Abstract
The circadian clock machinery orchestrates organism metabolism in order to ensure that development, survival and reproduction are attuned to diurnal environmental variations. For unknown reasons, there is a decline in circadian rhythms with age, concomitant with declines in the overall metabolic tissues homeostasis and changes in the feeding behavior of aged organisms. This disruption of the relationship between the clock and the nutrient sensing networks might underlie age-related diseases; overall, greater knowledge of the molecular mediators of and variations in clock networks during lifespan may shed light on the aging process and how it may be delayed. In this review we address the complex links between the circadian clock, metabolic (dys)functions and aging in different model organisms.
Keywords: aging, circadian clock, glucose metabolism, insulin, flies, mice, nutrients
Aging signaling pathways and control of nutrient metabolism
Whether aging might be regarded as a disease or as a consequence of development, in molecular terms it can be understood as a decline of the homeostatic mechanisms that ensure function of cells, tissues, organs and organ systems 1-4. During evolution, a set of interconnected signaling pathways were selected to sense environmental cues and modulate these fundamental homeostatic and physiological functions: proliferation/growth, repair/survival and reproduction. Thus, it should be no surprise that environmental conditions that influence longevity often exert their effects through such pathways 1-4.
Nutrient sensing pathways in model organisms
In yeast, nutrient signaling allows colonies to synchronize cell division and adapt their metabolism to the surrounding environment. In scarce nutrient conditions, 90-99% of the colony undergoes cell death in an altruistic fashion 5, allowing the remaining cells to survive on the restricted nutrients. This contributes to the main goal of a clonal population: to maximize the future replication of its shared genome, in this case by assuring that a small number of individuals will survive the harsh environmental conditions.
In multicellular organisms a set of tissues became specialized in nutrient sensing. For example, in C. elegans, nutrient signaling takes place primarily in neurons, the gonad and the intestine. As in higher animals, primary nutrient signaling pathways include insulin/insulin-like growth factor (IIS) and target of rapamycin (TOR), both of which are well-known to modulate lifespan 6, 7. Insulin-like peptides synthesized in response to nutrient sensation are bound by the insulin receptor in neurons. Loss of DAF-2, the only insulin/IGF-1 like receptor in the worm, enhances lifespan through signaling to the intestine and gonad 8. Germline loss, which mobilizes intestinal fat stores and increases autophagy, also promotes longevity 9, 10. Finally, reductions in TOR activity enhance lifespan and mimic the effects of caloric restriction 11. Altogether, these data show that modulation of nutrient signaling in development and reproduction also affects aging and lifespan in C. elegans.
Growth and reproduction are matched to the environment through long-range signals, primarily insulin, to provide whole-body coordination of energy consumption and storage. As with C. elegans, in Drosophila and mammal IIS signaling is integrated through AKT/PI3K to the TOR pathway to promote growth and proliferation 3, 4, 12, 13 (Figure 1). In Drosophila, reduction in the activity of TOR or IIS pathways leads to developmental delays and small-sized flies with reduced fecundity, but increased longevity 11, 13. Similar interventions in adult flies also enhance lifespan and slow the appearance of age related characteristics 11, 13. Furthermore, mice lacking ribosomal S6 protein kinase 1 (S6K1), a downstream component of the TOR pathway, have longer lifespan and exhibit resistance to age-related pathologies 14-17. Feeding worms, flies or mice with rapamycin, which reduces TOR signaling, also extends lifespan 16. Reduction of the anabolic hormones growth hormone (GH), IGF-1, or insulin, leads to long-lived mice and resistance to age related pathologies 18, 19. Mutant mice with reduced plasma IGF-1 are smaller in size and show lifespan extension 20. Similarly, mice mutant for insulin receptor substrate 1 (IRS1: a component of the IGF-1 pathway) show decreased insulin sensitivity but are long-lived 21 (Figure 1). In humans, increased insulin sensitivity, evidenced by reduction in plasma insulin, IGF-1 and plasma glucose levels, is a hallmark of increased longevity 4, 22. Indeed, individuals with Laron syndrome (see Glossary), who are deficient in GH receptor, show remarkable reduction in pro-aging signaling, cancer, and diabetes 23.
