Abstract
Rationale
The various α subtypes of GABAA receptors have been strongly implicated in alcohol reinforcement and consumption.
Objectives
The effects of the GABAA α1-preferring ligand, 3-propoxy-β-carboline hydrochloride (3-PBC), on seeking and self-administration responses were evaluated in two groups of baboons trained under a 3 component chained schedule of reinforcement (CSR).
Methods
Alcohol (4% w/v; n=5; alcohol group) or a preferred non-alcoholic beverage (n=4; control group) was available for self-administration only in component 3 of the CSR. Responses in component 2 provided indices of motivation to drink (seeking). 3-PBC (1.0 – 30.0 mg/kg) and saline were administered before drinking sessions under both acute and 5-day dosing conditions.
Results
Repeated, and not acute, doses of 3-PBC significantly decreased total self-administration responses (p<0.05), volume consumed (p<0.05) and g/kg of alcohol (p<0.05) in the alcohol group. In the control group, 5-day administration of 3-PBC significantly decreased total self-administration responses (p<0.05) but produced nonsignificant decreases in volume consumed. Within-session pattern of drinking was characterized by a high level of drinking in the first 20 min of the session for both groups, which was significantly (p<0.05) decreased by all doses of 3-PBC (1.0-18.0 mg/kg) only in the alcohol group. In contrast, the first drinking bout in the control group was only reduced at the highest doses of 3-PBC (10.0 and 18.0 mg/kg).
Conclusions
The results support the involvement of the GABAA α1 subtype receptor in alcohol reinforcement and consumption.
Keywords: Alcohol, 3-propoxy-β-carboline hydrochloride, 3-PBC, Self-Administration, Baboon
Introduction
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter and a primary inhibitor of dopamine (DA) neuronal activity in mesolimbic regions (Enoch 2008). The actions of GABA in the central nervous system are mediated by at least two receptors, GABAA and GABAB, which have different distributions in the brain (Chu 1990). The GABA system has been implicated in the maintenance of and relapse to chronic alcohol drinking (for recent reviews see Enoch 2008; Heilig et al. 2011; Lobo and Harris 2008). Alcohol modulates the GABA receptor complex allosterically to open the coupled chloride (CL-) channel and either hyperpolarize cells or potentiate the hyperpolarization produced by GABA (Blair et al. 1998; Koob 2004), subsequently modulating release of DA. GABAA receptors are important therapeutic targets given their involvement in many of the direct behavioral effects of alcohol including motor incoordination, sedation, tolerance, and withdrawal in laboratory animals (for reviews see Davies 2003; Korpi 1994; Nevo and Haman 1995) as well as alcohol reinforcement and consumption (for reviews see Chester and Cunningham 2002; Davies 2003).
GABAA receptors have a pentameric structure: five subunits, which form an ionophore. There are seven classes of subunits of GABAA receptors and multiple isoforms (e.g., α1-6, β1-3, γ1-3, δ, ε, π, θ) (for a review see D'Hulst et al. 2009). Co-expression of the α, β, and γ subunits is required for the formation of a GABAA receptor that has a benzodiazepine (BZ) binding site (Richter et al. 2012); and this basic combination, with variations in subunit isoform, is most prevalent in brain (Olsen and Sieghart 2008). In addition to their primary uses are anxiolytics and sleep aides, BZs are the standard treatment to alleviate alcohol withdrawal symptoms (Amato et al. 2011) which are thought to be due, in part, to a compensatory decrease in GABAergic inhibitory function that occurs after discontinuation of the chronic activation of GABA receptors by alcohol (Malcolm 2003). Activation of GABA/BZ receptor complex seems to play an important role in modulating alcohol reinforcing effects, as evidenced by reduction in alcohol self-administration (under limited access conditions) following acute pretreatment with GABA/BZ antagonists and inverse agonists (Chester and Cunningham 2002; Koob 2004).
GABA/BZ receptors containing α1, α2, α3, or α5 subunits appear to be especially relevant to inherited risks of alcohol. In humans, genetic variations in GABAA α1 and α2 subunits have been associated with alcohol dependence (Ittiwut et al. 2011; Johnson et al. 1992; Lydall et al. 2011) and with differences in the subjective effects of alcohol intoxication (Roh et al., 2010; Uhart et al. 2012) suggesting that these subunits may be particularly important in alcohol abuse and dependence. Rat strains specifically bred to for high alcohol drinking (HAD) and for alcohol preference (P) show elevations of GABAA receptors in the nucleus accumbens (Murphy et al. 2002) and recent studies in these inbred rat lines suggest that the GABAA α1 subunit is involved in modulation of a variety of alcohol-related behaviors including binge drinking (Yang et al. 2011), alcohol reinforcement (Harvey et al. 2002; June et al. 2003), and alcohol-induced loss of righting reflex (Boehm et al. 2006).
Isolation of the precise roles of the specific GABA receptor subtypes is currently being investigated using a series of β-carboline ligands that bind preferentially to the α1 receptor subtype (Yin et al. 2010; Namjoshi et al. 2011). One promising ligand, 3-propoxy-β-carboline hydrochloride (3-PBC), displays 10-fold selectivity for the α1 subtype over the α2 and α3 receptors, as well as over 150-fold selectivity for the α1 subtype over the α5 subtype (Harvey et al. 2002). Further, it shows a higher binding affinity (5.3 nM) than the prototypical -preferring BZ agonist zolpidem (29.6 nM). In behavioral studies, 3-PBC typically displays a GABAA competitive antagonist profile (Gourley et al. 2005; Lelas et al. 2002; Rowlett et al. 2003) while an in vitro study has reported low partial agonist efficacy at recombinant diazepam-sensitive receptors (i.e., BZ receptors containing α1, α2, or α3 subunits; Harvey et al. 2002), leading to a classification as a mixed BZ partial agonist/antagonist (Yin et al. 2010). In P rats, both systemic administration (parenteral, IP) and bilateral microinfusion of 3-PBC in the anterior and medial ventral palladium selectively produced marked reductions in alcohol-maintained responding (Harvey et al. 2002).
