Abstract
Monoclonal antibodies against mesothelin are being evaluated for the treatment of mesothelioma and multiple forms of cancers, and show great promise for clinical development for solid cancers. Antibodies against mesothelin have been shown to act via immunotoxin-based inhibition of tumor growth and induction of antibody-dependent cellular cytotoxicity (ADCC). However, complement-dependent cytotoxicity (CDC), considered an important additional mechanism of therapeutic antibodies against tumors, is inactive for such antibodies. Here, we used phage display antibody engineering technology and synthetic peptide screening to identify SD1, a human single-domain antibody to mesothelin. SD1 recognizes a conformational epitope at the C-terminal end (residues 539–588) of mesothelin close to the cell surface. To investigate SD1 as a potential therapeutic agent, we generated a recombinant human Fc (SD1-hFc) fusion protein. Interestingly, the SD1-hFc protein exhibits strong CDC activity, in addition to ADCC, against mesothelin-expressing tumor cells. Furthermore, it causes growth inhibition of human tumor xenografts in nude mice as a single agent. SD1 is the first human single-domain antibody targeting mesothelin-expressing tumors, shows potential as a cancer therapeutic candidate, and may improve current antibody therapy targeting mesothelin-expressing tumors.
Keywords: antibody dependent cell mediated cytotoxicity, complement-dependent cytotoxicity, mesothelin/MSLN, MORAb-009/amatuximab, phage display
Introduction
Mesothelin has been suggested as a therapeutic target because it is highly expressed in malignant mesotheliomas (1, 2) and other solid tumors such as cholangiocarcinoma, ovarian cancer, lung adenocarcinoma and breast cancer (3, 4, 5). The mesothelin (MSLN) gene encodes a ~71 kDa precursor protein that is processed to a ~31 kDa N-terminal protein and a ~ 40 kDa C-terminal membrane-bound mature mesothelin (3). A number of anti-mesothelin monoclonal antibodies (mAbs) have been developed. The mAb drugs, SS1P immunotoxin and MORAb-009 (also known as amatuximab), are currently being evaluated in clinical trials (3, 4). SS1P is an immunotoxin consisting of a murine anti-mesothelin Fv fused to a truncated Pseudomonas exotoxin that mediates cell killing (6). MORAb-009, a chimeric (mouse/human) antibody based on the murine SS1 Fv, elicits antibody-dependent cell-mediated cytotoxicity (ADCC) on mesothelin-bearing tumor cells (7). Recently, we and our collaborators generated two fully human mAbs (HN1 and m912) that recognize mesothelin (8, 9). HN1 recognizes an epitope overlapping the SS1 site in mesothelin, indicating that HN1 can be developed as a fully human version of SS1 Fv-based mAbs (such as MORAb-009).
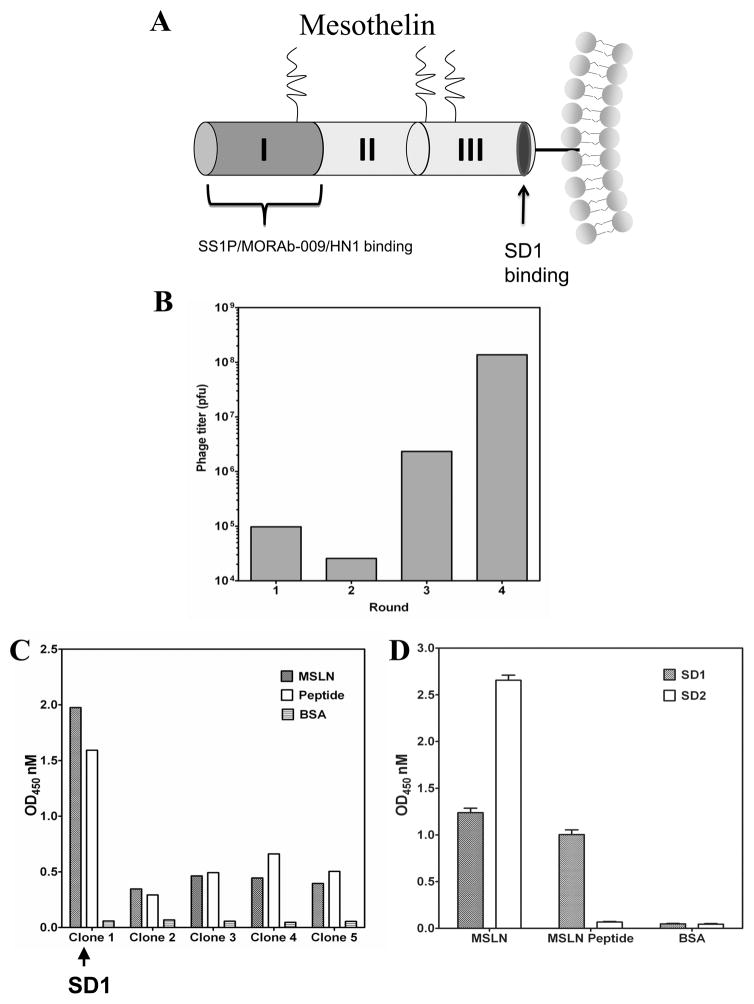
In our previous report, we proposed three distinct domains in cell-surface mature mesothelin (10): Regions I (residues 296–390), II (residues 391–486) and III (residue 487–598) (Fig. 1A). We experimentally established a minimum recognition sequence (named IAB; residues 296–359) in Region I for the binding of mucin MUC16/CA125. However, despite the fact that several mesothelin mAbs are now available, none have shown complement-dependent cytotoxicity (CDC) against tumor cells.
Figure 1.
Generation of a human single-domain antibody to the C-terminal end of mesothelin. (A) Design of the peptide used for screening human antibodies by phage display technology. (B) Phage panning on the C-terminal mesothelin peptide. (C) Monoclonal phage ELISA. The SD1 phage clone (Clone 1) bound both full-length mesothelin (MSLN) and the C-terminal peptide. (D) Monoclonal phage ELISA using two selected antibody phage clones (SD1 and SD2).
