Abstract
OBJECTIVE
To test whether cross-sectional or longitudinal measures of thigh muscle isometric strength differ between knees with and without subsequent radiographic progression of knee osteoarthritis (KOA), with particular focus on pre-osteoarthritic female knees (knees with risk factors but without definite radiographic KOA).
METHODS
Of 4796 Osteoarthritis Initiative participants, 2835 knees with Kellgren Lawrence grade (KLG) 0–3 had central X-ray readings, annual quantitative joint space width (JSW) and isometric muscle strength measurements (Good strength chair). Separate slope ANCOVA models were used to determine differences in strength between “progressor” and “non- progressor” knees, after adjusting for age, body mass index, and pain.
RESULTS
466 participant knees exceeded the smallest detectable JSW change during each of two observation intervals (year 2→4 and year 1→3) and were classified as progressors (213 women, 253 men; 128 KLG0/1, 330 KLG2/3); 946 participant knees did not exceed this threshold in either interval and were classified as non-progressors (588 women, 358 from men; 288KLG0/1, 658KLG2/3). Female progressor knees, including those with KLG0/1, tended to have lower extensor and flexor strength at year2 and at baseline than those without progression, but the difference was not significant after adjusting for confounders. No significant difference was observed in longitudinal change of muscle strength (baseline→year2) prior to radiographic progression. No significant differences were found for muscle strength in men, and none for change in strength concomitant with progression.
CONCLUSION
This study provides no strong evidence that (changes in) isometric muscle strength precedes or is associated with structural (radiographic) progression of KOA.
Keywords: Knee Osteoarthritis, Muscle strength, Radiography, Progression, Structure Modification
INTRODUCTION
Knee OA (KOA) causes severe functional limitations and reductions in the quality of life1 and has substantial impact on medical care expenditures 2. Biomechanical factors and excessive joint loading are known to play an important role in the onset and progression of KOA 3–5. Loss of thigh muscle strength, particularly the quadriceps, may adversely affect knee joint loading and biomechanics 6–13 and is an important contributor to knee pain and functional disability 14,15. Therefore, muscle (particularly quadriceps) strengthening has been recommended for the clinical management and treatment and potential prevention of knee OA 16–18. However, it is controversial whether muscle strengthening exercise has the potential to modify structural progression in KOA 13,18,19. It has been suggested that adequate quadriceps muscle strength may protect against incident symptomatic KOA, but not against incident radiographic KOA 13,20. Further, conflicting evidence exists, as to whether quadriceps strength is less in KOA patients with (radiographic) progression compared with those without progression 21–25. Thorstensson et al. 23 observed a relationship between reduced quadriceps strength and the onset of radiographic OA in pre-osteoarthritic knees (knees with risk factors for, but without established KOA at baseline), but not with worsening of the KL grade in those with established (definite radiographic) KOA at baseline. Other studies in cohorts with OA risk factors (but predominantly without definite radiographic KOA) also reported a relationship between muscle weakness and structural KOA progression, i.e. with femoral cartilage loss 26 and worsening of the femoro-tibial joint space narrowing (JSN) grade 25. The latter finding was specific to women and was not evident in men 25. Hence, it has been proposed that muscle strength may be a modifiable risk factor of KOA progression in women, but not in men, and that this may apply primarily to pre- osteoarthritic knees (Kellgren Lawrence grade [KLG] 0–1), but not to those with definite radiographic KOA (KLG≥2).
In the current study, we used data from the Osteoarthritis Initiative (OAI) cohort, in which isometric measurement of thigh muscle strength and quantitative measurement of knee joint space width (JSW) were obtained from fixed flexion radiographs 27, to test the following primary hypotheses:
Thigh isometric muscle strength is less in women (but not in men) with subsequent radiographic progression of KOA than in those without radiographic progression.
Differences in thigh isometric muscle strength between progressor vs. non-progressor knees are greater in female pre-osteoarthritic knees compared to female knees with definite radiographic KOA.
Secondary hypotheses were:
Longitudinal reduction in thigh isometric muscle strength during an interval preceding radiographic progression of KOA is greater in women (but not in men) with radiographic progression than in those without.
Differences in longitudinal reduction in thigh isometric muscle strength between progressor vs. non-progressor knees are greater in female pre-osteoarthritic knees compared to those with definite radiographic KOA.
On an exploratory basis, we also studied
whether cross-sectional differences or longitudinal reductions in thigh isometric muscle strength are stronger in pre-osteoarthritic knees of men compared to those with definite radiographic KOA,
whether (cross-sectional) differences between progressor and non-progressor knees can be identified two years early to the period of radiographic progression, and
whether longitudinal changes in thigh isometric muscle strength occur concomitant to the interval of progression.
To our knowledge, this is the first study to analyze longitudinal changes of isometric muscle strength during an interval before that of radiographic (structural) progression. This aspect is important, because in the longitudinal analysis every participant serves as his/her own control, and because a potential relationship between longitudinal change and subsequent structural progression would be more suggestive of potential benefits in modifying strength to reduce subsequent progression.
METHODS
The Osteoarthritis Initiative (OAI)
Clinical and imaging data were obtained from the Osteoarthritis Initiative (OAI), an ongoing multi-center longitudinal cohort study (http://www.oai.ucsf.edu/), designed to identify biomarkers of the onset and/or progression of knee osteoarthritis 28. The 4796 OAI participants were 45–79 years old (Table 1), with or at risk of symptomatic knee OA in at least one knee 28. Both knees were studied using fixed flexion radiography at baseline, one (Y1), two (Y2), three (Y3), and four year (Y4) follow-up; measures of muscle strength were obtained at baseline, Y2, and Y4 in a majority of participants.
Table 1.
