Expanding applications of nanomaterials, particularly carbonaceous nanoparticles (CNP), in new technologies, consumer products and biomedicine, imply their increasing levels of manufacturing. In occupational and environmental settings, the lung is the major portal of unintended CNP entry into the human body potentially leading to pulmonary damage, inflammation, oxidative stress, fibrosis, and granuloma formation.[1,2] In addition, there are numerous attempts to utilize nanoparticles for better delivery of drugs and nucleic acid-based therapeutics to disease sites in the lung, particularly to the lung epithelium. The inhalation of drug nano-formulations propelled the development of new strategies in therapy of several human lung diseases such as asthma, cystic fibrosis, chronic obstructive pulmonary disease, lung cancer, tuberculosis, etc.[3–6] Safety and lack of adverse health effects remain the major prerequisites for broader applications of these novel technologies. Toxicological assessments of nano-particles typically are performed on normal animals.[7] Thus possible effects of CNP on tumor growth have not yet been considered. The immune system safeguards the host from infections and malignancies. Recognition and undesirable interactions of CNP with cells of the immune system may lead to immunomodulation, hence increasing the host’s susceptibility to infections and cancer.[8] For instance, carbon nanotubes (CNT) can generate oxidative stress and inflammation that can interfere with the immune responses and, in turn, lead to enhanced susceptibility to and adverse outcomes from pulmonary infection.[9] Whether CNT-induced immunosuppression can augment tumor growth or alter tumor-host interactions is currently unknown. Here we report that pulmonary CNT aspiration renders the host susceptible to lung carcinoma formation in the murine metastasis/dissemination model. This effect was mediated by increased local and systemic accumulation of myeloid-derived suppressor cells (MDSC), as their depletion abrogated tumor-supporting activity of CNT in vivo.
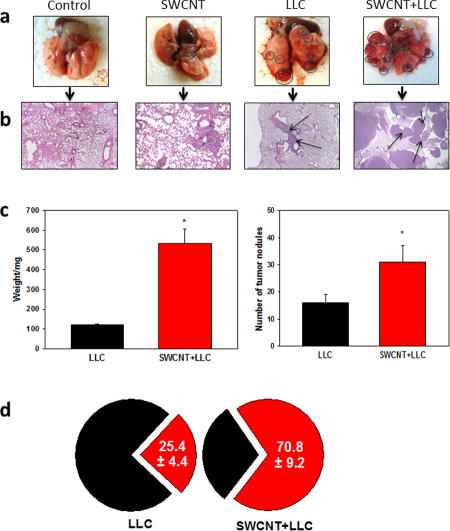
To determine whether Single Walled CNT (SWCNT) may affect tumor progression in vivo, we utilized syngeneic metastatic Lewis lung carcinoma (LLC) as an approved model of pulmonary metastatic disease in mice. Animals were pre-treated - via pharyngeal aspiration - with short pristine (short-cut) CNT (average size: 230 nm; 80 μg/mouse) to avoid possible pro-carcinogenic effects of CNT with high aspect ratios.[10] Of note, chemical cutting of CNT is associated with the appearance of carboxy- and hydroxy-functionalities on their surface.[11] Growth of transplanted tumor cells followed typical exponential kinetics with the appearance of numerous tumor nodules in the lung by day 21 after i.v. tumor cell inoculation. Morphologically, the lung tumors showed characteristic features of poorly-differentiated non-small cell carcinoma, such as nuclear pleomorphism and hyperchromasia, high nuclear/cytoplasmic ratio, brisk mitotic activity, extensive apoptosis and small areas of necrosis (Figure 1). Exposure of mice to SWCNT prior to tumor cell injection resulted in significant acceleration of tumor growth revealed by several parameters. First, the weight of the lungs in tumor-bearing animals, reflecting the overall tumor burden, increased up to 5-fold in SWCNT-treated mice compared to the control group of mice (without CNT exposure) (Figure 1c). Second, SWCNT pre-treatment caused significant, up to 2.5-fold, elevation of the number of visible pulmonary macro-metastasis (Figure 1a,c), as well as increasing the total area of metastatic nodules upon histopathology evaluation of the lung tissues (Figure 1b). In control tumor-bearing mice, the tumor nodules were of different size, but collectively comprised about 25% of tissue on the slides. Pre-treatment with SWCNT caused the appearance of significantly larger tumor nodules than in control group, resulting in up to a 3-fold increase in their collective area on tissue slides (Figure 1d). SWCNT did not change the typical morphological features of tumor, but accelerated tumor growth and the appearance of intratumoral necrosis zones, associated with rapid tumor growth (Figure 1). These data indicate that exposure of SWCNT promoted establishment and growth of pulmonary metastasis in mice in vivo.
