Abstract
Background
The relationship of primary care provider’s (PCP) CRC screening strategies to completion of screening is poorly understood.
Objective
To describe PCP test recommendation patterns, associated factors, and their relationship to patient test completion.
Design
This cross-sectional study used a PCP survey, in-depth PCP interviews, and electronic medical records.
Setting
Kaiser Permanente Northwest HMO.
Participants
132 PCPs and 49,259 eligible patients aged 51–75.
Measurements
Patterns related to PCP CRC screening recommendations, based upon frequency of recommending fecal occult blood testing (FOBT), flexible sigmoidoscopy (FS), and colonoscopy. We compared PCP demographics, CRC screening-test influences, concerns, decision making and counseling processes, and rates of patient CRC screening completion by PCP group.
Results
We identified four CRC screening-recommendation groups: a “Balanced” group (n=54; 40.9%) that recommended the tests nearly equally; an “FOBT” group (n=31; 23.5%) that largely recommended FOBT; an “FOBT& FS” (n=25; 18.9%); and a “Colonoscopy & FOBT” (n=22; 16.7%) group that recommended these tests nearly equally. Internal medicine (vs. family medicine) PCPs were more common in groups recommending endoscopy more frequently. The FOBT and FOBT&FS groups were most influenced by clinical guidelines. Groups recommending more endoscopy were most concerned that FOBT generates a lot of false positives and FOBT misses a lot of cancers. The FOBT and FOBT&FS groups were more likely to recommend a specific screening strategy compared to the Colonoscopy & FOBT and Balanced groups, which were more likely to let the patient decide. CRC screening rates did not differ by group.
Limitations
Small numbers within PCP groups
Conclusions
Specialty, the influence of guidelines, test concerns, and the “jointness” of the test selection decision distinguished CRC screening recommendation patterns. All patterns were associated with similar overall screening rates.
Keywords: colorectal cancer screening, primary care recommendations
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related death in the United States. (1) Early detection of high-risk pre-cancerous lesions through appropriate screening is associated with decreased incidence of and mortality from CRC. (2–4) The US Preventive Services Task Force (USPSTF) recommends that men and women of average risk begin screening for CRC at age 50. There is good direct evidence for the effectiveness of fecal occult blood testing (FOBT), fair direct evidence for the effectiveness of sigmoidoscopy, and indirect evidence for the combined use of FOBT and sigmoidoscopy, and colonoscopy alone, in reducing CRC mortality.(5)
The majority of the U.S. population at risk for CRC is not being screened.(6;7) More than half of adults age 50 and older in the US have received a CRC screening test, but only about 35–45% receive screening tests at recommended intervals. Physician recommendation for CRC screening(8–11) has consistently been shown to be a strong predictor of screening. However, clinicians likely utilize varying testing and counseling strategies to address CRC screening (12;13) and the nature of their recommendations, communication, and counseling may be important to patient screening completion.(9;14;15) Yet, surprisingly little is known about these screening recommendation strategies. A recent survey of US physicians revealed that 99% routinely recommend CRC screening, with 95% routinely recommending colonoscopy, 80% recommending FOBT, and 26% recommending sigmoidoscopy; other strategies are rarely recommended.(16) Just over half of PCPs report recommending two screening modalities, with the remainder fairly evenly split between reporting recommending three or one screening modalities.(16) Even less is known about what factors influence physicians’ strategies or the impact of different strategies on CRC screening use. Our objective was to describe the different CRC screening recommendation strategies reported by PCPs, factors associated with these strategies, and the association of each strategy with practice-level CRC screening rates among an insured patient population.
Methods
The study design and procedures were approved by the study site’s Institutional Review Board.
Study site and data sources
The study was conducted at Kaiser Permanente Northwest (KPNW), a not-for-profit health maintenance organization (HMO) in the Pacific Northwest with about 485,000 members. KPNW’s membership is similar to the local insured community.(17) Electronic records provided clinician and patient data. KPNW maintains a CRC screening clinical practice guideline based upon the recommendations of the USPSTF. (5) Each of the USPSTF-recommended CRC screening modalities is a covered benefit, although FOBT is encouraged in lower risk individuals.
Study design and participants
This cross-sectional study used a PCP survey, electronic health record data and in-depth interviews with PCPs. We identified PCPs who had active patient panels from January 2007-July 2009 and who had at least 20 patients eligible for CRC screening every six months during this period (N=195). In August 2009 each PCP meeting the criteria received up to 2 electronic survey copies via email, followed by up to 2 mailed paper questionnaires. The latter included an enclosed chocolate bar as a token of appreciation. 144 PCPs (73.8%) returned the survey. Of those, 132 (91.6%) completed all questions about their frequency of recommending the various CRC screening tests and are included in the analyses.
