Abstract
Rationale
Rats bred for high (HiS) and low (LoS) saccharin intake exhibit divergent behavioral responses to multiple drugs of abuse, with HiS rats displaying greater vulnerability to drug taking. Previous research indicates that this effect may be due to increased sensitivity to reward in HiS rats and to the aversive effects of acute drug administration in LoS rats.
Objective
The current study investigated whether HiS and LoS rats also exhibit different behavioral signs of withdrawal following one or repeated opiate exposures.
Methods
Emotional signs of opiate withdrawal were assessed with potentiation of the acoustic startle reflex and conditioned place aversion (CPA) in male and female HiS and LoS rats. Startle was measured before and 4 h after a 10 mg/kg injection of morphine on days 1, 2, and 7 of opiate exposure. CPA was induced with a two-day, naloxone-precipitated conditioning paradigm. Somatic signs of withdrawal and weight loss were used also measured.
Results
Male and female LoS rats exhibited lower startle potentiation than HiS rats on the seventh day of morphine exposure. LoS male rats also failed to develop a CPA to morphine withdrawal. No differences in physical withdrawal signs were observed between HiS and LoS rats, but males of both lines had more physical signs of withdrawal than females.
Conclusions
These results suggest that LoS rats are less vulnerable to the negative emotional effects of morphine withdrawal than HiS rats. A less severe withdrawal syndrome may contribute to decreased levels of drug taking in the LoS line.
Keywords: CPA, morphine, rat, saccharin preference, startle, withdrawal, selective breeding
Introduction
Animal models of drug abuse vulnerability have identified several behavioral traits that predict drug self-administration, including activity levels, impulsivity, novelty reactivity, and preference for sweetened dietary substances (see Carroll et al. 2009 for review). The latter has been extensively studied in rats selectively bred for high (HiS) and low (LoS) saccharin intake (Carroll et al. 2008). HiS and LoS rats display divergent behavioral responses to multiple drugs of abuse. For instance, HiS rats acquired cocaine (Carroll et al. 2002) and ethanol (Dess et al. 1998) self-administration at faster rates than LoS rats. The HiS line was also shown to be more resistant to extinction and more vulnerable to escalation and reinstatement of cocaine-seeking than LoS rats (Perry et al. 2006a). Because a history of drug abuse and preference for sweets is also linked in human subjects (Weiss 1982; Pomerleau et al. 1991; Kampov Polevoy et al. 1997; Kampov Polevoy et al. 2001; Janowsky et al. 2003), HiS and LoS rats are a useful model for studying how phenotypic differences in sweet preference contribute to or act as markers for addiction liability.
The behavioral overlap between compulsive consumption of highly palatable substances, such as sweets, and abused drugs is likely mediated by common neurobiological mechanisms (Avena et al. 2008; Mysels and Sullivan 2010). Of particular interest is the endogenous opioid system, as it is involved in both the positive and aversive effects of many abused drugs and highly palatable substances (Gosnell and Levine 2009; Wee and Koob 2010). Thus, in the current study we investigated whether the severity of withdrawal effects differs in HiS and LoS rats using a model of acute opiate (morphine) dependence. Since the development of addictive behavior relies on both the positive, rewarding effects of drugs of abuse as well as the negative emotional state that develops during withdrawal (Koob and Volkow 2010), characterizing the behavioral response of HiS and LoS rats to both the positive and negative emotional consequences of drug exposure is necessary to understand why HiS rats are addiction-prone while LoS rats are addiction-resistant.
Previous studies have shown that HiS rats are more responsive to the locomotor activating effects of cocaine (Wise and Bozarth 1987; Carroll et al. 2007). Additionally, in male rats, preference for an ethanol-paired flavor is apparent in HiS animals only (Dess et al. 2005). These studies suggest that HiS rats may be more sensitive to the rewarding effects of drugs. Another possibility is that LoS rats are more sensitive to the aversive effects of acute drug administration. Indeed, LoS rats show greater reactivity to stress such as reduced exploratory behavior in the open field test (Dess and Minor 1996) and greater stress-induced anorexia (Dess and Minor 1996) and analgesia (Dess et al. 2000).
Another variable of interest was potential sex differences in the withdrawal behaviors. While there are abundant findings indicating that females are more motivated than males during several phases of drug addiction (Anker and Carroll 2010; Becker and Hu 2008; Carroll et al. 2004; Carroll and Anker 2010), there are limited data regarding sex differences in the emotional signs of withdrawal from drugs of abuse. A number of studies have found increased physical signs of withdrawal in males from drugs such as ethanol (Devaud and Chadda 2001), morphine (Cicero et al. 2002), pentobarbital (Suzuki et al. 1985), and methaqualone (Suzuki et al. 1988). Similar findings have also been reported for phencyclidine (PCP) withdrawal in male vs. female monkeys (Perry et al. 2006) and for alcohol withdrawal in men vs. women (Deshmukh et al. 2003). These results raise the possibility that males are also more sensitive to the emotional effects of withdrawal; thus, analyzing for sex differences in the present studies will help answer this question.
To assess the emotional component of opiate withdrawal, male and female HiS and LoS rats were tested for potentiation of the acoustic startle reflex (“withdrawal-potentiated startle”) and CPA during withdrawal from acute morphine exposure. Both of these behavioral models are well-characterized measures of the negative affective component of the withdrawal syndrome. Startle is potentiated in response to both conditioned and unconditioned anxiogenic stimuli in rodents and humans, and the supposition that increased startle is a measure of anxiety is supported by evidence that it is blocked by anxiolytic drugs and inactivation of limbic system structures (Davis 2006). Potentiated startle following drug exposure is also attenuated by anxiolytic compounds and limbic system inactivation (Harris and Gewirtz. 2004; Harris et al. 2006; Cabral et al. 2009; Engelmann et al. 2009; Rothwell et al. 2009) as well as a second exposure to the drug (Engelmann et al. 2009; Rothwell et al. 2009; Radke et al. 2011) This latter finding supports the conclusion that startle potentiation following drug exposure is a withdrawal effect. CPA has also been widely used to assess the dysphoric or aversive aspects of withdrawal (Tzschentke 1998, 2007) and both of these models have been used to demonstrate that the expression of opiate withdrawal relies on structures of the extended amygdala and mesolimbic dopamine system (Kelsey and Arnold 1994; Bechara et al. 1995; Laviolette et al. 2002; Watanabe et al. 2002b; Harris et al. 2006; Radke et al. 2011; Radke and Gewirtz, 2012). The physical component of the withdrawal syndrome was also assessed by scoring somatic withdrawal signs after acute morphine exposure.
