The anxiety disorders have the highest prevalence of any group of psychiatric disorders (Kessler et al., 2005). Considering the early onset, chronic course (Bittner et al., 2007; Yonkers, Bruce, Dyck, & Keller, 2003), associated functional impairment, and high economic costs of anxiety disorders (Kessler & Greenberg, 2002), there is a critical need to discover pathophysiological markers and identify persons at risk for these conditions. There is growing evidence that abnormal error-related brain activity is a biomarker for anxiety disorders, and may reflect increased risk for these conditions.
In particular, the error-related negativity (ERN) is an increased negative deflection in the event-related potential (ERP) occurring approximately 50 ms after the commission of errors compared to correct responses in speeded reaction time tasks (Gehring, Goss, Coles, Meyer, & Donchin, 1993). Based on source localization (Mathalon, Whitfield, & Ford, 2003), and studies that combine ERP and fMRI (Debener et al., 2005), it appears likely that the ERN is generated in the anterior cingulate cortex (ACC)—a region of the medial prefrontal cortex implicated in the pathophysiology of anxiety disorders (Fitzgerald et al., 2005).
An increased ERN was originally reported among patients with obsessive-compulsive disorder (OCD; Gehring, Himle, & Nisenson, 2000)—a finding that has since been replicated several times (Stern et al., 2010; Weinberg, Riesel, & Hajcak, 2012). An increased ERN does not appear specific to OCD, however; similar findings have been reported in generalized anxiety disorder (GAD; Weinberg, Olvet, & Hajcak, 2010), as well as related personality traits such as negative emotionality/neuroticism (Hajcak, McDonald, Simons, 2004) and worry (Hajcak, McDonald, & Simons, 2003).
Neurodevelopmental studies suggest that ACC function and structure increase with age (Casey et al., 1997). Consistent with these data, the ERN may not reach adult-like levels until the late teen years (Davies, Segalowitz, & Gavin, 2004). However, the ERN can be elicited among very young children: one study found a robust ERN in children as young as 5 to 7 years old (Torpey, Hajcak, & Klein, 2009), suggesting that it may be useful as an early marker and in elucidating neurodevelopmental pathways of risk. Indeed, an increased ERN has been reported to be larger in youths with obsessive compulsive disorder (Hajcak, Franklin, Foa, & Simons, 2008) and in a heterogeneous group of clinically anxious youths (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006). Larger ERNs have also been associated with anxiety symptoms in non-clinical samples of youths, although this pattern did not emerge until early adolescence (Meyer, Weinberg, Klein, & Hajcak, 2012).
We have argued that the ERN is a plausible endophenotype for anxiety disorders (Hajcak, 2012; Olvet & Hajcak, 2008). There is evidence that the ERN is associated with externalizing disorders as well; however, it has been demonstrated that an ERN of reduced amplitude is elicited in individuals with externalizing disorders (Stieben et al., 2007). Fewer studies have examined associations between ERN amplitude and other internalizing disorders, such as depression, and the results have been equivocal (Compton et al., 2008; Holmes and Pizzagalli, 2008; Olvet, Klein, & Hajcak, 2010). In addition to being associated with disease, endophenotypes should be state-independent (i.e., apparent regardless of current disease state), heritable, and evident among non-affected individuals who are at high familial risk; furthermore, the endophenotype should not simply be due to medication status (Gottesman & Gould, 2003).
Consistent with the notion that the ERN is an endophenotype, it is trait-like and fairly stable over time, with high test-retest reliability over periods as long as two years (Weinberg et al., 2012, for review). Provoking significant anxiety and fear does not increase the amplitude of the ERN (Moser, Hajcak, & Simons, 2005). In addition, medication status is unrelated to the ERN among clinically anxious individuals (Stern et al., 2010), and reductions in anxiety following the successful treatment of anxiety disorders are not accompanied by reductions in the ERN (Hajcak et al., 2008). The ERN is also heritable, with estimates around .50 (Anokhin, Golosheykin, & Heath, 2008), and a recent study found increased ERNs among non-affected first-degree relatives of OCD patients (Riesel, Endrass, Kaufmann, & Kathmann, 2011). Collectively, these data indicate that an increased ERN satisfies criteria for an anxiety endophenotype; thus the ERN may be a heritable biomarker of increased risk for anxiety disorders.
