mustard greens leaf constituents. Subtrop Plant Sci. 2006;58:35–41. [Google Scholar]
- 24.Valpuesta V, Botella MA. Biosynthesis of l-ascorbic acid in plants: new pathways for an old antioxidant. Trends Plant Sci. 2004;9:573–577. doi: 10.1016/j.tplants.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Sideris CP, Young HY. Growth and chemical composition of Ananas comosus (L.) Merr. in solution cultures with different iron–manganese ratios. Plant Physiol. 1949;24:416–440. doi: 10.1104/pp.24.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]