GABA/BZ α1-preferring antagonists have been proposed as potential pharmacotherapies for treatment of human alcohol abuse disorders, based largely on data in rodents (Yin et al. 2010). While the studies in rodents are highly informative and provide a basis for the current studies, it is important to recognize that these studies were done in rodent lines selectively bred for alcohol preference and/or high alcohol consumption and genetics is only one factor in alcoholism risk. Chronic alcohol exposure induces compensatory adaptations in the GABA system, including decreases in α1 subunits in rats (Grobin et al. 1998; Ortiz et al. 1995) and nonhuman primate (Floyd et al 2004). Thus, it is important to examine the effects of potential treatment medications in outbred subjects, particularly in nonhuman primates who are closer in phylogenetic origin than rodents and will consume high levels of alcohol daily and over prolonged periods. Self-administration of alcohol over long periods (i.e., years) more closely models the long-term use characteristics of alcohol abuse in humans. The current study augments the data collected in rodents to provide cross-species validation and bridge the translational research gap between rodents and humans.
In the current studies, 3-PBC was administered before sessions consisting of a chained schedule of reinforcement (CSR) composed of distinct, sequential contingencies (“components”), each of which is correlated with a different stimulus (Kaminski et al. 2008; Weerts et al. 2006). The use of the chain schedule allows examination of drug effects on responding in the presence of alcohol-related stimuli that is maintained by conditioned reinforcement (i.e., responding that produces access to alcohol or “seeking”), as well as alcohol self-administration (consumption) within the same session. This study is the first to examine the effects of 3-PC on alcohol seeking behaviors. 3-PBC was administered under acute and repeated administration (5-days). In order to determine the specificity of effects on alcohol-related behaviors, repeated treatment with 3-PBC was also administered to baboons that self-administered a preferred, non-alcoholic beverage.
Methods and Materials
Subjects
Nine singly-housed adult male baboons (Papio anubis; Southwest Foundation for Biomedical Research, San Antonio, TX) weighing 27.2 kg kg (± 4.6 SD) served as subjects. Baboons were housed under conditions previously described (Kaminski et al. 2012). For the alcohol group (N=5), the reinforcer delivered was 4% alcohol w/v. For the control group (N=4), the reinforcer delivered was a preferred non-alcohol beverage (orange-flavored, sugar-free Tang®), diluted to a concentration that functioned as a comparable reinforcer (Duke et al. 2012). All baboons had extensive histories of self-administration of the reinforcer under the CSR. Baboons received standard primate chow (50-73 kcals/kg), fresh fruit or vegetables, and a children's chewable multivitamin daily. Drinking water was available ad libitum except during sessions. Facilities were maintained in accordance with USDA and AAALAC standards. The protocol was approved by the JHU Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (1996).
Apparatus
Each baboon's cage was modified to also function as the experimental chamber (for details, see Weerts et al. 2006) and contained (1) a panel with three colored “cue” lights, (2) an intelligence panel with 2 vertically operated levers, 2 different colored “jewel” lights, (3) a “drinkometer” connected to a calibrated 1000-ml bottle, and (4) a speaker, mounted above the cage, that presented auditory tones. A computer interfaced with Med Associates hardware and software remotely controlled the experimental conditions and data collection.
Drugs
All solutions for oral consumption were mixed using reverse osmosis (RO) purified drinking water. Ethyl alcohol (190 Proof, Pharmco-AAPER, Brookville CT) was diluted with RO water to 4% w/v alcohol. Orange-flavored, sugar-free, Tang® powder (Kraft Foods) was dissolved in RO water as described previously (Kaminski et al. 2012). 3-propoxy-β-carboline hydrochloride (3-PBC) was synthesized in the laboratory of Dr. James Cook (University of Wisconsin-Milwaukee; Yin et al. 2010). Doses of 3-PBC (1.0-32.0 mg/kg) were dissolved in a vehicle of 50% propylene glycol and 50% saline and administered via the intramuscular (IM) route (2-4 mls/injection). 3-PBC vehicle tests were completed using the same volume and procedures as detailed below.
Chained Schedule of Reinforcement (CSR) Procedure
For all sessions, fluids were available only via the drinkometer. The CSR procedures have been described in detail previously (Kaminski et al. 2008; Kaminski et al. 2012) and were identical for the alcohol and control groups. Daily sessions (7 d/week for both groups) were initiated at the same time (8:30 AM) and were signaled by a 3-sec tone. During component 1 (C1), a red cue light was illuminated and all instrumental responses were recorded but had no consequence. After 20 min, C1 was terminated and component 2 (C2) was initiated, as signaled by illumination of the yellow cue light. During the first link of C2 (C2-Link 1), the yellow jewel light over the left lever was continuously illuminated, and a concurrent fixed interval 10 min, fixed time 20 min (FI 10-min FT 20-min) schedule was in effect. In C2-Link 2, the jewel light over the lever flashed and a fixed ratio (FR 10) schedule was in effect on the left lever for transition to Component 3 (C3). If the FR 10 requirement was not completed, the session terminated without transitioning to C3 (i.e., no access to alcohol or the non-alcoholic beverage for the day). C3 began with the illumination of the blue cue light. A blue jewel light over the right lever was also illuminated, and drinks of the alcohol or the non-alcoholic beverage were available under an FR 10 schedule on the right lever followed by contact with the drinkometer spout. Fluid was delivered for the duration of spout contact or for a programmed duration (5 sec), whichever came first. C3 (and the session) ended after 120 min. Previously, we have demonstrated the Tang and alcohol concentrations used in the current study maintained similar breakpoints (i.e., functioned as equivalent reinforcers (see Duke et al 2012).
3-PBC Test Procedures
The CSR baseline criterion was defined as stable self-administration of alcohol or non-alcoholic beverage (i.e., ± 20%) for three consecutive CSR sessions. To evaluate acute effects of 3-PBC on alcohol-related behaviors and to verify the safety of the dose range, in experiment 1, doses of 3-PBC (1.0-30.0 mg/kg) or its vehicle were administered acutely in the alcohol group only. The CSR was established and CSR baseline criterion was met before each test dose of 3-PBC. Doses were tested in mixed order, with active doses tested no more than once per week. In addition, the results of experiment 1 were used to determine a safe dose range for repeated administration. In experiment 2, doses of 3-PBC (1.0-18.0 mg/kg) or vehicle were administered daily for 5 consecutive days to baboons in both groups. Only 4 of the 5 baboons from the alcohol group participated in Experiment 2; one baboon had been removed for health reasons unrelated to the study. For both experiments, doses of 3-PBC were administered 10 min before CSR sessions. For both experiments, stable baseline intake was sometimes disrupted after drug treatments and required additional time to stabilize before testing the next dose (e.g. 2 weeks).
