Abstract
The sigma-2 receptor, whose gene remains to be cloned, has been validated as a biomarker for tumor cell proliferation. Here we report the use of a novel photoaffinity probe, WC-21, to identify the sigma-2 receptor binding site. WC-21, a sigma-2 ligand containing both a photoactive moiety azide and a fluorescein isothiocyanate group, irreversibly labels sigma-2 receptors in rat liver; the membrane-bound protein was then identified as PGRMC1 (progesterone receptor membrane component-1). Immunocytochemistry reveals that both PGRMC1 and SW120, a fluorescent sigma-2 receptor ligand, colocalizes with molecular markers of the endoplasmic reticulum and mitochondria in HeLa cells. Overexpression and knockdown of the PGRMC1 protein results in an increase and a decrease in binding of a sigma-2 selective radioligand, respectively. The identification of the putative sigma-2 receptor binding site as PGRMC1 should stimulate the development of unique imaging agents and cancer therapeutics that target the sigma-2 receptor/PGRMC1 complex.
Introduction
Sigma-1 and sigma-2 receptors were initially characterized in the mid-1970's using radioligand binding studies 1. Radiolabeled benzomorphans such as [3H](+)-pentazocine bind potently and selectively to sigma-1 versus sigma-2 receptors. Characterization of the sigma-2 receptor has relied on radioligand binding studies using [3H]DTG in the presence of (+)-pentazocine or dextrallorphan to block binding to sigma-1 receptors 1. The sigma-1 receptor was purified, sequenced and cloned from guinea pig brain in 1996, and shows no sequence homology with any mammalian proteins cloned to date 2. Ligands binding to the sigma-1 receptor modulate the release of neurotransmitters; several of these compounds have shown promise as antipsychotics, antidepressants, and drugs blocking the effects of psychostimulants 3, 4. Recent studies indicate that the sigma-1 receptor is expressed in postsynaptic sites, regulates ion channels, and may function as a protein chaperone5-8. To date, the sigma-2 receptor has not been purified, sequenced or cloned.
Although its structure is not known, the sigma-2 receptor has been validated as a biomarker for tumor cell proliferation both in vitro and in vivo, and as a target for chemotherapy. Several studies have shown that: the sigma-2 receptor density in proliferative breast cancer cells is about 10-fold higher than in quiescent breast cancer cells 9; sigma-2 receptor expression is upregulated during the transition from quiescence to proliferation and down-regulated during the transition from proliferation to quiescence 10; sigma-2 receptor density can be used to determine the proliferative status of solid tumors 11; sigma-2 receptor ligands can serve as chemotherapeutics for treating solid tumors 12-14; and, sigma-2 receptor radioligands can be used to image tumors by positron emission tomography (PET) or single photon emission computed tomography (SPECT) 15-19. However, since the sigma-2 receptor gene has not been cloned, the exact role this receptor plays in tumor and normal cell proliferation is currently unknown. Our group has developed sigma-2 selective ligands, including [3H]RHM-1, [125I]RHM-4, and SW120 to directly identify, locate, and quantify this protein using a variety of receptor binding and molecular imaging techniques 13-15, 18, 20-22.
Progesterone receptor membrane component 1 (PGRMC1) is overexpressed in a variety of tumors in comparison with the corresponding normal cells, and thus represents an important biomarker for cancer progression and a potential target for anticancer drugs 23-25. PGRMC-1, which exists in phosphorylated and dephosphorylated states during cancer cell proliferation26, regulates cell growth and proliferation through interactions between its Cytochrome b5 binding domain and other potential binding partners which include Insig-1, PAIR-BP1, and P450 proteins23, 27-31. Recent studies suggest that PGRMC1 is a biomarker of cell proliferation and an excellent therapeutic target for inhibiting tumorigenesis24, 32, 33. Interestingly, proteomic studies also showed that PGRMC1 is expressed in high levels in the proliferative cells of human endometrium 34.
The purpose of the current study was to identify the protein or protein complex that contains the sigma-2 receptor ligand-binding site as the first step in determining the role of this receptor in cell proliferation, and defining a new target for the development of novel tumor imaging agents and cancer therapeutics. Here we describe a novel strategy developed for the identification of the sigma-2 receptor. The sigma-2 receptor photoaffinity probe, WC-21, an analogue of RHM-1, contains an azide moiety for the photo-affinity tagging of the sigma-2 receptor, and a fluorescein isothiocyanate group (FITC) for protein visualization. WC-21 was utilized to directly label the sigma-2 receptor binding site in rat hepatic membrane homogenates. Proteomic studies and consequent studies were carried out to determine the molecular identify of the sigma-2 receptor binding site and if it corresponded to any previously identified proteins. PGRMC1 was identified as the putative sigma-2 receptor binding site. The results of our studies provide strong experimental evidence supporting the localization of the putative sigma-2 receptor binding site within the PGRMC1 protein complex.