Study Measures
Patient panel outcomes
For the primary outcome, we assessed CRC screening rates for each PCP over six months among eligible paneled patients who were due or overdue for screening as of January 2009. First, we identified 122,661 patients aged 51–75 as of January 1, 2009 who were HMO members for at least 12 months prior to and 6 months after this date. To the extent feasible, we then limited this group to those who were at average risk for CRC, and therefore in whom any of the guideline-recommended CRC-screening methods was appropriate. Therefore, we excluded those who had any of the following: 1) colonoscopy within 10 years (n=35,067), 2) flexible sigmoidoscopy or double-contrast barium enema (DCBE) within 5 years (n=26,063), or 3) FOBT screening within the past 12 months (n=16,894), (n=44,637). We then excluded 4,150 patients because of medical conditions/medications suggesting they were inappropriate for CRC screening (including through FOBT). These included: 1) active CRC/GI risk factors (n=2,835) in the previous 12 months (referral for chronic diarrhea, esophageal reflux, iron deficiency, polyp follow-up/rectal surgery, diagnosis of prior CRC or adenomatous polyps, diagnosis of HIV/AIDS, 2) medical conditions (n=192) for which routine screening was not indicated (end-stage renal disease, hospice care, receipt of total colectomy), 2) use of medications (n=1,123) in the previous 4 months (plavix, warfarin) that elevated risk of a false positive FOBT (n=40,487). Finally, we limited the population to those who were members of the 132 PCP patient panels (final N=21,964; 166±68). CRC screening as an outcome was defined as the receipt of any of FOBT (stool guaiac or fecal immunochemical test), FS, colonoscopy, or DCBE from January 1-June 30 2009. We also assessed the incidence of screening by each procedure individually.
The secondary outcome was CRC screening among eligible patients, consistent with the Health Employer Data and Information Set (HEDIS) CRC screening quality measure. (18) This was defined as the receipt of any of FOBT during the measurement year [July 2008-June 2009], FS, or DCBE during that year or the four years prior, colonoscopy that year or the nine years prior among the study PCP’s 49,259 eligible patients (without a history of CRC or total colectomy, and with 24 months of prior membership) aged 51–80 from July 2008-June 2009 (373±117 per PCP).
PCP survey and demographic variables
Our PCP variables are based upon concepts identified in the Diagnostic Evaluation Model of CRC screening, i.e. that physician background and experience, cognitive and psychological representations, social support and influence, practice environment and patient characteristics affect physician screening intention, and that the latter two factors interact to directly affect screening behavior.(19)
Frequency of recommending CRC screening methods
We assessed these variables by asking providers’ about how often (on a scale of 1-never to 5-all the time) each possible CRC screening test or test combination (FOBT, FS, Colonoscopy, FOBT+FS, Other) was recommended to average-risk asymptomatic patients.
Influences on CRC screening method selection
These variables were assessed by a series of questions that elicited information about the degree to which (on a scale of 1-no influence to 5-strong influence) training/education, colleagues, personal experience with failed screening methods, trust in the recommendation and skill level of endoscopists, organizational guidelines and expectations, and experience working in the community influence PCP choice of a particular CRC screening exam.
Concerns about CRC screening methods
We assessed additional factors (concerns related to patient adherence, test performance, and associated complications) that might influence clinicians to recommend a specific CRC screening test. For each of FOBT, FS, and Colonoscopy we asked, “To what degree do you agree (from 1-strongly disagree to 5-strongly agree) with the statements (1) “it is unclear whom to screen and how often to screen, (2) “[the test] misses a lot of cancers”, and (3) “patients don’t tend to complete” [this test]. For FS and Colonoscopy we also included a survey item reflective of agreement with the statement “patients often have complications.”
Decision making about CRC screening
We asked questions about how often (on a scale of 1-never to 5-all the time) did certain aspects of PCP CRC screening communication occur with patients. Three questions related to the “jointness” of decision making were also included (i.e. how often they (1) let the patient decide which screening method to use; (2) recommended a specific method; and (3) came to a joint decision.
CRC screening counseling
Seven questions assessed how PCPs address different elements of CRC screening counseling (12). In factor analyses, 7 elements loaded on a single factor (60.6% of the variance explained) with all factors loadings greater than .67: benefits of screening; screening frequency; information about discomfort; accuracy; complications; and checking for patient understanding/confirming patient agreement with the method selected. We created a single counseling score by taking the mean of the responses to the 7 questions (Cronbach’s alpha=.89).
PCP demographic variables
We collected data on previous community practice experience (outside of KPNW, coded yes or no) and hours per week in clinical care (≥25 or <25 hours per week) by survey. PCP gender; age; years in practice at KPNW; primary care specialty [internal medicine [IM] or family practice [FP]; and patient panel size were extracted from electronic databases.