Data Analysis
The grand mean of the 3 days that preceded each test condition for each baboon was used as the baseline (BL) for comparison with vehicle and doses of 3-PBC, except when otherwise noted. In experiment 2, data analyzed were the last 3 of the 5 days of 3-PBC administration. Data were analyzed using separate statistical analysis of variance (ANOVA) for each Group (Alcohol or Control) with 3-PBC dose (BL, 0-30.0 mg/kg) as a repeated measure. Bonferroni's t-tests were used for pair-wise comparisons of BL with vehicle and 3-PBC doses. Total g/kg of alcohol was calculated based on individual body weights and total volume of alcohol consumed. Change in g/kg of alcohol consumed was calculated as test dose (vehicle or 3-PBC) – BL and doses of 3-PBC were compared to vehicle.
Temporal pattern of drinking was analyzed as number of drinks in sequential 20-min bins using two-way repeated measures ANOVA (Time × Dose) for each group given 5 days of 3-PBC dosing (experiment 2). Post hoc Bonferroni pairwise comparisons examined differences between vehicle and doses of 3-PBC.
Results
Stable, reliable drinking was observed in all baboons, in both groups. During criterion baseline (BL) sessions preceding test sessions, baboons in both groups reliably completed the CSR. The number of sessions required to satisfy the BL stability criterion varied. Following drug treatment, baseline intake was sometimes unsystematically disrupted and required 2 weeks or longer to meet the stability criterion. The volume of each drink, within the constraints described above, was under the control of the baboon. Average ml/drink (total volume consumed/number of drinks in the session) was approximately 30 ml/drink and did not vary systematically as a function of 3-PBC administration (data not shown). Few or no responses were recorded on the inactive operanda (all operanda in C1, right lever and drinkometer in C2, left lever in C3).
To determine if there were any differences in BL responding in the alcohol and control groups, BL session responding in experiment 2 was compared for the two groups (BL sessions of the alcohol group in experiment 1 are not included because corresponding control group sessions were not conducted). During BL sessions, systematic differences between the groups were not observed for C1 and C2 measures. In C3, both alcohol and the non-alcoholic beverage maintained self-administration responses (right lever responses; drink contacts) and high intake (ml). During the BL sessions preceding tests, the grand mean (± SEM) alcohol intake was 625.6 (31.2) ml and 1.13 (0.09) g/kg, comparable to intake which has previously been reported to produce blood-alcohol levels (BAL) of >.08% in baboons (Kaminski et al. 2008). The grand mean non-alcoholic beverage intake during BL sessions was 1000.0 (0.0) ml. Despite having previously demonstrated comparable reinforcement of 4% w/v alcohol and the non-alcoholic beverage under BL conditions via a progressive ratio procedure (Duke et al. 2012), volume of intake of the non-alcoholic beverage was greater than volume of alcohol t(4)=24.6, p<.001 under BL conditions.
Experiment 1: Effects of Acutely Administered 3-PBC on Seeking and Self-Administration Under the CSR
Acute administration of 3-PBC did not result in significant changes in any of the measures of seeking (C2-Link 1: FI responses, latency to complete the FI requirement; C2-Link 2: FR responses rate (r/s)) or self-administration (C3: FR responses, volume consumed, g/kg consumed) (Table 1). The data and unsystematic observation by laboratory personnel confirmed that administration of doses up to and including 30.0 mg/kg was safe and did not produce severe adverse effects. The highest dose (30.0 mg/kg) did, however, suppress daily food intake (i.e., technicians reported that a large proportion of daily free food ration was not consumed). As a result, this dose was not tested under 5-day administration conditions (experiment 2). Because 3-PBC did not systematically reduce seeking and self-administration in the alcohol group, it was not tested in the control group under acute administration conditions.
Table 1. Experiment 1.
Effects of acute 3-PBC (10-min pretreatment) on seeking (C2-L1 and C2-L2) and self-administration (C3) responses for alcohol under the CSR. BL is the grand mean of the 3 baseline days preceding each acute administration.
| 3-PBC Dose | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BL | 0.3 | 1.0 | 3.0 | 10.0 | 30.0 | F(5,15) | |||
| C2-L1 | Left Lev FI Resp | Mean | 92.4 | 130.6 | 66.6 | 38.6 | 256.2 | 205.8 | 0.57 |
| SEM | 47.5 | 80.2 | 47.5 | 18.0 | 206.9 | 155.0 | |||
| Left Lev FI Resp Latency (s) | Mean | 647.7 | 621.3 | 648.6 | 613.5 | 639.9 | 670.1 | 0.85 | |
| SEM | 17.9 | 11.3 | 21.7 | 3.4 | 20.9 | 41.9 | |||
| C2-L2 | Left Lev FR Resp Rate (r/s) | Mean | 2.2 | 3.2 | 2.7 | 1.0 | 2.3 | 3.2 | 1.88 |
| SEM | 0.3 | 0.8 | 0.9 | 0.4 | 0.5 | 0.6 | |||
| C3 | Right Lev FR Resp | Mean | 194.4 | 196.0 | 182.0 | 184.0 | 190.0 | 130.0 | 1.48 |
| SEM | 15.1 | 31.7 | 24.8 | 12.1 | 24.9 | 20.3 | |||
| Volume (ml) | Mean | 554.2 | 677.0 | 484.0 | 480.0 | 631.1 | 436.0 | 1.91 | |
| SEM | 61.0 | 147.2 | 57.1 | 60.4 | 83.2 | 79.4 | |||
| g/kg Alc Consumed | Mean | 1.03 | 1.27 | 0.88 | 0.88 | 1.10 | 0.75 | 1.65 | |
| SEM | 0.1 | 0.3 | 0.1 | 0.1 | 0.2 | 0.1 | |||
Data shown are group means (and SEM) for baseline (BL) and each 3-PBC dose (mg/kg). FI, Fixed Interval; FR Fixed Ratio; Lev, Lever; Resp, Response; Alc, Alcohol.