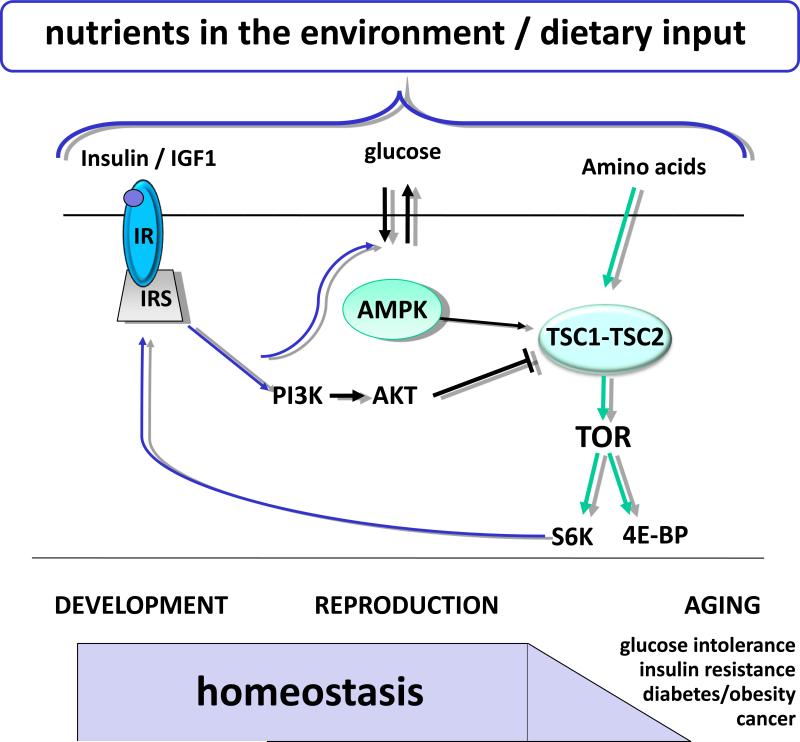
Figure 1. Nutrient sensing pathways crosstalk during lifespan.
Several distinct pathways sense dietary input from nutrients in the environment. Amino acids are sensed by the complex TSC1/TSC2 of the TOR pathway. This signal is relayed by a series of mediators, including TOR kinase to the downstream effectors S6K1 and 4E-BP. S6K and 4E-BP enhance translation, and hence, anabolism. Glucose is transported into the cell and converted from ADP and AMP to ATP, which ratios are sensed by the AMPK pathway. AMPK interacts with TSC1/TSC2 to ensure cell homeostasis. Circulating insulin and IGF (IIS) are sensed by the Insulin Receptor (IR) and transmitted to the Insulin Receptor Substrate (IRS, chico in flies). The activated IRS relays the signal to PI3K, which activates AKT, which in turn leads to transcriptional activation of target genes. AKT links the IIS pathway to the glucose sensors and TOR signaling and promotes anabolic processes. Reciprocally, signals from the TOR pathway are relayed via S6K to the IRS in the IIS pathway. As an organism's energy requirements vary throughout lifespan, these signaling pathways operate to maintain energy homeostasis during periods of high demand, such as development and reproduction. Decay in the relays of signals and/or crosstalk leads to aging and age related diseases like insulin resistance, glucose intolerance, diabetes, obesity, metabolic syndrome and cancer.
Dietary input and lifespan
The incidence of insulin resistance, diabetes, metabolic syndrome and cardiovascular diseases increases with age in humans 3, 4. Aging-related modifications in body composition that contribute to insulin resistance and metabolic syndrome are represented by abdominal obesity and sarcopenia 24. Abdominal obesity is observed in the elderly, despite a normal body mass index and visceral fat is associated with changes in proinflammatory cytokines, and interferes with insulin action. Sarcopenia contributes to the loss of skeletal muscle and to insulin resistance through reduction in energy expenditure 24.
Caloric restriction (CR) is the only intervention known to slow down aging across taxa 25, 26. While the positive effects of CR on longevity are clear in nematodes, flies, and inbred mouse strains 27, results on wild-caught mice 28, primates 29-31, and human populations 25, 26, 32, 33, suggest that the genetic background, the age of onset of CR, and the composition of the diet may affect the longevity outcomes in mammals. More studies are required to understand the effects of CR in lifespan and healthspan in higher vertebrates, as well as species-specific nutrient requirements throughout lifespan. This will also help to better understand which type of dietary restriction (DR) improves healthspan in each species 26. The mechanisms underlying CR effects are poorly understood. Evidence for involvement of insulin signaling was obtained in dilp (Drosophila insulin like peptides) mutant flies, which mimic the effects of CR 34. Flies fed a high fat diet (HFD) show alterations in insulin and glucose homeostasis, as well as other age related pathologies, similar to those observed in mammals. Down-regulation of the insulin-target of rapamycin (TOR) pathway ameliorates these effects 35.