Study PCPs were recruited for in-depth, semi-structured interviews by electronic mail and follow-up phone calls. Interviews were conducted in person, using an interview guide, (20) and analyzed by a trained qualitative research specialist (JS) blinded to PCP CRC screening recommendations or outcomes. All interviews were transcribed and content-analyzed using standard qualitative analysis techniques (20–25), and aided by the use of a qualitative research software program (26).
Statistical Analysis
PCP-reported frequency of recommending FOBT, FS & FOBT, and Colonoscopy were used in a cluster analysis to determine if groups of PCPs had similar patterns of recommending CRC screening methods. Responses to recommending FS only were not utilized in the cluster analysis because of the high degree of correlation with responses to recommending both FS & FOBT (r=.84 ). We used hierarchical cluster analysis with Ward’s Method and squared Euclidean distances in SPSS 15.0 to extract clusters. We based our decision on the number of clusters to retain in the final solution on the aglomeration schedule and interpretability. The hierarchical cluster analysis was followed with a K-means cluster analysis, using the final cluster centers from Ward’s method and a discriminant analysis predicting cluster group membership from the frequency of recommending variables to assess the fit of the final cluster solution. To validate the interpretation of the clusters we compared actual completed screening by each CRC screening method between the clusters using analysis of variance with post hoc tests. We used ANOVA and chi-square tests to compare the clusters on PCP demographics and panel characteristics, screening influences, test concerns, CRC screening “jointness” of decision variables, CRC screening counseling content scale score and PCP CRC screening rates. We considered p<0.05 to be statistically significant.
Results
PCP Clusters Based on Recommendations of CRC Screening Modalities
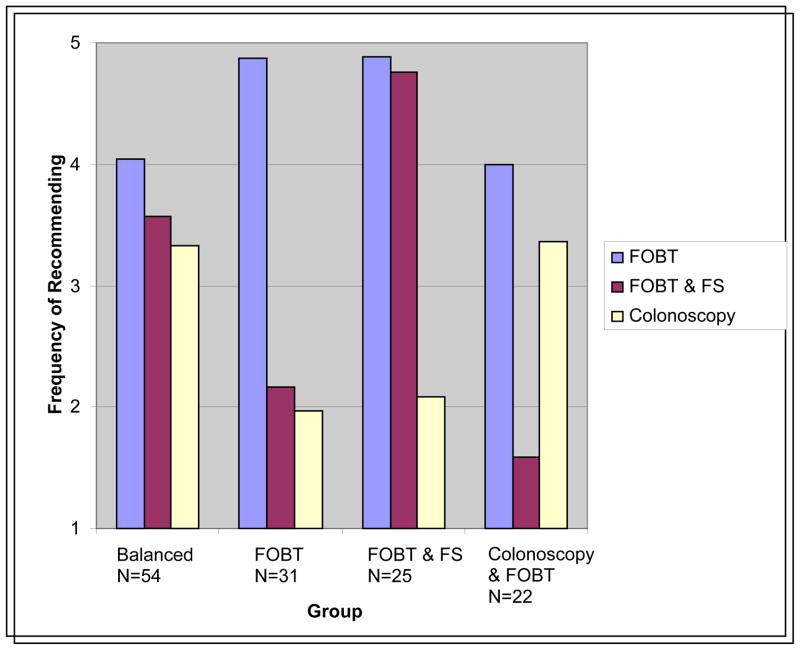
Hierarchical cluster analysis found 4 interpretable clusters based upon PCP-reported frequency of recommending FOBT, FOBT&FS, and Colonoscopy (Figure). The final cluster solution with K-means clustering fit the data well, with 97% of the cases correctly classified based on a discriminant analysis predicting cluster group membership. The “Balanced” cluster (n=54; 40.9%) recommended FOBT, FOBT & FS, and Colonoscopy screening methods nearly equally. The “FOBT” cluster (n=31; 23.5%) largely recommended FOBT and had the lowest frequency of recommending Colonoscopy. The “FOBT & FS” cluster (n=25; 18.9%) recommended FOBT and FOBT&FS nearly equally. The “Colonoscopy & FOBT” (n=22; 16.7%) cluster recommended these tests nearly equally and had the lowest frequency of recommending FS&FOBT.
Figure.
The four prevalent PCP CRC screening recommendation patterns
FOBT- fecal occult blood test, FS-flexible sigmoidoscopy
Table 1 presents the average percent of patients due or overdue for CRC screening by recommending-pattern cluster over 6 months. The clusters are significantly different in the rates of actual completed screening of FOBT, FOBT&FS, FS only, and Colonoscopy and any endoscopy in the directions consistent with interpretation and naming of the clusters.