Results
Photoaffinity labeling and protein identification
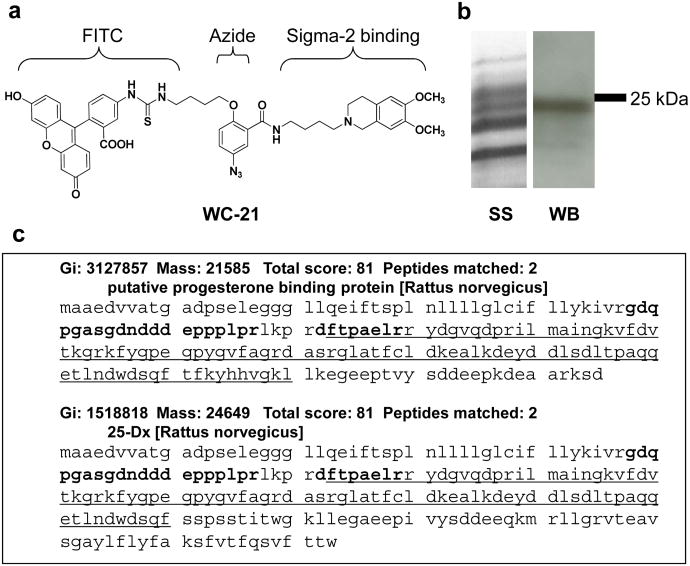
The synthesis of sigma-2 receptor photoaffinity probe WC-21, which contains an azide moiety for the photoaffinity tagging of the protein and a FITC group for protein visualization, is described in detail in the supporting online material (Supplementary Figs. S1- S3). WC-21 has high binding affinity for sigma-2 receptors (Ki = 8.7 nM) and relatively low binding affinity for sigma-1 receptors (Ki > 4,000 nM) (Fig. 1a). WC-21 was incubated with rat liver membrane homogenates, and the WC-21-protein complex photo-crosslinked. The protein supernatant was enriched and separated by gel electrophoresis (Fig. 1b). Western blot analysis revealed a dominant protein band at ∼24 kD that was labeled by FITC conjugated probe WC-21 (Fig. 1b). Labeling of this protein band with WC-21 could be blocked by well-characterized sigma-2 receptor ligands (Supplementary Fig. S4). Proteomic studies of the protein in the ∼24 kD band identified two proteins, the 22 kD putative progesterone binding protein and the 25 kD 25-Dx protein, that share the same genetic name, progesterone receptor membrane component 1 (PGRMC1) 23. Fig. 1c shows the protein sequence of the two peptides, gdqpgasgdndddeppplpr and dftpaelr, detected by mass spectrometry and the Cytochrome b5 domain (underlined) of PGRMC1. The gene for PGRMC1 is located on q22-q24 of the X chromosome.
Figure 1. Identification of the putative sigma 2 receptor binding site as PGRMC1 using a novel photoaffinity probe.
a, WC-21 contains a FITC group and a photoactive azide (N3) moiety, and displays high sigma-2 receptor affinity and selectivity; Ki = 8.7 ± 1 nM for sigma-2 receptor and Ki > 4,000 nM for sigma-1 receptor. b, Following UV cross-linking, ligand-labeled proteins were enriched and separated through SDS-PAGE; WC-21 cross-linked proteins can be visualized using an anti-FITC antibody; SS is silver staining and WB is western blot. c, Proteomic studies of the FITC positive band identified two proteins which shared the same genetic name: progesterone receptor membrane component 1 (PGRMC1). The protein structure shows the peptides (bold) detected by mass spectrometry as well as the Cytochrome b5 domain (underlined).
Three other proteins, glutathione-S-transferase alpha type 2, glutathione S-transferase mu 1(with 2 matched peptides), and NADH dehydrogenase (ubiquinone) flavoprotein 2 (with 1 matched peptide), were identified from the proteomics study. Based on the localization of sigma-2 receptors within the plasma membrane9, 35, 36, endoplasmic reticulum and mitochondria21, 22, these proteins were excluded as potential candidates since they are all intracellular enzymes which happen to overlap with PGRMC1 protein band.
The pharmacological profile of [125I]RHM-4 in HeLa cells
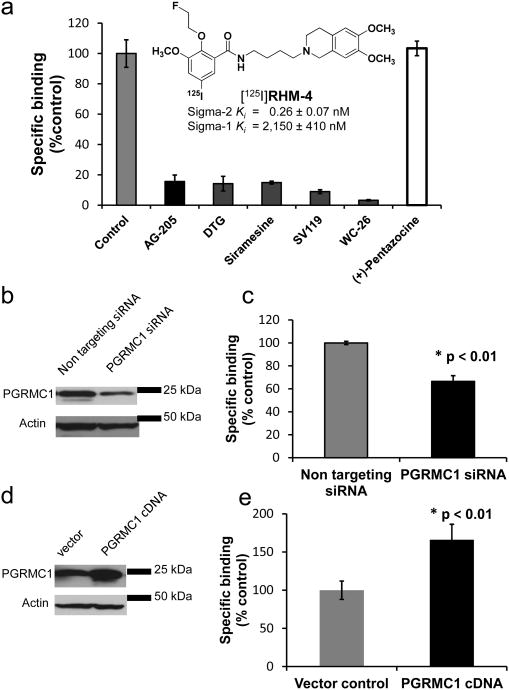
[125I]RHM-4 (Fig. 2a) is a useful radioligand for measuring sigma-2 receptor density in tumors and normal tissues 19. AG-205, a reported PGRMC1 ligand 33, 37, and the known sigma-2 receptor ligands, DTG, siramesine, SV119, and WC-26, readily displaced [125I]RHM-4 binding in HeLa cell membrane homogenates (Fig. 2a). Similar results were also observed in mouse mammary 66 tumor cell membrane homogenates (Supplementary Fig. S5).