Figure 1. Acute exposure to SWCNT accelerated formation and growth of lung carcinoma.
Pathogen-free female C57BL6/J mice received SWCNT (80 μg/mouse) by pharyngeal aspiration 48 h prior to Lewis lung carcinoma cells (LLC, 3×105 cells) via the tail vein and sacrificed 3 weeks later. The lungs were collected for visualization and counting of macroscopic tumor nodules (a,c), weight (c) and immunohistochemical (IHC) analysis of micrometastases after H&E staining (b,d). Lungs with tumor nodules (circled) and IHC of lung specimens with micrometastases (arrows) (x100) (b) are shown from a representative experiments. The average weight of the lungs and number of tumor nodules were calculated from 4 independent experiments with 7–8 mice/group in each experiment (c). The average collective areas of lung carcinoma on tissue slides are shown for LLC and SWCNT+LLC groups (d). *, p<0.05 (Student t-test, n=4).
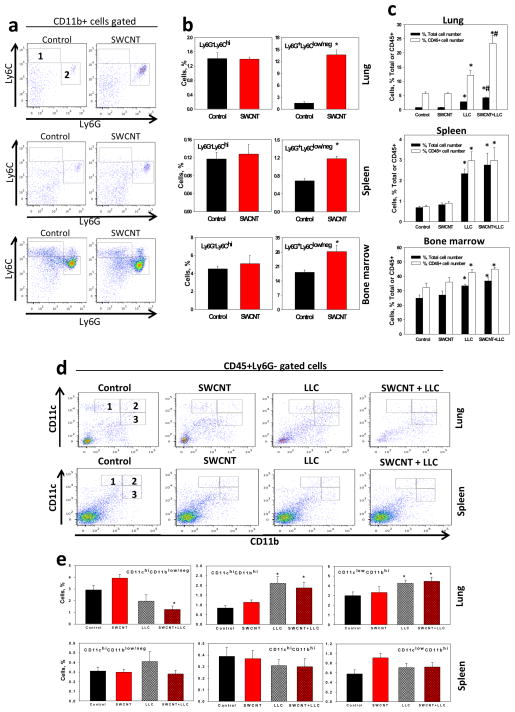
Next, we explored whether the tumor-supporting activity of CNT may be associated with the modulation of the tumor immunoenvironment. Myeloid-derived suppressor cells are one of the main cell populations responsible for regulating the host immune responses to tumors.[12] MDSC, among other myeloid regulatory cells, are accumulated in tumor-bearing hosts and support tumor growth and progression by inhibiting antitumor immunity, stimulating intratumoral neoangiogenesis, and sustaining tumor cell spreading and metastases.[13] Thus, we examined whether SWCNT exposure affected MDSC in the lungs. We found that SWCNT significantly up-regulated the generation and systemic homing of granulocytic MDSC. Elevated numbers of CD11b+Ly6G+Ly6Clow MDSC were detected in the lung, spleen and bone marrow of SWCNT-treated tumor-free animals 48 h after CNT administration (Figure 2a,b).
Figure 2. Single aspiration of SWCNT up-regulated accumulation of MDSC in tumor-free and tumor-bearing mice but did not up-regulate immature and regulatory dendritic cell subsets in tumor-bearing mice.