Table 1.
Percent of patients due and overdue for CRC screening screened by each method by PCP cluster
| Cluster | |||||
|---|---|---|---|---|---|
| Patient* Screening Rate by Method (%) | Balanced | FOBT | FOBT & Flex Sig | Colonoscopy & FOBT | p-value |
| FOBT only | 13.1a | 18.8a,b,c | 12.6b | 15.6c | <.001 |
| FOBT & FS | 1.6a,b | 0.4a,c | 1.9c,d | 0.1b,d | <.001 |
| FS only | 1.6a,b,c | 0.5a,d,e | 2.4b,d,f | 0.1c,e,f | <.001 |
| Colonoscopy | 3.3a,b | 1.7a,c | 2.0b | 3.3c | .006 |
| Any endoscopy† | 6.47ab | 2.58ac | 6.29cd | 3.54bd | <.001 |
| DCBE | 0.00 | 0.06 | 0.00 | 0.02 | .050 |
Means within a row with the same superscript (a,b,c,d) are significantly different from one another based on LSD post hoc tests
Mean % per PCP of patients due or over-due for CRC screening as of January 1, 2009 who were screened by June 30, 2009
Endoscopy- either FS, FS+FOBT, or colonoscopy
FOBT- fecal occult blood test, FS-flexible sigmoidoscopy, DCBE-double contrast barium enema
Physician Factors Associated with Recommendation Groups
Table 2 compares the demographic and patient panel characteristics of the PCP clusters. The groups were significantly different only with respect to specialty, with more PCPs in the Balanced cluster and Colonoscopy&FOBT cluster in IM (vs. FP). These two clusters also tended to have more community practice experience (p=.056).
Table 2.
PCP Demographic and Patient Panel Characteristics by Group
| Group | |||||
|---|---|---|---|---|---|
| Balanced | FOBT | FOBT & Flex Sig | Colonoscopy & FOBT | p-value | |
| Female (%) | 37.0 | 45.2 | 64.0 | 50.0 | .161 |
| Specialty (% IM)* | 63.0 | 29.0 | 44.0 | 50.0 | .024 |
| Community practice experience† (%) | 70.4 | 54.8 | 40.0 | 68.2 | .056 |
| ≥25 hrs/week clinical practice (%) | 85.2 | 71.0 | 76.0 | 77.3 | .457 |
| Age; mean (SD) | 48.7 (8.1) | 50.1 (8.4) | 49. (8.9) | 51.3 (5.4) | .591 |
| Years at site; mean (SD) | 11.7 (7.5) | 13.4 (7.3) | 11.2 (7.3) | 12.7 (7.1) | .651 |
| Patient panel Size; mean n (SD) | 2,437 (450) | 2357 (394) | 2316 (426) | 2259 (381) | .347 |
IM- internal medicine, the remainder of participants are in family practice
Prior medical practice outside of study site (Kaiser Permanente Northwest)
There were significant differences among the four clusters in physician-reported CRC screening test choice influences, test-related concerns, and “jointness” of decision-making, whereas the groups were similar in their reported communication (CRC counseling scale score) ( significant results in Table 3). Of the surveyed test choice influences (training/education, discussions with colleagues, personal experiences with failure of screening methods, trust in endoscopy specialists, organizational (KPNW) guidelines and expectations, and experience working in the community), only guidelines and expectations was a statistically significant influence. The FOBT cluster was most influenced by clinical guidelines, followed by the FOBT & FS cluster. The clusters did not differ in concerns about colonoscopy, but did differ in concerns about FOBT and FS. The most strongly endorsed concerns were that “FOBT generates a lot of false positives” (in the Balanced cluster) and that “FOBT misses a lot of cancers” (in the FOBT &FS and Balanced clusters). The FOBT cluster had the least concerns about being unclear whom to screen and how often to screen with FOBT, that FOBT misses a lot of cancers, and that FOBT generates a lot of false positives. The FOBT cluster also most strongly endorsed concerns about being unclear whom to screen and how often to screen with FS, and that FS patients often have complications.
Table 3.