Figure 2. The pharmacological profile of [125I]RHM-4 and sigma-2 receptor binding activity in PGRMC1 siRNA and cDNA treated HeLa cells.
a, Competitive binding studies of [125I]RHM-4 were carried out with the PGRMC1 ligand AG-205, sigma-2 receptor ligands DTG, WC-26, SV119, and siramesine or with the sigma-1 receptor ligand (+)-pentazocine. [125I]RHM-4 binding was blocked by AG-205 and the sigma-2 ligands but not by (+)-pentazocine. n = 2, sample in triplicate. b, Typical western blot confirming PGRMC1 protein expression knockdown in PGRMC1 specific siRNA treated HeLa cells relative to nontargeting siRNA treated controls compared to actin (loading control). c, The bar graph shows reduced sigma-2 receptor binding activity in the PGRMC1 knockdown cells; * p<0.01, Student's t-test, n=3. d, Typical western blot confirming increased PGRMC1 protein expression in PGRMC1 transfected HeLa cells relative to vector transfected controls. e, Bar graph showing increased sigma-2 receptor binding activity in PGRMC1 transfected cells; * p<0.01, Student's t-test, n=3. Error bars in a, c, d represent SEM.
Binding activity in PGRMC1 siRNA and cDNA treated HeLa cells
Because PGRMC1 is a known protein, a number of molecular biology tools are available to study the relationship between the expression of this protein and the binding of sigma-2 selective ligands in cancer cells. Knockdown studies using a PGRMC1-specific siRNA (Fig. 2b) reduced the binding of [125I]RHM-4 to HeLa cells (Fig. 2c), indicating that the PGRMC1 complex has binding properties similar to the sigma-2 receptor. Similar results were observed in Human Embryonic Kidney (HEK) 293T cells using the PGRMC1 specific siRNA and the sigma-2 selective fluorescent probe, SW120 (Supplementary Fig. S6). Cells treated with nontargeting siRNA were used as controls. Overexpression studies were also conducted in HeLa cells using a cDNA for the PGRMC1. HeLa cells transfected with the PGRMC1 cDNA displayed an increase in protein levels (Fig 2d) and a 60% increase in binding of [125I]RHM-4 (Fig 2e) over cells transfected with vector alone. Sigma-1 receptor binding activities, determined using radioligand [3H](+)-pentazocine binding were found to be not changed in the nontargeting siRNA and PGRMC1 siRNA treated cells, or in cells transfected with vector and PGRMC1 cDNA (Supplementary Fig. S7).
Sigma-2 receptor and PGRMC1 in mammary 66 and 67 cells
A good correlation between sigma-2 receptor binding of [125I]RHM-4 and the PGRMC1:actin ratio was observed in mouse mammary adenocarcinoma cells lines 66 and 67 after 6 (proliferative cells), 12 (early quiescent phase) and 18 (late quiescent phase) days in culture (Supplementary Fig. S8). These data are consistent with the expression of sigma-2 receptors in 66 and 67 cells 9.
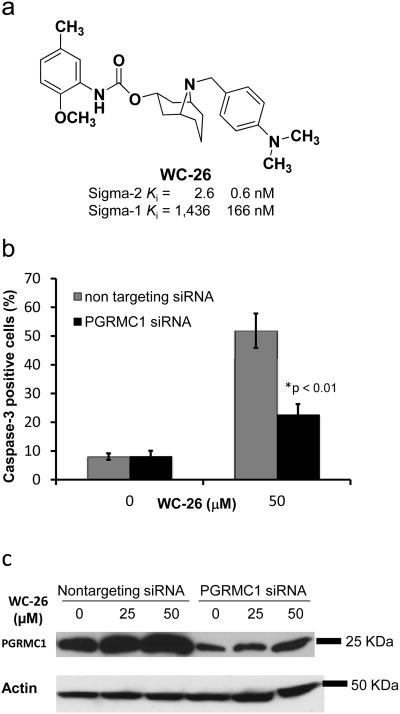
Caspase-3 activation and PGRMC1 upregulation by WC-26
Studies have shown that sigma-2 receptor ligands can induce caspase-3-dependent cell death 14, 38. In the current study, functional assays were conducted to examine the ability of the PGRMC1 to regulate caspase-3 activation by the sigma-2 receptor ligand, WC-26 (Fig. 3a). In HeLa cells, knocking down the PGRMC1 resulted in a blunting of the effect of WC-26 to induce caspase-3 activation relative to HeLa cells treated with the non-targeting siRNA (Fig. 3b). Also, previous studies have shown that AG-205, a PGRMC1 ligand, induced an upregulation of PGRMC1 in A549 lung cancer cells33, 37. The sigma-2 receptor ligand WC-26 induced an upregulation of PGRMC1 protein in a dose-dependent manner in nontargeting siRNA-treated cells (Fig. 3c). Only a minimal increase in PGRMC1 expression was observed in the PGRMC1 knockdown cells.