CDC has been suggested as an important additional mechanism for cancer therapeutic antibodies (11). The first approved mAb for cancer therapy, rituximab, is partially dependent on CDC for its anti-tumor activity (12, 13). It has been suggested that CDC may occur when the antibody binding site is close to the cell membrane (14). As evidence, ofatumumab, which binds much closer to the cell membrane of CD20 than rituximab, also has much higher CDC activity (14). However, a new anti-CD20 mAb (obinutuzumab or GA101) exhibits strong inhibition of cell growth in addition to ADCC, but no CDC (15,16). Almost all of the existing mesothelin mAbs recognize Region I, the N-terminal end of cell-surface mesothelin presumed to be located far from the cell membrane (10) (Fig. 1A). ADCC is the only mechanism that has been found to contribute to the activity of known anti-mesothelin mAbs. Therefore, we hypothesize that a more desirable anti-mesothelin mAb will be capable of causing additional anti-tumor activity (i.e., CDC, direct inhibition of tumor cell growth) as well as ADCC by targeting novel epitopes. To this end, antibodies recognizing a domain in mesothelin beyond Region I must be made and tested.
To generate antibodies with potential CDC against tumors, we surmised that they should bind Region III of mesothelin close to the cell surface as ofatumumab does. However, such mAbs have been challenging to generate because this region is poorly immunogenic. Our recent study using rabbit hybridoma technology produced around 8000 individual clones immunized by a full-length mesothelin protein. 96% of all positive clones were Region I-binders (like HN1 and SS1/MORAb-009). Only three were Region III binders. None bound the C-terminal end of mesothelin (Ho and Phung, unpublished data). This finding was consistent with our previous mouse hybridoma screening, in which almost all high-affinity binders bound Region I (17). Given that standard hybridoma technology failed to produce antibodies specific for the desired C-terminal end of mesothelin, we used phage display technology to identify new anti-mesothelin human mAbs. Epitopes close to the cell surface may be occluded and difficult to access by full-size IgG antibodies and large fragments such as Fabs. Therefore, we used a phage display library of smaller binders, human single-domain (VH) antibodies displayed on phage, and panned it against a peptide corresponding to the C-terminal end of mesothelin. After isolating the SD1 human antibody domain, we converted it to a human Fc fusion protein (SD1-hFc) for analysis. The SD1-hFc protein shows strong anti-tumor activity against tumor cells in vitro and inhibits xenograft tumor growth in nude mice, suggesting use for potential antibody therapeutics that could improve current mesothelin-targeted cancer therapy.
Materials and methods
Cell Culture
Human cholangiocarcinoma (CCA) lines (KMBC, Mz-ChA-1 and HuCCT-1) were obtained from Gregory J. Gores at the Mayo Clinic in Rochester, Minnesota (18). A431 (epidermal carcinoma), OVCAR3 (ovarian) and NCI-H226 (mesothelioma) were obtained from American Type Culture Collection (Manassas, VA). EKVX (human non-small cell lung cancer, or NSCLC) and OVCAR-8 (ovarian cancer) were obtained from National Cancer Institute (Development Therapeutics Program). L55 (NSCLC) was provided by Steven M. Albelda at the University of Pennsylvania (Philadelphia, PA). All tumor cell lines were grown as described (8, 18). A431/H9 is a transfected A431 cell line stably expressing human mesothelin (19). The HEK-293F cell line (Invitrogen, Carlsbad, CA) were grown in FreeStyle serum-free medium (Invitrogen). All cell lines were passaged only a few times (less than 1 month) after thawing of initial frozen stocks, which were generated right after obtaining cell lines, to reduce total number of passages to less than 15. All cell lines were tested and authenticated by morphology and growth rate and were Mycoplasma free.
Screening an engineered human antibody domain library
An engineered human (VH) antibody domain library named m8l showed an estimated diversity of 2.5×1010 (20). The C-terminal mesothelin peptide consisting of 50 amino acids (NH2-VQKLLGPHVEGLKAEERHRPVRDWILRQRQDDLDTLGLGLQGGIPNGYLV-COOH) was synthesized (GenScript, Piscataway, NJ). Full-length human mesothelin protein (MSLN) was prepared as described (10). The phage library was subjected to four rounds of panning on full-length human mesothelin (rabbit Fc-mesothelin fusion protein, rFc-MSLN) or the C-terminal mesothelin peptide coated on Nunc 96-well ELISA plates (Maxisorb, Nunc/Thermo Fisher Scientific, Rochester, NY) according to our lab protocol (21, 22). Randomly picked 384 phage clones at the end of each round of panning were analyzed for antigen binding by phage enzyme-linked immunosorbent (ELISA) assays.
Production of a SD1-human Fc fusion protein
The VH region encoding the SD1 human antibody domain fused with human IgGγ1 Fc and FLAG/His tag was PCR amplified with two primers (Forward: 5′-GTC ATC ACA ACT TCG ATA TCG CGG TGC AGC GGT GCA GTC TGG GGG AGG CTT GGT A-3′; reverse: 5′-GAA GTT GTG ATG ACT CCG GAG CCC TTA TCG TCA TCG TCC TTG TAG TCG CCG TGG-3′). The PCR product was inserted into the EcoRV and BspEI sites (underlined) of the vector, pVRC8400 (provided by Gary J. Nabel, the National Institute of Allergy and Infectious Diseases) (23, 24). The final plasmid (named pMH148) was transfected into HEK-293F cells and the protein was purified using protein A column (GE healthcare, Piscataway, NJ). We established a stable cell line by transfecting HEK-293F (Invitrogen) cells with pMH148. The stable line produced the SD1-hFc fusion protein with a high expression level (>70 mg/L) in culture supernatant.
Immunoprecipitation and western blot analysis
Cell lysate (1.5 mg) was incubated with 50 μg of SD1 or an irrelevant single-domain human Fc fusion protein in 500 μl of RIPA buffer (Cell signaling, Boston, MA) and rotated overnight at 4°C. Thirty μl of protein A beads were added (Sigma, St. Louis, MO) and rotated at 4°C for 2 hours. Beads were spun down and washed with RIPA buffer. Immune complexes were released from the beads after 5 minutes of boiling in 100 μl of 2× loading buffer. Western blot analysis was performed following a lab protocol (18).
ELISA
Direct ELISA and affinity measurement - direct binding and affinity of SD1-hFc were evaluated on the ELISA plates coated with mesothelin peptide, human mesothelin, or mouse mesothelin (mMSLN) following the procedures described previously (10).
Competition ELISA - various amounts of mesothelin peptide, an irrelevant 50 amino acid peptide, the HN1 human mAb or the SS1P immunotoxin (kindly provided by Ira Pastan, NCI) were mixed with 5 μg/ml of SD1-hFc and incubated at room temperature for 1 hour. The mixture was then transferred to an ELISA plate coated with 5 μg/ml of human mesothelin protein and incubated at room temperature for an additional hour following the procedure described above.