As an initial step in addressing the issue of ERN and risk for anxiety disorders, we examined the associations of ERN in six year-old children with two established measures of risk: maternal history of anxiety disorder (Last, Hersen, Kazdin, Orvaschel, & Perrin, 1991) and early child temperament (Klein, Dyson, Kujawa, & Kotov, in press). There is a large literature in youths and adults linking anxiety to negative emotionality (NE; Kotov, Gamez, Schmidt, & Watson, 2010). NE is a complex construct that includes anger, fear, and sadness (Rothbart, Ahadi, & Evans, 2000) and associations between fearful temperament, specifically, and anxiety have been found in children (Goldsmith & Lemery, 2000). Because of this, we hypothesized that offspring of parents with anxiety disorders and children with elevated levels of negative emotionality (NE), particularly fearfulness, in a laboratory assessment conducted at age 3 would exhibit increased ERNs at age 6. However, these hypotheses were tentative given the paucity of data on the early development of the association of ERN with anxiety.
Response-locked ERP studies have isolated another component associated with response monitoring: a large positivity known as the Error Positivity (Pe), that appears within 200 – 500 ms following an erroneous response (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Santesso, Segalowitz, & Schmidt, 2006a) and is independent of the ERN (Arbel & Donchin, 2009). Specifically, some source localization studies have demonstrated that, like the P300, the Pe is localized in more posterior regions than the ERN (Ullsperger & von Cramon, 2006).
Unlike the ERN, there is little evidence that the Pe is an endophenotype for anxiety disorders. Ruchsow et al. (2005) found no difference in the amplitude of the Pe in adults with OCD and healthy controls. Similar findings have been demonstrated in comparisons of anxious and non-anxious children (Ladouceur et al., 2006; Hajcak et al., 2008). However, Santesso, Segalowitz, and Schmidt (2006b) found that children high in obsessive-compulsive behaviors had a larger Pe compared to children low in these behaviors. Because the majority of the evidence suggests that there is no relationship between the Pe and anxiety disorders, it is hypothesized that the Pe will not be associated with NE in this sample.
Methods
Participants
Participants included 413 six year old children (54.5% male, 45.5% female) from a suburban community who were originally recruited and evaluated approximately three years earlier. Participants were originally recruited via a commercial mailing list. Eligible families had a child with no significant medical conditions or developmental disabilities, and at least one English-speaking biological parent. The mean ages of the children at the initial and follow-up assessments were 3.56 years (SD = .26) and 6.14 years (SD = .42) respectively. Most children were Caucasian (87.4%), came from two-parent homes (95.4%), and had at least one parent who had graduated from college (69.3%). After complete description of the study to the parents of the children, written informed consent was obtained. All procedures were approved by the University Institutional Review Board.
Age 3 Child Temperament and Parental Psychopathology Assessments
Child Temperament
The laboratory visit lasted approximately two hours, during which children participated in a standardized set of 12 laboratory episodes adapted from the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1995). The episodes, described in detail elsewhere (Olino, Klein, Dyson, Rose, & Durbin, 2010), were designed to elicit positive emotionality (PE), and various forms of negative emotionality (NE), including fear, sadness, and anger. For example, in Popping Bubbles, the child and experimenter played with a bubble-shooting toy. In Stranger Approach, the child was left alone briefly in the assessment room. A male accomplice entered the room and spoke to the child while slowly walking closer. In Box Empty, the child was given an elaborately wrapped box to open, under the impression that a toy was inside. After the child discovered that the box was empty, the experimenter returned with several small toys for the child to keep.
Episodes were videotaped for later coding. Coding procedures are described in detail elsewhere (Olino et al., 2010). NE and PE were scored from all 12 episodes. NE included displays of fear, anger, and sadness. Each instance of facial, vocal, and bodily anger, sadness, and fear was rated separately on a four-point intensity scale and then summed across each episode. These ratings were then averaged across the 12 episodes to yield composite scores for anger (α = .68), sadness (α = .81), and fear (α = .63). Interrater reliability, assessed using the intraclass correlation coefficient (ICC), for anger, sadness, and fear were .73, .79, and .64, respectively (N = 35). NE was calculated by summing the z-scored anger, sadness, and fear scales, yielding a composite score (α = .82; ICC = .74, N = 35). PE included displays of positive affect (PA; e.g., joy, enthusiasm) and interest/engagement. To rate PA, each instance of facial, vocal, and bodily positive affect was rated on a four-point intensity scale, and summed across episodes. The PA scores for each episode were averaged across the 12 episodes to yield a composite score (α = .87; ICC = .92, N = 35). A global rating of interest/engagement was made for each episode, based on all relevant behaviors during that episode. Ratings were then summed across episodes (α = .68; ICC = .75, N = 35). The composite PE score consisted of the summed z-scores of the PA and interest/engagement scores (α = .82; ICC = .89, N = 35).