The levels of monocytic CD11b+Ly6GnegLy6Chigh (area 1) and granulocytic CD11b+Ly6G+Ly6Clow/neg (area 2) MDSC in the lymphoid tissues and lungs in tumor-free C57Bl6/J mice 48 h after SWCNT (80 μg/mouse) or PBS (control group) pharyngeal aspiration were assessed by flow cytometry (a) and statistical analysis of data from 4 independent experiments each with 7–8 mice/group is shown as the mean ± SEM of accumulated polymorphonuclear MDSC *, p<0.05 (Student t-test, n=4) (b). Accumulation of MDSC in the lungs and lymphoid tissues in tumor-free and LCC-bearing mice pre-treated with SWCNT (80 μg/mouse) were assessed 21 days after administration of LLC cells (3×105 cells) or PBS (control groups). The results are shown as the percentage of MDSC among total cells (black bars) and CD45+ leukocytes (green bars). *, p<0.05 versus Control group; #, p<0.05 versus LLC group (One-way ANOVA, n=4) (c). Similarly, the levels of conventional (CD11chighCD11blow/neg) (area 1), immature (CD11chighCD11bhigh) (area 2) and regulatory (CD11clowCD11bhigh) (area 3) DC subsets in tumor-free and tumor-bearing mice were assessed in the lungs and spleens three weeks after LLC cell (3×105 cells) inoculation by flow cytometry (d) and distribution of these DC subsets are represented from experiment of 7–8 mice/group. The results are shown as the percentage of DC among total cells. *, p<0.05 versus Control group (One-way ANOVA, n=3) (e).
Based on the detected CNT induced marked acute accumulation of MDSC in lymphoid and non-lymphoid tissues, we hypothesized that the presence of MDSC in the lungs might support the local in growth of tumor cells. To test this, we first characterized MDSC in tumor-bearing mice. As anticipated, three weeks after tumor inoculation, control tumor-bearing mice showed a significant increase, up to 3–4-fold, in the amount of granulocytic MDSC in the lung, spleen and bone marrow as compared to non-tumor-bearing animals (Figure 2c). However, the levels of MDSC accumulation in lungs, but not in the lymphoid tissues, were additionally significantly increased up to 2-fold in SWCNT-pre-treated animals as compared to vehicle-treated controls (Figure 2c). This robust enhancement of MDSC accumulation by CNT pre-exposure was evident when either the total numbers of MDSC subsets were quantified or when their amounts were normalized by the total population of CD45+ leucocytes in the tissues with different levels of tumor burden (Figure 2c). The elevated levels of MDSC may be due to the overall increase of tumor burden in CNT-treated mice and/or initial up-regulation of MDSC accumulation after CNT inhalation, as shown above. However, significantly higher levels of MDSC in the lungs of CNT-pretreated mice support the latter mechanism. Alternatively, expansion of regulatory T cells (Tregs) in the tumor environment has been suggested to confer tumor growth and metastatic advantages by inhibiting antitumor immunity.[14] However, neither SWCNT exposure nor administration of LLC cells up-regulated accumulation of CD4+CD25+FoxP3+ regulatory T cells in mice, as shown, for example for splenic Treg cells (Figure S2). This is in line with the data in the literature where no alterations of Treg cells have been revealed for a number of different tumor models.[15, 16]
Growth of LLC cell-derived tumors may be associated with the accumulation of immunosuppressive regulatory dendritic cells (regDC) [17, 18] and exposure to CNT may alter DC in vivo resulting in systemic immune suppression.[19] Therefore, we next assessed different subsets of DC in control and treated mice. Based on the co-expression of CD11b and CD11c molecules[20] we confirmed that progression of lung metastases was accompanied by decreased levels of conventional CD11chighCD11blow/neg DC and increased accumulation of immature CD11chighCD11bhigh DC and regulatory CD11clowCD11bhigh DC in the lung cancer microenvironment (Figure 2d,e). Exposure of mice to SWCNT prior to tumor cell administration did not cause significant alterations in DC subsets, suggesting that down-regulation of conventional DC and up-regulation of immature DC and regDC cannot explain the strong tumor growth stimulation effect of CNT administration.