Experiment 2: Effects of Repeated Administration of 3-PBC on Seeking and Self-Administration Under the CSR
In the alcohol group, significant changes in C2 seeking measures (left lever responses) were not observed as a function of repeated administration of 3-PBC (Table 2). However, in the control group, the C2-L2 response rate was significantly decreased as a function of dose, with both 10.0 and 18.0 mg/kg differing significantly from BL. Although the number of C2-L1 FI responses was significantly increased in the control group, because this effect was also observed during vehicle administration; it does not appear to be directly related to 3-PBC effects, but may be related to the procedure for injections per se.
Table 2. Experiment 2.
Effects of repeated administration (5-day) of vehicle (V) and doses of 3-PBC (10-min pretreatment) on seeking (C2-L1, C2-L2) for alcohol (Alcohol Group) and a non-alcohol beverage (Control Group) under the CSR. All data are the mean of days 3-5 of each condition. BL is the grand mean of the 3 baseline days preceding each chronic condition.
| 3-PBC Dose | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Alcohol Group | BL | V | 1.0 | 3.0 | 10.0 | 18.0 | F(5,15) | ||
| C2-L1 | Left Lev FI Resp | Mean | 144.7 | 169.4 | 106.9 | 115.5 | 36.8 | 116.3 | 0.48 |
| SEM | 84.8 | 102.8 | 60.1 | 84.0 | 23.7 | 50.3 | |||
| Left Lev FI Resp Latency (s) | Mean | 630.2 | 606.2 | 695.8 | 712.3 | 697.8 | 739.0 | 1.60 | |
| SEM | 13.7 | 2.1 | 53.4 | 35.0 | 32.3 | 57.6 | |||
| C2-L2 | Left Lev FR Resp Rate (r/s) | Mean | 3.1 | 3.2 | 2.7 | 2.2 | 2.1 | 2.1 | 1.40 |
| SEM | 0.3 | 0.5 | 0.5 | 0.3 | 0.7 | 0.7 | |||
| C3 | Volume (ml) | Mean | 625.6 | 674.8 | 576.7 | 489.6 | 355.4 | 283.3 | 4.86 |
| SEM | 31.2 | 85.1 | 115.0 | 110.6 | 76.5 | 71.2 | |||
| g.kg Alc consumed | Mean | 1.13 | 1.12 | 1.00 | 0.90 | 0.67 | 0.48 | 3.85 | |
| SEM | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | |||
| Control Group | |||||||||
| C2-L1 | Left Lev FI Resp | Mean | 93.4 | 234.5 | 306.7 | 115.4 | 74.2 | 101.3 | 5.30 |
| SEM | 21.5 | 73.8 | 129.1 | 44.3 | 49.4 | 72.6 | |||
| Left Lev FI Resp Latency (s) | Mean | 606.8 | 610.7 | 616.4 | 710.1 | 711.0 | 676.9 | 0.96 | |
| SEM | 0.9 | 2.52 | 12.4 | 56.2 | 87.8 | 70.1 | |||
| C2-L2 | Left Lev FR Resp Rate (r/s) | Mean | 3.1 | 2.9 | 2.5 | 2.1 | 1.5 | 1.7 | 3.78 |
| SEM | 0.3 | 0.4 | 0.2 | 0.2 | 0.3 | 0.4 | |||
| C3 | Volume (ml) | Mean | 1000 | 1000 | 979.2 | 825.0 | 772.9 | 775.0 | 1.84 |
| SEM | 0.0 | 0.0 | 20.8 | 175.0 | 88.2 | 125.2 | |||
Data shown are group means (and SEM) for baseline (BL) and each 3-PBC dose (mg/kg). FI, Fixed Interval; FR, Fixed Ratio; Lev, Lever; Resp, Response; Alc, Alcohol
1An underlined F ratio indicates a significant (p<0.05 ANOVA); bolded numbers indicate a significant p<0.05) Bonferroni's post-hoc test compared to vehicle.
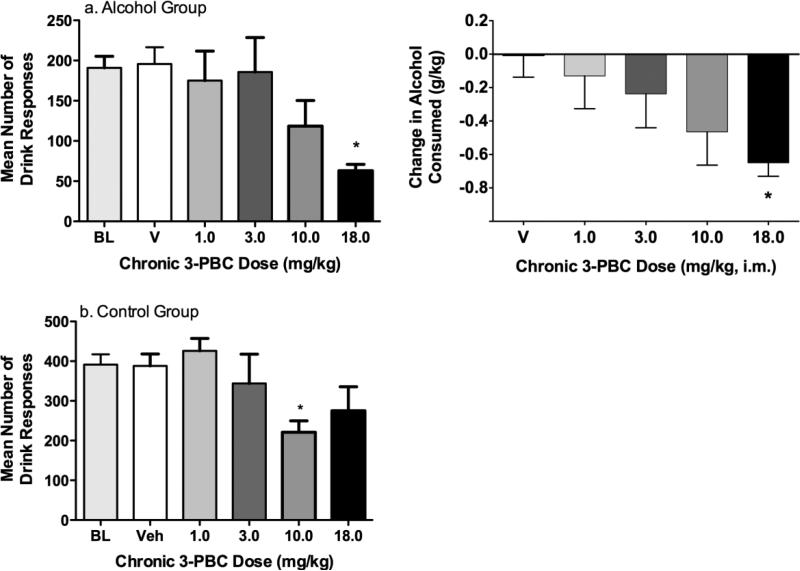
As shown in Fig 1a, in the alcohol group, 3-PBC dose-dependently decreased the number of right lever responses (i.e., self-administration responses) in C3 (F(4,12)=4.98, p<0.05), with a significant decrease relative to BL at 18.0 mg/kg. Both volume of alcohol consumed and g/kg consumed decreased as a function of 3-PBC dose (Table 2). Similarly, change in g/kg alcohol consumed (compared to BL) was significantly decreased as a function of dose (F(4,12)=3.53, p<0.05), with 18.0 mg/kg differing significantly from vehicle (Fig 1b).