Parental Psychopathology
Biological mothers and fathers of the children were interviewed during the age 3 assessment using the Structured Clinical Interview for DSM-IV, non-patient version (SCID-NP; First, Spitzer, Gibbon, & Williams, 1996). Two Masters-level raters conducted the interviews by telephone, which generally yields comparable results to face-to-face interviews (Rohde, Lewinsohn, & Seeley, 1997). When one parent could not be directly interviewed, diagnostic information on that parent was obtained by interviewing the co-parent using the family history method (Andreasen, Endicott, Spitzer, & Winokur, 1977). SCIDs were completed with 409 mothers and 350 fathers; family history information was available for an additional 56 fathers. Of the mothers, 104 (31.7%), 113 (34.5%), and 70 (21.3%) had a lifetime history of depressive, anxiety, and alcohol/drug use (abuse or dependence) disorders, respectively. Of the fathers, 53 (16.2%), 69 (21.0%), and 135 (41.2%) had a lifetime history of depressive, anxiety, and alcohol/drug use disorders, respectively. Based on audiotapes of 30 assessments (20 with mothers and 10 with fathers), inter-rater reliabilities (assessed with kappa) for presence/absence of a lifetime depressive, anxiety, and substance use disorder were .93, .91, and 1.00, respectively.
Age 6 Psychophysiological Assessment
Children returned to the laboratory for the psychophysiological assessment when they were approximately 6 years old. The assessment included a Go/No-Go Task, in which they were presented with a green equilateral triangle, in one of four different orientations, on each trial. Children were instructed to press a button only when the stimulus was a vertically aligned, upward pointing triangle, which occurred on 60% of the trials. Participants completed 4 blocks of 60 trials each and were told that they earned points which could be exchanged for money for every correct button press and for every correct withholding of a button press when they were presented with a triangle in a different orientation. Children were told they could win up to $5.00. Speed of response was emphasized. At the end of each block, the number of points won by the participant was displayed in white numbers. Following completion of the task, all children were told that they won the maximum number of points and received $5.00. Several practice blocks were given in order to ensure that the children understood the task. Further details about this task can be found in Torpey et al. (2012).
The EEG was recorded from 32 sites using the Active Two system (Biosemi, Amsterdam, The Netherlands). The signal was preamplified at the electrode with a gain of one; the EEG was digitized at 24-bit resolution with a sampling rate of 512 Hz using a low-pass fifth order sinc filter with a half-power cutoff of 104 Hz. Recordings were taken from 32 scalp electrodes based on the 10/20 system, as well as an electrode place on the tip of the nose. The electrooculogram was recorded from four facial electrodes: two 1 cm above and below the left eye, one 1 cm to the left of the left eye, and one 1 cm to the right of the right eye. Each electrode was measured online with respect to a common mode sense electrode that formed a monopolar channel. Offline analysis was performed using Brain Vision Analyzer software (Brain Products, Munich, Germany). All EEG data were re-referenced to the nose offline.
The EEG data were bandpass filtered with cutoffs of 0.1 and 30 Hz, and 1500 ms segments were extracted beginning 500 ms prior to correct and erroneous responses. Each trial was corrected for blinks and eye movements using the method developed by Gratton and colleagues (1983). Specific channels were rejected in each trial using a semi-automated procedure, with physiological artifacts identified by the following criteria: a step of more than 50 μV between sample points, a difference of 300 μV within a trial, and a maximum difference of less than 0.5 μV within 100-ms intervals. Additional physiological artifacts were identified using visual inspection. Trials were not included in ERP averages if the reaction time occurred outside of a 200 – 1,300 ms window. Only errors of commission were classified as incorrect (average number= 16.18 + 7.72) and were used to generate the ERN. The ERN was evaluated along the midline (Fz, Cz, and Pz) and defined as the average voltage in the window from 0 ms to 100 ms after the response. Additionally, error-specific activity in the time-range of the ERN was evaluated by subtracting the average voltage on correct trials from the average voltage on error trials (i.e., ΔERN). The Pe was also evaluated along the midline and was defined as the average voltage in the window 200 ms to 500 ms following the response on error trials.