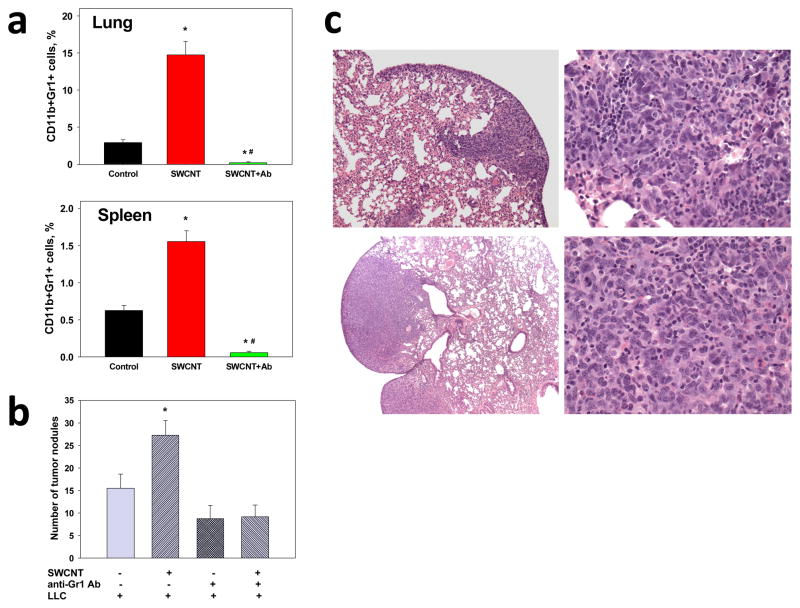
Assuming that acceleration of tumor formation and growth in the lung induced by CNT pre-exposure is due to up-regulation of MDSC, we reasoned that depletion of MDSC may lead to suppression of metastatic growth of tumors. We used anti-Gr-1 Ab[21] to deplete MDSC. We found that anti-Gr-1 Ab effectively prevented CNT-induced up-regulation of MDSC in the lungs and spleen (Figure 3a). Therefore, we tested whether anti-Gr-1 Ab pretreatment of animals would affect CNT-enhanced metastatic growth of lung tumors by suppressing the MDSC expansion. Remarkably, selective elimination of MDSC induced by the pre-treatment with anti-Gr-1 Ab, completely blocked CNT-mediated acceleration of the formation and growth of lung metastases (Figure 3b). Specifically, neither the number of macro-metastases (193.8% versus 108.1% increase in mice without and with MDSC depletion, respectively; p<0.05) nor the weight of the lungs (432.2% versus 93.8% increase in mice without and with MDSC depletion, respectively; p<0.05) were altered as a result of exposure to CNT. These results were confirmed by pathohistological examination of the lung tissues (Figure 3c) demonstrating the similar levels of micrometastasis formation in control and CNT-pretreated tumor-bearing mice after initial depletion of MDSC induced by pre-exposure to CNT. Sections in both groups show small nodules of poorly-differentiated non-small cell carcinoma with the largest measuring 1–2 mm in greatest dimension. At high power, tumor cells show nuclear pleomorphism and hyperchromasia, high nuclear/cytoplasmic ratio, brisk mitotic activity, extensive apoptosis and mild intratumoral and peritumoral leukocytic infiltration. These data provide strong evidence in favor of up-regulation of MDSC accumulation as an important mechanism contributing to CNT induced enhanced metastatic growth of lung carcinoma.
Figure 3. Depletion of SWCNT-induced MDSC suppressed metastatic growth of lung tumors.