Demographic data determined at year 2 follow-up, in participants with knees with and without radiographic progression (i.e. change in medial radiographic joint space width)
| Progressors | Non-progressors | Difference | P- | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | [95% CI] | value | |
| Women | n=213 | n=588 | |||||
| Age | 62.7 | 8.3 | 62.4 | 9.0 | 0.31 | [−1.07, 1.69] | 0.65 |
| BMI | 30 | 5.4 | 29.1 | 5.0 | 0.96 | [0.16, 1.77] | 0.025* |
| WOMACp | 3.3 | 3.9 | 2.3 | 2.9 | 1.04 | [0.54, 1.54] | 0.0004* |
| Men | n=253 | n=358 | |||||
| Age | 61.5 | 9.4 | 61.2 | 9.0 | 0.24 | [−1.24, 1.72] | 0.75 |
| BMI | 29.5 | 4.0 | 28.9 | 3.9 | 0.61 | [−0.03, 1.25] | 0.06 |
| WOMACP | 2.2 | 2.8 | 1.7 | 2.2 | 0.43 | [0.03. 0.82] | 0.036* |
SD = standard deviation, Diff = observed difference, CI = confidence interval for difference, BMI = body mass index, WOMACp = Western Ontario and McMaster Universities pain score [0–20]
Study design and sample selection
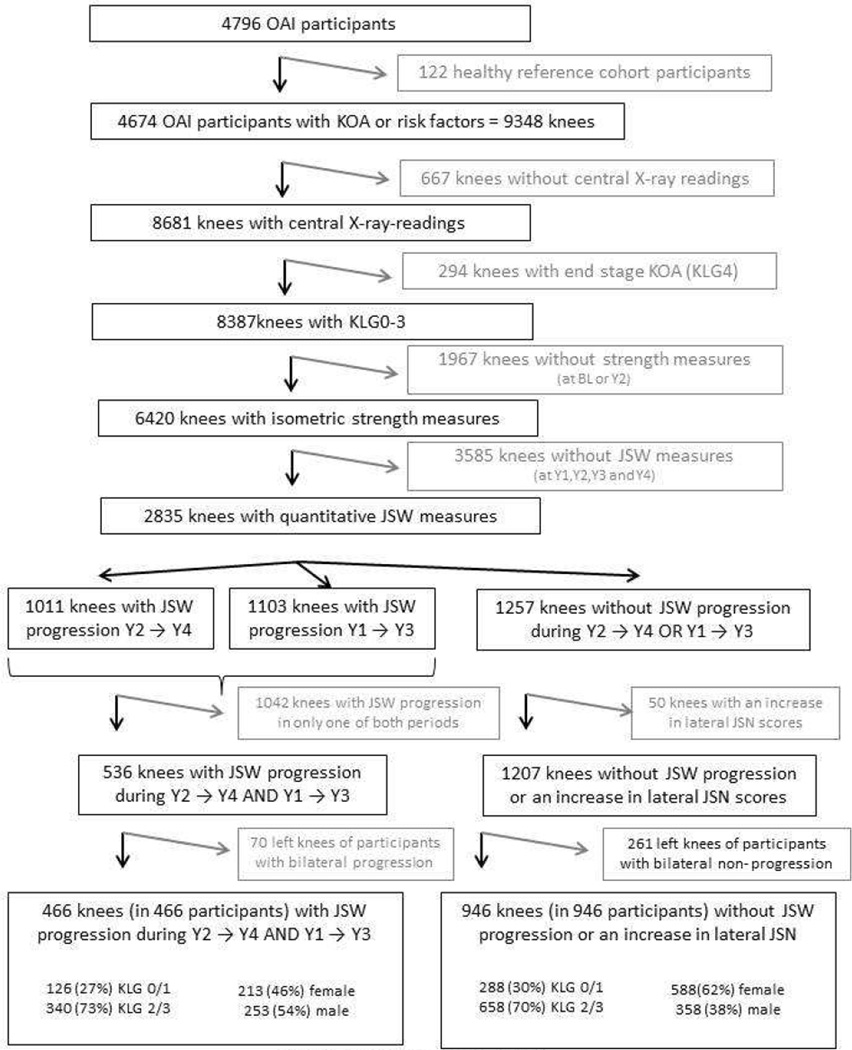
For this prospective, longitudinal case-control study, knees were selected as following (Fig. 1):
-
-
From the 4796 OAI participants, we excluded 122 healthy reference subjects without risk factors of KOA 28,29
-
-
Of these 4674 subjects, 1396 were from the progression subcohort and had both frequent symptoms (most days of the month within at least one of the past 12 months) and radiographic KOA (cKLG ≥2 in the site readings) in at least one knee 28. The remaining subjects were from the incidence subcohort and had either frequent symptoms or radiographic KOA (but not both), or neither frequent symptoms nor radiographic KOA, but risk factors of incident KOA28.
-
-
Of the 9348 knees of these 4674 participants, 8681 had central radiographic readings (from expert readers at Boston University) at baseline for radiographic classification 28. Please note that only knees with at least one follow-up visit and only knees with acceptable positioning, centering, tibial alignment, and radiographic exposure received central X-ray readings.
-
-
Of these 8681 knees, we excluded 294 with end stage radiographic KOA (KLG4) at baseline, because of a lack of a dynamic window for radiographic progression in subsequent time intervals.
-
-
Of the remaining 8387 knees (KLG0–3), 6420 (77%) had measurements of isometric extensor and flexor strength at baseline (BL) and at Y2 (Fig. 1). Please note that some measurements were lacking due to equipment issues and that subjects who recently had knee replacement surgery or were not (physically) able to complete the measurement also were not included. Further, in 367 subjects, strength measurements were taken at year 1 and 3 instead of baseline and year 2, because no valid measurement was obtained at baseline (or because the participants missed the baseline strength test), and the measurement therefore had to be repeated at the next visit.
-
-
Of the 6420 KLG 0–3 knees with central radiographic readings and isometric strength measurement (at baseline and year 2 follow-up), 3585 (56%) did not have complete data on JSW at Y1,Y2,Y3, and Y4 to determine/confirm radiographic progression (Fig. 1 and see below). Of these 3585 knees, 2720 did not get any measurement (due to limited funding), and 865 were drop outs (i.e. had some, but not all measurements). Of the 3585 knees, 439 were from the progression subcohort (16% of this subcohort) and 3146 were from the incidence cohort (48% of this subcohort).
-
-
To select progressor knees, we identified those with a reduction in the medial minimum JSW (mJSW) in fixed flexion radiographs 27 during Y2→Y4 that was larger than the smallest detectable change (SDC 30 = −328 µm). This threshold was determined based on reliability data from the OAI obtained by measuring the same images twice (kXR_quantJSW reliability_Duryea 0.1 and 1.1). Because these reliability data did not account for knee repositioning, we confirmed appropriate progressor classification by ascertaining that these knees also exceeded the SDC threshold during Y1→Y3. We felt this was crucial, because inconsistent positioning of knee relative to the film may result in differences in tibial rim distance and /or beam angle and may lead to false positive observations of progression in knees without change 31. However, the likelihood for a knee to be inconsistently positioned to falsely indicate progression during two independent intervals is much smaller..
-
-
To select controls without radiographic progression, we identified knees that did not show a reduction in mJSW larger than the SDC 27 during Y2→Y4 or during Y1→Y3. To exclude knees with potential progression in the lateral femoro-tibial compartment, we further excluded those with an increase in JSN grade (central radiographic readings) during Y2→Y4. JSN grade was used because of lack of validation of quantitative JSW measures in the lateral femoro-tibial compartment 32.