Similarly, CR improves insulin sensitivity and age related diseases in mice 36 and in rhesus monkeys 25, 29. As in flies, mice subjected to a HFD show TOR hyperactivation, which contributes to insulin resistance and obesity, both exacerbated during aging 17. Instead, mice null in ribosomal protein S6 kinase beta-1 (S6K1) do not show insulin resistance associated with HFD 17 (Figure 1). Further, preserved glucose tolerance and insulin sensitivity are common features of centenarians when compared to other age groups of elderly subjects 25, 32. Evidence in humans suggests that short term fasting protects against various cancers, possibly by downregulating the IIS/nutrient signaling pathway 37. Altogether, the evidence suggests that IIS/TOR pathways and dietary inputs cross-talk during the lifespan of an organism. Is this cross-talk, then, related to another major nexus between metabolism and diet, the circadian rhythm?
Circadian clock machinery and nutrient metabolism
Daily rotation of the Earth on its axis and yearly revolution around the Sun impose periodicity on most organisms. Molecular circadian clocks might have evolved to synchronize internal metabolic rhythms to predictable environmental cycles, in order to most advantageously time functions such as feeding, mating, and general patterns of activity 38. Moreover, a growing body of evidence suggests reciprocal links between nutrient sensing pathway and circadian clocks 39.
Circadian signaling in worms
C. elegans appear to use a different mechanism to generate daily rhythms than flies and mammals 40-42. Though most core circadian genes are conserved in C. elegans 40, 43, these genes generally function in timing the periodic moults in nematode larval development 41, 44, and are not expressed with any known periodicity in adulthood 42. Nevertheless, these animals do have detectable circadian rhythms that can be entrained by light:dark or warm:cool cycles. This rhythmicity is measurable in both larvae and adults, in parameters such as motility 45, 46, stress-resistance 47, 48, pathogen-resistance 49, food and oxygen consumption 50, and gene expression 42. Further, conserved signaling molecules are involved in the C. elegans circadian process. First, pigment dispersing factor (PDF) and its receptor, which in insects play a role in transducing circadian oscillations to downstream processes (a function analogous to that of VIP: Vasoactive Intestinal Peptide in mammals) 51, 52, have, been identified in C. elegans, and shown to be involved in circadian rhythmicity in locomotion 53. Second, circadian cycling of the levels of melatonin and its synthase has recently been identified 54, though not yet functionally characterized. Further downstream, profiling of genes expressed in rhythmic fashion during and after 12-hour light:dark or warm:cool cycles identified GO terms relating to a broad diversity of biological processes, chief among them metabolic processes, enriched in the genes that can be driven and/or entrained by cycling temperature in particular 42. Of note, this study found that light versus temperature cycling entrained distinct sets of genes (unlike the case in Drosophila, for example), suggesting that there could be multiple independent clock mechanisms or differential damping of genes entrained by different processes.
Circadian signaling in flies
Unlike in C. elegans, circadian clock genes and mechanisms seem to be largely conserved between Drosophila and humans. In flies and mammals, circadian clocks consist of transcriptional and translational feedback loops working in a cell autonomous manner (Figure 2). At the core of the Drosophila circadian clock there are four clock genes Clock (Clk), cycle (cyc, an ortholog of BMAL1 in mammals), timeless (tim), and period (per) 55. They interact in a negative feedback loop, whereby expression levels of per and tim are regulated by transcriptional activators encoded by Clk and cyc. This leads to periodic increase in the levels of PER and TIM proteins, which accumulate in cell nuclei, and repress CLK/ CYC activators, causing suppression of per and tim transcription 55. In addition to per and tim, CLK/ CYC heterodimers activate genes that participate in secondary clock feedback loops and a substantial number of clock output genes 55, 56.
Figure 2. The circadian clock network is conserved in flies and mammals.