Flow cytometry
To determine the binding of the SD1 antibody to cell surface associated mesothelin, flow cytometry analysis was performed according to our standard protocol (18). The average number of mesothelin sites per cell was measured on a FACSCalibur (BD Biosciences, San Jose, CA) using BD Quantibrite™ PE beads (BD Biosciences).
To investigate whether SD1 inhibits the binding of mesothelin to MUC16, 10 μg/ml of rFc-MSLN protein were pre-incubated with different concentration of SD1 or SS1P, and then incubated with MUC16-positive ovarian cancer cells (OVCAR3). Binding of rFc-MSLN to cell surface-associated MUC16 on OVCAR3 cells was analyzed following a flow cytometry protocol (10).
C1q and anti-mesothelin binding assays were conducted following an established protocol (14, 25). Briefly, A431/H9 or NCI-H226 cells were suspended at 1×106 cells/ml and incubated with different concentration of SD1-hFc, control human IgG or the HN1 human IgG on ice for 1 hour. After washing, cells were incubated with 20 μg/ml purified C1q (Complement Technologies, Tyler, TN) at 37C° for 0.5 hour. The cells were washed again and then incubated with FITC-labeled sheep anti-human C1q mAb (AbD Serotec, Raleigh, NC) for 0.5 hour on ice. At the end of the incubation, cells were washed and analyzed on a FACSCalibur.
ADCC and CDC
ADCC assay was performed by using human peripheral blood mononuclear cells (PBMC), purified human natural killer (NK) cells (26) and mouse NK cells (27), and measured by an LDH kit (Roche, Mannheim, Germany) according to a standard protocol (8). Human NK cells were isolated from the peripheral blood of healthy donors using the NK cells isolation kit (Miltenyi Biotec, Auburn, CA). Mouse NK cells were purified following a protocol as previously described (27). The purity of mouse NK cells was determined by an anti-CD49b conjugated with FITC (eBioscience, SanDiego, CA) on a FACSCalibur.
CDC activity was measured by LDH-releasing assay according to previous reports (14). Normal human sera were provided by the Department of Transfusion Medicine, NIH Clinical Center (Bethesda, MD) and mouse serum was freshly drawn before use. Percentage of specific lysis was calculated according to the following formula: % lysis = [experimental release-spontaneous release]/[maximum release- spontaneous release] × 100.
Xenograft anti-tumor testing in mice
Animal testing evaluating SD1-hFc was conducted using A431/H9 xenografts in nude mice following a well-established NCI protocol (7; 28). Four to six week old female athymic nude mice (NCI-Frederick Animal Production Area, Frederick, MD) were housed in micro-isolation cages during the course of the experiment. The animal protocol (LMB-059) was approved and mice were maintained as per institutional guidelines of the NIH. Three million A431/H9 cells were inoculated subcutaneously into the right flank of the mice. Tumor dimensions were determined using calipers and the tumor volume (mm3) was calculated by the formula: length (width) 2× 0.5. Treatment was initiated when tumors reached approximately 70 mm3 in size. The different treatment regimens included: PBS and SD1-hFc (50 mg/kg) via i.v. injection on days 7, 9, 11, 14, 17, 20 after tumor inoculation. Mice were sacrificed when tumors reached over 1000 mm3.
Results
Discovery and production of the SD1 human antibody
To find a new anti-mesothelin mAb targeting a site close to the cell surface, we designed a C-terminal mesothelin peptide for screening and used a library of small-size binders (VHs). Using the big-PI Predictor program, we predicted the GPI cleavage site was Ser598 (3, 4, 10). We designed a peptide (residues 539–588; NH2-VQKLLGPHVEGLKAEERHRPVRDWILRQRQDDLDTLGLGLQGGIPNGYLV-COOH) which is 10 amino acids away from the GPI cleavage site of mesothelin (Fig. 1A) for phage panning with an engineered human antibody domain phage display library (20) (Supplementary Table S1). After the fourth round of panning, phage titer was significantly increased (Fig. 1B) and more than 95% of clones were peptide binders. Phage clone SD1 was selected for further analysis because it bound not only the peptide but also full-length mesothelin (Fig. 1C). In comparison, we screened the same single-domain antibody phage library on full-length human mesothelin protein and found five different VH sequences. The clone SD2 sequence was found in 90% of all phage clones (total 384) analyzed at the end of the last round, indicating SD2 was highly enriched. By ELISA, SD2 was specific for full-length human mesothelin, but not the peptide (Fig. 1D). Since our aim was to isolate an antibody that binds to a site close to the cell surface, SD2 did not meet our criteria. We decided to choose SD1 for further analysis.
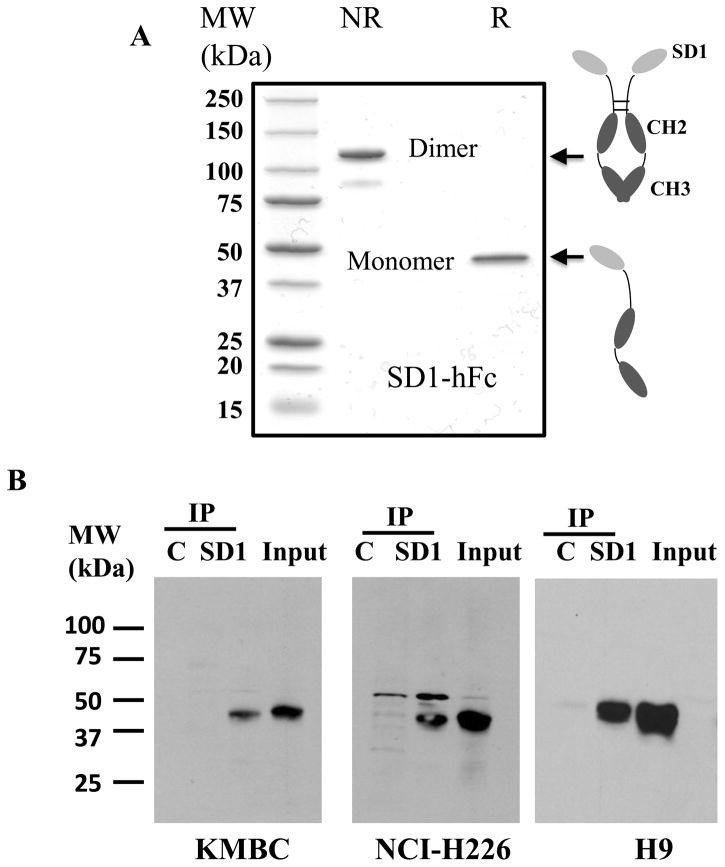
To investigate SD1 as a potential therapeutic, we converted it into a clinically relevant human Fc (hFc) fusion protein (Fig. 2A). For large-scale production, we established a stable cell line with a high expression level (>70 mg/L) in the culture supernatant of HEK-293F cells and the purity of SD1-hFc protein was greater than 95%
Figure 2.