Depletion of MDSC was carried out using Ly-6G/Ly-6C (Gr-1) antibody. (a) Analysis of CD11b+Gr-1+ MDSC in tumor-free C57BL6/J mice 48 h after SWCNT (80 μg/mouse) pharyngeal aspiration. Control animals received PBS aspiration and rat IgG Ab (i.p., 50 μg/day). The results are the mean of MDSC percentage among total cells ± SEM from two independent experiments with 6–7 mice per group in each experiment. *, p<0.05 versus Control group (One-way ANOVA); #, p<0.05 versus SWCNT group (One-way ANOVA). (b) Formation of visual macro-metastases in mice 3 weeks after LLC cell (3×105) administration and pre-treatment with SWCNT (80 μg/mouse). Although depletion of MDSC decreased formation of lung metastases, it completely blocked the stimulating effect of SWCNT aspiration on tumor formation. The results are the mean ± SEM from 3 independent experiments with 6–7 mice/group in each experiment. *, p<0.05 versus other groups (One-way ANOVA). (c) Histopathological analysis of micrometastases in the lungs of C57BL6/J mice treated with SWCNT (low panels) or PBS (control, upper panels) following by MDSC depletion and sacrificed 3 weeks after LLC cell (3×105) inoculation (x100 and x400). H&E stained slides demonstrated the presence of similar small nodules of poorly-differentiated non-small cell carcinoma in both groups with the similar levels of micrometastasis formation.
Several reports have alerted to the potential carcinogenicity of high aspect ratio CNT.[10, 22] In-vitro studies indirectly suggest the potential carcinogenicity of CNT based on their ability to induce DNA damage, and the activation of signal transduction pathways related to asbestos-induced lung cancer and mesothelioma.[23, 24] It has been also reported that prolonged exposure of human lung epithelial cell line to SWCNT in cultures induced malignant transformation of treated cells, which obtained the ability to form subcutaneous tumors in a xenograft model with immunodeficient mice.[25] However, the possibility of CNT’s action on host-tumor interactions and growth of tumors has not been considered. In this study, we employed non-pristine (chemically-cut) CNT containing carboxy- and hydroxy-functionalities on their surface. Our data indicate that non-pristine CNT promote metastatic tumor establishment and growth by altering the tissue microenvironment, specifically by facilitating MDSC accumulation. Notably, it has been shown that effects of CNT on cells, including (geno)toxicity in vitro and in vivo, may differ significantly for pristine and functionalized nanotubes being strongly dependent on the type of functional groups on their surface.[26] Overall, carboxylated and hydroxylated CNT may exert stronger modifying effects than pristine nanotubes.[27–29] It is likely that inflammatory MDSC induced by pulmonary exposure to CNT support the initial tumor cell homing in the lung and then are polarized into tumor-associated MDSC. The latter are known to block the development of anti-tumor immunity hence facilitate tumor growth by producing specific growth factors and cytokines that also stimulate intratumoral neoangiogenesis. However, possible effects of nanoparticles on systemic immune reaction invoked by tumor progression have not been addressed so far. Further studies of the specific pathways through which CNT and other nanoparticles may affect the immune cells in the tumor microenvironment and cause immunosuppression and unresponsiveness are warranted. This will lead to the design of tumor-specific theranostics platforms with modules capable of depleting or functionally suppressing MDSC (and other myeloid regulatory cells) to ensure effective immunosurveillance in the tumor microenvironment.
EXPERIMENTAL SECTION
Experimental procedures are provided in the Supporting Information section of the paper.
Supplementary Material
Acknowledgments
This work was supported by NIOSH OH008282, NTRC 927ZJHF, NIEHS R01ES019304, NIH RO1 CA154369.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Supporting Information is available on the WWW under http://www.small-journal.com or from the author.
