Abstract
Rationale and Objective
We sought to examine the impact of differing cocaine administration schedules and dosing on the magnitude of cocaine conditioned place preference (CPP), extinction, and stress- and cocaine-induced reinstatement of CPP.
Methods
First, in C57Bl/6J mice, we investigated whether total cocaine administration or pattern of drug exposure could influence the magnitude of cocaine CPP by conditioning mice with a fixed-low dose (FL; 7.5 mg/kg; 30 mg/kg total), a fixed-high dose (FH; 16 mg/kg; 64 mg/kg total), or an ascending dosing schedule (Asc; 2, 4, 8, and 16 mg/kg; 30 mg/kg). Next, we investigated if cocaine or saline is more effective at extinguishing preference by reconditioning mice with either a descending dosing schedule (Desc; 8, 4, 2, and 1 mg/kg) or saline. Finally, we examined if prior conditioning and re-conditioning history alters stress (~2-3 minutes forced swim test) or cocaine (3.5 mg/kg)-induced and reinstatement.
Results
We replicated and extended findings by Itzhak and Anderson (2011) demonstrating Asc conditioning produces a greater CPP than either the FL or FH conditioning schedules. The magnitude of extinction expressed was similar in the Desc reconditioned and saline groups. Moreover, only the saline, and not the Desc reconditioned mice, showed stress and cocaine-induced reinstatement of CPP.
Conclusions
Our results suggest that the schedule of cocaine administration during conditioning and reconditioning can have a significant influence on the magnitude of CPP and extinction of preference, and the ability of cocaine or a stressor to reinstate CPP.
Keywords: Addiction, cocaine, stress, reward, schedule, dose, pattern, Conditioned Place preference (CPP), reinstatement, extinction
INTRODUCTION
Conditioned reward studies routinely involve associating a distinct environment with a reward by using a fixed daily dose of a drug during training. However, a fixed pattern of drug administration does not mimic the progressive human pattern of drug intake frequently found in the clinical literature (Gawin 1991) and may fail to engage the same neural substrates and learning and memory processes (Ahmed et al. 2002; Ferrario et al. 2005; Koob and Kreek 2007; Porrino et al. 2002; Smirnov and Kiyatkin 2010; Vanderschuren and Everitt 2004; Zhou et al. 2011).
The conditioned place preference (CPP) paradigm has been increasingly used over the past few decades and is now one of the most commonly used behavioral reward models (Tzschentke 2007). CPP is a robust non-contingent model of drug reward and is frequently used to examine the motivational and conditioned rewarding properties of drugs of abuse. An important recent study by Itzhak and Anderson (2011) demonstrated that conditioning mice with one of two ascending dose schedules of cocaine led to a more robust expression of cocaine CPP than a fixed high dose of cocaine. Interestingly, the total cocaine administered was lower in the ascending groups (45 or 30mg/kg total) than in the fixed-high dose group (64mg/kg total). These findings suggest that the pattern, rather than the total cocaine administered, may be of primary importance in establishing a reliable animal model that replicates the increased intake over time reported in the clinical literature (Angarita et al. 2010). However, it is possible that the 16mg/kg dose used in that study was not optimal and a lower fixed dose of cocaine would have produced a more robust CPP (Mantsch et al. 2010). Moreover, the Itzhak and Anderson (2011) study also demonstrated that the pattern of drug administration – although in a descending dose pattern - may be similarly important during extinction training in providing resistance to subsequent cocaine-induced reinstatement of cocaine CPP.
Here we have replicated and extended key findings of the Itzhak and Anderson (2011) work. First, we replicated their findings in B6:129S F2 hybrid mice using C57BL/6J mice, demonstrating the ability of this dosage schedule to be relevant for inbred lines. Next, we extended their findings by demonstrating that an ascending schedule of cocaine conditioning produced a greater magnitude of cocaine CPP than a low-fixed dose cocaine administration schedule that resulted in the same overall level of cocaine exposure. Finally, we examined the ability of reconditioning with saline or a descending dose of cocaine to extinguish cocaine CPP. Our results are consistent with the previous report finding that a descending dose of cocaine is as effective as saline at extinguishing preference for the cocaine-paired context. However, reconditioning training with a descending dose of cocaine offered resistance to subsequent cocaine priming and stress-induced reinstatement of cocaine CPP.
METHODS
Subjects
Experiments were conducted on male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) aged 8–12 weeks and housed 2-5/cage with ad libitum access to food and water. Testing commenced at least 1 week after acclimation to the facilities. All procedures were approved by the Vanderbilt University Animal Care and Use Committees and were in accordance with the Animal Welfare Act. The number of mice used is reported in the figure legends.