Data Analysis
In order to be included in analyses, participants were required to have at least 6 artifact-free error trials (Olvet & Hajcak, 2009), which led to the exclusion of data from 85 out of 413 (20.6%) children in total. Additionally, the ERP data from one participant were lost due to technical error and the data from one additional participant were excluded due to values that were different from the grand mean of the data points by multiple standard deviations. A total of 326 subjects were retained in the final ERP analyses. Children whose data were included in the final analyses were not significantly different from those children whose data were excluded in terms of child age, sex, race/ethnicity, and proportion with at least one parent with a college degree.
Simultaneous linear multiple regression analyses were used to explore the relationships between ERP measures and parental psychopathology. Separate analyses were conducted to examine the impact of maternal and paternal psychopathology. In order to test the specificity of the association between parental anxiety disorders and ERN in offspring, parental mood and substance use disorders were also included in each analysis. This was also done when examining associations with the Pe.
Simultaneous regression analyses were also conducted to examine associations between child temperament and ERP components. The two broad temperament constructs of PE and NE were the predictor variables in one model. In order to examine whether the three NE facets differentially predict ERN and Pe, the second regression model included sadness, fear, and anger. As reported elsewhere (Torpey et al., 2012), the only significant association of parent and child demographic characteristics with the ERN variables was between child age and ΔERN at Cz. Hence, child age was included as a covariate in all analyses involving ΔERN.
Results
Typical patterns of behavioral performance on the Go/No-Go task were observed. Reaction times on error trials (M = 509.4 msec, SD = 86.9 msec) were significantly faster than reaction times on correct Go trials (M = 626.8 msec, SD = 72.5 msec; t (1,320) = −34.41, p < .001). Reaction times on correct trials that followed errors (M = 654.7 msec, SD = 117.9 msec) were significantly slower than the overall average of correct Go trials (t (1,320) = 6.26, p < .001).
As has been previously described (Torpey et al., 2012), errors were associated with a greater negativity than correct trials at all midline sites, with the minimum ERN at Fz. The difference between the error and correct trials (ΔERN) was maximal at Cz and the Pe was maximal at Pz. Hence, analyses focused on the ERN at Fz, ΔERN at Cz, and Pe at Pz.
Parental Psychopathology and Offspring ERN
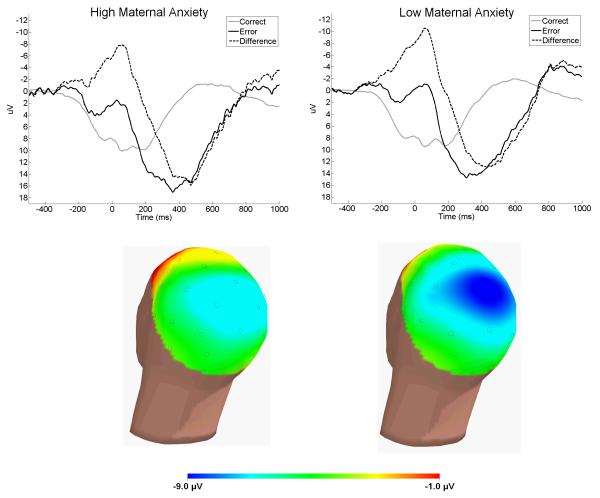
Table 1 and Figure 1 present the results of analyses examining ΔERN amplitude at Cz. Children whose mothers had a lifetime history of an anxiety disorder demonstrated a significantly smaller (i.e., less negative) ΔERN than children of mothers with no history of anxiety disorder. There were no significant associations with maternal mood and substance use disorders or with any form of paternal psychopathology.