References
- 1.Andujar P, Lanone S, Brochard P, Boczkowski J. Rev Mal Respir. 2011;28:e66. doi: 10.1016/j.rmr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE. Toxicol Appl Pharmacol. 2012;261:121. doi: 10.1016/j.taap.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy I, Vij N. Nanomedicine. 2010;6:23. doi: 10.1016/j.nano.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohenegger M. Curr Pharm Des. 2010;16:2484. doi: 10.2174/138161210791959890. [DOI] [PubMed] [Google Scholar]
- 5.Mitchison DA, Fourie PB. Tuberculosis (Edinb) 2010;90:177. doi: 10.1016/j.tube.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Conti DS, Bharatwaj B, Brewer D, da Rocha SR. J Control Release. 2012;157:406. doi: 10.1016/j.jconrel.2011.09.089. [DOI] [PubMed] [Google Scholar]
- 7.Yang K, Liu Z. Curr drug metab. 2012 doi: 10.2174/138920012802850029. [DOI] [PubMed] [Google Scholar]
- 8.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Endocrinol. 2010;151:458. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shvedova AA, Kisin ER, Porter D, Schulte P, Kagan VE, Fadeel B, Castranova V. Pharmacol Ther. 2009;121:192. doi: 10.1016/j.pharmthera.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, Yamashita K, Yoshikawa Y, Yasui H, Jiang L, Ohara H, Takahashi T, Ichihara G, Kostarelos K, Miyata Y, Shinohara H, Toyokuni S. Proc Natl Acad Sci USA. 2011;108:E1330. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, Tyurina YY, Shi J, Kisin ER, Murray AR, Franks J, Stolz D, Gou P, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA. Nat Nanotechnology. 2010;5:354. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condamine T, Gabrilovich DI. Trends Immunol. 2011;32:19. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Nat Rev. 2012;12:253. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teng MW, Ritchie DS, Neeson P, Smyth MJ. Curr Top Microbiol Immunol. 2011;344:61. doi: 10.1007/82_2010_50. [DOI] [PubMed] [Google Scholar]
- 15.Kimpfler S, Sevko A, Ring S, Falk C, Osen W, Frank K, Kato M, Mahnke K, Schadendorf D, Umansky V. J Immunol. 2009;183:6330. doi: 10.4049/jimmunol.0900609. [DOI] [PubMed] [Google Scholar]
- 16.Whiteside TL. Semin Cancer Biol. 2012 [Google Scholar]
- 17.Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. J Immunol. 2009;182:6207. doi: 10.4049/jimmunol.0803926. [DOI] [PubMed] [Google Scholar]
- 18.Shurin GV, Ouellette CE, Shurin MR. Cancer Immunol Immunother. 2012;61:223. doi: 10.1007/s00262-011-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tkach AV, Shurin GV, Shurin MR, Kisin ER, Murray AR, Young SH, Star A, Fadeel B, Kagan VE, Shvedova AA. ACS Nano. 2011;5:5755. doi: 10.1021/nn2014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Shurin GV, Gutkin DW, Shurin MR. Semin Cancer Biol. 2012 doi: 10.1016/j.semcancer.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Han Y, Guo Q, Zhang M, Cao X. J Immunol. 2009;182:240. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson K, Murphy F, Schinwald A, Duffin R, Poland CA. Nanomedicine (London, England) 2011;6:143. doi: 10.2217/nnm.10.139. [DOI] [PubMed] [Google Scholar]
- 23.Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, Schwegler-Berry D, Ducatman BS, Sbarra D, Hoover MD, Castranova V, Vallyathan V. Environ Health Perspect. 2008;116:1211. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, Kitajima S, Kanno J. J Toxicol Sci. 2008;33:105. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Luanpitpong S, Castranova V, Tse W, Lu Y, Pongrakhananon V, Rojanasakul Y. Nano Lett. 2011;11:2796. doi: 10.1021/nl2011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roda E, Coccini T, Acerbi D, Barni S, Vaccarone R, Manzo L. Histol Histopathol. 2011;26:357. doi: 10.14670/HH-26.357. [DOI] [PubMed] [Google Scholar]
- 27.Mu Q, Du G, Chen T, Zhang B, Yan B. ACS Nano. 2009;3:1139. doi: 10.1021/nn900252j. [DOI] [PubMed] [Google Scholar]
- 28.Coccini T, Roda E, Sarigiannis DA, Mustarelli P, Quartarone E, Profumo A, Manzo L. Toxicology. 2010;269:41. doi: 10.1016/j.tox.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Luo M, Deng X, Shen X, Dong L, Liu Y. J Nanosci Nanotechnol. 2012;12:274. doi: 10.1166/jnn.2012.5700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.