Nematodes appear to use non-conserved mechanism to generate circadian rhythms. Light inputs generate oscillations on gene expression and locomotion, among other variables, and PDF and melatonin might be mediators of enviromental cues. In higher species, the circadian clock is constituted by the CPN in flies and the SCN in mammals. In Drosophila there are four clock genes, which interact in a negative feedback loop: Clock (Clk), cycle (cyc, an ortholog of BMAL1 in mammals), timeless (tim), and period (per). CLK and CYC activate and regulate expression levels of per and tim This leads to periodic increase in the levels of PER and TIM proteins, which accumulate in cell nuclei, and repress CLK/ CYC activators, causing suppression of per and tim transcription. PDF is expressed in a subset of CPNs and affects circadian period. Increased nutrient sensing via dTOR signaling lengthens the circadian period through S6K and GSK3, which also affects TIM. In mammals, CLOCK and BMAL1 are transcriptional activators that bind the enhancer sequences of mPer (Per 1, Per 2 and Per 3) and mCry (Cryptochrome; Cry1 and Cry2). PER and CRY proteins accumulate in the cytoplasm and translocate to the nucleus to inhibit CLOCK:BMAL1. The CLOCK/BMAL1 complex also activates transcription of the nuclear receptors REV-ERBα/β and RORα/β/δ and γ. REV-ERB acts as negative regulators of ROR. ROR and REV-ERB proteins compete for binding sites in the promoter region of Bmal1 and regulate its transcription. SIRT1 interacts with CLOCK and deacetylates BMAL1 and PER. AMPK modulated levels of CRY1 and PER and regulates SIRT1. mTOR is also regulated in a circadian manner in the mouse SCN. VIP is expressed in a subset of neurons of SCN and affects the circadian period. These interactions ensure temporal homeostasis during development and reproduction.
The central clock in flies consists of ~150 central pacemaker neurons (CPNs) controlling rest/activity rhythms 57, similar to the suprachiasmatic nucleus (SCN) in mammals. In addition, peripheral clocks are located in most fly cells, such as sensory neurons, glia, fat, and excretory system; these oscillators are CPN-independent and serve tissue-specific functions 58. Some clock output genes are rhythmic in both central and peripheral clock cells, such as the enzymes involved in glutathione biosynthesis 59.
Circadian signaling in mammals
As in flies, the mammalian circadian clock is constituted by a coordinator center, the SCN, which integrates cues derived from peripheral clocks located in peripheral tissues (liver, adipose tissue, reproductive organs among others) with external light:dark cycles 38. At a molecular level, the “clock genes” constitute a similar network as that found in Drosophila. CLOCK and BMAL1 are transcriptional activators that bind the enhancer sequences of Per and Cry (Cryptochrome) 38. In turn, PER and CRY proteins accumulate in the cytoplasm and translocate to the nucleus to inhibit CLOCK:BMAL1 38. CLOCK/BMAL1 also activates transcription of the nuclear receptors (NRs) reverse transcript of erythroblastosis gene (REV-ERB) α/β and retinoic acid related (RAR) orphan receptor (ROR) α/β/δ and γ. REV-ERBα and REV-ERBβ act as negative regulators of ROR. ROR and REV-ERB proteins compete for ROR regulatory element (RRE) binding sites in the promoter region of Bmal1 and regulate its transcription 38, 60. SIRT1, a NAD-dependent deacetylase, interacts directly with CLOCK and deacetylates BMAL1 and PER 61. In addition, the nutrient sensor AMPK directly phosphorylates and destabilizes CRY1 62, and acts upstream of PER. AMPK can in turn regulate SIRT1 activity 63, participating with the core circadian clock machinery in an intricate nutrient sensing signaling loop that is now starting to be unraveled at the whole organism level (Figure 2).
Lessons from modulating circadian signaling pathways
Genetic manipulations in flies leading to increased dTOR signaling, specifically in a subset of the central pacemaker neurons, significantly lengthen the circadian period of locomotor activity, whereas mutations reducing AKT activity shorten the circadian period 64. In the fly brain, the modulation of circadian period is mediated by the S6K and activity of glycogen synthase kinase-3 (GSK3), which also affects core clock proteins 64. In the mouse SCN, TOR is also regulated by circadian function 65. Thus, conserved nutrient sensing pathways are integrated into circadian clock network, likely to ensure organism-wide temporal homeostasis and survival.
Another process integrated by the circadian clock network in mammals, and probably also in flies 66, is glucose homeostasis 38. In mammals, the hormones cortisol and melatonin also convey the rhythmic output of the SCN to peripheral oscillators and modulate glucose homeostasis 67-69. Circadian rhythmicity of cortisol secretion is implicated in nycthemeral changes of insulin sensitivity 67. Melatonin modulates glucose-stimulated insulin secretion 70, 71.
The circadian control of glucose homeostasis is demonstrated in mice with depletion of clock genes in the whole organism or in peripheral metabolic organs. Clock hypomorphs, Bmal1-/- and Per2-/- mice show impaired glucose metabolism 72-74. Furthermore, mouse models of diabetes and obesity, where glucose homeostatic mechanisms are altered, exhibit altered circadian behavior 73, 74. This bidirectional relationship between circadian disruption and metabolic pathologies also appears in humans. Diabetic patients exhibit dampened amplitude of rhythms of glucose tolerance and insulin secretion 75; conversely, a higher incidence of metabolic diseases and cardiovascular events is observed in shift workers 76, 77. Forced circadian misalignment (simulating shift work) has been shown to impact on neuroendocrine control of glucose metabolism 78. Moreover, genomic variations in clock genes have been linked to obesity and metabolic syndrome in humans 79, 80.