Place Conditioning Apparatus
The place conditioning apparatus used here was described in detail previously (Davis et al. 2008). Briefly, the two chamber place conditioning apparatus consists of a box insert that slides into the open field chamber (Med Associates; St. Albans, VT). The two compartments of the conditioning box were separated by a single removable guillotine door. One chamber has metal mesh flooring, which was always paired with saline, and the other chamber has bar flooring which was always paired with cocaine (Med Associates inc., product number ENV-3013-2). Each chamber was equipped with horizontal sensors and a 4cm null zone on each side of the guillotine door was used to increase the likelihood of mice being fully within one compartment when their presence was counted. Med Associates Activity Monitor software was used to track and score time spent in each compartment and distance traveled.
General Conditioning Procedures
All conditioning and testing was carried out in a dedicated CPP test room (Vanderbilt Murine Neurobehavior core: http://kc.vanderbilt.edu/mnlcore/) located in the same facility as the mouse housing room. Mice were kept on our regular animal facility light-cycle (lights on at 06:00 and lights off at 18:00). Mice were exposed to the CPP chamber for 20 minutes in all sessions. All conditioning days had 2 sessions/day, Session I was conducted between 10:00-12:00 Session II was conducted between 14:00-16:00. Saline was always given in Session I, unless otherwise noted, and saline-injected mice were always restricted to exploration of the mesh-flooring compartment (guillotine door in place). Cocaine was always administered in Session II and mice were restricted to exploration of the bar compartment (guillotine door in place). All mice were tested (described below) and allowed to free explore (described below) in a drug-free state, with unrestricted access to both compartments, and the were given between 12:00 and 14:00 to prevent prior experience with a drug given at the same time during the day on previous conditioning sessions from providing a temporal cue and unduly influencing behavior on a particular test. Previous testing by our group on this particular place conditioning apparatus has reliably produced a small but significant innate bias for the mesh flooring, however mice expressing a preference of greater than 33% for one side on the pre-test were removed from the study (1/48 miceBecause the lesser preferred bar flooring was always paired with cocaine,mice that express cocaine CPP were thus overcoming this small innate preference.
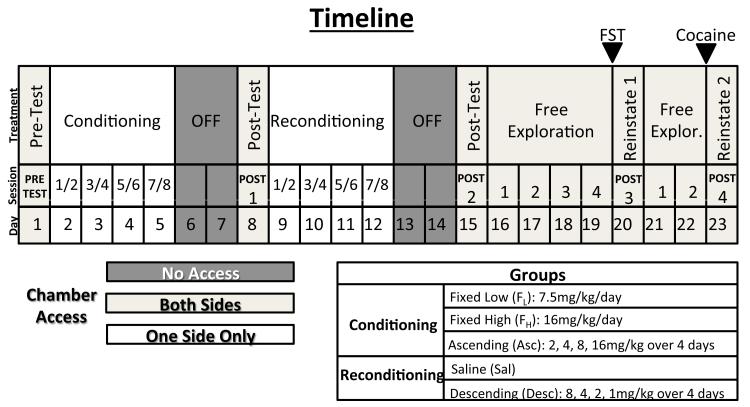
The schedule is shown in Fig. 1. On day 1, mice habituated to the chamber during a Pre-test in which access to both compartments is unrestricted (no guillotine door in place). Conditioning occurs on days 2-5, and two sessions are held on each of the four conditioning days (eight conditioning sessions total). Saline and cocaine were administered intraperitoneally (i.p.). All injections were given immediately prior to placing the animal in the conditioning chamber. At the end of each session, the chamber was thoroughly cleaned with 70% EtOH and detergent supplied by the mouse neurobehavioral core.
Fig. 1. Timeline of paradigm.
All testing days (Pre-test and Post-tests) were carried out at 12:00-14:00. ‘Conditioning’ represents days 2-5, with two sessions per day (morning session, saline, 10:00-12:00; afternoon session, cocaine, 14:00-16:00) during which mice were assigned to one of three groups (FL, FH, or Asc; described in the Groups box). ‘Off’ represents time in which mice are left undisturbed in home cages in housing facility, and are shown in dark grey in the timeline. ‘Post 1’ represents post-test 1 in which mice were tested for initial preference for the cocaine-paired side.; ‘Reconditioning’ represents days 9-12 during which two sessions of training was held per day (morning session, saline, 10:00-12:00; afternoon session, cocaine or saline, 14:00-16:00) and mice were assigned to one of two groups (Sal or Desc; described in the Groups box). ‘Post 2’ represents post-test 2 in which mice were tested for preference for the cocaine-paired side following reconditioning. ‘Free-exploration’ represents extinction sessions in which no injections were given (12:00-14:00). Stress administration/priming injections are denoted with arrows above treatment line. ‘FST’ represents administration of the ~2-3 minute forced swim stress, and ‘Cocaine’ represents the 3.5 mg/kg priming injection of cocaine. ‘Reinstate 1’ and ‘Reinstate 2’ represents the stress-induced (FST) reinstatement test (Post 3); and the cocaine-induced reinstatement (Post 4; both administered 12:00-14:00). Restricted access to either the mesh or bar chamber is shown in white. Unrestricted access to both compartments of the chamber, as occurs during testing and free exploration days, is shown in light grey. The ‘Groups’ box describes the schedule of conditioning and reconditioning.