To determine whether opiate withdrawal differs in the early stages of drug dependence, rats were tested after one, two, or seven morphine exposures. Given that HiS rats are more liable to acquire addictive behaviors than LoS rats, and that drug withdrawal may motivate drug taking, it was hypothesized that HiS rats would exhibit greater withdrawal behavior than LoS rats. Additionally, because somatic opiate withdrawal signs have previously been shown to be greater in male than female rats and mice (Kest et al. 2001; Cicero et al. 2002), it was hypothesized that males would also exhibit greater withdrawal signs than females in this study.
Materials and Methods
Subjects
Adult male and female rats (130 ± 2.4 days old) selectively bred at the University of Minnesota (Carroll et al. 2002) from Occidental HiS and LoS lines (Occidental College, Los Angeles, CA) were used in this study. The HiS and LoS lines were cultivated through breeding pairs based on extreme saccharin phenotype scores. The lines were started as outbred lines, defined as no mating closer than second cousins (Dess and Minor 1996; Lohmiller and Swing 2006). To maintain this status, we continue to avoid sibling, half-sibling, and first cousin mating and every four to six generations we purchase rats from the founding stock (i.e. Sprague-Dawley from Harlan, formerly Holtzman), test them for saccharin preference, and mate both males and females with our selectively bred rats. Thus, the rats are selectively outbred from Holtzman/Harlan Sprague-Dawley founding stock (Dess and Minor 1996, Dess 2000; Carroll et al. 2008). The HiS and LoS rats used in our experiments are selected before being tested for saccharin preference, and thus are selected not on their individual preference scores but on those of their parents.
Rats were bred and housed in plastic cages with ad libitum access to rat pellet chow (Purina Mills, Minneapolis, MN, USA) and water, at all times except during experimental sessions. The humidity, temperature (21–23 °C), and light–dark cycle (12 h–12 h; lights on at 6:00 AM) were all regulated. Prior to the beginning of the experiment, animals were transferred to individual housing and gently handled for two consecutive days. All procedures conformed to the eighth edition of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Academies Press 2011) and were approved by the University of Minnesota Institutional Animal Care and Use Committee under protocol numbers 1008A87754 and 0812A55002. All laboratory facilities were approved by the American Association for the Accreditation of Laboratory Animal Care.
Drugs
Morphine sulfate was purchased from Mallinckrodt (Hazelwood, MO). Naloxone hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO). Systemically administered drugs were dissolved in 0.9% saline and injected subcutaneously. Throughout the text, 0 mg/kg denotes groups that were given vehicle injection. All drug doses are expressed as the weight of the salt.
Experiment 1: Potentiated startle during morphine withdrawal
Acoustic startle was tested in four identical plastic cages (17 × 8.5 × 11 cm) resting on compression springs and located within individual ventilated sound-attenuating chambers. Cage movement resulted in displacement of a piezoelectronic accelerometer (Model ACH-01, Measurement Specialties, Valley Forge, PA) attached to each cage. Voltage output from the accelerometer was filtered and amplified by a custom-built signal processor, digitized on a scale of arbitrary units ranging from 0-1000 (National Instruments SCB100 and PCI-6071E boards), and recorded using Matlab (The MathWorks, Natick, MA). Startle amplitude was defined as the peak accelerometer voltage during the first 200 ms after onset of the startle stimulus. High frequency speakers (Radio Shack Supertweeters, range = 5-40 kHz) located 10 cm beside each cage delivered the startle stimuli, which were 50-ms bursts of filtered white noise (low pass: 22 kHz, rise-decay <5 ms) at intensities of 95 or 105 dB. Ventilating fans delivered background noise of approximately 60 dB.
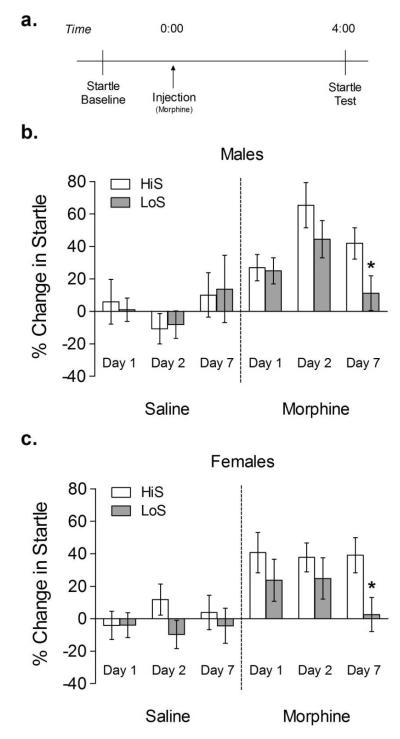
Each startle test session consisted of a 5 min acclimation period followed by presentation of 40 startle stimuli (10 blocks of 4 stimuli, 2 each at 95 and 105 dB in random order) with a 30 s fixed interstimulus interval. Activity levels were also monitored during the acclimation period and throughout the session. For each experiment, acoustic startle was first tested on 2 consecutive drug-free days. After the second day, average startle amplitudes were used to match animals into groups with similar overall mean startle amplitude. Rats were then injected with 0 or 10 mg/kg morphine sulfate for 7 consecutive days, and startle was tested 4 h after morphine injection on days 1, 2, and 7 (Harris and Gewirtz. 2004; Rothwell et al. 2009; Figure 1a). Each test day began at 9:00 AM with a pre-drug exposure, baseline startle session (pretest) and concluded with a final post-drug exposure startle session (posttest).
Fig 1. Startle potentiation during withdrawal from 1, 2, and 7 morphine exposures.