Interestingly, eating habits (timing and amount of food) may contribute to not only body weight gain and insulin sensitivity, but also to the circadian pattern of clock genes expression in the liver as well as the activity of nutrient sensing pathways like TOR, AMPK and SIRT1 81-83. A HFD with a time restricted feeding schedule, leads to reduced cholesterol levels and delayed onset of insulin resistance and glucose intolerance 81, 83. Time restricted feeding can restore the shifts induced in the oscillations of clock genes as well as the levels of expression of nutrient sensing molecules induced by a HFD 81, 83, 84. Such studies are relevant for human health since re-alignment of the clock through feeding could constitute a non-pharmacological treatment against metabolic diseases.
Aging molecular networks and the circadian clock
For reasons yet unknown, during aging there is a concomitant decline in circadian rhythms and the overall homeostasis of the organism. Because circadian clocks integrate a wide range of physiological functions, better knowledge of the molecular mediators and variations in clock networks during lifespan might lead to a better understanding of the aging process and how it may be delayed.
Circadian rhythms and aging in worms
Genome wide expression analysis in C. elegans identified a set of light and temperature driven oscillating transcripts, suggesting that these transcripts might be under circadian control 42. Moreover, the GO term “multicellular organismal aging” is enriched in the light-driven and light-entrained transcript sets. Further, the aging-relevant transcription factors pha-4, skn-1, sod-3 and daf-16 oscillate in warm:cool cycles (but not in light:dark cycles); however none can be entrained to continue free-running oscillation in the absence of periodic stimulus. The physiological relevance of these observations remains unclear. However, extended-longevity phenotypes are well known to correlate with stress-resistant in C. elegans 85; as such, the observation that resistance to osmotic, oxidative, and pathological challenges varies in circadian fashion 47 suggests a circadian modulation of pathways relevant for lifespan determination.
Circadian rhythms and aging in flies and mammals
In flies and mammals, including human, robust high-amplitude circadian rhythms are observed in young individuals but lose their strength with age 86-88. Daily rhythms in hormone levels, body temperature, sleep/wake cycles, and other physiological and behavioral variables are diminished during aging 88, 89. In mammals, disruption of circadian rhythms with age might be associated with a reduced sensitivity of SCN to entrainment signals 90, 91. For example, aged mice are less sensitive to light entrainment than young ones and have reduced Per2 expression 92, 93, reduced responsiveness to melatonin 94 and serotonin in the SCN 95.
Studies performed in aging mammals reported an either reduced or normal expression of different clock genes, depending on the organs and species examined 96. More recent evidence, both in mammals and flies, suggest aging is associated with reduced expression of specific clock genes in peripheral clocks but not in the Central Pacemaker Neurons (CPNs) or SCN 86, 96, 97: Moreover, aging affects differentially the re-entrainment responses of central and peripheral circadian clocks 90, 91. Altogether, these data indicate that synchrony between all the clocks in the body and the CPN/SCN as well as within the central clock might be crucial to prevent aging.
Possible mediators of the aging circadian network
In mammals, disruption of circadian rhythms with age might be associated with a reduced sensitivity of SCN to entrainment signals 90, 91. For example, aged mice are less sensitive to light entrainment than young ones and have reduced Per2 expression 92, 93, reduced responsiveness to melatonin 94 and serotonin in the SCN 95. Clock -/- and Bmal1 -/- mice, on top of impaired glucose homeostasis, exhibit premature age-associated disorders and reduced lifespan 72, 87, 98, 99, as well as increased sensitivity to genotoxic stress 100. Although, downstream effectors remain largely unknown, a likely candidate is the redox system, which oscillates daily and affects BMAL1 activity 93, 101. Indeed, Bmal1 -/- mice suffer from chronic redox stress 100, which could explain the sensitivity to genotoxic stress and short lifespan. A recent study showed that reactive oxygen species (ROS) oscillate in a circadian manner in wild-type mouse brains, though exhibit a stochastic behavior in Bmal1 -/- brains 101. Likewise, in Drosophila, ROS levels oscillate in wild-type heads and accumulate to higher levels in aging per-/- flies 102, 103. Therefore, ROS species are plausible candidates to mediate the effects of decaying clocks on the impairments of brain function and locomotion that are observed in mutant and aged mice 87, 104. Melatonin, which has antioxidant neuroprotective effects 105, oscillates daily 67, 106, 107, and decreases with age 69, 95, 107, 108, is another putative link between the decline of brain function and circadian clock. Nevertheless, there is no conclusive data regarding locomotor impairment, memory loss, mood and/or sleep disorders in mice mutant for clock genes 87. Furthermore, given the role of clock genes in growth/proliferation and cancer 100, other possible mediators of clock genes are the cell cycle checkpoint genes, which could explain high cancer susceptibility, and hence short lifespan in mice mutant for certain clock genes’.