Figure 3. Caspase-3 activation and PGRMC1 upregulation induced by the sigma-2 receptor ligand, WC-26.
a, Chemical structure of WC-26, a selective sigma-2 receptor ligand. b, Knockdown of PGRMC1 in HeLa cells resulted in decreased caspase-3 activation induced by WC-26. c, WC-26 stimulated PGRMC1 expression in the same manner as AG-205 34, a PGRMC1 ligand; * p<0.01, Student's t-test, n=3. Error bars in b represent SEM.
Intracellular localization of PGRMC1 and sigma-2 receptors
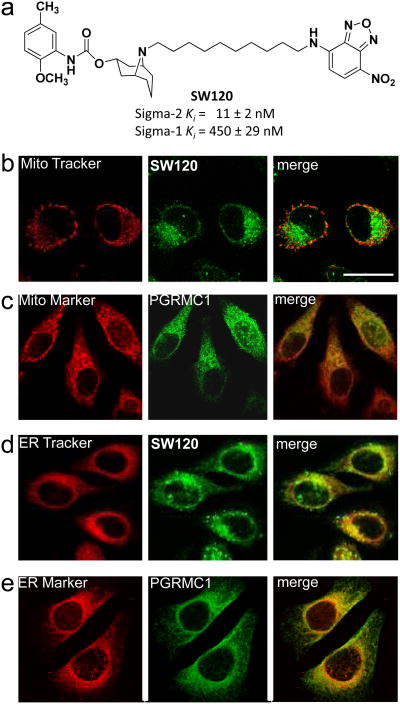
The intracellular localization of PGRMC1 and sigma-2 receptors was compared using confocal microscopy. PGRMC1 proteins were visualized with an anti-PGRMC1 antibody, whereas sigma-2 receptors were visualized using the fluorescent probe, SW12021 (Fig. 4a). Both the sigma-2 receptor probe (Fig. 4b, d) and anti-PGRMC1 antibody (Fig. 4c, e) colocalized with markers of the mitochondria (Fig. 4b, c) and the endoplasmic reticulum (Fig. 4d, e). An additional set of high magnification images are shown in Supplementary Fig. S9.
Figure 4. Intracellular co-localization of PGRMC1 and sigma-2 receptors in HeLa cells using confocal microscopy.
a, Sigma-2 receptors labeled with SW120 and PGRMC1 partially colocalized with mitochondria markers (b, c) and endoplasmic reticulum markers (d, e) in HeLa cells. For imaging sigma-2 receptors, live cells were incubated with SW120 and either MitoTracker or ER-Tracker and then imaged by confocal microscopy. For imaging PRGMC1, cells were fixed and incubated with goat anti-PGRMC1 antibody followed by a FITC-conjugated secondary antibody (Mito-marker or ER-marker). Antibody stained HeLa cells were then coverslipped and imaged by confocal microscopy. Scale bar represents 20 μm.
Discussion
In recent years, considerable effort has been focused on the identification of cancer biomarkers which could be used in early detection, patient prognosis, and prediction of response to different types of chemo- and radiotherapy. In many regards, the sigma-2 receptor possesses a unique ability to serve as both a diagnostic and therapeutic biomarker of solid tumors. The 10-fold higher density of sigma-2 receptors in proliferating breast cancer cells versus quiescent breast cells in vitro and in vivo9, 11 suggest that measuring the sigma-2 receptor status of solid tumors should provide an assessment of the proliferating:quiescent cell ratio in solid tumors, which could be used by an oncologist to select between certain types of chemotherapy (e.g., cell cycle specific versus cell cycle nonspecific) and radiation therapy (hyperfractionated versus conventional radiation therapy). Furthermore, preclinical studies of sigma-2 receptor agonists have shown promise as chemotherapeutic agents since they are capable of killing tumor cells via a variety of mechanisms including caspase- and non-caspase-mediated apoptosis. However, the widespread acceptance of the sigma-2 receptor as a cancer biomarker has been hampered by the fact that the molecular identity of this protein was not known.
The goal of the current study was to complete a series of steps aimed at determining the molecular identity of the sigma-2 receptor. The first step involved the development of a sigma-2 selective photoaffinity probe which could irreversibly tag sigma-2 receptors in rat liver membrane homogenates, the tissue source used in sigma-2 receptor binding assays. The photoaffinity labeling and mass spectrometry sequence analysis indicated that a strong candidate protein for the sigma-2 receptor was PGRMC1. A review of the literature revealed a number of similarities between the expression of PGRMC1 in cancer cells and normal tissues and the density of sigma-2 receptors in the same cancer cells and tissues. The availability of siRNA, cDNA, and monoclonal antibodies for PGRMC1 provided the tools needed to study the relationship between the expression of this protein and the binding of sigma-2 selective ligands in cancer cells. The intracellular localization of PGRMC1 and sigma-2 receptors in mitochondria and endoplasmic reticulum, reduction in sigma-2 receptor binding and caspase-3 mediated cell death in PGRMC1 siRNA treated HeLa cells, and the increase in sigma-2 receptor binding upon overexpressing the PGRMC1 clearly demonstrate that sigma-2 receptor ligands bind to the PGRMC1 protein complex. Therefore, this study is the first to report that the putative sigma-2 receptor ligand binding site is located within the PGRMC1 protein complex. In addition, the observations that AG-205, a reported PGRMC1 ligand, displaces [125I]RHM-4 from HeLa cell membrane homogenates, and that both AG-205 and the sigma-2 receptor ligand WC-26 induce an upregulation of the PGRMC1 protein in lung cancer cells 33, 37 and HeLa cells further support our conclusions.