Rats were injected with saline or morphine (10 mg/kg) for 7 consecutive days and tested for startle on days 1, 2, and 7. Startle was tested immediately before and 4 h after morphine injection. White bars represent HiS rats and gray bars represent LoS rats. *p < 0.05 compared to HiS group on the same day.
Experiment 2: CPA to morphine withdrawal
CPA was induced using a previously established conditioning paradigm from our laboratory (Rothwell et al., 2009). The place conditioning apparatus consisted of a rectangular plastic cage (40 × 20 × 20 cm) divided into two sides by a central partition. Each side had a distinct floor texture and wall color: metal bars paired with white walls and wire mesh paired with black striped walls. The rat’s position within the conditioning chamber was monitored by an overhead video camera connected to a computer running ANY-Maze software (Stoelting, Wood Dale, IL).
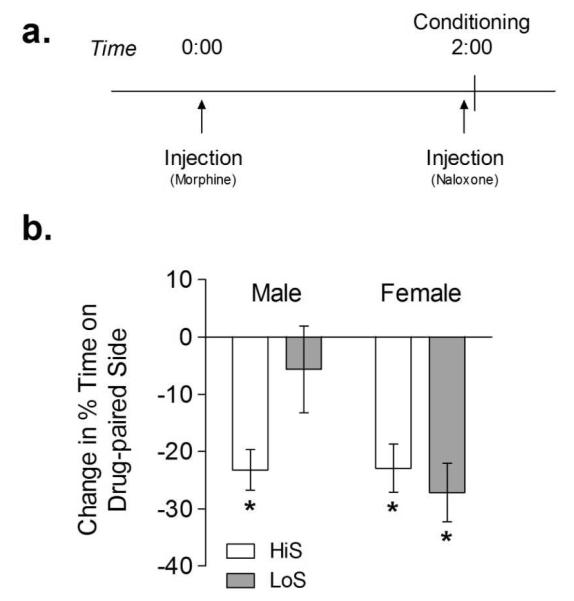
All test and conditioning sessions were conducted at 12:00 PM over four consecutive days. Rats were acclimated to the conditioning room for 10 min before each experimental session. The experiment began with a 10 min baseline session in which rats were free to move between both sides of the conditioning chamber. Rats with > 75% baseline preference for one side were excluded from further study (4 HiS and 3 LoS in total). The side of the chamber paired with drug treatment was counterbalanced within each experiment, yielding an unbiased procedure in which rats spent on average 50% of the baseline session on the drug-paired side. Two 30 min conditioning sessions, in which rats were injected with 0 or 10 mg/kg morphine at 0 h and 1 mg/kg naloxone at 2 h and then immediately confined to one side of the conditioning chamber, followed the baseline session (Figure 3a). All rats received 0 mg/kg on the first conditioning day and 10 mg/kg on the second conditioning day since naloxone given 24 h after morphine can still precipitate withdrawal signs. We used naloxone to reliably induce withdrawal at the time of context exposure, since we have found this approach produces more robust conditioning (Rothwell et al. 2009). A final 10 min test session, in which rats were free to move between both sides of the chamber, was conducted 24 h after the second conditioning session.
Fig 3. Conditioned place aversion to morphine withdrawal.
Rats were conditioned in a two-day procedure in which they were injected with saline or morphine (10 mg/kg) followed by naloxone (1 mg/kg) and then immediately confined to one side of the conditioning apparatus. The percentage of time spent on the drug-paired side of the apparatus during baseline and test was used to calculate change in % time spent on the drug-paired side. White bars represent HiS rats and gray bars represent LoS rats. *Significant difference between baseline and test (p < 0.01).
Experiment 3: Somatic signs during morphine withdrawal
Somatic signs of withdrawal were tested in a rectangular, clear plastic cage (40 × 20 × 20 cm) topped with a clear plastic lid. Rats were habituated to the cages for 10 min over 2 days. On test day rats were injected with morphine (10 mg/kg) at 0 h followed by naloxone (1 mg/kg) at 2 h and then immediately placed in the testing chamber for 10 min. Videos were scored later by an observer blind to experimental groups. The weighted scale of Gellert and Holtzman (1978) was used to score both graded and checked signs. Graded signs included escape attempts, abdominal constrictions, and wet dog shakes. Checked signs (present or absent) include diarrhea, chewing or teeth chatter, swallowing, profuse salivation, chromodacryorrhea, ptosis, abnormal posture, and irritability upon handling. Naloxone was used to precipitate withdrawal since we rarely observe somatic signs during spontaneous withdrawal from the dose of morphine used in these studies.
Saccharin preference testing
Phenotype score was derived from a 24 h two-bottle test (see Badia Elder et al. 1996 for details) in which consumption of 0.1% saccharin solution was assessed relative to previously attained 24 h water intake, divided by body weight to control for sex/age of the rats [saccharin score = (saccharin ml − water baseline ml)/ body weight × 100]. Table 1 shows group numbers and saccharin scores, measured two weeks after the final drug exposure to prevent saccharin experience from affecting subsequent behaviors. This time period for saccharin phenotype score assessment is a standard component of our i.v. drug self-administration procedures. In such instances, the housing of animals is transferred from experimental housing to individual bins for saccharin preference assessment following i.v. self-administration testing, and the two week interim allows for adequate environmental acclimation and drug washout. Therefore, we followed the same procedure in the present study to maintain consistency across all studies conducted with the HiS and LoS rats in our laboratory.
Table 1.