During conditioning Session II on days 2-5, mice were evenly divided based on pre-test preference data and placed into one of 3 groups that differed in cocaine schedules: fixed (low) dose- (FL; 7.5 mg/kg/day for 4 days), n=16, 30 mg/kg total intake; fixed (high) dose- (FH; 16 mg/kg/day for 4 days), n=15, 64 mg/kg total intake; or ascending dose (Asc; 2 mg/kg on day 2; 4 mg/kg on day 3; 8 mg/kg on day 4; and 16 mg/kg on day 5), n=15 30 mg/kg total intake.
The FH dose was chosen for our study based on previous work showing that this dose produced significant cocaine CPP (Itzhak and Anderson 2011) and 16 mg/kg was also the highest dose used on the Asc dose schedule. The FL dose was chosen based on studies showing that maximal cocaine CPP in C57Bl/6J mice is achieved using a fixed cocaine dose <15 mg/kg cocaine (Mantsch et al. 2010; Miner 1997; Schindler et al. 2010). Further, this FL schedule using 7.5 mg/kg dose allowed us to investigate, in addition to schedule of reinforcement, whether total amount of cocaine administered (30 mg/kg in both FL and Asc groups) is a contributing factor in the magnitude of observed cocaine CPP. Finally, this Asc schedule was based on previous work showing that this schedule and higher Asc dosages of 3, 6, 12, and 24 mg/kg produced cocaine CPP of comparable magnitude (Itzhak and Anderson 2011).
Expression of Cocaine CPP
On day 8, ~72 hours after the last conditioning session, mice were tested for the expression of cocaine CPP (Post-test 1). During the post-test, mice were treated as they were for the pre-test with unrestricted access to both compartments and no injection given. Mice were placed on mesh side at start of test, and locomotor activity and time spent in the cocaine- and saline- paired chambers were recorded.
Extinction Procedures
Reconditioning
On days 9-12 (Fig. 1), all mice were divided into two reconditioning groups, descending (Desc) and saline (Sal), with six subgroups. The first reconditioning group was exposed to a descending schedule of cocaine dosing (Desc; 8 mg/kg on day 9; 4 mg/kg on day 10; 2 mg/kg on day 11; and 1 mg/kg on day 12), resulting in 3 descending subgroups based on prior conditioning history: fixed-low (7.5 mg/kg) dose-descending (FL-Desc) subgroup, fixed-high (16 mg/kg) dose-descending (FH-Desc) subgroup, and ascending (2, 4, 8, 16 mg/kg) –descending (Asc-Desc) subgroup. The second group of mice was reconditioned with saline, resulting in 3 additional reconditioning subgroups based on prior conditioning history fixed-low (7.5 mg/kg) dose -saline (FL-Sal) subgroup, fixed-high (16 mg/kg) dose –saline (FH-Sal) subgroup, and an ascending (2, 4, 8, 16 mg/kg)-saline (Asc-Sal) subgroup. As with the first round of conditioning, all mice were exposed both to a morning (Session I; saline) and afternoon (Session II; descending cocaine schedule or saline) reconditioning sessions, for a total of 8 sessions over 4 days.
Post-test 2 (Following Reconditioning)
On day 15, ~72 hours after the last reconditioning session, all mice were re-tested for expression of cocaine CPP, in a manner identical to that described in the Expression of Cocaine CPP section (above).
Reinstatement Tests
Free Exploration
To ensure extinction of cocaine CPP, mice were exposed to two rounds of free exploration sessions (extinction sessions) with one session per day on days 16-19 and again on days 21 and 22. During free exploration, mice were given access to both sides of the chamber and no injection was administered. Such sessions provided additional data on the rate of extinction. Similar to ‘test’ sessions, locomotor activity and time spent in the cocaine- and saline-paired chambers were recorded.
Stress-induced Reinstatement
On day 20, ~24 hours after the final session of the first round of free exploration, mice were exposed to a ~2-3 minutes forced swim (FS) in warm (22-28OC) water. At the end of the FST, mice were towel dried and placed immediately into the mesh-side of the chamber, with access to both compartments (post-test3). Locomotor activity and time spent on the cocaine- and saline-paired sides were recorded.
Cocaine-induced Reinstatement
On day 23, ~24 hours after the final session of the second round of free exploration, mice were administered a 3.5 mg/kg ‘priming’ dose of cocaine and immediately placed into the mesh side of the chamber, with access to both compartments (post-test 4). Locomotor activity and time spent on the cocaine- and saline-paired sides were recorded.