The identification that the putative sigma-2 receptor binding site resides within the PGRMC1 protein complex is significant for a variety of reasons. First, it provides a link between receptor binding studies using sigma-2 radioligands and a known protein, the PGRMC1, which has been validated as a biomarker of cancer and cell proliferation. Second, it provides a gene whose expression can be examined across a wide panel of tumor cells. The genetic mutations and single-nucleotide polymorphism (SNP) of PGRMC1 can also be readily examined in assessing the possible roles of this gene in normal cell function and in malignant transformation. Finally, the results reported here provide an important scientific bridge between the sigma-2 receptor and the PGRMC1, and make available a variety of radioligands, fluorescent probes, and potential small molecule ligands to study the sigma-2 receptor/PGRMC1 complex in solid tumors and cancer cells using a variety of experimental techniques.
Methods
Chemical synthesis of the compounds and radiotracers
The tritiated compound, [3H]RHM-1, was made by American Radiolabeled Chemicals, Inc. (St. Louis, MO, USA) via O-alkylation of the corresponding phenol precursor 17, 20; the specific activity of the radioligand was 80 Ci/mmol. [3H](+)-pentazocine was purchased from Perkin-Elmer (Boston, MA, USA). [125I]RHM-4 was prepared by an iododestannylation reaction of the corresponding tributyltin precursor 13, 19. SV119, SW120 and WC-26, and siramesine were synthesized by our group according to published methods 12, 21, 39, 40. The synthesis of the novel FITC conjugated probe, WC-21 (Supplementary Figures S1-S3), is described in the supplementary online methods. AG-205 was purchased from TimTec (Newark, DE, USA). DTG and (+)-pentazocine were purchased from Sigma Aldrich (Milwaukee, WI, USA). Haloperidol was purchased from Tocris Bioscience (St. Louis, MO, USA). PGRMC1 recombinant protein was purchased from Assay Designs, Inc. (Ann Arbor, MI, USA).
Cell membrane and hepatic membrane homogenates
Human HeLa and HEK 293T cells were grown in Minimum Essential Media (MEM) containing 10% fetal bovine serum, 2 mM L-glutamine, 1% nonessential amino acids, and 1 × penicillin/streptomycin at 37 °C in a humidified a 5% CO2/95% air atmosphere. The mouse mammary tumor 66 cells were grown as previously described 9. The cells were harvested from T75 flasks after 6, 12, or 18 days in culture. For binding assays, cells were mechanically scraped, rinsed with ice-cold PBS, transferred to a 15 mL conical centrifuge tube and centrifuged for 10 min at 200 × g. The cell pellet from each T75 flask was resuspended in 1 mL of 50 mM Tris-HCl at pH 8.0, and homogenized using an Ultra-Turrax T8 polython homogenizer (IKA Works, Inc, Wilmington, NC, USA) at speed 3 for 20 seconds. Hepatic cells membrane homogenates were prepared from the liver of male Sprague-Dawley rats as previously described 20.
Photoaffinity labeling and protein identification
For photoaffinity labeling, 100 nM of WC-21 was incubated in a 96 well plate with rat liver membrane homogenates (∼ 300 μg of protein in a volume of 150 μL) for 1 h, then the reactions were harvested and filtered into 96 well filter plate after being washed three times. The washed filters were irradiated with 254 nm ultraviolet light for 2 min at a distance of 1 cm; the protein-loaded filters were then punched out, pooled and crushed by homogenization in 2% sodium dodecyl sulfate (SDS) solution in a 15 mL centrifuge tube, following a centrifugation of 10 minutes at speed of 800 × g, the supernatant was mixed with protein gel sample buffer and boiled for 2 min.
For western blot analysis of the WC-21-labeled protein band, lysates containing 35 μg of protein were run on a 12% acrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad Laboratories, Hercules, CA, USA). The position of the predominant band of the ligand-labeled protein in the gel was determined using a horseradish peroxidase-conjugated goat anti-FITC antibody (Biodesign International Inc., Saco, ME, USA) at a 1:1,000 dilution (Supplementary Fig. S3).