Significant across group differences in CRC screening test influences, concerns, decision-making and communication
| Cluster | |||||
|---|---|---|---|---|---|
| CRC screening element Mean (SD) |
Balanced | FOBT | FOBT & FS | Colonoscopy & FOBT | p-value |
| Influences* | |||||
| KP Guidelines Influence | 3.83ab (0.97) | 4.52a (0.68) | 4.42b (0.83) | 4.14 (0.99) | .003 |
| Test concerns† | |||||
| FOBT unclear who & how often to screen | 1.98a (0.85) | 1.43ab (0.63) | 2.16b (1.18) | 1.86 (0.73) | .012 |
| FOBT misses a lot of cancers | 3.26a (1.00) | 2.29abc (0.82) | 3.33b (0.82) | 2.86c (1.08) | <.001 |
| FOBT generates a lot of false positives | 3.51abc (0.85) | 2.84a (0.93) | 2.92b (1.04) | 2.95c (0.90) | .003 |
| FS unclear whom & how often to screen | 2.26ac (0.947) | 3.00ab (1.20) | 2.08bd (0.91) | 2.85cd (1.04) | .001 |
| FS patients often have complications | 2.02ad (0.67) | 2.52ab (0.93) | 1.84bc (0.62) | 2.43cd (0.75) | .001 |
| “Jointness” of decision making ‡ | |||||
| Let the patient decide | 3.37a,b (0.78) | 2.90a,c (1.04) | 2.78b,d (1.00) | 3.43c,d (0.93) | .017 |
| Recommend a specific method | 3.78a,b (0.83) | 4.19a (0.75) | 4.46b,c (0.59) | 3.81c (0.68) | .001 |
| Joint decision | 3.87 (0.59) | 3.90 (0.91) | 4.26 (1.01) | 4.05 (0.83) | .232 |
| Communication§ | |||||
| Counseling Scale Score | 3.95 (0.65) | 3.94 (0.82) | 3.96 (0.67) | 3.90 (0.45) | .991 |
Influences (Scale 1 no influence to 5 strong influence)
Test Concerns (Scale 1 strongly disagree to 5 strongly agree)
Decision making- How often does each of the following occur (Scale 1- never to 5-all the time)?
Communication- Counseling scale score based upon mean score of all the items: How often does each of the following occur in CRC screening discussion (Scale 1- never to 5-all the time)?: explain benefit of screening, recommended frequency, information about discomfort, accuracy, complications, check for patient understanding and confirm patient agreement with method selected
-Means within a row with the same superscript (a,b,c,d) are significantly different from one another based on LSD post hoc tests
FOBT- fecal occult blood test, FS-flexible sigmoidoscopy
The clusters differed in reported “jointness” of decision-making. The FOBT and FOBT&FS clusters more frequently reported recommending a specific screening strategy, and the Colonoscopy&FOBT and Balanced clusters more frequently reported letting the patient decide (consistent with the interpretation of these latter clusters).
Post hoc analyses (data not shown) comparing FP with IM PCPs found that (among all PCPs) IM PCPs had more concerns about FOBT (3.17 vs. 2.75; p= 0.014) and FS missing a lot of cancers (3.35 vs. 3.01; p=0.024), and FOBT having false positives (3.33 vs. 2.94; p=0.014). FP PCPs had more concerns that colonoscopy patients often have complications (2.58 vs. 2.31; p=0.022). Personal experience was a stronger influence for IM PCPs (2.90 vs. 2.29; p=0.003).
A total of 20 PCPs were interviewed (results by cluster in Table 4). Content analysis from the provider interviews supports the quantitative findings, including the influence of KPNW guidelines, the tendency for the FOBT and FOBT&FS clusters to be more likely to recommend a specific strategy; and the more mutual or “joint” approach to decision-making of the Balanced cluster. Additionally, the qualitative data support that influence and interpretation of recommendations from “local” organizational experts are also factors in shaping providers’ test preferences and approach with patients, particularly for the FOBT and FOBT&FS clusters. Furthermore, PCPs in the FOBT&FS cluster were most concerned about overburdening the system with colonoscopies, which may contribute to their more frequent recommendations of non-colonoscopy screening methods to patients. While the Colonoscopy&FOBT group described recommending yearly FOBT, this group also strongly stated the importance and need for some additional scoping, and preferred the completeness of colonoscopy to that of FS. Providers in the Balanced group described feeling “more free” to discuss and recommend all screening options with patients, allowing them to engage in a more patient-driven approach rather than recommending a specific strategy based on resource constraints or test concerns.
Table 4.
Qualitative Interview Data: PCP Cluster CRC Counseling & Decision-Making by Screening Test (N=20)
| Interviewees by Cluster | FOBT Beliefs | Flexible sigmoidoscopy Beliefs | Colonoscopy Beliefs | CRC screening counseling/ decision-making approach |
|---|---|---|---|---|
| Balanced (n=9) (FP=4; IM=5) |
|
|
|
|
| FOBT (n=3) (FP=2; IM=1) |
|
|
|
|
| FOBT&FS (n=5) (FP=3; IM=2) |
|
|
|
|
| Colonoscopy & FOBT (n=3); (IM=1; FP=2) |
|
|
|
|
FOBT-fecal occult blood test, FS-flexible sigmoidoscopy, IM- internal medicine, FP-family practice
Association of CRC Screening Recommendation Clusters with Overall CRC screening rates
The mean CRC screening rates per PCP among those due and overdue, using the CRC screening HEDIS measure (by any method) by cluster, are displayed in Table 5. The FOBT cluster trended towards the highest screening rate among those due or overdue and the Balanced cluster trended toward the highest HEDIS rate but the differences among clusters were not statistically significant.