Statistical Analysis
When determining whether a cocaine-dosing schedule had any significant effect on the expression of CPP, a two-way Analysis of Variance (ANOVA) was used [schedule (FL, FH, Asc) x test (pre, post)]. A two-way repeated measures ANOVA was used to determine if reconditioning had an effect on the magnitude of extinction [schedule history (FL-Sal, FL-Desc, FH-Sal, FH-Desc Asc-Sal, and Asc-Desc) x test [data analyzed as extinction ratio (x test/post-test 1, where x represents: post-test 2, free exploration day 1, and free exploration day 3)]. A three-way ANOVA was used to determine if prior schedule history had an effect on stress-induced reinstatement [conditioning schedule (FL, –FH, or Asc) x reconditioning schedule (Sal or Desc)x test (post-test 2, last day of free exploration or stress reinstatement)]. To determine if previous schedule history had an effect on cocaine-induced reinstatement, a three-way ANOVA was used [conditioning schedule (FL, FH, or Asc) x reconditioning schedule (Sal or Desc) x test (last day of free exploration or cocaine reinstatement)]. In all experiments, locomotor activity was also analyzed using a one-way ANOVA. A P value < 0.05 was considered significant. When an interaction effect was significant, Bonferroni (for two-way ANOVA) or Student Newman-Keuls (SNK; for three-way ANOVA) post hoc tests were performed. All statistical analyses were performed using Prism GraphPad v.6.0.
RESULTS
Experiment 1: Conditioning with an ascending dosing schedule results in a higher magnitude of cocaine CPP than conditioning with fixed dosage schedules
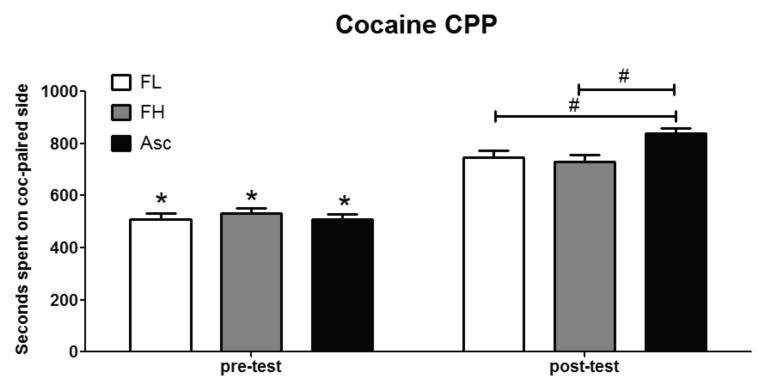
This experiment was designed to investigate how different schedules of cocaine and total intake influence the magnitude of CPP. The magnitude of CPP was calculated as the total amount of seconds spent on the cocaine-paired side. No differences were found on locomotor activity between groups (data not shown). The magnitude of CPP (seconds spent on the cocaine-paired side), as the dependent variable, showed significant differences between the groups on pre-test compared to post-test 1 [F(1,44) = 66.24, P < 0.05; Fig. 2] and an interaction of test and treatment [F(2,43) = 3.02, P < 0.05; Fig. 2]. A Bonferroni post hoc analysis revealed that all three groups showed a significant increase in time-spent on the cocaine-paired side on the preference test (post-test 1), suggesting all three dosing schedules effectively establish cocaine CPP (P < 0.05). However, the magnitude of CPP expressed on post-test 1 in the ascending dose (Asc) group (838 ± 21 seconds) was significantly higher than that of the fixed low (FL) group (746 ± 18 seconds; P < 0.05) and the fixed high (FH) group (731 ± 25 seconds; P < 0.05). No significant differences were observed between the FL and FH groups.
Fig. 2. Mice in the Ascending dosing schedule display a higher magnitude of cocaine conditioned place preference (cocaine CPP) compared to the FL or FH groups.
All 3 groups showed a significant (*p < 0.05 when difference is within group) increase in time (seconds) spent on the cocaine-paired side on the post-test compared to pre-test. The Ascending (Asc) group showed a significantly higher magnitude of cocaine CPP (#p < 0.05 when difference is between groups) compared to the fixed low (FL) and fixed high (FH) groups. Data are represented as mean ± SEM, *p > 0.05, n = 15-16 mice per group.
Our results also suggest that total cocaine dosing was not a factor. By the completion of the 4 conditioning sessions, the Asc group and the FL groups had both received 30 mg/kg cocaine, yet the magnitude of preference expressed in the Asc group was significantly higher than the FL group. Likewise, the FH group received more than twice as much cocaine (total intake = 64 mg/kg) compared to the FL group (total intake = 30 mg/kg), yet the magnitude of preference expressed was not significantly different between the two fixed dose groups. These findings suggest that the conditioning pattern was more important than the total amount of cocaine administered.