Production and analysis of the SD1 human Fc fusion protein. (A) SDS-PAGE analysis of purified SD1-hFc (4 μg of protein per lane). NR: non-reducing; R: reducing; (B) Immunoprecipitation of endogenous mesothelin protein in A431/H9 (16), NCI-H226 and KMBC cell extracts. SD1 was used to pull down endogenous mesothelin protein in the cell lysate. C: an irrelevant VH single-domain human Fc fusion; IP: immunoprecipitation; Input: western blot on whole cell lysates before immunoprecipitation.
The SD1 human antibody binds cancer cell surface-associated mesothelin
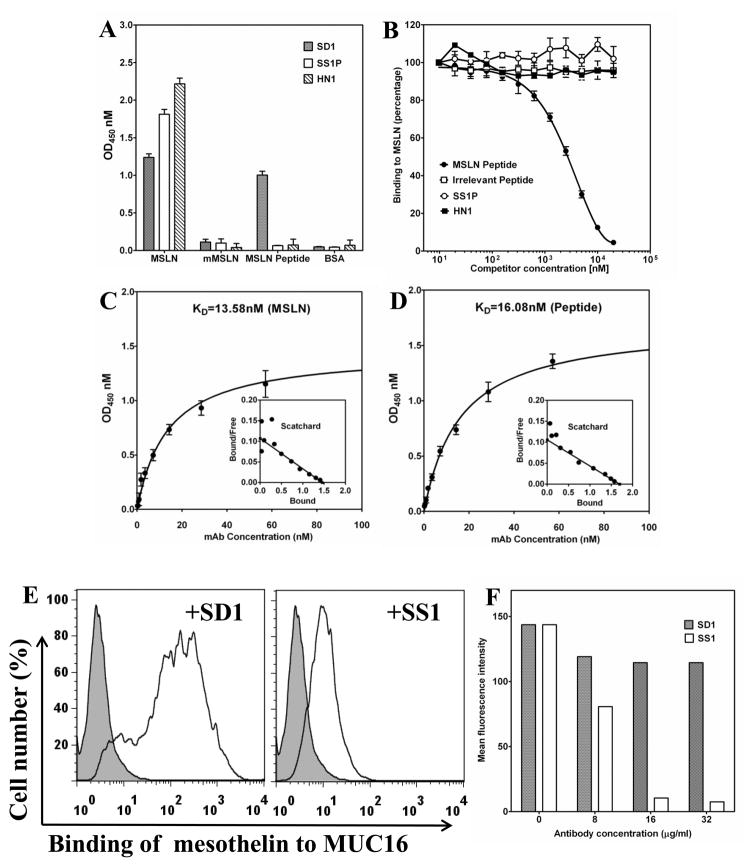
To analyze the binding properties of SD1 to mesothelin protein in cancer cells, we perform Western blot and pull-down assays using cancer cell lysates. Initial Western blot analysis of various cancer cell lysates using SD1-hFc could not detect a mesothelin band under reducing conditions even in high mesothelin-expressing cell lines such as A431/H9 and NCI-H226 (data not shown), indicating that SD1-hFc did not recognize denatured mesothelin protein. By conducting pull-down assays to detect endogenous mesothelin proteins in solution (Fig. 2B), SD1-hFc successfully pulled down mature mesothelin protein from three different cancer cell lines (A431/H9, NCI-H226 and KMBC). The molecular weight of mature mesothelin (~ 40 kDa) was consistent with previous studies (18, 29). In ELISA assays, SD1-hFc bound both full-length human mesothelin protein and peptide (Fig. 3A) and did not bind full-length mouse mesothelin protein, BSA or other irrelevant proteins. As expected, SS1P and HN1 bind only full-length human mesothelin, not the C-terminal peptide.
Figure 3.
Binding properties of SD1-hFc. (A) Direct ELISA. MSLN: full-length human mesothelin protein (MSLN); mMSLN: mouse mesothelin. (B) Competition ELISA. (C) The dissociation equilibrium KD of SD1-hFc to the human mesothelin protein was 13.58 nM and (D) 16.08 nM for the peptide. (E, F) Recombinant mesothelin protein bound to MUC16 in the presence of SD1.
To evaluate whether SD1-hFc recognizes the C-terminal end, we pre-incubated SD1-hFc, HN1, or SS1P with peptide (residues 539–588) and tested binding of the antibody-peptide mixture to human mesothelin coated on an ELISA plate. Competition ELISAs (Fig. 3B) showed that the C-terminal peptide blocked the binding of SD1-hFc, not SS1P or HN1, to full-length human mesothelin. We also measured the kinetics of SD1 binding using full-length mesothelin protein and the C-terminal peptide. SD1-hFc binds to human mesothelin protein with dissociate equilibrium (KD) of 13.59 nM (Fig. 3C), and to the peptide with a KD of 16.08 nM (Fig. 3D). The equilibrium constants and Scatchard plots were determined by using Prism (version 3.02) for Windows (GraphPad software, San Diego, CA) (10). To determine whether SD1 blocks the binding of mesothelin to MUC16, we pre-incubated SD1 or SS1 with mesothelin, added the mixture to MUC16-positive OVCAR3 cells, and then performed flow cytometry analysis. We found that unlike SS1, SD1 did not inhibit the mesothelin-MUC16 interaction (Fig. 3E and F) indicating the SD1 binding site does not overlap the SS1 site.