Table 5.
Percent of patients due or overdue for CRC screening* who were screened over six months* and HEDIS† CRC screening measure by PCP group
| Group | |||||
|---|---|---|---|---|---|
| Screening Rate by Method (%) (95% CI) | Balanced | FOBT | FOBT & Flex Sig | Colonoscopy & FOBT | p-value |
| CRC screening among those due and overdue* | 19.8 (18.3–21.3) | 21.7 (19.8–23.7) | 19.1 (16.9–21.4) | 19.3 (16.9–21.7) | .256 |
| CRC screening HEDIS measure† | 63.9 (61.8 – 64.3) | 62.9 (60.8–65.1) | 61.7 (57.6–65.8) | 62.2 (58.8–65.7) | .660 |
Patients due or over-due for CRC screening in January 2009 who were screened by FOBT, FS, Colonoscopy or DCBE by June 30, 2009
Consistent with the Health Employer Data and Information Set (HEDIS) CRC screening measure the percentage of patients aged 51–80 without a history of CRC or total colectomy, with 24 months of prior membership during July 2008-June 2009 who had FOBT that year, FS or DCBE during that year or the four years prior, Colonoscopy that year or the nine years prior. FOBT- fecal occult blood test, FS-flexible sigmoidoscopy, DCBE-double contrast barium enema, CI- confidence interval
Discussion
Our analyses of self-reported CRC screening test recommendations made by PCPs in a large integrated care setting demonstrated that recommendations fell into four primary patterns. These patterns were associated with types of CRC screening tests completed by patients. However, the groups did not differ significantly in their overall practice-level CRC screening rates. This finding is compatible with previous studies that found that the most important consistent predictor of CRC screening is provider recommendation (27). Given the literature and the findings from the current study, it is reasonable to conclude that provider recommendation to screen, and not the specific nature of the recommended screening test, is the major driver of screening. Many patients likely ultimately follow through on their physician’s CRC screening test recommendations, no matter which test is recommended. Thus, the important and simple message for health care practitioners is to enthusiastically recommend CRC screening in a way that works in the context of current practice standards within individual practice settings. These primary findings are unique. We were unable to compare our primary findings to others’ because we were unable to identify other research that compared PCP-reported specific CRC screening recommendation strategies to actual screening rates. One national study of PCP screening practices revealed that recommending FOBT and colonoscopy was the most common reported practice pattern (50.3%), followed by colonoscopy only in 15% and FOBT, FS, and colonoscopy in 14%, but did not relate these patterns to overall practice screening rates. (16) Our data also suggest that clinicians will be the major drivers of the mix of CRC screening tests used and therefore ultimately of the cost-effectiveness of CRC screening in the community (as patient test completion appears to largely follow PCP recommendation patterns).
We found that concerns about FOBT, along with the influence of guidelines, appear to be the strongest factors differentiating those groups recommending more FOBT from those recommending more endoscopy. Another study actually found that provider concerns about FOBT accuracy reduced the likelihood of CRC screening. (28) Test concerns will be important leverage points for policy and practice leaders to influence the mix of CRC screening tests recommended in the future. For example, to increase the use of fecal tests clinicians will likely want to see improved sensitivity and specificity, with this information clearly conveyed through influential clinical practice guidelines and organizational procedures.
We are unable to determine the direction of cause and effect between our findings related to the decision-making style and pattern of screening recommendations, but our qualitative data strongly suggest that, in the cases of the Balanced and FOBT&Colonoscopy groups, the decision making style (belief in patient choice) is a primary factor leading to the pattern of testing. In the latter group patient “choice” is more purposefully limited by the PCPs, (should the patient desire endoscopy) because of the PCP’s test beliefs (i.e. their preference for colonoscopy over flexible sigmoidoscopy). In the cases of the FOBT and FOBT&FS groups, the PCPs appear to use their decision-making style (recommending a specific screening method) to get the patient to be screened using the test the PCP prefers.