Table 1. Simultaneous Regression Analyses Examining Associations of Parental Psychopathology with ΔERN at Cz, ERN Amplitude at Fz, and Pe Amplitude at Pz.
| ΔERN at Cz | ERN at Fz | Pe at Pz | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variables Entered | b | Standard Error |
t | b | Standard Error |
t | b | Standard Error |
t |
| N = 322 | N = 322 | N = 322 | |||||||
| Maternal Depression | −.91 | 1.10 | −.82 | −.60 | .99 | −.60 | 1.35 | 1.49 | .90 |
| Maternal Anxiety Disorder | 3.04 | 1.10 | 2.77** | 2.96 | .99 | 3.00** | −.75 | 1.48 | −.50 |
| Maternal Substance Use Disorder | 1.05 | 1.24 | .84 | .11 | 1.12 | .10 | 2.69 | 1.68 | 1.59 |
| Child Age (used only for analyses including ΔERN) |
−3.06 | 1.16 | − 2.64** |
||||||
| Overall Model: Total R-squared | .05** | .03* | .01 (p = ns) | ||||||
| N = 320 | N = 320 | N = 320 | |||||||
| Paternal Depression | −.68 | 1.44 | −.47 | −.72 | 1.29 | −.55 | 1.62 | 1.93 | .84 |
| Paternal Anxiety Disorder | 1.22 | 1.31 | .93 | 1.62 | 1.17 | 1.38 | −.06 | 1.75 | −.03 |
| Paternal Substance Use Disorder | −.51 | 1.04 | −.49 | −.38 | .93 | −.41 | .70 | 1.39 | .51 |
| Child Age (used only for analyses including ΔERN) |
−2.76 | 1.17 | −2.36* | ||||||
| Overall Model: Total R-squared | .02 (p = ns) | .01 (p = ns) | .00 (p = ns) | ||||||
p ≤ .05
p ≤ .01
Figure 1.
Response-locked ERPs for errors (black), correct responses (grey), and the error minus correct difference (dotted) at Cz (top)—the scalp topography of the error minus correct difference in the time-range of the ERN is plotted below, separately for participants with no history of maternal anxiety disorder (left) and a history of maternal anxiety disorder (right).
Similar results were evident for ERN amplitude at Fz (see Table 1). Children of mothers with a history of an anxiety disorder exhibited a smaller (i.e., less negative) ERN than children of mothers with no lifetime anxiety disorder. There were no significant associations with maternal mood or substance use disorders or with paternal psychopathology.
Child Temperament and ERN
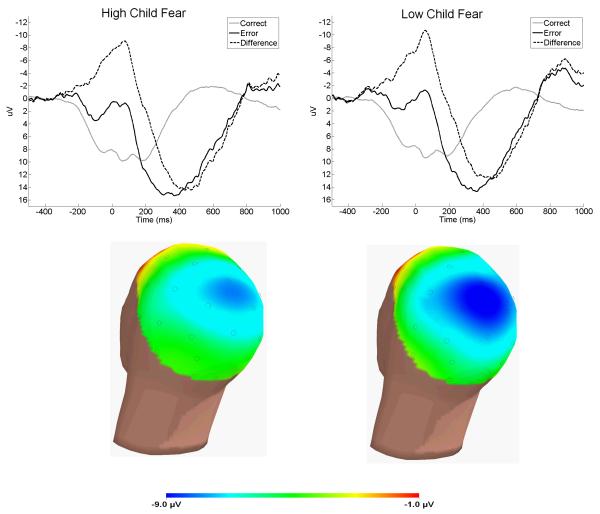
Table 2 and Figure 2 present the results of analyses examining ΔERN amplitude at Cz. Child NE was significantly associated with ΔERN, with higher NE predicting a smaller (i.e., less negative) ΔERN. However, child PE was not associated with ΔERN. Of the three forms of child NE, only fear was uniquely and significantly associated with ΔERN, with higher levels of fear predicting a smaller (i.e., less negative) ΔERN.
Table 2. Simultaneous Regression Analyses Examining Associations of Child Temperament with ΔERN at Cz, ERN Amplitude at Fz, and Pe Amplitude at Pz.