Experiment 2: Reconditioning with either a descending cocaine dosing schedule or saline results in a similar magnitude of extinction
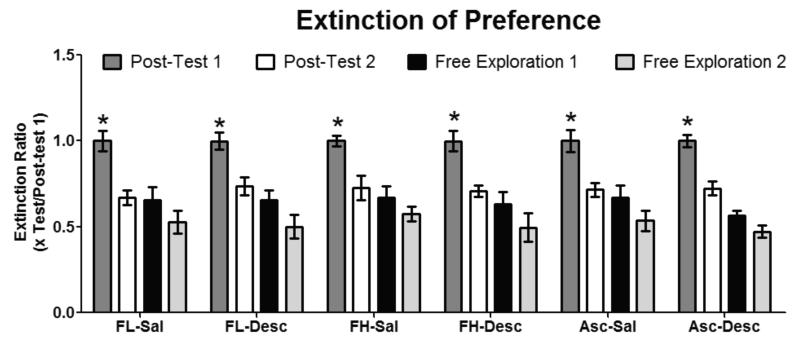
This experiment was designed to investigate whether reconditioning mice with a descending schedule of cocaine following CPP induction would be as effective as traditional extinction training in which saline is used to extinguish cocaine CPP. No differences were found on locomotor activity between any of the groups (data not shown) The magnitude of extinction (x test/ post-test 1) was calculated as the ratio of post-test 1 cocaine-side preference remaining on post-test 2 (post-reconditioning), on free exploration day 1, or free exploration day 2. A two-way repeated measure ANOVA [conditioning history x time; repeated measures: post-test 1 (preference test), post-test 2, free exploration day 1, and free exploration day 2] was employed to investigate statistical differences. The outcome of extinction training on preference expressed (ratio of seconds spent on the cocaine-paired side during x test/ post-test 1), as the dependent variable, showed significant differences in time [F(3,59) = 32.40, P < 0.05 for all days and conditions, Fig. 3] but not treatment, and no significant interaction was observed. However, reconditioning with the descending (8, 4, 2, 1 mg/kg) cocaine dosing schedule was equally effective as saline at extinguishing preference (Fig. 3) on the initial extinction test, as no significant differences were observed between extinction training conditions in any of the subgroups examined [ FL-Sal , FH-Sal, Asc-Sal, FL-Desc, FH-Desc, and Asc-Desc)].
Fig. 3. In extinguishing cocaine CPP, both saline and a descending dosing schedule of cocaine are equally effective.
All mice showed a significant within group decrease in preference for the cocaine-paired side on the post-test following reconditioning (Post-test 2) compared to initial preference observed in Post-test 1. No significant differences were found between groups on the ratio of preference remaining for the cocaine-paired side on the post-test following reconditioning (Post-test 2/Post-test 1). Data are represented as mean ± SEM, *p > 0.05, n = 7-8 mice per group.
Experiment 3: Extinction training by reconditioning with a descending cocaine dosing schedule attenuates stress-induced reinstatement of cocaine CPP
Previously, Itzhak and Anderson (2011) demonstrated that a low-dose (3.5 mg/kg) priming injection of cocaine could reinstate cocaine CPP, but only in mice that had been reconditioned with saline, regardless of initial conditioning experience. To assess the impact of reconditioning on stress-induced reinstatement, we utilized a moderate forced swim (FS) stressor to reinstate CPP (stress-induced reinstatement; Kreibach and Blendy 2004; Mantsch et al 2010). We hypothesized that reconditioning (with the Desc schedule or saline) and prior conditioning experience (FL, FH, and Asc) would affect the magnitude of stress-induced reinstatement.
A 3-way ANOVA was used to analyze the effect of forced swim stress on cocaine CPP with conditioning group, reconditioning group and reinstatement test group as the variables, and with outcome of stress on preference expressed (seconds spent on the cocaine-paired side), as the dependent variable. There was no significant three-way interaction (P > 0.05). There was a significant main effect of reinstatement test group (prior free exploration day compared to test immediately following stressor) [F(1,40) = 23.30, P < 0.05], but no main effect of conditioning group (FL, FH, or Asc) or reconditioning group (Desc or Sal). .There was a significant interaction of reconditioning group x reinstatement test [F(1,40) = 6.91, P<0.05], indicating reduced stress-induced reinstatement of CPP in mice exposed to the descending cocaine schedule during reconditioning. No significant interactions between conditioning and reinstatement test day or conditioning and reconditioning (P > 0.05) were observed. No differences were found on locomotor activity between groups (data not shown).
Experiment 4: Reconditioning with descending cocaine dosing schedule, but not saline reconditioning, attenuates cocaine-induced reinstatement of CPP
We used a 3.5 mg/kg cocaine priming dose to induce reinstatement as in Itzhak and Anderson (2011), and we extended their study by (1) also examining a FL group, and (2) assessing if cocaine-primed reinstatement occurs in mice that had previously undergone a stress-induced reinstatement test.