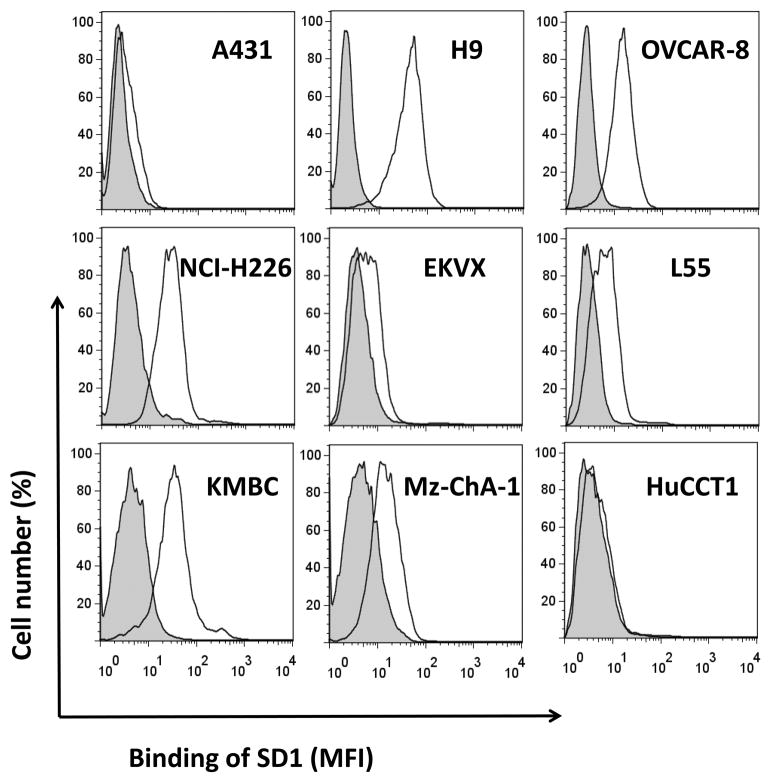
To analyze whether SD1 is suitable for cancer therapy, we determined whether SD1-hFc binds native mesothelin molecules on human tumor cells. We performed flow cytometric analysis on a panel of mesothelin-expressing cancer cells and experimentally measured the average number of mesothelin sites per cell using the QuantiBRITE fluorescence quantitation system (Supplementary Table S2). SD1-hFc binds A431/H9, but not A431, indicating that binding on cell surface-associated mesothelin is highly specific (Fig. 4). We tested the binding of SD1-hFc on a panel of native human tumor cell lines. SD1-hFc strongly bound human ovarian cancer (OVCAR-8), mesothelioma (NCI-H226), and cholangiocarcinoma cell lines (KMBC). It moderately bound human cholangiocarcinoma cell line (Mz-ChA-1) and weakly bound human NSCLC cell lines (EKVX and L55). It did not bind to cholangiocarcinoma cells, HuCCT1, which is a mesothelin-negative line (18). SD1 recognizes a conformational epitope of native mesothelin close to the cancer cell surface and binds cell-surface associated native mesothelin proteins with high affinity and excellent specificity.
Figure 4.
Flow cytometry analysis of the SD1-hFc protein on mesothelin-expressing cancer cells. MFI: Mean Fluorescence Intensity.
Anti-tumor activity of SD1-hFc: CDC and ADCC
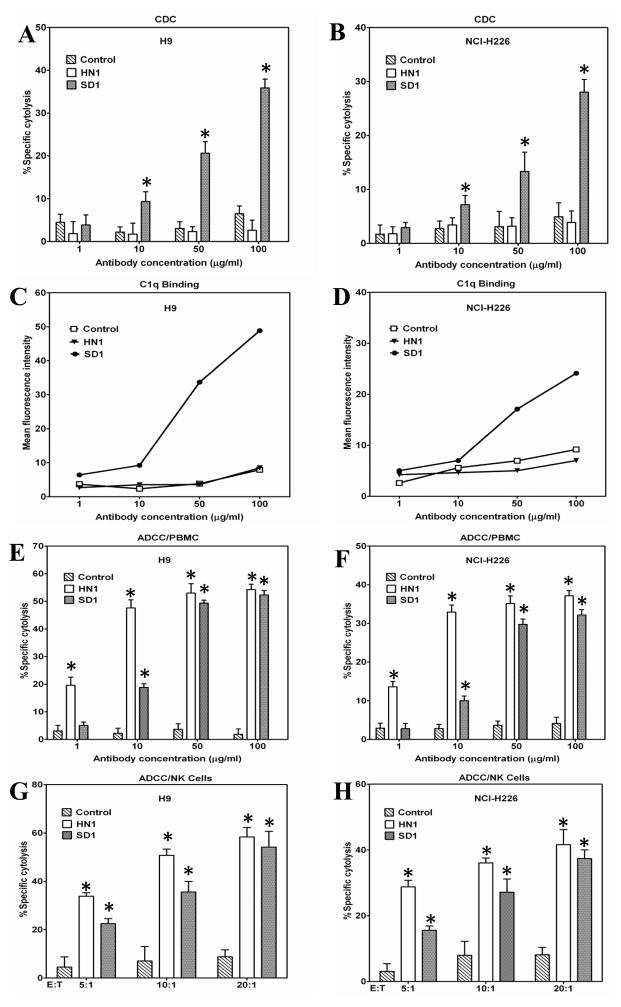
To evaluate the anti-tumor activity of SD1-hFc against cancer cells, we tested the cytotoxic activity in A431/H9 and NCI-H226 cell models in the presence of human serum as a source of complement. A human VH single-domain antibody isolated from the same phage library specific for an irrelevant tumor cell surface antigen was used as a control in the present study. In CDC assays, SD1-hFc exerted potent CDC activity by killing 40% of A431/H9 (Fig. 5A) and more than 30% of NCI-H226 mesothelioma cell lines (Fig. 5B) and showed no activity on the mesothelin-negative A431 cell line (data not shown). In contrast, the control single-domain antibody and HN1 showed no activity at the same concentrations, indicating that targeting to the C-terminal epitope of SD1 is capable of inducing CDC. A previous study showed that MORAb-009 showed no significant CDC activity against tumor cells (7). To analyze the role of complements in the anti-tumor activity of SD1-hFc, we used flow cytometry to determine C1q binding to cancer cells reacted with anti-mesothelin human mAbs following a well-established protocol for characterization of rituximab, ofatumumab and other anti-CD20 therapeutic mAbs (14, 25). As shown in Figure 5C and D, the C1q complement bound to A431/H9 or NCI-H226 cells in the presence of SD1-hFc. However, no C1q binding was found in the presence of HN1 or an irrelevant human single-domain antibody control. Moreover, the binding of C1q to cancer cells is associated with the binding of SD1-hFc in a dose-response manner. These results demonstrate that the C-terminal end binder SD1-hFc, not the N-terminal end binder HN1, can recruit C1q to the mesothelin-expressing cell surface. The HN1 human IgG has an extra CH1 domain in its Fc portion. To determine whether the CH1 domain in the HN1 IgG may interfere with its potential CDC activity on tumor cells, we generated a new HN1single chain Fv(scFv)-Fc fusion protein by fusing the HN1 scFv to the same Fc sequence used in SD1-hFc (Supplementary Fig. S3A). When comparing SD1-hFc with HN1(scFv)-hFc in CDC assays, we found that HN1(scFv)-hFcdid not show any CDC activity (Supplementary Fig. S3B and C). Therefore, it is unlikely that the CDC activity observed with SD1 treatment was related to its shorter Fc portion.