Figure 1.
Schematic of the selection process of knees with and without radiographic progression:
X-ray = radiography, KOA = knee osteoarthritis, KLG = Kellgren and Lawrence grade; BL = baseline, Y1 = 1 year follow-up, Y2 = 2 year follow-up, Y3 = 3 year follow-up, Y4 = 4 year follow-up, JSW = medial minimum joint space width measured with fixed flexion radiography, JSN = joint space narrowing.
Measurement of Isometric Muscle strength
Isometric muscle strength was measured using the “Good Strength Chair” (Metitur Oy, Jyvaskyla, Finland) 33–35 [http://www.oai.ucsf.edu/datarelease/forms.asp; release 0.2.2. and 3.2.1.]). After two warm-up trials with 50% effort, the maximal isometric extensor and flexor force (N) was measured with the knee at an angle of 60°. The maximum of three measurements was used, for which satisfactory reliability has been reported 36.
Statistical analyses
For cross sectional comparisons, we used both isometric “strength” and isometric “strength normalized to body weight” 37,38. Comparison between knees with and without progression was performed using two-sided, unpaired Welch’s t-tests first, separately for male/female, and KLG 0/1 and 2/3 knees. Next, we used a separate slope ANCOVA model adjusting for age and body mass index (BMI). Because we have recently shown that knees with frequent knee pain demonstrate lower quadriceps strength than contra-lateral knees without knee pain with the same radiographic (KLG) stage 35, and because knees with frequent pain at baseline are known to exhibit greater rates of cartilage loss than those without pain 39, our analyses were also adjusted for WOMAC pain as a confounder. Data were visually inspected for normality by using probability plots. In case of critical results, the Kolmogoroff-Smirnov test was used to check whether data were normally distributed. The homogeneity of variances was tested by using the tests of Hartley and Bartlett 40. 95% confidence intervals (CIs) based on unadjusted means were computed for the differences between progressor and non-progressor knees, and a p-value <0.05 was selected to indicate statistical significance. These computations were performed using STATISTICA 10.0 41. An additional (confirmatory) logistic regression analysis is shown in Online appendix I.
RESULTS
Demographics
The baseline demographic data of the 1790 OAI participants, of whom 2835 KLG0–3 knees (Fig. 1) had complete data on isometric muscle strength and mJSW from Y1 through Y4 (56% women; age 62.2±8.9 y; BMI 29±4.6 kg/m2; WOMAC pain score 2.3±3.2) was similar to that of the OAI (excluding the healthy reference cohort and KLG4 knees): 58% women (57%/59% progression/incidence subcohort), age 61.3±9.2y (61.4±9.1/61.3±9.2); BMI 29±4.8 kg/m2 (30±4.928±4.6); WOMAC pain score 2.4±3.3 (4.1±4.0/ 1.7±2.7). Of these, 1257 knees (in 946 participants) neither showed mJSW progression during Y2→Y4 nor during Y1→Y, and of these 1207 did not show an increase in lateral JSN scores during Y2→Y4 (Fig 1). After excluding left knees in subjects in whom both knees were not progressing (n=261), 946 non-progressor knees (of 946 participants) were available as controls: 152 (16%) were KLG0, 136 (14%) KLG1, 481 (51%) KLG2, and 177 (19%) KLG3 (Fig. 1). 536 knees (of 466 participants) showed progression during both Y2→Y4 and Y1→Y3. After excluding left knees in subjects in whom both knees were progressing (n=70), 466 progressor knees (of 466 participants) were available as cases: 57 (12%) were KLG0, 69 (15%) KLG1, 210 (45%) KLG2, and 130 (28%) KLG3 (Fig. 1).
Women with radiographic progression had a similar age to those without progression, but were heavier and had stronger knee pain than women without progression (Table 1). 27% of the female progressor knees were baseline KLG0/1, and 73% KLG2/3. Of the non-progressor knees 27% were KLG0/1 and 73% KLG2/3. Men with radiographic progression also had a similar age compared with non-progressor knees, were somewhat heavier, and had stronger knee pain non-progressors (Table 1). 27% of the male progressor knees were KLG0/1 and 73% KLG2/3; 36% of the non-progressor knees were KLG0/1 and 64% KLG2/3.
Cross sectional (primary) analysis of isometric muscle strength prior to radiographic progression
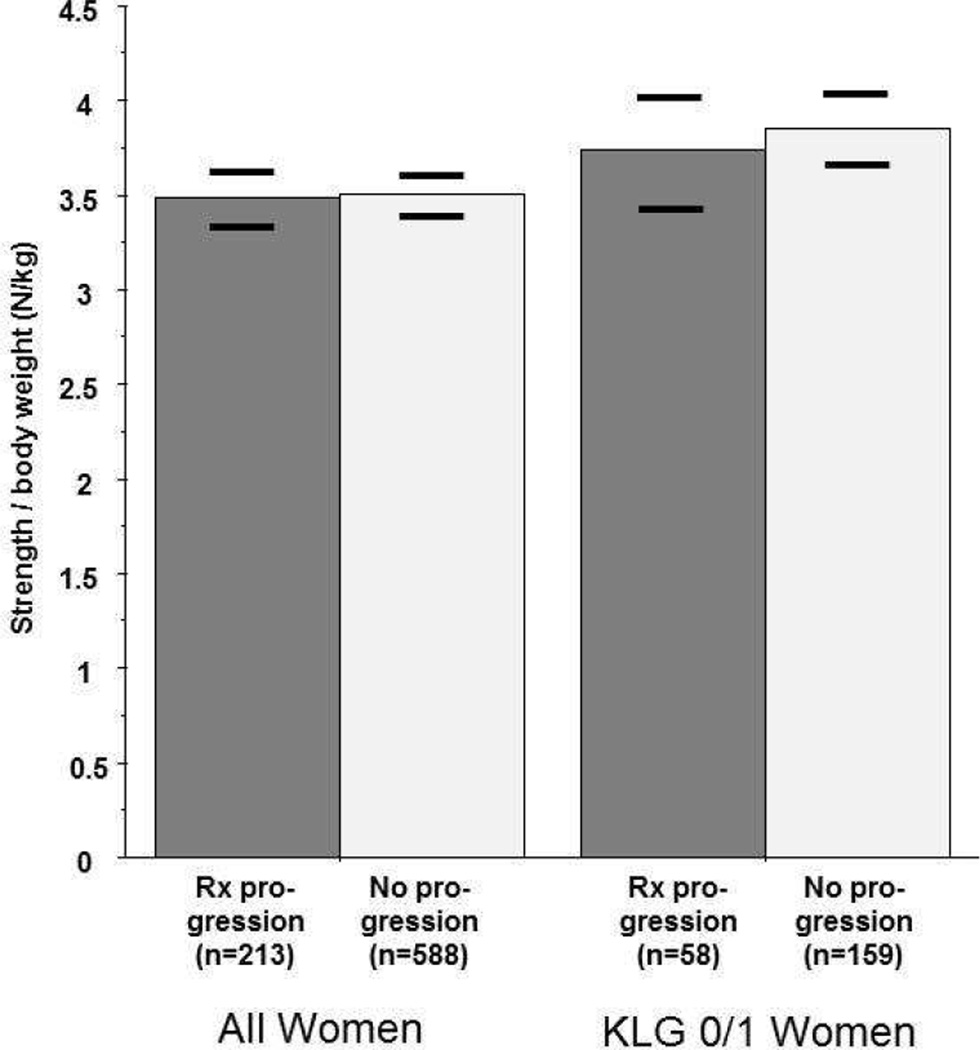