Finally, the PCP groups did not differ in the extent to which they included all the possible counseling elements about CRC screening. All groups often provided information about the benefits and recommended frequency of screening, and gave less information about test discomfort, accuracy, and complications. Other research supports this common communication pattern.(29;30) It is possible that multiple counseling approaches, thoughtfully delivered in the context of complex factors such as physician-patient relationship and history, perceived patient SES, literacy, numeracy, and PCP perceptions of patient desired counseling style, do achieve similar results as long as the clinician enthusiastically recommends CRC screening. Other studies have found that patient perception of clinician “spending sufficient time”, providing an “adequate explanation” (31) or good “information”,(32) assessing patient understanding during CRC screening counseling,(33) and answering patients questions adequately (14) have been positively associated with receipt of CRC screening. In contrast, discussing pros and cons and eliciting patient preferences,(12) and providing screening choices (14) reduced screening likelihood. Our results need to be interpreted with caution. The PCPs in our study indicated that they frequently included many of the CRC screening counseling elements. Other studies have found that clinicians often do not include many of the elements of CRC screening informed decision making (12), and overestimate the elements that they do include. (34)
This study has several limitations. The study site, as an integrated group practice that is culturally strongly guideline-focused, may have less variation in practice than other environments. The findings may not therefore be completely generalizable. In particular, there were few PCPs who reported mostly recommending colonoscopy, and thus we had a limited ability to evaluate that practice pattern. The study included a small number of PCPs in each group, especially for the qualitative data gathering. Also, the study’s retrospective design introduces several weaknesses. For the primary outcome, we attempted to distinguish true CRC screening from diagnostic testing but we may not have been completely successful in this regard. However, this study has multiple strengths, such as a very high PCP participation rate, clinically documented screening versus patient report, the ability to validate clinician stated recommendations through data on specific screening test use, and enough variation in CRC screening practice to generate clearly identifiable practice patterns.
In conclusion, although the Balanced recommendation style was the most common, the other three styles were common as well; patterns were primarily distinguished by specialty of the PCP, the influence of guidelines and test concerns, (especially related to FOBT test performance) and decision making style. Each of the four physician recommendation patterns identified here appeared to be equally effective in accomplishing CRC screening. As more information becomes available about the relative cost-effectiveness of older versus newer technologies, communicating this information to clinicians in a manner that considers their diverse influences, test-related concerns, and decision-making styles will be important for improving community CRC screening rates.
Acknowledgments
Funders: This project was supported by grant # R01CA132709 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The funding organization was not involved in the design or conduct of the research; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of this manuscript. Dr. Feldstein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Prior presentations: The findings were presented at the Society of General Internal Medicine Annual meeting in Minneapolis, Minnesota, April 28, 2010.
Contributors: We would like to thank the many primary care clinicians at Kaiser Permanente Northwest who gave of their valuable time to respond to the study survey and the many physician leaders and staff who assisted with the project: Michael Kositch MD, Thomas Hickey MD, Maureen Wright MD, Heather Block, Stephanie Schoap, and Andre Smith. We would also like to thank Leslie Bienen for editorial assistance, Dixie Sweo for administrative support, and Mary Rix for project management.
Reference List
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004 Jan;54(1):8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Flehinger BJ, Schottenfeld D, Miller DG. Screening for colorectal cancer with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst. 1993 Aug 18;85(16):1311–8. doi: 10.1093/jnci/85.16.1311. [DOI] [PubMed] [Google Scholar]
- 3.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000 Nov 30;343(22):1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 4.Atkin WS, Cuzick J, Northover JM, Whynes DK. Prevention of colorectal cancer by once-only sigmoidoscopy. Lancet. 1993 Mar;341(8847):736–40. doi: 10.1016/0140-6736(93)90499-7. [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force, Agency for Healthcare Research and Quality. Topic Page. Rockville, MD: 2009. Mar, Screening for Colorectal Cancer. Available from: URL: http://www.ahrq.gov/clinic/uspstf/uspscolo.htm. [Google Scholar]