Experimental group information.
| Experiment | Group | N | Saccharin Phenotype Score ± SEM |
|---|---|---|---|
| Startle | |||
| HiS male | 0 mg/kg | 8 | 10.8 ± 1.6 |
| 10 mg/kg | 10 | 14.5 ± 1.3 | |
| HiS female | 0 mg/kg | 9 | 35.5 ± 3.6 |
| 10 mg/kg | 10 | 37.6 ± 3.5 | |
| LoS male | 0 mg/kg | 6 | 12.9 ± 5.7 |
| 10 mg/kg | 11 | 6.7 ± 1.0 | |
| LoS female | 0 mg/kg | 10 | 13.7 ± 2.9 |
| 10 mg/kg | 11 | 20.0 ± 3.0 | |
| CPA | |||
| HiS male | 9 | 11.7 ± 1.6 | |
| HiS female | 7 | 27.6 ± 4.1 | |
| LoS male | 9 | 11.6 ± 2.4 | |
| LoS female | 9 | 22.2 ± 2.6 | |
| Somatic Signs | |||
| HiS male | 6 | 13.4 ± 2.4 | |
| HiS female | 6 | 43.0 ± 7.4 | |
| LoS male | 5 | 6.9 ± 2.0 | |
| LoS female | 6 | 26.7 ± 5.1 |
Data Analysis
Throughout the text and figures all data are expressed as mean ± SEM. Startle data were collapsed across both intensities (95/105 dB) before further statistical analyses were conducted, as the magnitude of withdrawal-potentiated startle does not depend on startle stimulus intensity (Harris and Gewirtz 2004). In each experiment, one-way analysis of variance (ANOVA) was conducted to verify similar baseline startle amplitude between experimental groups. Changes in startle after experimental treatment were calculated as percent change from baseline on the same day, that is percent change = [(test-baseline)/baseline] × 100 (Harris and Gewirtz 2004). For the CPA experiments, one-way ANOVA was used to verify that the experimental groups spent similar amounts of time on the drug-paired side of the chamber during the baseline session. CPA data are expressed as the change in percent time spent on the drug-paired side of the chamber from baseline to test.
Data from all experiments were analyzed using mixed factorial ANOVA, with repeated measures on within-subject factors. For main effects or interactions involving repeated measures, the Huynh– Feldt correction was applied to control for violations of the sphericity assumption. Significant interactions were followed with a priori t-tests for simple effects. Data were evaluated for outliers with the Grubb’s extreme studentized deviate test with a significance level of α = 0.05. All statistical analyses were conducted using SPSS (version 17.0) with a Type I error rate of α = 0.05 (two-tailed).
Results
Experiment 1: Potentiated startle during morphine withdrawal
No differences in baseline startle were observed between HiS and LoS rats. Startle was potentiated in rats injected with 10 mg/kg morphine (main effect of treatment F1,67 = 34.819, p < 0.001) (Figure 1). A four-way (line × sex × treatment × day) ANOVA with repeated measures on day also revealed a significant main effect of line (F1,67 = 5.255, p < 0.05), day × treatment interaction (F2,134 = 4.547, p < 0.05), and day × treatment × sex (F2,134 = 3.488, p < 0.05) interaction. Post hoc analyses revealed that startle was significantly increased following morphine on day 1 (F1,65 = 15.29, p < 0.001), day 2 (F1,65 = 33.50, p < 0.001) and day 7 (F1,65 = 4.418, p < 0.05). Both male (t19 = 2.108, p < 0.05) and female (t19 = 2.415, p < 0.05) LoS rats exhibited lower startle potentiation on day 7 than HiS rats.
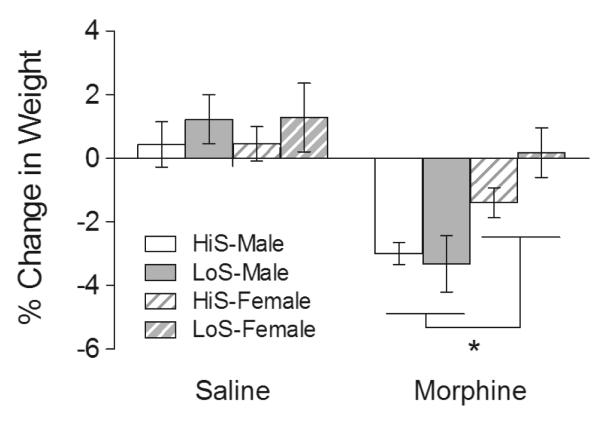
We also observed that males lost more body weight over the 7 days of morphine exposure than females, regardless of line (Figure 2). A three-way (line × sex × treatment) ANOVA of body weight on day 7 expressed as a percent of the weight on day 1 revealed a significant main effect of sex (F1,67 = 5.714, p < 0.05), main effect of treatment (F1,67 = 25.422, p < 0.001), and sex × treatment interaction (F1,67 = 5.352, p < 0.05).
Fig 2. Weight loss following 7 days of repeated morphine exposure.
Rats were injected with saline or morphine (10 mg/kg) for 7 consecutive days. Body weight was measured prior to each injection and the % change in weight between day 1 and day 7 was calculated. Shaded white bars represent HiS male rats, shaded gray bars represent LoS male rats, striped white bars represent HiS female rats, and striped gray bars represent LoS female rats. *Significant main effect of sex (p < 0.05).
A three-way (line × sex × treatment) ANOVA on saccharin preference scores revealed significant main effects of line (F1,56 = 24.394, p < 0.001) and sex (F1,56 = 45.841, p < 0.001) and a significant line × sex interaction (F1,56 = 13.579, p < 0.001) (Table 1). No effects of treatment were observed. Records of scores from 11 females (7 LoS 0 mg/kg, 2 HiS 10 mg/kg, and 2 HiS 0 mg/kg) were physically lost and could not be included in this analysis.
Experiment 2: CPA to morphine withdrawal
Following conditioning with 10 mg/kg morphine and 1 mg/kg naloxone, rats displayed a significant aversion to the drug-paired side of the conditioning chamber (main effect of session, F1,32 = 49.859, p < 0.001) (Figure 3). A three-way (line × sex × session) ANOVA with repeated measures on session also revealed a significant session × line × sex interaction (F1,32 = 6.240, p < 0.05). Post hoc analyses revealed that CPA (a significant difference between baseline and test) was present in HiS male (t8 = 7.572, p < 0.001), HiS female (t8 = 4.210, p < 0.01), and LoS female (t8 = 5.332, p < 0.001) rats. CPA was not observed in LoS male rats (t8 = 0.750, p > 0.05). A two-way (line × sex) ANOVA revealed a significant main effect of sex (F1,30 = 24.69, p < 0.001) on saccharin preference scores (Table 1).