Although female knees with progression showed slightly lower extensor strength (at Y2) than those without progression, this difference (−7.8N [95% CI: −20.6/+5.0N]) was not significant before or after adjustment for age, BMI and WOMAC pain scores (Table 2). The difference was greater and reached statistical significance when strength was individually normalized to body weight (−0.2N/kg [95% CI: −0.39/−0.01N/kg], p=0.038). However, this relationship was attenuated when adjusting for WOMAC pain (p=0.09), age (p=0.66), or BMI (p=0.46), and no difference was observed when adjusting for all three covariates (p=0.38; Fig. 2). Female progressor knees also showed lower flexor strength (per weight) than those without progression, but the difference failed to remain significant after adjustment for WOMAC pain, age, or BMI (Table 2). In female KLG0/1 knees, the difference between progressor and non-progressor knees was somewhat stronger for extensor strength (−19.5N (95% CI −43.3/+4.3N) and flexor strength (−11.7N; 95% CI 23.8/+0.4N) than in female KLG 2/3 knees (Table 2), but it was not statistically significant (Table 2; Fig. 2). These results were confirmed by logistic regression analysis that treated progression as the outcome and isometric muscle strength as the predictor (Online appendix I).
Table 2. Cross-sectional results in women:
Thigh muscle strength in knees with and without radiographic progression (i.e. change in minimum medial radiographic joint space width [JSW] between year2 and 4 follow-up).
| Time | Progressor | Non-progr. | Difference | P1 | P2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | [95% CI] | ||||
| All women | (N=213) | (N=588) | |||||||
| E Max | Y2 | 261.3 | 80.9 | 269.1 | 80.9 | −7.8 | [−20.6, +5.0] | 0.24 | 0.68 |
| F Max | Y2 | 98.8 | 42.1 | 105.8 | 42.1 | −6.9 | [−13.4, −0.5] | 0.029* | 0.25 |
| E Norm | Y2 | 3.41 | 1.23 | 3.61 | 1.23 | −0.2 | [−0.39, −0.01] | 0.038* | 0.38 |
| F Norm | Y2 | 1.30 | 0.61 | 1.42 | 0.61 | −0.12 | [−0.22, −0.03] | 0.007* | 0.13 |
| KLG 0/1 women | (N=58) | (N=159) | |||||||
| E Max | Y2 | 267.7 | 83.9 | 287.2 | 76.7 | −19.5 | [−43.3+4.3] | 0.12 | 0.90 |
| F Max | Y2 | 102.3 | 35.0 | 114.1 | 41.7 | −11.7 | [−23.8, +0.4] | 0.041* | 0.93 |
| E Norm | Y2 | 3.64 | 1.29 | 3.97 | 1.22 | −0.32 | [−0.7, +0.05] | 0.10 | 0.57 |
| F Norm | Y2 | 1.40 | 0.56 | 1.57 | 0.62 | −0.17 | [−0.36, +0.01] | 0.053 | 0.72 |
| KLG 2/3 women | (N=155) | (N=429) | |||||||
| E Max | Y2 | 258.9 | 82.8 | 262.4 | 81.5 | −3.5 | [−18.5,+11.6] | 0.65 | 0.85 |
| F Max | Y2 | 97.5 | 39.9 | 102.7 | 41.8 | −5.2 | [−12.8, +2.4] | 0.17 | 0.23 |
| E Norm | Y2 | 3.32 | 1.15 | 3.48 | 1.21 | −0.16 | [−0.38, +0.06] | 0.16 | 0.62 |
| F Norm | Y2 | 1.26 | 0.55 | 1.36 | 0.6 | −0.1 | [−0.21, 0.00] | 0.049* | 0.13 |
| All women | (N=213) | (N=588) | |||||||
| E Max | BL | 282.3 | 91.1 | 285 | 91.4 | −2.65 | [−17.0,+11.7] | 0.72 | 0.71 |
| F Max | BL | 111.6 | 44.8 | 115.1 | 44.9 | −3.57 | [−10.6, +3.48] | 0.32 | 0.22 |
| E Norm | BL | 3.67 | 1.28 | 3.81 | 1.35 | −0.15 | [−0.35, +0.06] | 0.16 | 0.69 |
| F Norm | BL | 1.46 | 0.65 | 1.54 | 0.65 | −0.08 | [−0.18.+0.02] | 0.12 | 0.17 |
SD = standard deviation; diff = difference, CI = 95% confidence interval for difference of unadjusted means; E max = maximum isometric extensor force determined with the ,,Good strength chair“; E max = maximum (isometric) extensor force or strength (unit = N = newton); norm = force divided by individual body weight, F = maximum (isometric) flexor force; Y2 = measurement at year2 follow-up, BL = measurement at baseline;
p-value1 = significance level of unadjusted difference,
p-value2 = significance level of difference after adjusting for age, body mass index, and WOMAC pain scores, E = extensor, max = maximal isometric force; F = flexor, Norm = force divided by individual body weight;
statistically significant at p<0.05 (without adjustment for multiple comparisons)
Figure 2.
Adjusted means of year 2 extensor isometric muscle strength (normalized to the individual body weight) in knees with radiographic progression (between year 2 and year 4 [and between year 1 and year 3]), and in knees without radiographic progression (between year 2 and year 4 [or between year 1 and year 3]). The horizontal bars show the lower and upper 95% confidence interval of the adjusted means, respectively.
Rx = radiography, KLG = Kellgren and Lawrence grade.