Figure 5.
SD1-hFc caused CDC and ADCC against mesothelin-expressing cancer cells. (A, B) CDC. A431/H9 (A) and NCI-H226 (B) cells were incubated with increasing concentrations of SD1-hFc in the presence of normal human serum (NHS, 20% vol/vol) as a source of complement The complement C1q bound H9 (C) and NCI-H226 (D) cells in the presence of SD1-hFc, not HN1 or a control hFc fusion protein. (E, F, G, H) ADCC. Freshly isolated PBMC were incubated with target cells A431/H9 (E) and NCI-H226 (F) cells at ratio of 50:1, in the presence of SD1-hFc with increasing concentrations. (G, H) Purified human NK cells incubated with target cells A431/H9 (G) or NCI-H226 (H) cells at different E:T ratios with 50 μg/ml of SD1-hFc. CDC and ADCC activity were determined by LDH assay (*: p < 0.05).
In addition to CDC activity, we tested ADCC activity of SD1-hFc against tumor cells. High levels of cytotoxicity were found using SD1-hFc with human peripheral blood mononuclear cells (PBMC) at different concentrations or purified human NK cells with various ratios between effector cells and target cells (E:T). SD1 exhibited significant ADCC activity by killing more than 40% against A431/H9 cells (Fig. 5E) and more than 30% of NCI-H226 mesothelioma cells (Fig. 5F). No activity was found on mesothelin-negative A431 cells (data not shown). NK cells played an important role in SD1-mediated ADCC. Using purified human NK cells, SD1 killed almost 20% cells even with the lower E: T ratio of 5:1 (Fig. 5G and H). Taken together, SD1-hFc has strong CDC and ADCC anti-tumor activity against mesothelin-expressing tumor cells in vitro.
Anti-tumor activity in mice
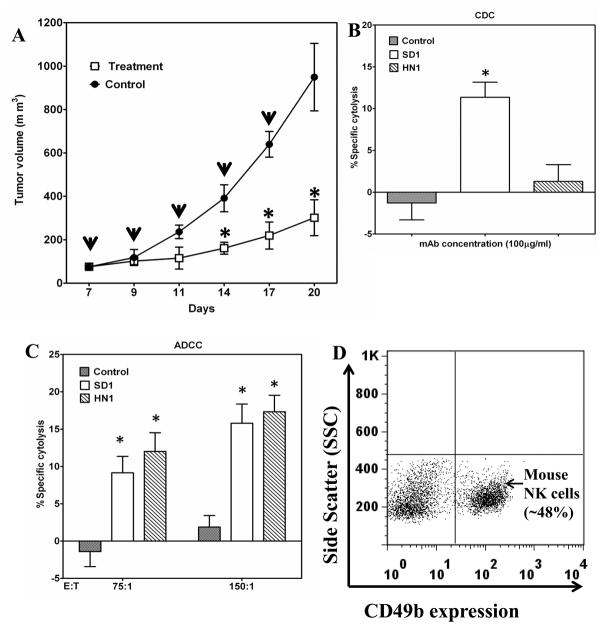
To evaluate the anti-tumor activity of SD1-hFc in vivo, we used immunodeficient mice bearing tumor xenografts following an established protocol used to evaluate MORAb-009 in preclinical studies (7). Athymic nude mice bearing A431/H9 tumors were treated with 50 mg/kg of SD1-hFc (Fig. 6A). The number of mesothelin sites in A431/H9 is comparable to that of malignant mesothelioma cells endogenously expressing mesothelin and their implantation in mice consistently results in aggressive tumor growth. Twenty days after inoculation of tumor cells, the average tumor size in mice treated with SD1-hFc alone was significantly reduced (average 300 mm3) compared to the control group (average 1000 mm3), demonstrating that SD1-hFc is very active as a single agent.
Figure 6.
Strong anti-tumor effect of SD1-hFc on tumor growth. (A) A431/H9 cells were inoculated in the flank of nude mice to establish tumors of approximately 70 mm3 in size. From day 7, mice were treated with SD1-hFc (50 mg/kg) or PBS every other day. The down-pointing arrows indicate the day of injections. Average tumor size for each treatment group was calculated on days 7–20. (*: p < 0.05). (B) A431/H9 cells were incubated with 100 μg/mL of SD1-hFc in the presence of normal mouse serum (30% vol/vol) as a source of mouse complement. CDC activity was measured by LDH assay (*: p < 0.05). (C) A431/H9 cells were incubated with 100 μg/mL of SD1-hFc in the presence of purified mouse NK cells as a source of mouse effector cells. Mouse ADCC activity was measured by LDH assay (*: p < 0.05). (D) The purity of mouse NK cells.
To evaluate SD1-induced CDC in mice, we examined CDC activity using mouse sera and found that SD1-hFc killed 11% of A431/H9 cells in the presence of 30% mouse serum freshly drawn from nude mice (Fig. 6B), indicating that mouse complement was 10-fold less active than human complement (Fig. 5) for SD1-hFc, consistent with previous studies involving human Fc (30). SD1 was able to induce ADCC with purified mouse NK cells and killed mesothelin-positive cancer cells (H9) with different E:T ratios (Fig. 6C). The purity of mouse NK cells was around 50% (Fig. 6D). Taken together, SD1-hFc caused growth inhibition of tumor xenografts in nude mice. Both CDC and ADCC may contribute to the anti-tumor activity of SD1-hFc observed in vivo.
Discussion
In the present study, we used phage display to develop an engineered antibody domain, called SD1, recognizing an epitope at the C-terminus of mesothelin. This epitope does not overlap with any previously developed mesothelin therapeutic antibodies in preclinical or clinical development. SD1-hFc shows strong CDC and ADCC activity against mesothelin-expressing cancer cells in vitro and causes growth inhibition of human tumor xenografts in nude mice in vivo. Our results suggest that the SD1 single-domain antibody represents a new class of anti-mesothelin mAbs and is a promising therapeutic candidate for novel mesothelin-targeted therapy.