Fig. 1.
Experiment 2: The effects of repeated (5 day) administration of 3-PBC on self-administration in C3 of the CSR in (a) the Alcohol Group and (b) the Control Group. Data shown are the group means (± SEM) of right (self-administration) responses (left panels) and for the alcohol group, change in g/kg of alcohol consumed (right panel). * indicates p<0.05 for pairwise comparison for each dose vs. baseline
In the control group, 3-PBC produced a significant decrease in the number of right lever operant responses during C3 (F(4,12)=6.18, p<0.05) with a significant decrease relative to BL at 10 mg/kg (Fig 1a). A nonsignificant decrease in the volume consumed (Table 1) was also obtained in the control group. Individual data showed that 3-PBC decreased volume consumed for all 4 baboons at 10.0 and 18.0 mg/kg, while lower doses (1.0 & 3.0 mg/kg) produced a decrease in only one of the baboons.
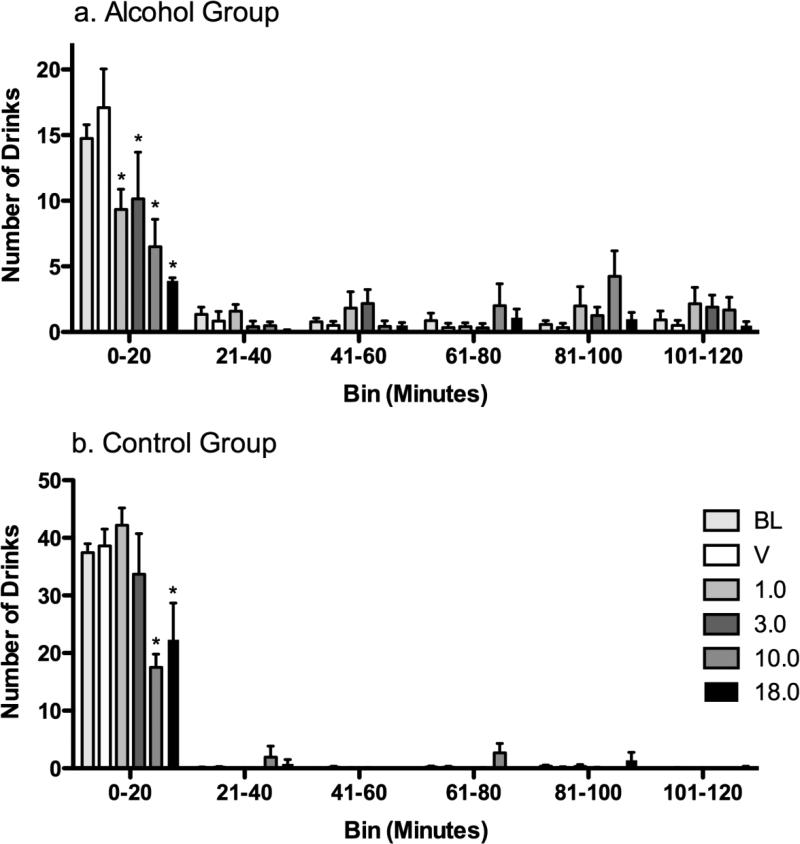
During BL, in both the alcohol and control groups, the majority of drinks occurred in the first 20 min of availability, followed by a lower rate across the subsequent 20-min bins. Despite this general similarity, a greater proportion of drinks occurred during the first 20-min bin in the control group (> 95%) compared to the alcohol group (> 75%) (fig 2). All doses (1.0-18.0 mg/kg) of 3-PBC significantly (p < .05) decreased the number of drinks during the first 20 min in the alcohol group, with larger decreases observed at the higher (10.0 and 18.0 mg/kg) doses. In the control group, only the highest doses (10 and 18.0 mg/kg) significantly decreased drinking during the first 20 min of drinking.
Fig. 2.
Experiment 2: The effects of repeated (5 day) administration of 3-PBC on the pattern and number of drinks per 20-min interval of the 120-min self-administration period (C3) in the (a) Alcohol Group and (b) the Control Group. Data shown are group mean drinks (+ SEM) for each successive time bin of availability of alcohol or the non-alcoholic beverage, and * indicates p<0.05 for pairwise comparison for each 3-PBC dose vs. vehicle within each time bin
Discussion
Targets for therapeutic agents to treat alcohol abuse and dependence include the reduction of alcohol intake and attenuation of the motivation or desire to consume alcohol. In the current model, this would be reflected in decreases in alcohol self-administration responses and g/kg intake (consumption in C3), and disruption of responses directed towards obtaining the opportunity to drink (seeking in C2). Several important findings were identified in the current study. First, repeated (5 day), but not acute, administration of the GABAA α1-preferring ligand 3-PBC reduced ongoing alcohol self-administration in baboons with long-term alcohol self-administration experience. Second, 3-PBC did not disrupt the established pattern of alcohol seeking and self-administration, but reduced the magnitude of intake, particularly during the initial drinking bout. Third, 3-PBC also suppressed responding maintained by a non-alcoholic beverage, albeit at higher doses than required to suppress alcohol. Each of these findings is discussed below.
The finding that 3-PBC decreased alcohol-maintained responding and consumption in primates provides further evidence of a role of α1 GABAA subtype in alcohol abuse and dependence. The involvement of the GABAA receptor in the behavioral actions of alcohol is complex, with a different subtypes differentially involved in the various effects of alcohol. Studies with subtype-preferring compounds and in modified mouse models have shown that several of the subtypes (α1, α2, α3, α5) may have involvement in alcohol reinforcement (Atack 2003; Cook et al. 2005; Stephens et al 2005; for a review see Kumar et al 2008). 3-PBC exhibits binding preference for the GABAA α1 receptor (Cox et al. 1999; Huang et al. 2000). The current findings are consistent with studies in rats selectively bred for high alcohol preference (P) or high alcohol drinking (HAD). Specifically, administration of the α1-preferring ligands 3-PBC or β-carboline-3-carboxlate-t-butylester (βCCt) decreased ongoing alcohol consumption in P rats when administered systemically or via microinfusion into the ventral palladium (Harvey et al 2002; June et al 2002). Taken together with the current findings, the α1 GABAA subtype-preferring ligand 3-PBC reduces alcohol-maintained behaviors and daily alcohol intake in both genetically predisposed animals and outbred animals with long-term drinking experience.