| ΔERN at Cz | ERN at Fz | Pe at Pz | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variables Entered | b | Standard Error |
t | b | Standard Error |
t | b | Standard Error |
t |
| N = 326 | N = 326 | N = 326 | |||||||
| Child PE | .05 | 2.12 | .03 | 1.88 | 1.86 | 1.01 | 1.15 | 2.79 | .41 |
| Child NE | 4.56 | 1.92 | 2.37* | 3.56 | 1.73 | 2.06* | .06 | 2.59 | .02 |
| Child Age (used only for analyses including ΔERN) |
−2.69 | 1.18 | −2.28* | ||||||
| Overall Model: Total R-squared | .03* | .02† | .00 (p = ns) | ||||||
| Child Sadness | −.95 | 1.94 | −.49 | .50 | 1.75 | .29 | −2.98 | 2.60 | −1.15 |
| Child Fear | 3.09 | 1.45 | 2.14* | 2.41 | 1.30 | 1.85† | 3.26 | 1.94 | 1.68† |
| Child Anger | 2.47 | 1.63 | 1.52 | .93 | 1.46 | .64 | −.46 | 2.17 | −.21 |
| Child Age (used only for analyses including ΔERN) |
−2.70 | 1.16 | −2.33* | ||||||
| Overall Model: Total R-squared | .04* | .02 (p = ns) | .01 (p = ns) | ||||||
p ≤ .10
p ≤ .05
Figure 2.
Response-locked ERPs for errors (black), correct responses (grey), and the error minus correct difference (dotted) at Cz (top)—the scalp topography of the error minus correct difference in the time-range of the ERN is plotted below, separately for participants below (left) or above (right) the mean on laboratory measures of temperamental fear.
Similar results were evident for ERN amplitude at Fz (see Table 2): higher levels of child NE were associated with a smaller (i.e., less negative) ERN, whereas child PE was not correlated with ERN. Of the three forms of child NE, there was a trend for a unique association between greater fearfulness and a smaller (i.e,. less negative) ERN.
Unique Effects of Maternal Anxiety and Child NE/Fearfulness
Hierarchal linear regression analyses were conducted to determine whether the effects of maternal anxiety disorder and child NE on children’s ERNs were independent or if one variable accounted for the effects of the other. In the model predicting ΔERN amplitude at Cz, we entered child age on the first step, and maternal anxiety disorder and child NE simultaneously on the second step. After controlling for child age, maternal anxiety and child NE both had significant unique associations with ΔERN amplitude (overall model: R-squared = .06, p = .001; maternal anxiety: b = 2.89, S.E. = 1.03, t = 2.80, p < .01; child NE: b = 4.52, S.E. = 1.90, t = 2.38, p < .05). In the model predicting ERN amplitude at Fz, we entered maternal anxiety disorder and child NE simultaneously. Again, both variables had significant unique associations with ERN amplitude (overall model: R-squared = .04, p < .01; maternal anxiety: b = 2.76, S.E. = .93, t = 2.95, p < .01; child NE: b = 3.44, S.E. = 1.72, t = 2.00, p < .05).
We also conducted a hierarchal linear regression analysis to examine the unique effects of maternal anxiety disorder and child fearfulness on children’s ΔERN amplitude at Cz. ERN at Fz was not examined as the association with child fear had only achieved a trend level of significance in the analysis reported above. We entered child age on the first step, and maternal anxiety disorder and child fear simultaneously on the second step. After controlling for child age, maternal anxiety and child fear both had significant unique associations with ΔERN amplitude (overall model: R-squared = .06, p = .001; maternal anxiety: b = 2.99, S.E. = 1.03, t = 2.90, p < .01; child fear: b = 3.28, S.E. = 1.39, t = 2.36, p < .05).
Parental Psychopathology, Child Temperament, and Offspring Pe
Table 1 and Figure 1 present the results of analyses examining Pe amplitude at Pz. There were no significant associations between offspring Pe amplitude and any form of maternal (F(3, 318) = 1.20, p = ns) and paternal psychopathology F(3, 316) = .39, p = ns). Table 2 and Figure 2 present the results of analyses examining Pe amplitude at Pz. There were no significant associations between Pe amplitude and child PE or child NE (F(2, 323) = .09, p = ns). There were also no significant associations with any of the three forms of child NE (F(3, 322) = 1.27, p = ns).
Discussion
Both maternal history of anxiety disorders (Last et al., 1991) and temperamental NE (Kotov et al., 2010), particularly fearfulness (Goldsmith & Lemery, 2000), have been linked to increased risk for anxiety disorders. In the current study, both variables were assessed in a community sample of 413 three year-olds, and were related to ERN and Pe measured at age six. Both maternal history of anxiety disorders and high NE independently and additively predicted a smaller ERN. Moreover, the effect for temperamental NE was driven by fearfulness, rather than sadness or anger. There were no significant associations with Pe amplitude. To our knowledge, these are the first data linking variation in the ERN among children to known risk factors for the later development of anxiety. This finding is consistent with the notion that variation in error-related brain activity may be a trait-like biomarker of risk for anxiety (Weinberg et al., 2012).