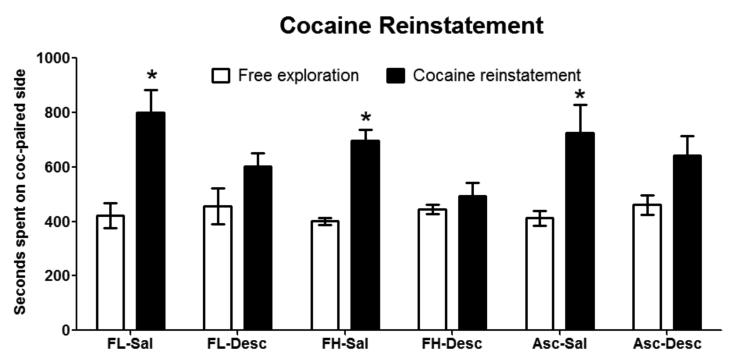
A 3-way ANOVA was used to analyze the effect of a cocaine priming dose on cocaine CPP with conditioning group, reconditioning group and reinstatement test group as the variables, and with outcome of cocaine prime on preference expressed (seconds spent on the cocaine-paired side), as the dependent variable. There was no significant three-way interaction (P > 0.05). There were a significant main effects of reinstatement test group (prior free exploration day compared to test immediately following stressor) [F(1,40) = 17.14, P < 0.05] and reconditioning (Sal compared to Desc) schedule [F(1,40) = 3.98, P < 0.05], but there was no significant main effect of conditioning (FL, FH, or Asc) schedule. There was a significant interaction of test x reconditioning [F(1,40) = 6.89, P < 0.05]. No significant interaction of conditioning schedule and testing day or conditioning schedule and reconditioning schedule (P > 0.05) was observed. No differences were found on locomotor activity (data not shown). A SNK post hoc analysis reveals that reconditioning with a descending dose of cocaine (compared to saline) has a significant effect on cocaine prime-induced reinstatement (Fig. 5; P < 0.05).
Fig. 5. Reconditioning with descending cocaine doses blocked reinstatement of preference for the cocaine-paired side followinga priming injection of cocaine (3.5 mg/kg).
Saline reconditioned mice all showed a significant within group increase in time spent on the cocaine-paired side during the cocaine-induced reinstatement test compared to time spent on the cocaine-paired side during the free exploration session the previous day. None of the Descending reconditioned mice show a significant increase in time spent on the cocaine-paired side during the cocaine-induced reinstatement test compared to time spent on the cocaine-paired side during the free exploration session the previous day. Data are represented as mean ± SEM, *p > 0.05, n = 7-8 mice per group.
DISCUSSION
In the present study, we found that conditioning with ascending doses of cocaine (Asc; 2, 4, 8, and16 mg/kg) resulted in higher levels of CPP compared to both low (FL; 7.5mg/kg)- and high (FH; 16mg/kg)-fixed conditioning doses of cocaine. During reconditioning, administration of either saline or descending doses of cocaine (Desc; 8, 4, 2, 1 mg/kg) lead to similar levels of cocaine extinction, regardless of conditioning history. Our results demonstrate that reconditioning with descending levels of cocaine, but not saline, attenuates cocaine- and stress-induced reinstatement following the reconditioning sessions. Our results are in line with studies showing that human addicts take drugs in which the binge phase involves increasing amounts of cocaine consumption (Angarita et al. 2010; Gawin and Ellinwood 1989).
This study aimed to replicate and expand on the important recent work by Itzhak and Anderson (2011). Notably, we have expanded these data in four ways: (1) The previous study utilized an intercrossed strain of mouse (C57BL/6J + 129S1/SvlmJ) while all experiments described here used the inbred C57BL/6J strain. (2) As described below, we incorporated a FLD group, which received equal total cocaine administration as the AscD group. (3) We replicated the effect of a ‘priming’ dose of cocaine on reinforcement of drug seeking, and demonstrated that the descending dosage schedule of reconditioning also protects against stress-induced cocaine seeking. This is crucial additional data, as priming- versus stress-induced reinstatement of drug seeking involve differential circuitry. (4) Finally, we utilized a slightly biased CPP paradigm in these experiments. While Itzhak and Anderson (2011) did not observe significant bias toward either the smooth-floored or rough-floored compartments of their CPP apparatus, we routinely observe a small but significant innate preference for the mesh-floored side of our apparatus (57.3% initial preference for this side in the experiments described here, P < 0.001). In order to minimize bias, we assigned saline injections to this initially preferred side, and cocaine was administered with exclusive access to the bar-floored side of the apparatus. Thus, mice must overcome this initial bias in order to show CPP. Additionally, we excluded any mice that demonstrated a greater than 33% bias towards one side of the apparatus during the pretest (1/48 mice).
Conditioning mice with an ascending dosing schedule of cocaine results in higher levels of expressed preference for the cocaine-associated environment than either FLD or FHD cocaine administration. Consistent with our findings, the Itzhak and Anderson (2011) study demonstrated that even accounting for higher, lower, and similar average daily dosing of cocaine during conditioning could not explain the differences they observed in the magnitude of cocaine CPP. One potential partial explanation of their findings is that the dose employed in their study was relatively high (16 mg/kg) and may be accompanied by more aversive properties. Indeed, other studies have demonstrated that administering fixed doses lower than 16 mg/kg results in more robust CPP (Mantsch et al. 2010). However, we found that even when total cocaine dosing in the FLD group (30 mg/kg total) was less than half the total dosing in the FHD group (64 mg/kg total), the magnitude of cocaine CPP was similar. Importantly, we found that although the ascending schedule group had the same total dosing as the FLD group and less than half that of the FHD group, the magnitude of CPP observed in the AscD group was significantly higher. Our results suggest that the schedule of dosing, but not the dose itself, may be a more important predictor of the magnitude of the CPP that is expressed. Recent studies have begun to explore the importance of using different dosing schedules of cocaine to better model addiction (Itzhak and Anderson 2011; Morgan et al. 2009; Zimmer et al. 2011).