Longitudinal (secondary) analysis of isometric muscle strength prior to radiographic progression
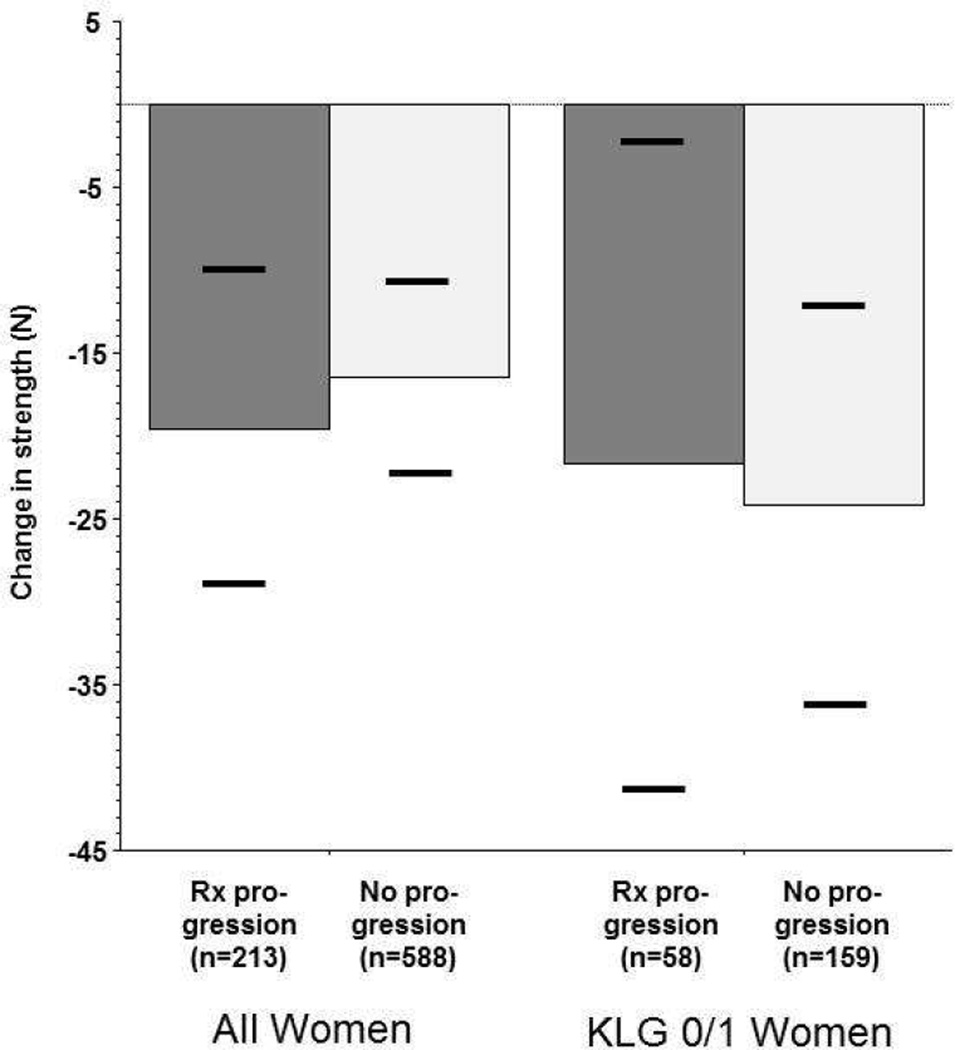
The observed longitudinal loss of extensor muscle strength in the interval prior to radiographic progression (BL→Y2) was greater in female knees with radiographic progression than in those without (−21.0 vs. −15.9N). However, the variability of change between subjects was large and the difference (−5.15N; 95% CI: −16.1/+5.8N) did not reach statistical significance before or after adjusting for confounders (Table 3, Fig. 3). The observed loss of flexor strength also was greater in female knees with progression than in those without, but again the difference was not significant (Table 3). These observations also applied to KLG 0/1 and KLG 2/3) strata (Table 3; Fig. 3). Again, these results were confirmed by logistic regression analysis (Online appendix I).
Table 3. Longitudinal results in women:
Longitudinal thigh muscle strength change in knees with and without radiographic progression (i.e. change in minimum medial radiographic joint space width [JSW] between year 2 and 4 follow-up).
| Time | Progressor | Non-progr. | Difference | P1 | P2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | [95% CI] | ||||
| All women | (N=213) | (N=588) | |||||||
| E Max | BL→Y2 | −21.04 | 67.26 | −15.9 | 70.73 | −5.15 | [−16.1,+5.8] | 0.35 | 0.99 |
| F Max | BL→Y2 | −12.72 | 34.94 | −9.35 | 34.95 | −3.37 | [−8.9,+2.1] | 0.23 | 0.83 |
| KLG 0/1 women | (N=58) | (N=159) | |||||||
| E Max | BL→Y2 | −23.5 | 73.5 | −22 | 74.1 | −1.5 | [−23.9, +20.9] | 0.90 | 0.30 |
| F Max | BL→Y2 | −14.8 | 34.6 | −10.5 | 33.3 | −4.3 | [−14.5,+5.8] | 0.41 | 0.83 |
| KLG 2/3 women | (N=155) | (N=429) | |||||||
| E max | BL→Y2 | −20.1 | 65.0 | −13.6 | 69.4 | −6.5 | [−19.1,+6.1] | 0.30 | 0.60 |
| F max | BL→Y2 | −11.93 | 35.14 | −8.92 | 35.58 | −3.01 | [−9.5, +3.5] | 0.36 | 0.69 |
| All women | (N=181) | (N=520) | |||||||
| E Max | Y2→Y4 | 1.03 | 68.48 | −6.66 | 56 | 7.69 | [−2.4,+17.8] | 0.18 | 0.92 |
| F Max | Y2→Y4 | −1.62 | 29.30 | −4.52 | 28.19 | 2.89 | [−1.9, +7.7] | 0.25 | 0.66 |
Progr. = progressor; Y4 = year 4 follow up; for other abbreviations, please seeTable 2
Figure 3.
Adjusted means of change in extensor isometric muscle strength during a time period (baseline to year2 follow-up) preceeding that of radiographic progression or non-progression (year2 to year4 [and year1 to year3]. The horizontal bars show the lower and upper 95% confidence interval of the adjusted means, respectively.
Rx = radiography, KLG = Kellgren and Lawrence grade.