In the present study, high doses of 3-PBC also produced a decrease in self-administration of a palatable non-alcoholic beverage, which suggests that 3-PBC effects may not be specific to alcohol. Although 3-PBC typically displays a BZ antagonist-like profile in most behavioral tasks, in an in vitro analysis 3-PBC exhibited a low partial agonist efficacy at recombinant diazepam-sensitive receptors (Harvey et al 2002). The decreased component 2 FR (C2-L2) response rate in the control group is consistent with a rate-suppressing agonist effect. However responding was not also suppressed during the FI link of C2 in the control group (and, in fact, was increased at lower doses), suggesting that a general rate-decreasing effect cannot account for the non-specific effects obtained.
Non-specific effects have also been reported in other studies. For example, α1 receptor knockout mice consumed less ethanol in a two-bottle choice procedure, but also less saccharin, when compared to wild-type mice (Blednov et al. 2003; June et al. 2007). α1-GABAA receptor knockout mice also showed decreased operant responding for both ethanol and sucrose (June et al. 2007). However, Harvey et al. (2002) reported that only the highest dose IP administered dose (20 mg/kg) of 3-PBC significantly suppressed saccharin maintained responding and did so throughout the drinking period. As 3-PBC does bind to other α-receptor subtypes to some degree, the authors suggested there may be a saturation of all α-receptor subtypes following the highest dose. Similarly, in the present study, acute administration of the highest dose of 3-PBC (30 mg/kg) in the alcohol group disrupted daily food intake and repeated administration of the highest doses (10 and 18 mg/kg) suppressed responding for a highly preferred beverage during the initial 20 minutes of the session in the control group. The highest dose tested under the 5-day dosing conditions in the present study (18 mg/kg) is roughly equivalent (via interspecies conversion, Dews 1976; Mordenti and Chappell 1989) to 78 mg/kg in the rat, a dose that is substantially higher than that evaluated by Harvey et al. (2002). Thus, it is possible that receptor saturation may account for the decrease in non-alcohol beverage in the present study.
This is the first study to examine the effects of 3-PBC on responses to gain access to alcohol (seeking). 3-PBC did not significantly decrease seeking during either C2 link in the alcohol group. Alcohol-related cues maintain seeking behavior even under conditions of alcohol abstinence and are highly resistant to change. For example, studies in rats have shown that stimuli previously paired with alcohol continue to maintain responding for many sessions (e.g., Ciccocioppo et al. 2001; Zironi et al. 2006). Likewise, in Kaminski et al. (2008), presentation of alcohol-related cues in the CSR maintained C2 responding for an extended period (i.e., 30 consecutive sessions) after water was substituted for alcohol in C3 (i.e., during extinction). In addition, when alcohol was available for consumption in C3, high levels of seeking responses were maintained (> 600 responses in C2 Link 2) under conditions in which the response requirement was progressively increased to obtain the daily supply of alcohol (Kaminski et al. 2008). In the alcohol group, then, the strength of C2 seeking responding, which is maintained by transition to C3 where alcohol is available for consumption, appears to have mitigated the C2 (seeking) response suppression observed in the control group. This suggests that 3-PBC suppression of alcohol-maintained responding obtained in C3 is a function of changes in the reinforcing effects of alcohol upon consumption.
Within each daily session, in both the alcohol and control groups, the majority of baseline drinking occurred within the first 20 min of the 2 h of availability. Drinks in the first 20-min was tightly clustered (i.e., a single drinking bout). 3-PBC did not disrupt this pattern of intake, but dose-dependently decreased the number of drinks in this initial drinking bout in the alcohol group. Consistent with the results of Harvey et al. (2002), after the initial suppression of intake, 3-PBC, did not reduce the low levels of alcohol drinking during later portions of the session. As a result, the effects of 3-PBC on self-administration measures for the entire 2-hr drinking period differed from BL only at the highest doses of 3-PBC. The suppression of alcohol intake during the first drinking bout of the CSR, with baseline levels of alcohol intake later in the session, is similar to that previously reported for naltrexone (Kaminski et al. 2012). Naltrexone is one of the current FDA-approved treatments to reduce drinking and promote abstinence in alcohol dependent persons and numerous clinical trials have demonstrated its effectiveness for treatment of alcohol dependence (Johnson 2008). Studies have suggested that naltrexone's clinical effective is due, in part, to preventing drinking episodes from becoming a full-fledged relapse to heavy drinking (Anton et al. 1999; O'Malley and Froehlich 2003; Pettinatti et al. 2006).
There are a number of strengths of the current study that increase the translational value of these findings. First, recent reviews have emphasized the importance of animal models with sufficient alcohol intake to achieve a biologically-relevant blood alcohol level (BAL) of 0.08 mg/dL or more for better medications development (Egli 2005; Grant and Bennett 2003). In the alcohol group, baboons drank approximately 1 g/kg/day of alcohol. Mean BAL of 88.2 mg/dl (>0.08%) were previously determined in these same baboons after comparable alcohol intake (mean volume 0.93 g/kg) (Kaminski et al. 2008). A BAL of 0.08% is defined as intoxication with regard to NIAAA definitions and for driving violations in most of the USA. Second, the current procedure models key elements of human problematic drinking. In humans, drinking to intoxication (e.g., 0.8 to 1 g/kg, BAL > 0.08%) within a single drinking period (binge) and regular drinking at this level across days (heavy drinking) is characterized as problem drinking with higher risk for alcoholism (Rethinking Drinking, NIH). The baboons in the current study had long-term self-administration experience (i.e., years) under the CSR with either alcohol or, for the control group, the non-alcoholic beverage. Thus, based on NIAAA definitions, baboon drinking in the current study models problem drinking in humans. Third, our study is the first to show that a GABAA α1-preferring ligand reduces alcohol self-administration behaviors and g/kg consumption in long-term heavy drinking primates under a CSR. Use of the CSR is novel, as it allows within the same session evaluation of drug effects on responding in the presence of alcohol-related stimuli maintained by conditioned reinforcement, as well as alcohol self-administration (consumption), and thus provides a measure of the motivation to drink. Fourth, the inclusion of the control group allowed the examination of specificity of 3-PBC effects on alcohol-related behaviors.