Western blot analysis with a horseradish peroxidase (HRP) conjugated anti-FITC antibody revealed the predominant protein band at ∼24 kD was labeled by WC-21 (Fig. 1b). This protein band labeling could be blocked by sigma-2 receptor ligands, such as DTG, haloperidol and RHM-1 (Supplementary Fig. 3). The 24 kD band on the silver stained gel was excised and trypsinized; the trypsinized protein solution was further confirmed as tagged with WC-21 an antibody specific for FITC. Proteomic study of the protein solution indentified two proteins, the 22 kDa putative progesterone binding protein and the 25 kDa 25-Dx protein, which share the same genetic name, progesterone receptor membrane component 1 (PGRMC1)
For sequencing studies, proteins were enriched by SDS polyacrylamide gel electrophoresis (SDS-PAGE) on 4-15% gradient gels using ∼100 μg of protein per loading well. Ten gel pieces containing proteins ranging from 20-26 kD molecular weight were excised and subjected to electro-elution with Midi GeBAflex-tube (Gerard Biotech, Oxford, OH, USA). The eluted protein samples were dialyzed with 7,000 MWCO SnakeSkin pleated dialysis tubing (Pierce Biotechnology, Inc. Rockford, IL, USA) and lyophilized; the enriched protein sample was further separated with two 12% gels. One gel was silver stained for protein band visualization using a mass spectrometry compatible kit from Bio-Rad Laboratories (Hercules, CA, USA) and the other gel was transferred to a PVDF membrane for western blot analysis with a horseradish peroxidase conjugated anti-FITC antibody (Fig. 1B). The FITC positive protein band was found at molecular weight of 24 kD, which was determined using the protein standards. The 24 kD band was excised and trypsinized. MALDI-mass spectrometry analysis of the trypsinized proteins was performed by the Proteomics & Mass Spectrometry facility of the Donald Danforth Plant Science Center (St. Louis, MO, USA) to identify the proteins.
PGRMC1 siRNA treatment
HeLa cells were plated on 6-well plates at a cell density of 1.5 × 105 cells/well. 24 h after plating, the cells were treated with either nontargeting siRNA (catalogue number D-001210-02-20, Thermo Scientific Dharmacon, Inc., Lafayette, CO, USA) or human PGRMC1 siRNAs (catalogue number L-010642-00-0020, Thermo Scientific Dharmacon, Inc., Lafayette, CO, USA) mixed with DharmaFECT 2 Transfection Reagent (Thermo Scientific Dharmacon, Inc., Lafayette, CO, USA) to yield a final concentration of 50 nM. siRNA transfection was performed according the siRNA transfection protocol outlined by Dharmacon. 48 h after siRNA treatment, cells were collected for the sigma-2 receptor binding assay. The silencing of PGRMC1 was confirmed by western blot analysis.
Overexpression of PGRMC1
HeLa cells were plated in 100 mm dishes at 1.5 × 106/dish. 24 h after plating, the cells were transfected with the pCMV6-XL4 vector only or human PGRMC1 cDNA (OriGene Technologies, Inc., Rockville, MD) using a Fugene 6 transfection reagent (Roche Applied Science, Indianapolis, IN). Transfection was carried out according to the guidelines of the manufacturer. For transfection of HeLa cells, 18 μL of Fugene 6 and 10 μg human PGRMC1 cDNA were used. After 24 h, the cells were collected for the sigma-2 receptor binding assays. The overexpression of PGRMC1 was confirmed by western blot analysis.
Caspase-3 activation assay
WC-26 induced caspase-3 activation in PGRMC1 siRNA treated HeLa cells was quantified by flow cytometry using a FACScan (BD Biosciences, San Jose, CA, USA) equipped with an air-cooled argon laser. HeLa cells were fixed in 70% ethanol, washed twice with phosphate buffered saline (PBS), and incubated with rabbit anti cleaved-caspase-3 antibody (Cell Signaling Technology, Danvers, MA, USA) at a 1:50 dilution in BD Perm/WashTM buffer (BD Biosciences, San Jose, CA, USA) for 2 h at 37 °C . The cells were washed three times with PBS, and then incubated with phycoerythrin (PE) conjugated donkey anti rabbit IgG (BD Biosciences Pharmingen,) (1:300 dilution) for 1 h at room temperature. After washing with PBS, 5 μL of 7-amino-actinomycin D (7-AAD, BD Biosciences, San Jose, CA, USA) was added at a concentration of 0.25 μg of 7-AAD/1×106 cells. The PE- conjugated antibody was excited at 488 nm, and the emission collected using a 570 nm filter. The 7-AAD was excited at 488 nm, and the emission collected using a 650 nm long-pass filter.
Immunocytochemistry of sigma-2 receptors and PGRMC1
MitoTracker Red CMXRos and ER-Tracker™ Red dye were purchased from Invitrogen Corporation (Carlsbad, CA, USA). HeLa cells were plated in 35 mm diameter glass-bottom dishes at 2 × 105 cells/dish for 24 h. The live cells were incubated with 30 nM SW120 and either the mitochondria tracker, MitoTracker Red CMXRos (20 nM), or the endoplasmic reticulum tracker, ER-Tracker™ Red (500 nM), for 2 h at 37 °C. The labeled cells were then coverslipped and imaged by confocal microscopy as previously described21, 22. The images were acquired using a confocal laser scanning microscope (LSM5 Pascal, Carl Zeiss, Thirnwood, NY, USA). SW120 was excited using the 488 nm line from an argon laser, and the emission collected through a 505-530 nm band-pass filter. MitoTracker and ER-Tracker were excited using the 543 nm line from a helium–neon laser, and the emission collected through a 560 nm long-pass filter.