Exploratory comparisons
The observed extensor and flexor isometric strength at Y2 tended to be greater in men with radiographic progression than in those without, particularly in those with KLG2/3 (Supplement Table I), but the difference was not significant. After normalization to body weight, extensor and flexor strength per unit body weight strength results were similar between male progressor and non-progressor knees, but were still somewhat (but not significantly) elevated in KLG 2/3 progressor knees (Supplement Table I). Across all male knees, the loss of extensor and flexor isometric muscle strength in the period preceding radiographic progression was slightly less in knees with subsequent radiographic progression than in those without, but the difference did not reach statistical significance (Supplement Table II). In male KLG 0/1 knees, the loss of extensor muscle strength tended to be greater, and in KLG 2/3 less in progressor vs. non-progressor knees, but again the differences did not reach statistical significance (Supplement Table II). At baseline, (two years prior to the interval of progression), the difference between progressor and non-progressor knees was even smaller than that at Y2 and was not significant (Table 2 and Supplement Table I).
Of the 466 progressor knees, 408 also had Y4, and of the 946 non-progressor knees, 852 also had Y4 isometric muscle strength data available. In the interval during which progression was evaluated (Y2→Y4), female progressor knees displayed a very small gain in extensor strength (+1.03N), whereas non-progressor knees lost a little extensor strength (−6.66N). Progressor knees (−1.62N) and non-progressor knees (−4.52N) both lost flexor strength, but neither difference attained statistical significance (Table 2 and Supplement Table I). In male knees, the change in strength during Y2→Y4 did not differ between progressor and non- progressor knees (Table 3; Suppl. Table II).
DISCUSSION
To our knowledge, this is the first study to look at longitudinal changes of isometric muscle strength before an interval of radiographic (structural) progression The objective was to test whether thigh isometric muscle strength is less (and shows greater longitudinal change) in women with subsequent radiographic progression of KOA than in those without. We further tested whether differences in strength (and those of longitudinal change in strength) between progressor vs. non-progressor knees were stronger in female pre-osteoarthritic knees (KLG 0/1) than in female knees with definite radiographic KOA (KLG 2/3). To accommodate analysis of several muscle strength measures and time points/periods without risk of severely accumulating possible type 1 errors, care was taken to clearly define primary, secondary and exploratory analysis.
Although women with progression tended to have lower, and men with progression greater thigh isometric muscle strength prior to the interval of radiographic progression than those without progression, the variation between subjects and the overlap between progressors and non-progressors was large. Thus, the mean differences did not reach statistical significance after adjustment for confounding by age, BMI and WOMAC pain, despite the relatively large sample. Although reductions in thigh isometric muscle strength tended to be greater between female KLG0/1 progressor vs. non-progressor than between female KLG 2/3 progressor vs. non-progressor knees, this difference also did not reach statistical significance. Further, cross sectional differences in muscle strength two years prior to the observation period of radiographic progression, and longitudinal changes in muscle strength concomitant to the period of progression also did not differ significantly between progressor and non-progressor knees in women or men, or in KLG 0/1 and KLG 2/3 strata.
A strength of the study is that a large sample was available for selecting radiographic progressor and non-progressor knees. This resulted in comparatively narrow confidence intervals that reduce the risk of the study being underpowered for detecting clinically relevant differences in isometric muscle strength between knees with and without radiographic progression. Further, one observation period (Y2→Y4) was used to define progression, and another independent one was used to confirm it, which tat did not share the same measurement time points (Y1→Y3). This was done to reduce the risk of misclassification due to positioning error or variability between radiographs31,42. Progressor knees are known to represent only a very limited subsample of KOA participants, even when being determined with highly sensitive methods such as subregional MRI 43. Further, by excluding knees that showed an increase in the OARSI JSN grade in the lateral compartment, we ensured that participants with lateral progression were not classified as non-progressor controls, as may happen when only medial mJSW is used as a criterion. Although the specific threshold that was used for defining medial radiographic progression was at the lower end of those reported in a meta-analysis 44, we believe that applying this threshold to two independent (albeit overlapping) observation periods in the same individual provided a conservative and robust approach for identifying radiographic “progressors”. In subjects with bilateral progression (or non-progression), only one knee per person was used as a case (or control), because observations in contralateral knees may be correlated and cannot be expected to provide fully independent information 45.
A particular strength of our study design was its focus on muscle strength changes during an interval that preceded that of radiographic progression. In this particular analysis, every participant served as his/her own control, and a significant relationship between longitudinal change and subsequent structural progression would have been suggestive of potential benefits in modifying strength to reduce subsequent progression. Cross-sectional analyses were not only performed immediately preceding the period of potential progression, but also two years earlier, which is important from a prognostic view point. Further, we were able to stratify women vs. men, and knees with established radiographic KOA (KLG 2/3) vs. pre-osteoarthritic knees with risk factors of knee OA (KLG 0/1), for which differences in the relationship with structural progression of KOA have been proposed 13. This aspect also is important because there are known challenges in detecting a relationship between a potential risk factor and structural progression, if the same risk factor also is involved in the onset of structural (radiographic) disease 46. This challenge exists because of a potential alternative non-causal path between the exposure (muscle strength) and ROA progression that has been addressed as collider bias 46. Yet, there is no convincing evidence that low muscle strength is a risk factor for incident radiographic KOA 13,20,25, and analyzing the relationship between strength and radiographic progression in a subcohort that does not have radiographic KOA alleviates this issue. Yet, we were unable to confirm in such a (KLG 0/1) subcohort that knees with radiographic progression have lower thigh isometric muscle strength than those without. With regard to those with pre-existing radiographic KOA (KLG2/3), we may not be able to proof a lack of causality between change in muscle strength and progression, but we can claim that isometric measurement of muscle strength does not help in empirically predicting who will exhibit structural progression and who will not.
Our findings do not rule out the possibility that muscle strength predicts structural progression in the femoro-patellar joint 24; but this relationship could not be examined here, because the OAI did not provide quantitative radiographic measures of the femoro-patellar joint. Another limitation was that we were unable to adjust for differences in knee alignment and laxity between joints 22, since these covariates have not yet been determined in the OAI cohort. However, attempts were made to account for differences in age, BMI, and WOMAC pain. To account for variability in body dimensions, isometric muscle strength was normalized to body weight based on previous recommendations 37,38. Although it is unclear whether muscle strength linearly scales with body weight 13, our results for normalized and non-normalized strength comparisons were similar, and using both approaches should sufficiently cover various potential relationships. Another potential limitation of the study is that strength was determined under isometric conditions, and not isokinetically 13 or using functional tests, such as the one-leg rise time 23. Isometric strength may be less related to the physiologically relevant biomechanical protection of the knee than other measures of strength, which involve more coordinative components. Also, it has been suggested that isokinetic strength was a better predictor of pain and disability scores than isometric strength 47; however, for the ease of implementation, the OAI did not provide isokinetic measurements of muscle status.