Experiment 3: Somatic signs during morphine withdrawal
A greater number of somatic signs were observed in male than female rats, regardless of line (Table 2). A two-way (line × sex) ANOVA revealed a significant main effect of sex (F1,19 = 11.42, p < 0.01) only. Somatic signs observed included ptosis, teeth chattering, abnormal posture, diarrhea, and abdominal constrictions. A two-way (line × sex) ANOVA revealed significant main effects of sex (F1,19 = 24.75, p < 0.001) and line (F1, 19 = 5.273, p < 0.05) on saccharin preference scores (Table 1).
Table 2.
Somatic signs of withdrawal.
| Group | Withdrawal Score ± SEM |
|---|---|
| HiS male | 8.57 ± 1.78 |
| HiS female | 3.00 ± 0.63 |
| LoS male | 9.50 ± 2.02 |
| LoS female | 5.00 ± 1.13 |
Discussion
In Experiment 1 both male and female LoS rats failed to show potentiated startle after repeated morphine exposures. In Experiment 2 male LoS rats did not develop a CPA following a single treatment with morphine and naloxone. The observation of complementary results with two different behaviors in addition to spontaneous and naloxone-precipitated withdrawal supports the conclusion that the LoS rats, and male LoS rats in particular, exhibited decreased anxiety and aversion during withdrawal from an acute opiate exposure. Furthermore, the lack of a line difference on days 1 and 2 in Experiment 1 suggests that LoS rats developed tolerance to the effects of the drug after repeated exposures. This behavior can be considered abnormal, as the strength of opiate withdrawal typically escalates over time (Schulteis et al. 1997; 1999; 2003; Azar et al. 2003; Harris et al. 2004).
The literature on withdrawal responses in rodent models of drug abuse vulnerability is sparse and conflicting. Much of the confusion can be resolved by distinguishing between emotional and physical signs of withdrawal. Physical and emotional withdrawal signs rely on distinct neural mechanisms (Bozarth and Wise 1984; Koob et al. 1992; Higgins and Sellers 1994; Radke et al. 2011), and it is the latter that are considered to play a role in motivating drug taking (Koob and Le Moal 1997). The experiments conducted here used two different measures of the emotional component of opiate withdrawal. We also examined somatic signs and weight loss over seven days of morphine exposure as measures of physical withdrawal effects.
Few studies have examined emotional withdrawal behaviors, such as potentiated startle and CPA in animals with high- vs. low-vulnerability to drug abuse and, as far as we are aware, this is the first study to look at the emotional signs of opiate withdrawal. In agreement with our results, a nicotine withdrawal-induced place aversion was found in Lewis rats, an inbred strain with enhanced drug-taking liability (Kosten and Ambrosio 2002), but not the low-vulnerable Fischer 344 line (Suzuki et al. 1999). A number of studies have used the startle reflex to look at ethanol withdrawal in rodent models of ethanol preference, including alcohol-preferring vs. alcohol-nonpreferring rats, two lines of high- vs. low-alcohol-drinking rats, and high vs. low-alcohol-preferring mice (Chester et al. 2003; Chester et al. 2004; Chester et al. 2005; Chester and Barrenha 2007). Some of these studies report increases in startle in alcohol-preferring (Chester et al. 2004) and non-preferring (Chester et al. 2003) lines while others report decreases in alcohol-preferring (Chester et al. 2003; Chester et al. 2005; Chester and Barrenha 2007) and non-preferring (Chester et al. 2005; Chester and Barrenha 2007) lines during withdrawal. A similar study conducted in HiS and LoS female rats observed higher startle in the LoS line 48 h after two weeks of continuous ethanol exposure (Dess et al. 2005).
The lack of consistency in these studies may be specific to alcohol withdrawal and to the potentially varied effects of different doses and exposure regimens in the numerous lines that have been studied. It should also be noted that our studies measured percent change in startle before vs. after morphine exposure and ensured that baseline startle levels were similar between treatment groups, while in the ethanol withdrawal experiments cited above startle was compared only after ethanol exposure. This leaves open the possibility that some of the reported differences in startle between ethanol-naïve and ethanol-exposed animals could have been reflected in baseline, pre-withdrawal measures. In any case, it will be important for future studies of high- and low-vulnerable animals to analyze multiple emotional measures of withdrawal to fully determine how emotional signs of withdrawal are differentially expressed in high- vs. low-vulnerable groups. Because these behavioral signs may be mediated by different neural mechanisms, inclusion of multiple measures of withdrawal in future studies could be used to determine the neural circuits involved in increased proclivity for drug taking.
We did not observe a main effect of sex in either the startle or CPA studies. Because of this lack of a sex difference in our preliminary results and our hesitance to introduce an additional confound to the experiments (e.g.: repeated vaginal lavage is rewarding and could sensitize the females’ response to morphine; Walker et al. 2002), the estrous cycle of the female rats was not monitored in either study. However, CPA was observed in LoS females but not LoS males. This result suggests that LoS males in particular may be less vulnerable to morphine withdrawal than LoS females. While relatively little work has been done investigating sex differences in the emotional signs of drug withdrawal, there is some evidence that male rats exhibit greater anxiety during withdrawal from ethanol (Gatch and Lal 2001; Varlinskaya and Spear 2004; Overstreet et al. 2004) and male monkeys (vs. female) decrease responding for food during PCP withdrawal (Perry et al. 2006b). Our results cannot corroborate these findings, but further research should be done to determine whether reduced withdrawal in LoS males generalizes to classes of drugs other than opiates.
Our analysis of body weight loss over seven days of morphine exposure and somatic withdrawal signs during precipitated withdrawal revealed no differences between HiS and LoS rats. Other studies that use physical signs of withdrawal as their measure of interest have found that the low-vulnerable animals exhibit more severe withdrawal signs (Suzuki et al. 1992; Metten et al. 1998). We did find greater weight loss and more somatic signs in males than in females, which is consistent with previous reports of physical withdrawal from morphine (Craft et al. 1999; Kest et al. 2001; Cicero et al. 2002) and other drugs (Suzuki et al. 1992).
While we found greater withdrawal in HiS rats, a number of other studies have found that rodent lines with low vulnerability for drug taking display increased aversion to an acute drug exposure (Froehlich et al. 1988; Stewart et al. 1991; Stewart et al. 1996; Lancellotti et al. 2001; Schramm-Sapyta et al. 2006; Shram et al. 2006). It is important to note that while acute administration of a drug of abuse can be aversive, these effects are not necessarily the same as aversion caused by drug withdrawal. This distinction is important, since the two likely play different roles in motivating drug taking. It will be useful for future studies on rodent models of drug abuse vulnerability to differentiate between the various negative effects of drug exposure (i.e., acute aversive effects vs. emotional signs of withdrawal vs. physical signs of withdrawal).