For imaging PRGMC1, fixed HeLa cells were incubated with goat anti-PGRMC1 antibody at a 1:100 dilutions in the blocking serum overnight at 4 °C. After washing, the cells were incubated with FITC-conjugated donkey anti-goat IgG secondary antibody for 1 h. Rabbit anti-COX IV antibody (Cell signaling, Danvers, MA) and rabbit anti-GRP78 BiP antibody (Abcam, Cambridge, MA) were used for imaging Mito-marker and ER-marker, respectively. The secondary antibody used for imaging Mito-marker and ER-marker is TRITC-conjugated donkey anti-rabbit IgG. The FITC and TRITC antibody labeled cells were then coverslipped and imaged by confocal microscopy.
Supplementary Methods for receptor binding assay, western blot analysis of PGRMC1, real time PCR for PGRMC1 mRNA expression and synthesis of the novel photoaffinity probe WC-21 and the 1H NMR and 13C NMR spectra for compounds 2, 3, 4, 5 and WC-21 (Supplementary Figures S10-S19) can be found in the Supplementary Information.
Supplementary Material
Acknowledgments
Supported by CA 102869, American Cancer Society MRSG 08-019-01CDD, VA Merit Award 1136919, and by the McDonnell Center for Systems Neuroscience at Washington University. We thank Dr. Leslie M. Hicks, Ms. Jeanne Speichinger and Ms. Rebecca White for help with the MALDI-mass spectrometry analysis and protein sequencing.
Footnotes
Competing financial interests: Isotrace Technologies, LLC, St. Charles, MO has a licensing agreement with Washington University School of Medicine for the commercialization of fluorine-18 labeled sigma-2 receptor radiotracers developed in the laboratory of RH Mach. RH Mach has no financial interest in Isotrace Technologies, nor is he a paid consultant for the company. Therefore, the authors declare are no competing financial interests.
Author contributions: J.X., C.Z., K.T.W., and R.H.M. conceived and coordinated the experiments and wrote the manuscript. W.C. and D.Zhou designed and synthesized WC-21, WC-26 and synthesized siramesine under the supervision of R.H.M.; S.V., Z.T. and D.Zeng synthesized compounds SV119, SW120 and [125I]RHM-4 under the supervision of R.H.M.. J.X. designed and carried out the protein purification and photoaffinity labeling study. F.P., J.M.R., and F.Z. performed the cell culture and western blot analysis under the supervision of C.Z.. K.C.C. designed and performed the caspase-3 flow cytometry study under the supervision of R.S.H.. F.J. and D.S. designed and performed the PGRMC1 knockdown experiments with HEK 293T cells under the supervision of W.G.H..
References
- 1.Hellewell SB, et al. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 2.Hanner M, et al. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- 4.Takebayashi M, Hayashi T, Su TP. Nerve growth factor-induced neurite sprouting in PC12 cells involves sigma-1 receptors: implications for antidepressants. J Pharmacol Exp Ther. 2002;303:1227–1237. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, et al. Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering Rats. J Pharmacol Exp Ther. 2009;332:1054–1063. doi: 10.1124/jpet.109.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavlyutov TA, Epstein ML, Andersen KA, Ziskind-Conhaim L, Ruoho AE. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neuroscience. 2010;167:247–255. doi: 10.1016/j.neuroscience.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, Bowen WD. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J Biol Chem. 2008;283:28198–28215. doi: 10.1074/jbc.M802099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mach RH, et al. Sigma 2 receptors as potential biomarkers of proliferation in breast cancer. Cancer Res. 1997;57:156–161. [PubMed] [Google Scholar]
- 10.Al-Nabulsi I, et al. Effect of ploidy, recruitment, environmental factors, and tamoxifen treatment on the expression of sigma-2 receptors in proliferating and quiescent tumour cells. Br J Cancer. 1999;81:925–33. doi: 10.1038/sj.bjc.6690789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler KT, et al. Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br J Cancer. 2000;82:1223–1232. doi: 10.1054/bjoc.1999.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu W, et al. New N-substituted 9-azabicyclo[3.3.1]nonan-3alpha-yl phenylcarbamate analogs as sigma2 receptor ligands: synthesis, in vitro characterization, and evaluation as PET imaging and chemosensitization agents. Bioorg Med Chem. 2009;17:1222–1231. doi: 10.1016/j.bmc.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornick JR, et al. The novel sigma-2 receptor ligand SW43 stabilizes pancreas cancer progression in combination with gemcitabine. Mol Cancer. 2010;9:298. doi: 10.1186/1476-4598-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashiwagi H, et al. Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy. Mol Cancer. 2007;6:48. doi: 10.1186/1476-4598-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mach RH, Dehdashti F, Wheeler KT. PET Radiotracers for Imaging the Proliferative Status of Solid Tumors. PET Clin. 2009;4:1–15. doi: 10.1016/j.cpet.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mach RH, Wheeler KT. Development of molecular probes for imaging sigma-2 receptors in vitro and in vivo. Cent Nerv Syst Agents Med Chem. 2009;9:230–245. doi: 10.2174/1871524910909030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu Z, et al. Carbon-11 labeled sigma2 receptor ligands for imaging breast cancer. Nucl Med Biol. 2005;32:423–430. doi: 10.1016/j.nucmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Tu Z, et al. Fluorine-18-labeled benzamide analogues for imaging the sigma2 receptor status of solid tumors with positron emission tomography. J Med Chem. 2007;50:3194–3204. doi: 10.1021/jm0614883. [DOI] [PubMed] [Google Scholar]