Age related SCN derangement
In humans and rhesus monkeys, the circadian timing system shows progressive age-related derangements 88. Overt degeneration in the human SCN occurs later than the functional changes in circadian organization 87: reduction in SCN volume and neuron number begin at 80 years of age, whereas behavioral and sleep pattern changes are reported in 50 to 60 year-old subjects 109. Considering that SCN oscillators drive metabolic pathways through autonomic nervous system innervation to the pancreas and liver 110, the progressive deterioration of neuronal networks in the circadian system may lead to increasing destabilization of the array of rhythms that underlies the maintenance of organism metabolic homeostasis (Figure 3).
Figure 3. The circadian clock network and nutrient sensing pathways cross-talk during lifespan.
In mammals, the circadian coordinator center, the suprachiasmatic cell nuclei (SCN), integrates light/dark cycles and nutrient cues from the environment. The SCN then relays signals to peripheral clocks. Nutrient sensing pathways in the SCN cross-talk with peripheral clocks, in a yet undefined manner. The crosstalk between nutrient sensing pathways and clock network leads to the oscillation of metabolites, ROS and hormones. These oscillations constitute individual “body time” and lead to the overall physiological homeostasis of the organism during development and reproduction. Desynchronization, disruption or decay of the clock network is associated with aging and age-associated diseases.
Effects of aging on the circadian clock-dependent changes in peripheral nutrient metabolism
Energy requirements are among the physiological variables the most robustly change both during the day and throughout the life of an organism. An example of crosstalk between the nutrient sensing pathways, clock genes and the environment is observed in feeding behavior and the fluctuations of metabolic hormones, which are altered during physiological aging 87, 107, and in metabolic diseases 67, 68, 110.
Declining clock genes expression in aging flies may have multiple metabolic consequences
In Drosophila, clock proteins are expressed in the fat body, an organ combining the fat and liver functions of mammals. The fat body clock has been linked to circadian rhythm in feeding and flies lacking clocks in these tissues display increased food consumption and higher sensitivity to starvation 111, 112. Overall, the input of neuronal clocks in the brain and that of peripheral clocks in metabolic tissues appear coordinated to provide effective energy homeostasis 111, 112. More specifically, basic metabolite levels are coordinated in a clock-dependent manner and that clock function is required to maintain lipid homeostasis 66. However, the important question of how declining expression of clock genes may affect metabolism in aging flies remains to be addressed. This role may be substantial, though: the clock in the Drosophila fat body drives rhythmic expression of genes involved in metabolism, detoxification, and hormone regulation 113. Restricted feeding (RF) drives rhythmic gene expression in the fat body but not in the brain clocks. Limiting food to a time of day when consumption is low leads to desynchronization of internal rhythms and to reduced egg production, showing that the circadian clock interacts with metabolic physiology to influence reproductive fitness. Further, clocks are also important for rhythmic expression of xenobiotic-metabolizing enzymes in flies 114 and mammals 115.
Additionally, in mammals, there are daily circadian oscillations in hormones such as leptin, insulin and glucagon, which affect blood glycemia 67, 68, 110. However, if and how these oscillations change throughout lifespan, and which molecular signaling pathways are involved, remains to be addressed. It is possible that decaying clocks affect the rhythmic oscillations of metabolic enzymes, through the action of melatonin and cortisol, leading to age-associated insulin resistance, diabetes, or glucose intolerance.
Diet effects on circadian rhythms
Like the fat bodies in flies, the role of mammalian pancreatic and liver clocks in aging might be substantial. However, these effects may be indirect: the environment (i.e. diet, stress insults) can affect aging through feeding, and the feeding regime can affect circadian rhythms 116, 117. In fact, before they become obese, mice fed on a HFD start feeding during the day73. A HFD modifies circadian synchronization to light 117. Recent data on a diurnal rodent, Arvicanthis ansorgei, indicates that a hypocaloric diet can also affect the circadian system, in particular Per2 light induced expression in the SCN 118.