It is important to note, however, that the direction of these effects were opposite to what would be predicted based on existing research, which has generally found that increased anxiety relates to a larger ERN (Gehring et al., 2000; Stern et al., 2010; Weinberg et al., 2012; Weinberg et al., 2010; Hajcak et al., 2008; Ladouceur et al., 2006). However, existing studies on ERN and anxiety have generally assessed much older participants. Even in studies among youth, the ERN has been related to anxiety in samples that average 13.3, 10.2, and 11.42 years of age (Hajcak et al., 2008; Ladouceur et al., 2006; Meyer et al., 2012). The average age in the current study was 6.1 years.
One possibility is that the relationship between ERN and anxiety-related traits changes over the course of development. Along these lines, we recently examined the ERN among 8 to 13 year-olds, and found that increased parent-reported symptoms of anxiety were associated with a larger (i.e., more negative) ERN—but only among older children (i.e., 11-13 year-olds; Meyer et al., 2012). In fact, among younger children the pattern of results was opposite, albeit not significant: higher parent-reported anxiety was related to a smaller (i.e., less negative) ERN among 8-10 year-olds (Meyer et al., 2012).
There are at least two possible explanations for this type of developmental change. First, these findings could reflect differential involvement of dorsal versus rostral ACC across development, and as a function of anxiety. For instance, hypoactive rostral ACC has been reported in relation to anxiety in some studies (Cunningham, Bhattacharyya, & Benes, 2002); in addition, more dorsal ACC activation may uniquely differentiate adults from children during response monitoring (Velanova, Wheeler, & Luna, 2008). Thus, the relative ratio of dorsal to rostral ACC activity may increase across development, and anxiety may differentially relate to subregions of the ACC. Future longitudinal studies using fMRI would be necessary to more directly test this possibility.
Another reason that the relationship between ERN and anxiety may vary across development could have to do with the changing nature of anxiety itself. Younger participants may focus much more on external threats to safety, and not self-monitor as a function of anxiety. On the other hand, older youth may begin to monitor themselves for signs of danger. We have argued that the ERN most likely relates to more ‘cognitive’ forms of anxiety, such as anxious apprehension (Weinberg et al., 2010; Moser et al., 2005), which does not appear to develop until middle childhood or later (Vasey, Crnic, & Carter, 1994). The present findings suggest that as the nature of anxiety changes over development from preschool to early adolescence, this may be reflected in a shift from a reduced to an enhanced ERN.
Consistent with this possibility, one study found that behavioral inhibition (BI) measured in childhood predicted a larger ERN measured in adolescence; moreover, ERN moderated the relationship between BI and anxiety in adolescence: the combination of high BI in childhood and a larger ERN in adolescence was associated with anxiety disorder status (McDermott et al., 2009). Participants from the current study are currently being followed longitudinally, so it will be possible to prospectively evaluate the relation between ERN at age 6 and subsequent anxiety disorders. An intriguing possibility is that participants with smaller ERNs at age 6 may have larger ERNs in early adolescence, and this trajectory of change may capture variability in risk for anxiety disorders. Finally, it is possible that the ERN differentially relates to anxiety disorder risk versus status, being smaller among high-risk individuals and larger among those with currently diagnosed disorders. We are evaluating this possibility currently.
Of note, the increased negativity on error compared to correct trials was maximal at central recording sites (i.e., Cz); although a centrally-maximal ERN is consistent with previous work on the ERN, the overall scalp distribution of this effect was somewhat more parietal than frontal. As we continue to evaluate these subjects, we will be able to determine whether the scalp distribution of the ERN enhancement becomes more frontal across development.
Acknowledgements
This work was supported by National Institute of Mental Health grants NIMH Grant RO1 MH 069942 (Klein) and NIMH Grant RO3 MH 082113 (Hajcak).
The authors would like to thank Jennie Park and Flannery Murphy for their assistance with data collection.
Footnotes
Conflict of interest statement: no conflicts declared.