Extinction training with saline is a common procedure in reinstatement studies. Our data, however, demonstrate that reconditioning mice with a descending dose of cocaine during extinction is just as effective as saline in the magnitude of extinction that is achieved, regardless of the original conditioning schedule used. Over time, the rate of extinction is also different between groups; mice reconditioned with a descending dose of cocaine, regardless of conditioning history, and mice conditioned with an ascending dosing schedule of cocaine and reconditioned with saline showed a progressive decrease in preference for the cocaine-paired side over time. Our data suggest the typical model employed in cocaine CPP paradigms of conditioning with a fixed dose of cocaine and extinction training with saline is a less effective approach to extinguishing preference for the cocaine-paired environment. Our results are consistent with a few recent studies (Itzhak and Anderson 2011; Marks et al. 2010; Panlilio et al. 2007), suggesting that when the expectation of reward is successively lowered (devalued), as may occur over the course of reconditioning with a descending dose of cocaine, an animal can learn and adjust their behavior. Although this idea runs counter to the compulsive and perseverative nature of drug-seeking behavior, to our knowledge no study has directly addressed the hypothesis that operant cocaine-seeking behavior can be attenuated with extinction training by reconditioning with a descending schedule of cocaine administration and future studies should investigate the potential of this paradigm to alter the rate of extinction in contingent self-administration models.
It is important to note that this descending dosage paradigm reduces the length of time between the final drug administration and the post-test when compared to saline-treated animals. Thus, some of the apparent protective nature of the descending paradigm may be due in part to incubation of drug craving within the saline-treated group (Grimm, et al. 2001). Future studies will be needed to match the time of the last descending dose of cocaine with the final cocaine dose in saline-extinguished animals to determine if the protective effect of descending reconditioning is preserved. The descending dosage paradigm also could alleviate withdrawal symptoms, and this decrease in withdrawal may play a role in the observed protective effect. Further studies will be necessary in order to determine if withdrawal severity plays a role in the protective nature of the descending dosage schedule. In addition, further studies will be necessary to isolate whether the descending dose reconditioning paradigm is useful for reduction in reinstatement in an independent context than the initial conditioning cocaine doses.
In mice extinguished with a descending dose of cocaine, some resistance to reinstatement was observed. The ability to extinguish CPP by administering decreasing doses of cocaine in the cocaine-paired context, may prove to be a more useful method of preventing relapse. Indeed, our results replicating and extending the work of Itzhak and Anderson (2011) suggest many new avenues for investigation that may focus on questions of whether recall and reconsolidation of addiction memories are more labile when descending doses of the drug are present. Interestingly, d-amphetamine is clinically being explored as a therapeutic tool for the treatment of cocaine dependence (Rush et al. 2009). Taken together, our results suggest a powerful influence of the pattern of drug exposure, but not the amount of drug, on the magnitude of preference expressed for a conditioned reward. Our findings may also have significant implications for the development of addiction therapies.
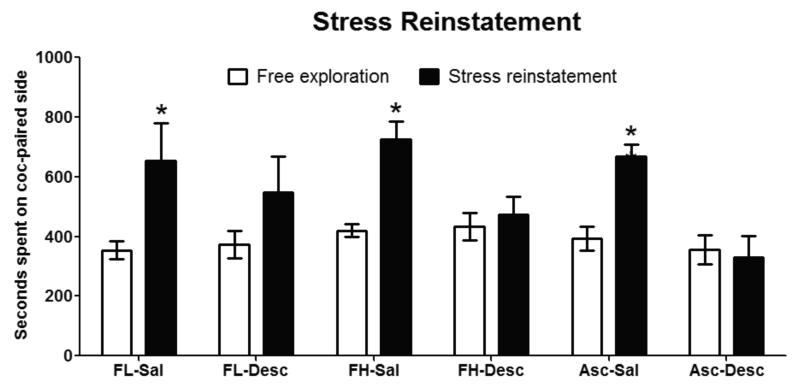
Fig. 4. Reconditioning with descending cocaine doses blocked reinstatement of preference for the cocaine-paired side following a forced swim stressor.
Saline reconditioned mice all showed a significant within group increase on time spent on the cocaine-paired side during the stress-induced reinstatement test compared to time spent on the cocaine-paired side during the free exploration session the previous day. None of the Descending schedulereconditioned mice show a significant increase in time spent on the cocaine-paired side during the stress-induced reinstatement test compared to time spent on the cocaine-paired side during the free exploration session the previous day. Data are represented as mean ± SEM, *p > 0.05, n = 7-8 mice per group.