As in Drosophila, mice under RF have a deranged peripheral clock without affecting the SCN pacemaker 81, 84, 116. Nevertheless, RF improves the overall health, in particular, metabolic homeostasis 81, 83, 84. Depending on the age of administration, intermittent RF increased both mean and maximum lifespan in inbred mice 119. It is yet to be determined if this is the case for all types of diets and what the influence of the genetic background may be. Mice placed under CR live longer and show resistance to age associated diseases 25-27. These mice also consume most of their food within a few hours, however their peripheral clocks are well synchronized with the SCN 116. Considering that mice under RF have a deranged peripheral clock and lifespan extension requires further research, it has been speculated that this synchrony observed in mice under CR could be the source of lifespan extension 116. Though synchrony appears important for lifespan extension, the integrity of the SCN, which governs the feeding behavior, and the time of feeding appear important for metabolic homeostasis and healthspan.
Further, αMUPA mice may be a very useful model for elucidating the links between aging, caloric intake and circadian rhythms because they show spontaneous reduction in appetite, live as long as CR mice, and show higher amplitude in circadian rhythms 116, 120.
The cross-talk between circadian clock circuitry and metabolic pathways could represent a fundamental hinge in the pathophysiological mechanisms of the aging process. The negative effects on human health deriving from alterations of the correct timing of the endogenous circadian clock (chronodisruption) are an important cause of diseases (metabolic, degenerative, cancer) leading to premature aging 121 (Figure 3). Recent studies have aimed to determine individual “body time” by measuring the oscillation of metabolites in individual blood samples 122, potentially paving the way to individualized health interventions. Overall, such metabolic oscillators may link circadian rhythmicity with nutrient sensing pathways, and impairment of these links may underlie or aggravate many of the pathologies associated with aging.
Concluding remarks
Model organism data and epidemiological evidence discussed here indicate that disruption of circadian rhythms has detrimental effects on health and can lead to metabolic diseases such diabetes and obesity. At the same time, circadian clocks are tightly coupled to cellular metabolism and respond to light and feeding cycles. A regular lifestyle in terms of exposure to daylight, physical exercise, sleep/wake and feeding patterns has beneficial impact on our health probably because it helps to maintain such synchrony. Thus, it will be important to determine why circadian rhythms diminish during aging, and whether such decline could be reversed to boost temporal tissue homeostasis. Ongoing 123 and future studies that include understanding the impact of alterations in the biological clock system on chromatin remodeling, gene expression, translation, signaling, and function of individual cell types and peripheral tissues will advance our knowledge towards personalized therapeutic approaches. In the meantime progress in circadian research appears to provide scientific validation of the maxim “Early to bed, early to rise, makes a man healthy, wealthy and wise”.
Outstanding Questions BOX.
What are the main factors that determine individual body time? Can genetic and environmental factors be quantified? Can restricted feeding improve temporal homeostasis?
Is there a differential role for TOR in the SCN or in peripheral clocks?
How is the clock system altered in mutants for IIS signaling? How does the IIS pathway influence metabolite oscillations and body time?
How does declining expression of clock genes affect metabolism in aging organism?
What is the role of chromatin remodeling factors in determining body time?
Acknowledgments
We apologize to all those researchers who are not cited due to space limits. We thank Antonio Musarò for comments on the paper. MV is supported by the Foundation for Liver Research and by the Italian Ministry of Health. JMG supported by NIH 1R21AG038989 and R21-NS-075500 grants. ZP is supported by NIH 1K99AG04248701. GM is supported by RC1203ME46 grant from the Italian Ministry of Health.