Finally, while we did observe that saccharin preference scores generally followed the pattern observed in previous studies (i.e., higher scores in the HiS line and in female rats; Carroll et al. 2008), it is worth noting that HiS and LoS rats’ scores did not differ in the CPA experiment. In fact, the saccharin preference scores of the HiS and LoS rats were nearly identical (Table 1). Although on the whole the HiS and LoS phenotypes have been very stable over the years, saccharin preference scores can vary considerably among individuals (Carroll et al. 2008). Selection of a small number of subjects will therefore occasionally produce groups that deviate from the mean. Importantly, differences in drug intake can still be observed even when the results of a single saccharin preference test are inconclusive (Carroll et al. 2008). Because we have continued to observe robust differences in saccharin preference in our most recent experiments (Holtz and Carroll 2011; Radke, Holtz and Carroll unpublished data), the lack of a difference in the current studies is most likely a type II error. Nevertheless, because phenotypic and genetic drift can occur in a population over time, it will be important for future studies to verify the link between sweet preference, drug taking, and withdrawal signs by comparing these measures within subjects. Future studies with HiS and LoS rats should also aim to reduce the variability in saccharin preference scores (Beebe-Center et al. 1948) by measuring intake over multiple days.
Diminished anxiety/aversion during withdrawal in the LoS rats, especially following repeated exposures, may help explain their decreased levels of drug self-administration. Withdrawal is thought to come to motivate drug taking by establishing a cycle in which individuals administer drug to offset or relieve the negative emotional withdrawal state (Solomon and Corbit 1974; Koob and Le Moal 1997; Baker et al. 2004). In rats, conditioned withdrawal has been shown to increase opiate self-administration (Kenny et al. 2006) and high anxiety is predictive of cocaine intake (Dilleen et al. 2012). If LoS rats do not experience negative affect during withdrawal, or experience it to a lesser degree, the drive to take drugs may be lessened. This, combined with a decreased sensitivity to the rewarding effects of the drug could lead to lower overall levels of drug taking. It is particularly interesting that the LoS male rats, the only group that did not develop CPA to morphine withdrawal, are the least likely to take drugs of abuse in a self-administration paradigm (Carroll et al. 2008). Decreased withdrawal may also partially explain the lower levels of reinstatement observed in LoS rats (Perry et al. 2006a), an idea which is supported by examples from the human literature demonstrating that withdrawal severity predicts higher rates of relapse to nicotine, cannabis, or cocaine (West et al. 1989; Kampman et al. 2001; Ferguson et al. 2006; Budney et al. 2008). Since withdrawal is especially important in motivating drug taking following chronic, repeated exposure it will be important for future studies to assess whether the effects seen here hold up after higher doses or longer exposures to morphine. It will also be important to investigate the relationship between negative emotional withdrawal signs and drug intake in other models of drug abuse vulnerability. Doing so will help determine whether the emotional component of withdrawal is predictive of drug intake more generally.
We currently know little about how HiS and LoS rats differ on a neural level, but information about the brain mechanisms responsible for anxiety and aversion during opiate withdrawal may provide clues. Opiate withdrawal-induced anxiety and aversion, measured with withdrawal-potentiated startle and CPA respectively, rely on similar neural mechanisms. Expression of both withdrawal effects requires structures such as the central nucleus of the amygdala, bed nucleus of the stria terminalis, and shell of the nucleus accumbens (Kelsey and Arnold 1994; Aston-Jones et al. 1999; Watanabe et al. 2002a; Watanabe et al. 2002b; Nakagawa et al. 2005; Harris et al. 2006; Cabral et al. 2009; Radke and Gewirtz 2012) as well as increased noradrenergic signaling (Aston-Jones et al. 1999; Harris and Gewirtz 2004; Rothwell et al. 2009) and decreased dopaminergic signaling (Laviolette et al. 2002; Radke et al. 2011; Radke and Gewirtz 2012). One intriguing explanation for reduced drug reward and withdrawal in LoS rats stems from our recent finding of lower levels of orexin in the lateral hypothalamus and perifornical area of female LoS rats (Holtz et al. 2012). Because orexin neurons project to dopaminergic neurons in the VTA and are functionally interconnected with extended amygdala noradrenergic and corticotropin-releasing factor systems (Fadel and Deutch 2002; Smith and Aston-Jones 2008), decreased orexin signaling in LoS rats could influence the expression of opiate withdrawal through one or more pathways. In support of this idea, acute withdrawal from morphine increases orexin gene expression (Zhou et al. 2006), and physical signs of opiate withdrawal are attenuated in orexin knock-out mice (Georgescu et al. 2003) and mice given an orexin type-1 receptor antagonist (Sharf et al. 2008).
In summary, our results indicate that LoS male and female rats are less susceptible to morphine withdrawal than HiS rats when withdrawal-potentiated startle and CPA, behavioral measures of anxiety and aversion, respectively, are used. No differences in physical withdrawal signs were observed between HiS and LoS rats, although males of both lines displayed more somatic signs of withdrawal and weight loss than females. As one of the first studies to examine withdrawal following an acute drug exposure in HiS and LoS rats, these findings will help shape our understanding of how variations in the positive and negative effects of drugs of abuse relate to individual differences in vulnerability to drug-taking.
Acknowledgements
We thank Sofiya Hupalo and Jacob Leslie for technical assistance, Gail Towers for animal husbandry, Dr. Mark Thomas for the use of the place conditioning apparatus, and Dr. Andrew Harris for assistance measuring somatic withdrawal signs. This work was funded by NIDA grants K05 DA015267 and P20 DA024196 (MEC) and the University of Minnesota (JCG).
Footnotes
Conflict of Interest The authors declare no conflict of interest in regards to this work.