Previously, we have shown that alcohol seeking behaviors maintained by alcohol-associated cues are highly resistant to change, and are sensitive to duration of abstinence and alcohol availability (Kaminski et al 2006; 2012; Weerts et al 2006). In the present study, 3-PBC did not decrease C2 seeking measures, but did produce time-dependent changes in alcohol self-administration behaviors in C3. As indicated previously, ideal therapeutic agents for alcohol abuse and dependence would reduce alcohol seeking and self-administration in the current model. Thus, the present results suggest that, like naltrexone, GABAA α1-preferring ligands, such as 3-PBC, may have clinical utility in reducing the severity of drinking episodes when they do occur, but are less likely to affect the motivation or desire to consume alcohol. With the recent development of ligands selective for each of the α subtypes, future research can further clarify the role of the GABA receptors in alcohol abuse and dependence.
Acknowledgments
This research was supported by NIH/NIAAA R01AA15971 (Weerts), MH046851 (Cook), and The Lynde and Harry Bradley Foundation (Cook).
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors have no conflicts to disclose.
References
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev. 2011;6:CD008537. doi: 10.1002/14651858.CD008537.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156:1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Atack JR. Anxioselective compounds acting at the GABAA receptor benzodiazepine binding site. Curr Drug Targets CNS Neural Disord. 2003;2:213–232. doi: 10.2174/1568007033482841. [DOI] [PubMed] [Google Scholar]
- Blair LA, Levitan ES, Marshall J, Dionne VE, Barnard EA. Single subunits of the GABAA receptor form ion channels with properties of the native receptor. Science. 1988;242:577–579. doi: 10.1126/science.2845583. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Alva H, Creech K, Findlay G, Harris RA. GABAA receptor alpha 1 and beta 2 subunit null mutant mice: behavioral responses to ethanol. J Pharmacol Exp Ther. 2003;305(3):854–63. doi: 10.1124/jpet.103.049478. [DOI] [PubMed] [Google Scholar]
- Boehm SL, Ponomarev I, Blednov YA, Harris RA. From gene to behavior and back again: new perspectives on GABAA receptor subunit selectivity of alcohol actions. Adv Pharmacol. 2006;54:171–203. doi: 10.1016/s1054-3589(06)54008-6. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. GABA(A) receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26(3):131–43. doi: 10.1016/s0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- Chu DC, Albin RL, Young AB, Penney JB. Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neurosci. 1990;34:341–57. doi: 10.1016/0306-4522(90)90144-s. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Weiss F. Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol Clin Exp Res. 2001;25:1414–1419. doi: 10.1097/00000374-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Cook JB, Foster KL, Eiler WJ, 2nd, McKay PF, Woods J, 2nd, Harvey SC, Garcia M, Grey C, McCane S, et al. Selective GABAA α5 benzodiazepine inverse agonist antagonizes the neurobiological actions of alcohol. Alcohol Clin Exp Res. 2005;29:1390–1401. doi: 10.1097/01.alc.0000175073.94575.86. [DOI] [PubMed] [Google Scholar]
- Cox ED, Diaz-Arauzo H, Huang Q, Reddy MS, Harris B, McKernan RM, Skolnick P, Cook JM. Synthesis and evaluation of analogues of the partial agonist 6-(propyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester (6-PBC) and the full agonist 6-(benzyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester (Zk 93423) at wild type and recombinant GABA(A) receptors. J Med Chem. 1998;41:2537–2552. doi: 10.1021/jm970460b. [DOI] [PubMed] [Google Scholar]
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28(4):263–74. [PMC free article] [PubMed] [Google Scholar]
- Dews PB. Interspecies differences in drug effects: behavioral. In: Usdin E, Forrest IS, editors. Psychotherapeutic Drugs, Part I. Marcel Dekker; New York: 1976. pp. 175–214. [Google Scholar]
- D'Hulst C, Atack JR, Kooy RF. The complexity of the GABAA receptor shapes unique pharmacological profiles. Drug Discover Today. 2009;14:866–875. doi: 10.1016/j.drudis.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Duke AN, Kaminski BJ, Weerts EM. Baclofen effects on alcohol seeking, self-administration and extinction of seeking responses in a within-session design in baboons. Addict Biol. 2012 Mar 28; doi: 10.1111/j.1369-1600.2012.00448.x. doi: 10.1111/j.1369-1600.2012.00448.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90:95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addict Biol. 2005;10:309–10. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Gourley SL, DeBold JF, Yin W, Cook J, Miczek KA. Benzodiazepines and heightened aggressive behavior in rats: reduction by GABAA/α1 receptor antagonists. Psychopharmacol. 2005;178:232–240. doi: 10.1007/s00213-004-1987-3. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–55. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacol. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma CR, Cook JM, June HL. The GABA(A) receptor alpha(1) subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22(9):3765–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12(11):670–84. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, He XH, Ma CR, Liu RY, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM. Pharmacophore/receptor models for GABA(A) /BzR subtypes (α1β3γ2, α5β3γ2, α6β3γ2) via a comprehensive ligand-mapping approach. J Med Chem. 2000;43(1):71–95. doi: 10.1021/jm990341r. [DOI] [PubMed] [Google Scholar]