We isolated the SD1 domain by phage panning on a C-terminal 50-residue peptide of mesothelin and showed the antibody binds native mesothelin proteins in cancer cells. This region has never been accessed by any known anti-mesothelin antibodies. Our data demonstrate that CDC triggered by SD1-hFc depends on the specific new epitope because the HN1 human IgG (specific for the N-terminus of mesothelin, Region I, far from the cell surface) does not exhibit CDC activity and cannot recruit C1q to cancer cells. Furthermore, the HN1(scFv)-hFc that contains the same Fc sequence of SD1-hFc does not exhibit CDC activity. We also tested SD1 and HN1 in cell proliferation assays and found that none of these antibodies exhibited direct inhibition of tumor cell growth (data not shown). It remains uncertain if any anti-mesothelin antibodies can directly inhibit tumor cell proliferation. Taken together, our results indicate that CDC likely contributes to the anti-tumor activity of SD1.
MORAb-009 is the only anti-mesothelin antibody currently being evaluated in clinical trials (31, 32, 33, 34) and should be compared to SD1. MORAb-009 is a mouse/human chimeric IgG antibody containing mouse Fv with improved affinity (35) while SD1 is a fully human VH single-domain antibody isolated from a naïve human antibody phage library. SD1 recognizes the C-terminus of cell surface-associated mesothelin while MORAb-009 and other existing antibodies including HN1 recognize Region I. SD1 exhibited strong CDC activity while a previous study indicated that MORAb-009 did not (7). Interestingly, SD1 caused 70% growth inhibition of tumor xenografts in nude mice as a single agent while MORAb-009 showed about 40% growth inhibition (7). Future studies are necessary to directly compare SD1 with MORAb-009 in both in vitro and in vivo assays.
Human single-domain antibodies are attractive candidates for cancer therapy. However, human VHs are typically prone to aggregation (36). In the present study, we fused the SD1 VH to CH2 and CH3 of human IgGγ1 and produced SD1-hFc as a dimeric IgG-like protein in mammalian HEK-293F cells. We also produced recombinant immunotoxins based on SD1 and showed it could inhibit proliferation of mesothelin-positive tumor cells in a dose-dependent manner (Supplementary Table S2 and Supplementary Fig. S1). All recombinant proteins were properly folded for in vitro and in vivo assays. Currently, SD1 and SD2 are the only two single-domain antibodies targeting mesothelin that have been identified. To determine whether the SS1 VH alone in MORAb-009 is functional, we also constructed VH single-domain molecules based on SS1 as well as HN1. We expressed and compared the VH domains of SD1, SS1 and HN1 using mammalian cell display technology (37). Only SD1 VH, not the VH domain of SS1 or HN1, bound mesothelin (Supplementary Fig. S2)
We noticed the discrepancy between calculated and actual molecular weights of the SD1-hFc and HN1(scFv)-hFc on SDS-PAGE gels (Supplementary Fig. S3). Similar discrepancies were previously described in other studies (38, 39, 40, 41). We ran commercially available human IgG proteins on a SDS-PAGE gel (Supplementary Fig. S3D) and found a molecular weight of a dominant human IgG band of 250 kDa, instead of the 150 kDa theoretical value. This discrepancy may not be rare in human IgG or Fc fusion molecules although the precise mechanism is unclear. To evaluate whether N-glycosylation on human Fc may affect the actual molecular weight, we treated human Fc fusion proteins with PNGase F and found that enzymatic deglycosylation only modestly reduced molecular masses (Supplementary Fig. S3E and F). This discrepancy may be due to the Y-shaped structure of IgG molecules, which it is distinct from other globular proteins (41).
Although our in vivo animal testing using a xenograft tumor model in nude mice showed strong anti-tumor activity of SD1-hFc, it would be interesting to conduct complement depletion studies to further validate the role of CDC in vivo. Future studies may further improve the anti-tumor activity of SD1 by affinity maturation of the Fv portion (21) and by enhancing ADCC via glycoengineering of the Fc portion (15, 16), or by combining the SD1 antibody therapy with chemotherapy (7).
In summary, we generated the first human single-domain antibody against mesothelin-expressing tumors and showed it has strong anti-tumor activity in vitro and in vivo by targeting an epitope close the cancer cell surface via CDC as well as ADCC. Such a binding site has not been accessed by any known anti-mesothelin antibodies. Development of the SD1 human antibody may lead to novel antibody therapies targeting mesothelioma and other mesothelin-expressing tumors.
Supplementary Material
Acknowledgments
Financial support:
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI), Center for Cancer Research (Z01 BC 010891 and ZIA BC 010891) and by the 2011 NCI Director’s Intramural Innovation Award (Principal Investigator Award) to M Ho. This work was also possible due to a Mesothelioma Applied Research Foundation Craig Kozicki Memorial Grant to M Ho. M Ho is a Zijiang Lecture Professor of East China Normal University. Z Tang is on the NIH Graduate Partnerships Program.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. We thank the NIH Fellows Editorial Board for editorial assistance.
Footnotes
Disclosure of potential conflicts of interest:
No potential conflicts of interest were disclosed.