References
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: Evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2010;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Anon . In: Guide for the care and use of laboratory animals. Eighth ed Council NR, editor. National Academies Press; Washington DC: 2011. [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Ann NY Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003;70:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- Badia Elder N, Kiefer SW, Dess NK. Taste reactivity in rats selectively bred for high vs. low saccharin consumption. Physiol Behav. 1996;59:749–755. doi: 10.1016/0031-9384(95)02131-0. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bechara A, Nader K, van der Kooy D. Neurobiology of withdrawal motivation: evidence for two separate aversive effects produced in morphine-naive versus morphine-dependent rats by both naloxone and spontaneous withdrawal. Behav Neurosci. 1995;109:91–105. doi: 10.1037//0735-7044.109.1.91. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrin. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe-Center JG, Black P, Hoffman AC, Wade M. Relative per diem consumption as a measure of preference in the rat. J Comp Physiol Psych. 1948;41:239–251. doi: 10.1037/h0058409. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984;224:516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: Severity and contribution to relapse. J Subst Abuse Treat. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Ruggiero RN, Nobre MJ, Brandao ML, Castilho VM. GABA and opioid mechanisms of the central amygdala underlie the withdrawal-potentiated startle from acute morphine. Prog Neuro-Psychoph. 2009;33:334–344. doi: 10.1016/j.pnpbp.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Higher locomotor response to cocaine in female (vs. male) rats selectively bred for high (HiS) and low (LoS) saccharin intake. Pharmacol Biochem Behav. 2007;88:94. doi: 10.1016/j.pbb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104:S70–S78. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD. Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference. Alcohol Clin Exp Res. 2007;31:1633–1644. doi: 10.1111/j.1530-0277.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Further evidence of an inverse genetic relationship between innate differences in alcohol preference and alcohol withdrawal magnitude in multiple selectively bred rat lines. Alcohol Clin Exp Res. 2003;27:377–387. doi: 10.1097/01.ALC.0000056619.98553.50. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res. 2004;28:677–687. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Effects of chronic alcohol treatment on acoustic startle reactivity during withdrawal and subsequent alcohol intake in high and low alcohol drinking rats. Alcohol Alcoholism. 2005;40:379–387. doi: 10.1093/alcalc/agh172. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Gender-linked differences in the expression of physical dependence in the rat. Pharmacol Biochem Behav. 2002;72:691–697. doi: 10.1016/s0091-3057(02)00740-2. [DOI] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA, Bartok RE, Walpole TI, King SJ. Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology (Berl) 1999;143:1–7. doi: 10.1007/s002130050911. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Deshmukh A, O’Reilly A, Pfefferbaum A, Rosenboom MJ, Sassoon S, Sullivan EV. Alcoholic men endorse more DSM-IV withdrawal symptoms than alcoholic women matched in drinking history. J Stud Alcohol. 2003;64:375–379. doi: 10.15288/jsa.2003.64.375. [DOI] [PubMed] [Google Scholar]
- Dess NK, Arnal J, Chapman CD, Siebel S, VanderWeele DA, Green KF. Exploring adaptations to famine: rats selectively bred for differential intake of saccharin differ on deprivation-induced hyperactivity and emotionality. Int J Comparative Psychol. 2000;13:34–52. [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–278. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Dess NK, Minor TR. Taste and emotionality in rats selectively bred for high versus low saccharin intake. Anim Learn Behav. 1996;24:105–115. [Google Scholar]
- Dess NK, O’Neill P, Chapman CD. Ethanol withdrawal and proclivity are inversely related in rats selectively bred for differential saccharin intake. Alcohol. 2005;37:9–22. doi: 10.1016/j.alcohol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcoholism: clinical and experimental research. 2001;25:1689–1696. [PubMed] [Google Scholar]
- Dilleen R, Pelloux Y, Mar AC, Molander A, Robbins TW, Everitt BJ, Danney JW, Belin D. High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology (Berl) 2012;222:89–97. doi: 10.1007/s00213-011-2626-4. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Radke AK, Gewirtz JC. Potentiated startle as a measure of the negative affective consequences of repeated exposure to nicotine in rats. Psychopharmacology (Berl) 2009;207:13–25. doi: 10.1007/s00213-009-1632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interations: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J Consult Clin Psychol. 2006;74:1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- Froehlich J, Harts J, Lumeng L, Li T. Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav. 1988;31:215–222. doi: 10.1016/0091-3057(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Lal H. Animal models of the anxiogenic effects of ethanol withdrawal. Drug Dev Res. 2001;54:95–115. [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–546. [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrott M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell BA, Levine AS. Reward systems and food intake: role of opioids. Int J Obes (Lond) 2009;33:S54–58. doi: 10.1038/ijo.2009.73. [DOI] [PubMed] [Google Scholar]
- Harris AC, Atkinson DM, Aase DM, Gewirtz JC. Double dissociation in the neural substrates of acute opiate dependence as measured by withdrawal-potentiated startle. Neuroscience. 2006;139:1201–1210. doi: 10.1016/j.neuroscience.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC. Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 2004;171:140–147. doi: 10.1007/s00213-003-1573-0. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Sellers EM. Antagonist-precipitated opioid withdrawal in rats: evidence for dissociations between physical and motivational signs. Pharmacol Biochem Behav. 1994;48:1–8. doi: 10.1016/0091-3057(94)90489-8. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. Baclofen has opposite effects on escalation of cocaine self-administration: Increased intake in rats selectively bred for high (HiS) saccharin intake and decreased intake inthose selected for low (LoS) saccharin intake. Pharmacol Biochem Behav. 2011;100:275–283. doi: 10.1016/j.pbb.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Zlebnik NE, Carroll ME. Differential orexin/hypocretin expression in addiction-prone and -resistant rats selectively bred for high (HiS) and low (LoS) saccharin intake. Neuroscience Letters. 2012;522:12–15. doi: 10.1016/j.neulet.2012.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky D, Pucilowski O, Buyinza M. Preference for higher sucrose concentrations in cocaine abusing-dependent patients. J Psychiatr Res. 2003;37:35–41. doi: 10.1016/s0022-3956(02)00063-8. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, Mulholland EM, Jawad AF, Parikh GA, Mulvaney FD, Weinrieb RM, O’Brien CP. Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addict Behav. 2001;15:52–59. doi: 10.1037/0893-164x.15.1.52. [DOI] [PubMed] [Google Scholar]