- Ittiwut C, Yang BZ, Kranzler HR, Anton RF, Hirunsatit R, Weiss RD, et al. GABRG1 and GABRA2 Variation Associated with Alcohol Dependence in African Americans. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01637.x. Epub 2011/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatment for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KJ, Sander T, Hicks AA, van Marle A, Janz D, Mullan MJ, et al. Confirmation of the localization of the human GABAA receptor alpha 1-subunit gene (GABRA1) to distal 5q by linkage analysis. Genomics. 1992;14(3):745–8. doi: 10.1016/s0888-7543(05)80178-8. [DOI] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, et al. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28(12):2124–37. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- June HL, Sr, Foster KL, Eiler WJ, 2nd, Goergen J, Cook JB, Johnson N, Mensah-Zoe B, Simmons JO, June HL, Jr, Yin W, Cook JM, Homanics GE. Dopamine and benzodiazepine-dependent mechanisms regulate the EtOH-enhanced locomotor stimulation in the the GABAA alpha1 subunit null mutant mice. Neuropsychopharm. 2007;32:137–152. doi: 10.1038/sj.npp.1301097. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Goodwin AK, Wand G, Weerts EM. Dissociation of alcohol-seeking and consumption under a chained schedule of oral alcohol reinforcement. Alcohol Clin Exp Res. 2008;32:1–9. doi: 10.1111/j.1530-0277.2008.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Duke AN, Weerts EM. Effects of naltrexone on alcohol drinking patterns and extinction of alcohol seeking in baboons. Psychopharmacology. 2102;223:55–66. doi: 10.1007/s00213-012-2688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER. Role of GABAA receptors in the actions of alcohol and in alcoholism: recent advances. Alcohol Alcohol. 1994;29(2):115–29. [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelas S, Rowlett JK, Spealman RD, Cook JM, Ma CR, Li XY, Yin WY. Role of GABAA/benzodiazepine receptors containing α1 and α5 subunits in the discriminative stimulus effects of triazolam in squirrel monkeys. Psychopharmacology. 2002;161:180–188. doi: 10.1007/s00213-002-1037-y. [DOI] [PubMed] [Google Scholar]
- Lobo IA, Harris RA. GABA(A) receptors and alcohol. Pharmacol Biochem Behav. 2008;90(1):90–4. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydall GJ, Saini J, Ruparelia K, Montagnese S, McQuillin A, Guerrini I, et al. Genetic association study of GABRA2 single nucleotide polymorphisms and electroencephalography in alcohol dependence. Neurosci Lett. 2011;500(3):162–6. doi: 10.1016/j.neulet.2011.05.240. [DOI] [PubMed] [Google Scholar]
- Malcolm RJ. GABA systems, benzodiazepines, and substance dependence. J Clin Psychiatry. 2003;64(Suppl 3):36–40. [PubMed] [Google Scholar]
- Mordenti J, Chappell W. The use of interspecies scaling in toxicokenetics. In: Yacobi A, Kelly J, Batra V, editors. Toxicokenetics and New Drug Development. Pergamon Press; New York: 1989. pp. 42–96. [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32(5):363–88. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Namjoshi OA, Gryboski A, Fonseca GO, Van Linn ML, Wang Z, Deschamps JR, Cook JM. Development of a Two-Step Route to 3-PBC and βCCt, Two Agents Active against Alcohol Self-Administration in Rodent and Primate Models. J Org Chem. 2011;76:4721–4727. doi: 10.1021/jo200425m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int. 1995;26(4):305–36. doi: 10.1016/0197-0186(94)00139-l. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56(1):141–8. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley SS, Froehlich JC. Advances in the use of naltrexone: an integration of preclinical and clinical findings. Recent Dev Alcohol. 2003;16:217–245. [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, et al. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 2001;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O'Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Richter L, de Graaf C, Sieghart W, Varagic Z, Mörzinger M, de Esch IJP, Ecker GF, Ernst M. Diazepam-bound GABAA receptor models identify new benzodiazepine binding-site ligands. Chem Biol. 2012 Mar 25; doi: 10.1038/nchembio.917. 2012, doi 10.1038 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh S, Matsushita S, Hara S, Maesato H, Matsui T, Suzuki G, et al. Role of GABRA2 in moderating subjective responses to alcohol. Alcohol Clin Exp Res. 2011;35(3):400–7. doi: 10.1111/j.1530-0277.2010.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Spealman RD, Lelas S, Cook JM, Yin W. Discriminative stimulus effects of zolpidem in squirrel monkeys: role of GABAA/α1 receptors. Psychopharmacology. 2003;165:209–215. doi: 10.1007/s00213-002-1275-z. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Cook JM, Duke AN, Platt DM. Selective antagonism of GABAA receptor subtypes: An in vivo approach to exploring the therapeutic and side-effects of benzodiapine-type drugs. CNS Spectrums. 2005;10:40–48. doi: 10.1017/s1092852900009895. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Guzman F, Cook JM. β-Carboline-3-carboxylate-t-butyl ester: a selective BZ1 benzodiazepine receptor antagonist. Life Sci. 1984;35:2227–2236. doi: 10.1016/0024-3205(84)90464-8. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Pistocakova J, Worthing L, Atack JR, Dawson GR. Role of GABAA alpha5-containing receptors in ethanol reward: the effects of targeted gene deletion, and a selective inverse agonist. Eur J Pharmacol. 2005;5:240–250. doi: 10.1016/j.ejphar.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Uhart M, Weerts EM, McCaul ME, Guo X, Yan X, Kranzler HR, Li N, Wand GS. GABRA2 markers moderate the subjective effects of alcohol. Addict Biol. 2012 Apr 13; doi: 10.1111/j.1369-1600.2012.00457.x. doi: 10.1111/j.1369-1600.2012.00457.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. Environmental cues, alcohol seeking, and consumption in baboons: effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res. 2006;30:2026–2036. doi: 10.1111/j.1530-0277.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Yang AR, Liu J, Yi HS, Warnock KT, Wang M, June HL, Jr, Puche AC, Elnabawi A, Sieghart W, Aurelian L, June HL., Sr Binge Drinking: In Search of its Molecular Target via the GABA(A) Receptor. Front Neurosci. 2011;5:123. doi: 10.3389/fnins.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Majumder S, Clayton T, Petrou S, VanLinn ML, Namjoshi OA, et al. Design, synthesis, and subtype selectivity of 3,6-disubstituted beta-carbolines at Bz/GABA(A)ergic receptors. SAR and studies directed toward agents for treatment of alcohol abuse. Bioorg Med Chem. 2010;8(21):7548–64. doi: 10.1016/j.bmc.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH. Context is a trigger for relapse to alcohol. Behav Brain Res. 2006;167:150–155. doi: 10.1016/j.bbr.2005.09.007. [DOI] [PubMed] [Google Scholar]