Previous studies on the relationship between baseline muscle strength and radiographic progression (or other measures of structural change) have yielded contradictory results 21–26,37,38. It has been suggested recently that these discrepancies my results from studying men and women and knees with and without definite radiographic KOA in pooled cohorts, and that a relationship might only exist in women without evident radiographic KOA 13. Our data suggest a trend towards female progressors having slightly less thigh isometric strength (and slightly greater longitudinal isometric strength loss) prior to radiographic progression. This trend was somewhat stronger in KLG 0/1 than in KLG 2/3 knees, but was not significant in either group. The opposite trend was observed in men, particularly in those with definite radiographic KOA (KLG2/3). However, given the lack of statistical significance in this relatively large cohort, our findings do not strongly support the concept that thigh muscle status is associated with subsequent radiographic progression in KOA. Along those lines, a recent interventional trial was unable to identify structural benefits of strength training on KOA 19,48. It may well be that in some knees, lack of muscle strength contributes to radiographic progression by providing less biomechanical protection 13, whereas in others, an increase in muscle strength may increase dynamic joint loading 22, which in turn leads to greater structural progression and cartilage loss 4. However, as a general concept, there is no strong evidence that (change in) isometric muscle strength is related to structural outcomes of KOA, and we suggest that this relationship, if existent at all, is very small at best across general KOA cohorts.
In conclusion, despite a relatively large sample and a very robust definition of radiographic progression in KOA, using two independent time intervals, this study provides no strong evidence that (change in) thigh isometric muscle strength is associated with radiographic progression in a subsequent time interval. Particularly, such a relationship could not be detected in a subsample of female pre-osteoarthritic knees with risk factors of KOA that did not exhibit definite radiographic KOA.
Supplementary Material
Acknowledgment
The authors thank the readers of the fixed flexion radiographs at Boston University for the central KL grading, the OAI investigators, clinic staff and OAI participants at each of the OAI clinical centers for their contributions in acquiring the publicly available clinical and imaging data, the team at the OAI coordinating center.
Role of the funding source: The study and data acquisition was funded by the OAI, a public- private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2- 2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners of the OAI include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. The sponsors were not involved in the design and conduct of this particular study, in the analysis and interpretation of the data, and in the preparation, review, or approval of the manuscript. However, this manuscript received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- the conception and design of the study: FE, WW
- acquisition of data: KK, JD
- analysis and interpretation of data: FE, WH, KK, JD, WW
- Drafting the article: FE
- Revising the article critically for important intellectual content: FE, WH, KK, JD, WW
- Final approval of the version ubmitted: FE, WH, KK, JD, WW
- Statistical expertise: WH, WW
- Collection and assembly of data: WH, WW
Competing Interest Disclosure:
Felix Eckstein is CEO of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry. He provides consulting services to MerckSerono, Novartis, Sanofi Aventis, Bioclinica, Perceptive, and Abbot, and has received speaker honoraria Medtronic, and Synthes.
Wolfgang Hitzl and Jeff Duryea have no relevant conflicts.
C. Kent Kwoh has provided consulting services to Novartis and has received research support from Astra-Zeneca
Wolfgang Wirth has a part time employment with Chondrometrics GmbH; Wolfgang Wirth is a co-owner of Chondrometrics GmbH.
References
- 1.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med. 2011;154:217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60:3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 3.Englund M. The role of biomechanics in the initiation and progression of OA of the knee. Best Pract Res Clin Rheumatol. 2010;24:39–46. doi: 10.1016/j.berh.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Bennell KL, Bowles KA, Wang Y, Cicuttini F, Davies-Tuck M, Hinman RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis. 2011;70:1770–1774. doi: 10.1136/ard.2010.147082. [DOI] [PubMed] [Google Scholar]
- 5.Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–1726. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 6.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009;42:2294–2300. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Jefferson RJ, Collins JJ, Whittle MW, Radin EL, O'Connor JJ. The role of the quadriceps in controlling impulsive forces around heel strike. Proc Inst Mech Eng H. 1990;204:21–28. doi: 10.1243/PIME_PROC_1990_204_224_02. [DOI] [PubMed] [Google Scholar]
- 8.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91(Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hortobagyi T, Garry J, Holbert D, Devita P. Aberrations in the control of quadriceps muscle force in patients with knee osteoarthritis. Arthritis Rheum. 2004;51:562–569. doi: 10.1002/art.20545. [DOI] [PubMed] [Google Scholar]
- 10.Lattanzio PJ, Petrella RJ, Sproule JR, Fowler PJ. Effects of fatigue on knee proprioception. Clin J Sport Med. 1997;7:22–27. doi: 10.1097/00042752-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Skinner HB, Wyatt MP, Hodgdon JA, Conard DW, Barrack RL. Effect of fatigue on joint position sense of the knee. J Orthop Res. 1986;4:112–118. doi: 10.1002/jor.1100040115. [DOI] [PubMed] [Google Scholar]
- 12.Radin EL, Yang KH, Riegger C, Kish VL, O'Connor JJ. Relationship between lower limb dynamics and knee joint pain. J Orthop Res. 1991;9:398–405. doi: 10.1002/jor.1100090312. [DOI] [PubMed] [Google Scholar]
- 13.Segal NA, Glass NA. Is quadriceps muscle weakness a risk factor for incident or progressive knee osteoarthritis? Phys Sportsmed. 2011;39:44–50. doi: 10.3810/psm.2011.11.1938. [DOI] [PubMed] [Google Scholar]
- 14.McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993;52:258–262. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998;57:588–594. doi: 10.1136/ard.57.10.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Bennell KL, Hunt MA, Wrigley TV, Lim BW, Hinman RS. Muscle and exercise in the prevention and management of knee osteoarthritis: an internal medicine specialist's guide. Med Clin North Am. 2009;93:161–177. doi: 10.1016/j.mcna.2008.08.006. xii. [DOI] [PubMed] [Google Scholar]
- 18.Roos EM, Herzog W, Block JA, Bennell KL. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol. 2011;7:57–63. doi: 10.1038/nrrheum.2010.195. [DOI] [PubMed] [Google Scholar]
- 19.Mikesky AE, Mazzuca SA, Brandt KD, Perkins SM, Damush T, Lane KA. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Rheum. 2006;55:690–699. doi: 10.1002/art.22245. [DOI] [PubMed] [Google Scholar]