References
- 1.Chang K, Pai LH, Batra JK, Pastan I, Willingham MC. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res. 1992;52:181–6. [PubMed] [Google Scholar]
- 2.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93:136–40. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho M. Advances in liver cancer antibody therapies: a focus on glypican-3 and mesothelin. Biodrugs. 2011;25:275–84. doi: 10.2165/11595360-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchou J, Wang LC, Selven B, Zhang H, Conejo-Garcia J, Borqhaei H, et al. Mesothelin, a novle immunotherapy target for triple negative breast cancer. Breast Cancer Res Treat. 2012;133:799–804. doi: 10.1007/s10549-012-2018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 7.Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 8.Ho M, Feng M, Fisher RJ, Rader C, Pastan I. A novel high-affinity human monoclonal antibody to mesothelin. Int J Cancer. 2011;128:2020–30. doi: 10.1002/ijc.25557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Y, Xiao X, Zhu Z, Streaker E, Ho M, Pastan I, et al. A novel human monoclonal antibody that binds with high affinity to mesothelin-expressing cells and kills them by antibody-dependent cell-mediated cytotoxicity. Mol Cancer Ther. 2009;8:1113–8. doi: 10.1158/1535-7163.MCT-08-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneko O, Gong L, Zhang J, Hansen JK, Hassan R, Lee B, et al. A binding domain on mesothelin for CA125/MUC16. J Biol Chem. 2009;284:3739–49. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–7. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 13.Cragg MS, Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti- CD20 reagents. Blood. 2004;103:2738–43. doi: 10.1182/blood-2003-06-2031. [DOI] [PubMed] [Google Scholar]
- 14.Pawluczkowycz AW, Beursken FJ, Beum PV, Lindorfer MA, van de Winkel JG, Parren PW, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183:749–58. doi: 10.4049/jimmunol.0900632. [DOI] [PubMed] [Google Scholar]
- 15.Mossner E, Brunker P, Moser S, Punterner U, Schmidit C, Herter S, et al. Increasing the efficacy of CD20 antibody by therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH, et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood. 2011;117:4519–29. doi: 10.1182/blood-2010-07-296913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onda M, Willingham M, Nagata S, Bera TK, Beers R, Ho M, et al. New monoclonal antibodies to mesothelin useful for immunohistochemistry, fluorescence-activated cell sorting, Western blotting, and ELISA. Clin Cancer Res. 2005;11:5840–6. doi: 10.1158/1078-0432.CCR-05-0578. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Feng M, Kim H, Phung Y, Kleiner DE, Gores GJ, et al. Mesothelin as a potential therapeutic target in human cholangiocarcinoma. J Cancer. 2010;1:141–9. doi: 10.7150/jca.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–20. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Zhu Z, Feng Y, Xiao X, Dimitrov DS. Construction of a large phage-displayed human antibody domain library with a scaffold based on a newly identified highly soluble, stable heavy chain variable domain. J Mol Biol. 2008;382:779–89. doi: 10.1016/j.jmb.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho M, Kreitman RJ, Onda M, Pastan I. In vitro antibody evolution targeting germline hot spots to increase activity of an anti-CD22 immunotoxin. J Biol Chem. 2005;280:607–17. doi: 10.1074/jbc.M409783200. [DOI] [PubMed] [Google Scholar]
- 22.Ho M, Pastan I. In vitro antibody affinity maturation targeting germline hotspots. Methods Mol Biol. 2009;525:293–308. doi: 10.1007/978-1-59745-554-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barouch DH, Yang ZY, Kong WP, Korioth-Schmitz B, Sumida SM, Truitt DM, et al. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol. 2005;79:8828–34. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ofek G, McKee K, Yang Y, Yang ZY, Skinner J, Guenaga FJ, et al. Relation-ship between antibody 2F5 neutralization of HIV-1 and hydrophobicity of its heavy chain third complementarity-determining region. J Virol. 2010;84:2955–62. doi: 10.1128/JVI.02257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Shi S, Qian W, Zhao L, Zhang D, Hou S, et al. Development of novel tetravalent anti- CD20 antibodies with potent antitumor activity. Cancer Res. 2008;68:2400–8. doi: 10.1158/0008-5472.CAN-07-6663. [DOI] [PubMed] [Google Scholar]
- 26.Xiang X, Feng M, Felder M, Connor JP, Man YG, Patankar MS, et al. HN125: A novel immunoadhesin targeting MUC16 with potential for cancer therapy. J Cancer. 2011;2:280–91. doi: 10.7150/jca.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhary A, Hilton MB, Seaman S, Haines DC, Stevenson S, Lemotte PK, et al. TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer Cell. 2012;21:212–26. doi: 10.1016/j.ccr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Hansen JK, Xiang L, Kawa S, Onda M, Ho M, et al. A flow cytometry method to quantitate internalized immunotoxins shows that taxol synergistically increase cellular immunotoxins uptake. Cancer Res. 2010;70:1082–9. doi: 10.1158/0008-5472.CAN-09-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho M, Bera TK, Willingham MC, Onda M, Hassan R, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–5. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 30.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, et al. Complement activation determines the therapeutic activity of Rituximan in vivo. J Immunol. 2003;171:1581–7. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 31.Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2012;16:6132–8. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov [homepage on the Internet] Identi er: NCT01417000. [Google Scholar]
- 33.ClinicalTrials.gov [homepage on the Internet] Identi er: NCT00738582. [Google Scholar]
- 34.Kelly RJ, Sharon E, Pastan I, Hassan R. Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther. 2012;11:517–25. doi: 10.1158/1535-7163.MCT-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowdhury PS, Pastan I. Improving antibody affinity by mimicking somatic hypermutation in vitor. Nat Biotechnol. 1999;17:568–72. doi: 10.1038/9872. [DOI] [PubMed] [Google Scholar]
- 36.Arbabi-Ghahroudi M, Tanha J, MacKenzie R. Isolation of monoclonal antibody fragments from phage display libraries. Methods Mol Biol. 2009;502:341–64. doi: 10.1007/978-1-60327-565-1_20. [DOI] [PubMed] [Google Scholar]
- 37.Ho M, Nagata S, Pastan I. Isolation of anti-CD22 Fc with high affinity by Fv display on human cells. Proc Natl Acad Sci U S A. 2006;103:9637–42. doi: 10.1073/pnas.0603653103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Y, Li F, Zha D, Potgieter TI, Mithcell T, Moore R, et al. Purification process development of a recombinant monoclonal antibody expressed in glycoengineered Pichia pastoris. Protein Expr Purif. 2011;76:7–14. doi: 10.1016/j.pep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Jung ST, Reddy ST, Kang TH, Borrok MJ, Sandlie I, Tucker PW, et al. Aglycosylated IgG variants expressed in bacteria that selectively bind FcgammaRI potentiate tumor cell killing by monocyte-dendritic cells. Proc Natl Acad Sci U S A. 2010;107:604–9. doi: 10.1073/pnas.0908590107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan CE, Lim AP, Chan AH, MacAry PA, Hanson BJ. Optimized expression of full-length IgG1 antibody in a common E. coli strain. PLoS One. 2012;5:e10261. doi: 10.1371/journal.pone.0010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stork R, Zettlitz KA, Muller D, Rether M, Hanisch FG, Kontermann RE. N-glycosylation as novel strategy to improve pharmacokinetic properties of bispecific single-chain diabodies. J Biol Chem. 2008;283:7804–12. doi: 10.1074/jbc.M709179200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.