- 19.Tu Z, et al. Radiosynthesis and biological evaluation of a promising sigma(2)-receptor ligand radiolabeled with fluorine-18 or iodine-125 as a PET/SPECT probe for imaging breast cancer. Appl Radiat Isot. 2010;68:2268–2273. doi: 10.1016/j.apradiso.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, et al. [3H]N-[4-(3,4-dihydro-6,7-dimethoxyisoquinolin-2(1H)-yl)butyl]-2-methoxy-5 -methylbenzamide: a novel sigma-2 receptor probe. Eur J Pharmacol. 2005;525:8–17. doi: 10.1016/j.ejphar.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 21.Zeng C, et al. Characterization and evaluation of two novel fluorescent sigma-2 receptor ligands as proliferation probes. Molecular Imaging. 2010 in press. [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng C, et al. Subcellular localization of sigma-2 receptors in breast cancer cells using two-photon and confocal microscopy. Cancer Res. 2007;67:6708–6716. doi: 10.1158/0008-5472.CAN-06-3803. [DOI] [PubMed] [Google Scholar]
- 23.Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Peluso JJ, Liu X, Gawkowska A, Lodde V, Wu CA. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol Cell Endocrinol. 2010;320:153–161. doi: 10.1016/j.mce.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohe HJ, Ahmed IS, Twist KE, Craven RJ. PGRMC1 (progesterone receptor membrane component 1): A targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol Ther. 2009;121:14–19. doi: 10.1016/j.pharmthera.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neubauer H, et al. Membrane-initiated effects of progesterone on proliferation and activation of VEGF in breast cancer cells. Climacteric. 2009;12:230–239. doi: 10.1080/13697130802635637. [DOI] [PubMed] [Google Scholar]
- 27.Intlekofer KA, Petersen SL. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience. 2011;172:55–65. doi: 10.1016/j.neuroscience.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suchanek M, Radzikowska A, Thiele C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat Methods. 2005;2:261–267. doi: 10.1038/nmeth752. [DOI] [PubMed] [Google Scholar]
- 29.Szczesna-Skorupa E, Kemper B. Progesterone receptor membrane component 1 inhibits the activity of drug-metabolizing cytochromes P450 and binds to cytochrome P450 reductase. Mol Pharmacol. 2011;79:340–350. doi: 10.1124/mol.110.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T, et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 31.Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone's antiapoptotic action. Endocrinology. 2006;147:3133–3140. doi: 10.1210/en.2006-0114. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed IS, Rohe HJ, Twist KE, Craven RJ. Pgrmc1 (progesterone receptor membrane component 1) associates with EGFR and regulates erlotinib sensitivity. J Biol Chem. 2010;285:24775–24782. doi: 10.1074/jbc.M110.134585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed IS, Rohe HJ, Twist KE, Mattingly MN, Craven RJ. Progesterone receptor membrane component 1 (Pgrmc1): a heme-1 domain protein that promotes tumorigenesis and is inhibited by a small molecule. J Pharmacol Exp Ther. 2010;333:564–573. doi: 10.1124/jpet.109.164210. [DOI] [PubMed] [Google Scholar]
- 34.Chen JI, et al. Proteomic characterization of midproliferative and midsecretory human endometrium. J Proteome Res. 2009;8:2032–2044. doi: 10.1021/pr801024g. [DOI] [PubMed] [Google Scholar]
- 35.Gebreselassie D, Bowen WD. Sigma-2 receptors are specifically localized to lipid rafts in rat liver membranes. Eur J Pharmacol. 2004;493:19–28. doi: 10.1016/j.ejphar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Vilner BJ, John CS, Bowen WD. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995;55:408–413. [PubMed] [Google Scholar]
- 37.Yoshitani N, et al. A structure-based strategy for discovery of small ligands binding to functionally unknown proteins: combination of in silico screening and surface plasmon resonance measurements. Proteomics. 2005;5:1472–1480. doi: 10.1002/pmic.200401032. [DOI] [PubMed] [Google Scholar]
- 38.Jonhede S, Petersen A, Zetterberg M, Karlsson JO. Acute effects of the sigma-2 receptor agonist siramesine on lysosomal and extra-lysosomal proteolytic systems in lens epithelial cells. Mol Vis. 2010;16:819–827. [PMC free article] [PubMed] [Google Scholar]
- 39.Vangveravong S, Xu J, Zeng C, Mach RH. Synthesis of N-substituted 9-azabicyclo[3.3.1]nonan-3alpha-yl carbamate analogs as sigma2 receptor ligands. Bioorg Med Chem. 2006;14:6988–6997. doi: 10.1016/j.bmc.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 40.Perregaard J, Moltzen EK, Meier E, Sanchez C. Sigma ligands with subnanomolar affinity and preference for the sigma 2 binding site. 1. 3-(omega-aminoalkyl)-1H-indoles. J Med Chem. 1995;38:1998–2008. doi: 10.1021/jm00011a019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.