- 6.Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev. 2007;(1):CD001216. doi: 10.1002/14651858.CD001216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1623–30. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 8.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004 May 15;100(10):2093–103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 9.Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005 Sep;43(9):939–44. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- 10.Brenes GA, Paskett ED. Predictors of stage of adoption for colorectal cancer screening. Prev Med. 2000 Oct;31(4):410–6. doi: 10.1006/pmed.2000.0729. [DOI] [PubMed] [Google Scholar]
- 11.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005 Jul;41(1):23–9. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Ling BS, Trauth JM, Fine MJ, Mor MK, Resnick A, Braddock CH, et al. Informed decision-making and colorectal cancer screening: is it occurring in primary care? Med Care. 2008 Sep;46(9 Suppl 1):S23–S29. doi: 10.1097/MLR.0b013e31817dc496. [DOI] [PubMed] [Google Scholar]
- 13.Dunn AS, Shridharani KV, Lou W, Bernstein J, Horowitz CR. Physician-patient discussions of controversial cancer screening tests. Am J Prev Med. 2001 Feb;20(2):130–4. doi: 10.1016/s0749-3797(00)00288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafata JE, Divine G, Moon C, Williams LK. Patient-physician colorectal cancer screening discussions and screening use. Am J Prev Med. 2006 Sep;31(3):202–9. doi: 10.1016/j.amepre.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McQueen A, Bartholomew LK, Greisinger AJ, Medina GG, Hawley ST, Haidet P, et al. Behind closed doors: physician-patient discussions about colorectal cancer screening. J Gen Intern Med. 2009 Nov;24(11):1228–35. doi: 10.1007/s11606-009-1108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006–2007. Am J Prev Med. 2009 Jul;37(1):8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeborn DK, Pope C. Promise and Performance in Managed Care: The Prepaid Group Practice Model. Baltimore, MD: Johns Hopkins University Press; 1994. [Google Scholar]
- 18.Colorectal cancer screening: percentage of adults 50 to 80 years of age who had appropriate screening for colorectal cancer. HEDISR 2009: Healthcare Effectiveness Data & Information Set. 2010;1 & 2 [Google Scholar]
- 19.Myers RE, Turner B, Weinberg D, Hauck WW, Hyslop T, Brigham T, et al. Complete diagnostic evaluation in colorectal cancer screening: research design and baseline findings. Prev Med. 2001 Oct;33(4):249–60. doi: 10.1006/pmed.2001.0878. [DOI] [PubMed] [Google Scholar]
- 20.Erlandson DA, Harris EL, Skipper BL, Allen SD. Doing Naturalistic Inquiry: A Guide to Methods. Newbury Park, Calif: Sage Publications, Inc; 1993. [Google Scholar]
- 21.Patton MQ. Qualitative research and evaluation methods. 3. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- 22.Seidman I. Interviewing as qualitative research: A guide for researchers in education and social sciences. New York: Teachers college Press; 1991. [Google Scholar]
- 23.Denzin N, Lincoln Y. The Sage Handbook of Qualitative Research. 3. Thousand Oaks, CA: Sage Publications; 2005. [Google Scholar]
- 24.Strauss AL, Corbin JM. Basics of qualitative research: Grounded theory procedures and techniques. Newbury Park, CA: Sage Publications; 1990. [Google Scholar]
- 25.Wolcott HF. Transforming Qualitative Data: Description, Analysis, and Interpretation. Thousand Oaks, Calif: Sage Publications, Inc; 1994. [Google Scholar]
- 26.ATLAS.ti Visual Qualitative Data Analysis [computer program]. Version 5.9. Berlin: 1997. [Google Scholar]
- 27.Holden DJ, Harris R, Porterfield DS, Jonas DE, Morgan LC, Reuland D, et al. Evidence Report/Technology Assessment No 190 AHRQ Publication No 10-E-002. Rockville, MD: Agency for Healthcare Research and Quality; Feb, 2010. Enhancing the Use and Quality of Colorectal Cancer Screening. Feb. Report No.: AHRQ 190. [Google Scholar]
- 28.Dulai GS, Farmer MM, Ganz PA, Bernaards CA, Qi K, Dietrich AJ, et al. Primary care provider perceptions of barriers to and facilitators of colorectal cancer screening in a managed care setting. Cancer. 2004 May 1;100(9):1843–52. doi: 10.1002/cncr.20209. [DOI] [PubMed] [Google Scholar]
- 29.Wackerbarth SB, Tarasenko YN, Joyce JM, Haist SA. Physician colorectal cancer screening recommendations: an examination based on informed decision making. Patient Educ Couns. 2007 Apr;66(1):43–50. doi: 10.1016/j.pec.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canada RE, Turner B. Talking to patients about screening colonoscopy--where conversations fall short. J Fam Pract. 2007 Aug;56(8):E1–E9. [PubMed] [Google Scholar]
- 31.Carcaise-Edinboro P, Bradley CJ. Influence of patient-provider communication on colorectal cancer screening. Med Care. 2008 Jul;46(7):738–45. doi: 10.1097/MLR.0b013e318178935a. [DOI] [PubMed] [Google Scholar]
- 32.O’Malley AS, Forrest CB, Feng S, Mandelblatt J. Disparities despite coverage: gaps in colorectal cancer screening among Medicare beneficiaries. Arch Intern Med. 2005 Oct 10;165(18):2129–35. doi: 10.1001/archinte.165.18.2129. [DOI] [PubMed] [Google Scholar]
- 33.Ling BS, Klein WM, Dang Q. Relationship of communication and information measures to colorectal cancer screening utilization: results from HINTS. J Health Commun. 2006;11(Suppl 1):181–90. 181–90. doi: 10.1080/10810730600639190. [DOI] [PubMed] [Google Scholar]
- 34.Wolf MS, Baker DW, Makoul G. Physician-patient communication about colorectal cancer screening. J Gen Intern Med. 2007 Nov;22(11):1493–9. doi: 10.1007/s11606-007-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]