- 20.Segal NA, Torner JC, Felson D, Niu J, Sharma L, Lewis CE, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61:1210–1217. doi: 10.1002/art.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandt KD, Heilman DK, Slemenda C, Katz BP, Mazzuca SA, Braunstein EM, et al. Quadriceps strength in women with radiographically progressive osteoarthritis of the knee and those with stable radiographic changes. J Rheumatol. 1999;26:2431–2437. [PubMed] [Google Scholar]
- 22.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138:613–619. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 23.Thorstensson CA, Petersson IF, Jacobsson LT, Boegard TL, Roos EM. Reduced functional performance in the lower extremity predicted radiographic knee osteoarthritis five years later. Ann Rheum Dis. 2004;63:402–407. doi: 10.1136/ard.2003.007583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amin S, Baker K, Niu J, Clancy M, Goggins J, Guermazi A, et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60:189–198. doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segal NA, Glass NA, Torner J, Yang M, Felson DT, Sharma L, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage. 2010;18:769–775. doi: 10.1016/j.joca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding C, Martel-Pelletier J, Pelletier JP, Abram F, Raynauld JP, Cicuttini F, et al. Two-year prospective longitudinal study exploring the factors associated with change in femoral cartilage volume in a cohort largely without knee radiographic osteoarthritis. Osteoarthritis Cartilage. 2008;16:443–449. doi: 10.1016/j.joca.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Duryea J, Neumann G, Niu J, Totterman S, Tamez J, Dabrowski C, et al. Comparison of radiographic joint space width with magnetic resonance imaging cartilage morphometry: analysis of longitudinal data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken ) 2010;62:932–937. doi: 10.1002/acr.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging-the Osteoarthritis Initiative. Nat Rev Rheumatol. 2012:10. doi: 10.1038/nrrheum.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckstein F, Yang M, Guermazi A, Roemer FW, Hudelmaier M, Picha K, et al. Reference values and Z-scores for subregional femorotibial cartilage thickness--results from a large population-based sample (Framingham) and comparison with the non-exposed Osteoarthritis Initiative reference cohort. Osteoarthritis Cartilage. 2010;18:1275–1283. doi: 10.1016/j.joca.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruynesteyn K, Boers M, Kostense P, van der LS, van der HD. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann Rheum Dis. 2005;64:179–182. doi: 10.1136/ard.2003.018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Graverand MP, Vignon EP, Brandt KD, Mazzuca SA, Piperno M, Buck R, et al. Head-to- head comparison of the Lyon Schuss and fixed flexion radiographic techniques. Long-term reproducibility in normal knees and sensitivity to change in osteoarthritic knees. Ann Rheum Dis. 2008;67:1562–1566. doi: 10.1136/ard.2007.077834. [DOI] [PubMed] [Google Scholar]
- 32.Buckland-Wright JC, Macfarlane DG, Lynch JA, Jasani MK, Bradshaw CR. Joint space width measures cartilage thickness in osteoarthritis of the knee: high resolution plain film and double contrast macroradiographic investigation. Ann Rheum Dis. 1995;54:263–268. doi: 10.1136/ard.54.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc. 1997;45:1439–1445. doi: 10.1111/j.1532-5415.1997.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 34.Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year- old men and women. Age Ageing. 1994;23:132–137. doi: 10.1093/ageing/23.2.132. [DOI] [PubMed] [Google Scholar]
- 35.Sattler M, Dannhauer T, Hudelmaier M, Wirth W, Sanger AM, Kwoh CK, et al. Side differences of thigh muscle cross-sectional areas and maximal isometric muscle force in bilateral knees with the same radiographic disease stage, but unilateral frequent pain - data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2012;20:532–540. doi: 10.1016/j.joca.2012.02.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralnik J, Willcox BJ, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54:737–742. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 37.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 38.Slemenda C, Heilman DK, Brandt KD, Katz BP, Mazzuca SA, Braunstein EM, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Eckstein F, Cotofana S, Wirth W, Nevitt M, John MR, Dreher D, et al. Greater rates of cartilage loss in painful knees than in pain-free knees after adjustment for radiographic disease stage: data from the osteoarthritis initiative. Arthritis Rheum. 2011;63:2257–2267. doi: 10.1002/art.30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neter J, Wassermann W, Kutner M. Applied Statistical Models. 1990. Regression, Analysis of Variance, and Experimental Design; pp. 614–619. [Google Scholar]
- 41.Hill T, Lewicki P. Statistics: Methods and Applications. Tulsa, USA: StatSoft; 2011. [Google Scholar]
- 42.Cline GA, Meyer JM, Stevens R, Buckland-Wright C, Peterfy C, Beary JF. Comparison of fixed flexion, fluoroscopic semi-flexed and MTP radiographic methods for obtaining the minimum medial joint space width of the knee in longitudinal osteoarthritis trials. Osteoarthritis Cartilage. 2006;14(Suppl A):A32–A36. doi: 10.1016/j.joca.2006.02.023. Epub;%2006 May 8.:A32-A36. [DOI] [PubMed] [Google Scholar]
- 43.Buck RJ, Wyman BT, Le Graverand MP, Hudelmaier M, Wirth W, Eckstein F. Osteoarthritis may not be a one-way-road of cartilage loss - comparison of spatial patterns of cartilage change between osteoarthritic and healthy knees. Osteoarthritis Cartilage. 2010;18:329–335. doi: 10.1016/j.joca.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Ornetti P, Brandt K, Hellio-Le Graverand MP, Hochberg M, Hunter DJ, Kloppenburg M, et al. OARSI-OMERACT definition of relevant radiological progression in hip/knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:856–863. doi: 10.1016/j.joca.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryant D, Havey TC, Roberts R, Guyatt G. How many patients? How many limbs? Analysis of patients or limbs in the orthopaedic literature: a systematic review. J Bone Joint Surg Am. 2006;88:41–45. doi: 10.2106/JBJS.E.00272. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Niu J, Felson DT, Choi HK, Nevitt M, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken ) 2010;62:1527–1532. doi: 10.1002/acr.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madsen OR, Bliddal H, Egsmose C, Sylvest J. Isometric and isokinetic quadriceps strength in gonarthrosis; inter-relations between quadriceps strength, walking ability, radiology, subchondral bone density and pain. Clin Rheumatol. 1995;14:308–314. doi: 10.1007/BF02208344. [DOI] [PubMed] [Google Scholar]
- 48.Bennell KL, Hinman RS. A review of the clinical evidence for exercise in osteoarthritis of the hip and knee. J Sci Med Sport. 2011;14:4–9. doi: 10.1016/j.jsams.2010.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.