- Kampov Polevoy AB, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry. 1997;154:269–270. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- Kampov Polevoy AB, Tsoi MV, Zvartau EE, Neznanov NG, Khalitov E. Sweet liking and family history of alcoholism in hospitalized alcoholic and non-alcoholic patients. Alcohol Alcoholism. 2001;36:165–170. doi: 10.1093/alcalc/36.2.165. [DOI] [PubMed] [Google Scholar]
- Kelsey JE, Arnold SR. Lesions of the dorsomedial amygdala, but not the nucleus accumbens, reduce the aversiveness of morphine withdrawal in rats. Behav Neurosci. 1994;108:1119–1127. doi: 10.1037//0735-7044.108.6.1119. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A. Assessment of acute and chronic morphine dependence in male and female mice. Pharmacol Biochem Behav. 2001;70:149–156. doi: 10.1016/s0091-3057(01)00600-1. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–268. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrino. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Lancellotti D, Bayer BM, Glowa JR, Houghtling RA, Riley AL. Morphine-induced conditioned taste aversions in the LEW/N and F344/N rat strains. Pharmacol Biochem Behav. 2001;68:603–610. doi: 10.1016/s0091-3057(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Nader K, van der Kooy D. Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res. 2002;129:17–29. doi: 10.1016/s0166-4328(01)00327-8. [DOI] [PubMed] [Google Scholar]
- Lohmiller JJ, Swing SP. Reproduction and breeding. In: Suckow A, Weisbroth SH, Franklin CL, editors. The laboratory rat. 2nd edn Elesvier Academic Press; Burlington, MA: 2006. pp. 147–162. [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Mysels DJ, Sullivan MA. The relationship between opioid and sugar intake: review of evidence and clinical applications. J Opioid Manag. 2010;6:445–452. doi: 10.5055/jom.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, Kaneko S. Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience. 2005;134:9–19. doi: 10.1016/j.neuroscience.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawal from ethanol. Pharmacol Biochem Behav. 2004;78:459–464. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Dess NK, Morgan AD, Carroll ME. Escalation of iv cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology (Berl) 2006a;186:235–245. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- Perry JL, Normile LM, Morgan AD, Carroll ME. Sex differences in physical dependence on orally self-administered phencyclidine (PCP) in rhesus monkeys (Macaca mulatta) Exp Clin Psychopharmacology (Berl) 2006b;14:68–78. doi: 10.1037/1064-1297.14.1.68. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Garcia AW, Drewnowski A, Pomerleau OF. Sweet taste preference in women smokers: comparison with nonsmokers and effects of menstrual phase and nicotine abstinence. Pharmacol Biochem Behav. 1991;40:995–999. doi: 10.1016/0091-3057(91)90118-l. [DOI] [PubMed] [Google Scholar]
- Radke AK, Rothwell PE, Gewirtz JC. An anatomical basis for opponent process mechanisms of opiate withdrawal. J Neurosci. 2011;31:7533–7539. doi: 10.1523/JNEUROSCI.0172-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Gewirtz JC. Increased dopamine receptor activity in the nucleus accumbens shell ameliorates anxiety during drug withdrawal. Neuropsychopharmacology. 2012;37:2405–2415. doi: 10.1038/npp.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Thomas MJ, Gewirtz JC. Distinct profiles of anxiety and dysphoria during spontaneous withdrawal from acute morphine exposure. Neuropsychopharmacology. 2009;34:2285–2295. doi: 10.1038/npp.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Opiate withdrawal signs precipitated by naloxone following a single exposure to morphine: potentiation with a second morphine exposure. Psychopharmacology (Berl) 1997;129:56–65. doi: 10.1007/s002130050162. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol. 1999;10:235–242. doi: 10.1097/00008877-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Morse AC, Liu J. Repeated experience with naloxone facilitates acute morphine withdrawal: potential role for conditioning processes in acute opioid dependence. Pharmacol Biochem Behav. 2003;76:493–503. doi: 10.1016/j.pbb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, DiLeone RJ. Orexin mediated the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64:175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Stewart R, McBride W, Lumeng L, Li TK, Murphy J. Chronic alcohol consumption in alcohol-preferring P rats attenuates subsequent conditioned taste aversion produced by ethanol injections. Psychopharmacology (Berl) 1991;105:530–534. doi: 10.1007/BF02244375. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Murphy J, McBride WJ, Lumeng L, Li TK. Place conditioning with alcohol in alcohol-preferring and-nonpreferring rats. Pharmacol Biochem Behav. 1996;53:487–491. doi: 10.1016/0091-3057(95)02102-7. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Maed J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in Lewis and Fischer 344 inbred rat strains. Eur J Pharmacol. 1999;369:159–162. doi: 10.1016/s0014-2999(99)00086-2. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Koike Y, Misawa M. Sex differences in physical dependence on methaqualone in the rat. Pharmacol Biochem Behav. 1988;30:483–488. doi: 10.1016/0091-3057(88)90484-4. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Koike Y, Yoshii T, Yanaura S. Sex differences in the induction of physical dependence on pentobarbital in the rat. Jpn J Pharmacol. 1985;39:453–459. doi: 10.1254/jjp.39.453. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Motegi H, Otani K, Koike Y, Misawa M. Susceptibility to, tolerance to, and physical dependence on ethanol and barbital in two inbred strains of rats. Gen Pharmacol. 1992;23:11–17. doi: 10.1016/0306-3623(92)90040-q. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Review on CPP: Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol. 2002a;88:399–406. doi: 10.1254/jjp.88.399. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yamamoto R, Maeda A, Nakagawa T, Minami M, Satoh M. Effects of excitotoxic lesions of the central or basolateral nucleus of the amygdala on naloxone-precipitated withdrawal-induced conditioned place aversion in morphine-dependent rats. Brain Res. 2002b;958:423–428. doi: 10.1016/s0006-8993(02)03468-6. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GJ. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G. Food fantasies of incarcerated drug users. Int J Addict. 1982;17:905–912. doi: 10.3109/10826088209056337. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med. 1989;19:981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]