References
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: Reliability and validity. Archives of General Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45:524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Arbel Y, Donchin E. Parsing the componential structure of post-error ERPs: A principal component analysis of ERPs following errors. Psychophysiology. 2009;46:1179–1189. doi: 10.1111/j.1469-8986.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- Bittner A, Egger HL, Erkanli A, Costello EJ, Foley DL, Angold A. What do childhood anxiety disorders predict? Journal of Child Psychology and Psychiatry. 2007;48:1174–1183. doi: 10.1111/j.1469-7610.2007.01812.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Lin M, Vargas G, Carp J, Fineman SL, Quandt LC. Error detection and posterror behavior in depressed undergraduates. Emotion. 2008;8:58–67. doi: 10.1037/1528-3542.8.1.58. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. Journal of Comparative Neurology. 2002;45:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. Journal of Neuroscience. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorders-Non-patient edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Sciences. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Sciences. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS. Linking temperamental fearfulness and anxiety symptoms: A behavioral-genetic perspective. Biological Psychiatry. 2000;48:1199–1209. doi: 10.1016/s0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- Goldsmith H, Reilly J, Lemery K, Longley S, Prescott A. Laboratory Temperament Assessment Battery: Preschool Version. 1995 Unpublished manuscript. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G. What we’ve learned from mistakes: Insights from error-related brain activity. Current Directions in Psychological Science. 2012;21:101–106. [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. American Journal of Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain and Cognition. 2004;56:189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Greenberg PE. The economic burden of anxiety and stress disorders. In: Davis KL, Charney DS, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. Lippincott Williams and Wilkins; Philadelphia, PA: 2002. pp. 981–992. [Google Scholar]
- Klein DN, Dyson MW, Kujawa AJ, Kotov R. Temperament and internalizing disorders. In: Zentner M, Shiner R, editors. Handbook of Temperament. Guilford Press; New York, NY: in press. [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychological Bulletin. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Last CG, Hersen M, Kazdin A, Orvaschel H, Perrin S. Anxiety disorders in children and their families. Archives of General Psychiatry. 1991;48:928–934. doi: 10.1001/archpsyc.1991.01810340060008. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biological Psychology. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2:152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Simons RF. The effects of fear on performance monitoring and attentional allocation. Psychophysiology. 2005;42:261–268. doi: 10.1111/j.1469-8986.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: Associations in a large community sample. Journal of Abnormal Psychology. 2010;119:468–478. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Klein DN, Hajcak G. Depression symptom severity and error-related brain activity. Psychiatry Research. 2010;179:30–37. doi: 10.1016/j.psychres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: Evidence from unaffected first-degree relatives. American Journal of Psychiatry. 2011;168:317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Comparability of telephone and face-to-face interviews assessing Axis I and II disorders. American Journal of Psychiatry. 1997;154:1593–1598. doi: 10.1176/ajp.154.11.1593. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Grön G, Reuter K, Spitzer M, Hermle L, Kiefer M. Error-related brain activity in patients with obsessive-compulsive disorder and in healthy controls. Journal of Psychophysiology. 2005;19:298–304. [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses in 10-year-old children and young adults. Developmental Science. 2006a;9:473–481. doi: 10.1111/j.1467-7687.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Developmental Neuropsychology. 2006b;29:431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Stern ER, Liu Y, Gehring WJ, Lister JJ, Yin G, Zhang J, Fitzgerald KD, Himle JA, Abelson JL, Taylor SF. Chronic medication does not affect hyperactive error responses in obsessive-compulsive disorder. Psychophysiology. 2010;47:913–920. doi: 10.1111/j.1469-8986.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa A, Klein DN. Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Developmental Psychobiology. 2012;54:139–150. doi: 10.1002/dev.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Klein DN. The impact of motivational influences on error-related brain activity in young children. Developmental Neuropsychology. 2009;34:749–761. doi: 10.1080/87565640903265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. How does error correction differ from error signaling? An event-related potential study. Brain Research. 2006;1105:102–109. doi: 10.1016/j.brainres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Crnic KA, Carter WG. Worry in childhood: A developmental perspective. Cognitive Therapy Research. 1994;18:529–549. [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulated and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological Psychology. 2010;85:472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, Hajcak G. Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation Emotion. 2012;36:84–100. [Google Scholar]
- Yonkers KA, Bruce SE, Dyck IR, Keller MB. Chronicity, relapse, and illness-course of panic disorder, social phobia, and generalized anxiety disorder: Findings in men and women from 8 years of follow-up. Depression and Anxiety. 2003;17:173–179. doi: 10.1002/da.10106. [DOI] [PubMed] [Google Scholar]