Acknowledgments
The behavioral data presented in this manuscript were generated at the Vanderbilt Murine Neurobehavioral Laboratory: http://kc.vanderbilt.edu/mnlcore/. We would like to thank Dr. John Allison for his generous assistance and expertise.
This work was supported by National Institutes of Health Grants NS07491 and MH065215-08 (KLC) and AA019455 AND DA019112 (DGW).
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nature neuroscience. 2002;5:625–6. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Angarita GA, Pittman B, Gueorguieva R, Kalayasiri R, Lynch WJ, Sughondhabirom A, et al. Regulation of cocaine self-administration in humans: lack of evidence for loading and maintenance phases. Pharmacology, biochemistry, and behavior. 2010;95:51–5. doi: 10.1016/j.pbb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bourin M, Petit-Demouliere B, Dhonnchadha BN, Hascoet M. Animal models of anxiety in mice. Fundamental & clinical pharmacology. 2007;21:567–74. doi: 10.1111/j.1472-8206.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- Carr GD, Phillips AG, Fibiger HC. Independence of amphetamine reward from locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology. 1988;94:221–6. doi: 10.1007/BF00176849. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Dickinson SD, Grahame NJ, Okorn DM, McMullin CS. Genetic differences in cocaine-induced conditioned place preference in mice depend on conditioning trial duration. Psychopharmacology. 1999;146:73–80. doi: 10.1007/s002130051090. [DOI] [PubMed] [Google Scholar]
- Davis AR, Shields AD, Brigman JL, Norcross M, McElligott ZA, Holmes A, et al. Yohimbine impairs extinction of cocaine-conditioned place preference in an alpha2-adrenergic receptor independent process. Learn Mem. 2008;15:667–76. doi: 10.1101/lm.1079308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biological psychiatry. 2005;58:751–9. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–6. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Ellinwood EH., Jr Cocaine dependence. Annual review of medicine. 1989;40:149–61. doi: 10.1146/annurev.me.40.020189.001053. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Anderson KL. Changes in the magnitude of drug-unconditioned stimulus during conditioning modulate cocaine-induced place preference in mice. Addict. Biol. 2011;17(4):706–16. doi: 10.1111/j.1369-1600.2011.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. cAMP response element binding protein is required for stress but not cocaine-induced reinstatement. The Journal of Neuroscience. 2004;24(30):6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Archives of general psychiatry. 2001;58:334–41. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. The American journal of psychiatry. 2007;164:1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–78. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KR, Kearns DN, Christensen CJ, Silberberg A, Weiss SJ. Learning that a cocaine reward is smaller than expected: A test of Redish’s computational model of addiction. Behavioural brain research. 2010;212:204–7. doi: 10.1016/j.bbr.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner LL. Cocaine reward and locomotor activity in C57BL/6J and 129/SvJ inbred mice and their F1 cross. Pharmacology, biochemistry, and behavior. 1997;58:25–30. doi: 10.1016/s0091-3057(96)00465-0. [DOI] [PubMed] [Google Scholar]
- Morgan D, Liu Y, Oleson EB, Roberts DC. Cocaine self-administration on a hold-down schedule of reinforcement in rats. Psychopharmacology. 2009;201:601–9. doi: 10.1007/s00213-008-1328-z. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. Blocking of conditioning to a cocaine-paired stimulus: testing the hypothesis that cocaine perpetually produces a signal of larger-than-expected reward. Pharmacology, biochemistry, and behavior. 2007;86:774–7. doi: 10.1016/j.pbb.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, et al. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. The Journal of neuroscience. 2002;22:7687–94. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addictive behaviors. 1997;22:157–67. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR. Cocaine effects during D-amphetamine maintenance: a human laboratory analysis of safety, tolerability and efficacy. Drug and alcohol dependence. 2009;99:261–71. doi: 10.1016/j.drugalcdep.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Li S, Chavkin C. Behavioral stress may increase the rewarding valence of cocaine-associated cues through a dynorphin/kappa-opioid receptor-mediated mechanism without affecting associative learning or memory retrieval mechanisms. Neuropsychopharmacology. 2010;35:1932–42. doi: 10.1038/npp.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain research. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Current psychiatry reports. 2007;9:388–95. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Smirnov MS, Kiyatkin EA. Cocaine action on peripheral, non-monoamine neural substrates as a trigger of electroencephalographic desynchronization and electromyographic activation following i.v. administration in freely moving rats. Neuroscience. 2010;165:500–14. doi: 10.1016/j.neuroscience.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction biology. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–9. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Litvin Y, Piras AP, Pfaff DW, Kreek MJ. Persistent increase in hypothalamic arginine vasopressin gene expression during protracted withdrawal from chronic escalating-dose cocaine in rodents. Neuropsychopharmacology. 2011;36:2062–75. doi: 10.1038/npp.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Dobrin CV, Roberts DC. Brain-cocaine concentrations determine the dose self-administered by rats on a novel behaviorally dependent dosing schedule. Neuropsychopharmacology. 2011;36:2741–9. doi: 10.1038/npp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]