Glossary
- αMUPA mice
these animals carry a transgene encoding urokinase-type plasminogen activator, a member of the plasminogen/plasmin system that functions in fibrinolysis and extracellular proteolysis. These mice spontaneously consume less food when fed ad libitum and live longer compared with wild-type control mice. αMUPA mice are obesity resistant and share many similarities with calorically restricted animals 116, 120
- Caloric restriction (CR)
the reduction of caloric intake (typically by 20–40% of ad libitum consumption) while maintaining adequate nutrient intake 26
- Dietary restriction (DR)
restriction of one or more components of intake (typically macronutrients) with no reduction in total caloric intake 26
- Chronotherapy
use of circadian or other rhythmic cycles in the application of therapy
- Glucose intolerance
a pre-diabetic state of hyperglycemia that is associated with insulin resistance and increased risk of cardiovascular pathology
- Insulin sensitivity
a physiological condition in which cells respond to the normal actions of the hormone insulin. Insulin-sensitive cells are able to take in glucose, amino acids and fatty acids
- Melatonin
a hormone secreted by the pineal gland in the brain that promotes sleep, and helps to regulate circadian rhythms and temporal homeostasis
- Metabolic syndrome
a combination of medical disorders that, together, increase the risk of developing cardiovascular disease and diabetes. These include obesity, raised triglycerides, reduced HDL cholesterol, raised blood pressure and raised fasting plasma glucose
- Laron syndrome
an autosomal recessive disorder, also known as Growth Hormone Receptor Deficiency, characterized by an insensitivity to growth hormone (GH), caused by a splice site mutation in exon 6 of the growth hormone receptor gene. Phenotypically, this syndrome causes short statue with normal body proportions in childhood but child-like proportions in adults. Individuals have reduced TOR expression and low plasma insulin, which leads to increased insulin sensitivity. These could be the causes of the observed resistance to diabetes and cancer in this individuals23
- Restricted feeding (RF)
animals are fed ad libitum but food is provided for only a short time (e.g. 2 h) during each circadian cycle. RF can change phase of clock oscillations in liver but not in the SCN leading to desynchronization
- Sarcopenia
age related degenerative loss of muscle mass and strength
- Serotonin
a monoamine neurotransmitter that is biochemically derived from tryptophan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yeoman M, et al. Insights into CNS ageing from animal models of senescence. Nat Rev Neurosci. 2012;13:435–445. doi: 10.1038/nrn3230. [DOI] [PubMed] [Google Scholar]
- 2.Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- 3.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22:R741–752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Barzilai N, et al. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabrizio P, et al. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J Cell Biol. 2004;166:1055–1067. doi: 10.1083/jcb.200404002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapierre LR, Hansen M. Lessons from C. elegans: signaling pathways for longevity. Trends Endocrinol Metab. 2012 doi: 10.1016/j.tem.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panowski SH, Dillin A. Signals of youth: endocrine regulation of aging in Caenorhabditis elegans. Trends Endocrinol Metab. 2009;20:259–264. doi: 10.1016/j.tem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Libina N, et al. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- 9.Lapierre LR, et al. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol. 2011;21:1507–1514. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang MC, et al. Fat metabolism links germline stem cells and longevity in C. elegans. Science. 2008;322:957–960. doi: 10.1126/science.1162011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapahi P, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partridge L, et al. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp Gerontol. 2011;46:376–381. doi: 10.1016/j.exger.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, et al. Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood. 2011;117:1228–1238. doi: 10.1182/blood-2010-05-287821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shima H, et al. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. Embo J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem Soc Trans. 2011;39:460–465. doi: 10.1042/BST0390460. [DOI] [PubMed] [Google Scholar]
- 17.Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 18.Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012;67:652–660. doi: 10.1093/gerona/gls086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinciguerra M, et al. mIGF-1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart. Aging Cell. 2012;11:139–149. doi: 10.1111/j.1474-9726.2011.00766.x. [DOI] [PubMed] [Google Scholar]
- 20.Panici JA, et al. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. Faseb J. 2010;24:5073–5079. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selman C, et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. Faseb J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 22.Fontana L, et al. Growth factors, nutrient signaling, and cardiovascular aging. Circ Res. 2012;110:1139–1150. doi: 10.1161/CIRCRESAHA.111.246470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guevara-Aguirre J, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinciguerra M, et al. Regulation of muscle atrophy in aging and disease. Adv Exp Med Biol. 2010;694:211–233. doi: 10.1007/978-1-4419-7002-2_15. [DOI] [PubMed] [Google Scholar]
- 25.Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011;585:1537–1542. doi: 10.1016/j.febslet.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trepanowski JF, et al. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: a summary of available findings. Nutr J. 2011;10:107. doi: 10.1186/1475-2891-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontana L, et al. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper JM, et al. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kastman EK, et al. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J Neurosci. 2012;32:11897–11904. doi: 10.1523/JNEUROSCI.2553-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willcox DC, et al. The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr. 2009;28(Suppl):500S–516S. doi: 10.1080/07315724.2009.10718117. [DOI] [PubMed] [Google Scholar]
- 33.Sebastiani P, et al. Whole genome sequences of a male and female supercentenarian, ages greater than 114 years. Front Genet. 2011;2:90. doi: 10.3389/fgene.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gronke S, et al. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birse RT, et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonkowski MS, et al. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee C, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:124ra127. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzoccoli G, et al. Clock genes and clock-controlled genes in the regulation of metabolic rhythms. Chronobiol Int. 2012;29:227–251. doi: 10.3109/07420528.2012.658127. [DOI] [PubMed] [Google Scholar]
- 39.Peek CB, et al. Nutrient sensing and the circadian clock. Trends Endocrinol Metab. 2012;23:312–318. doi: 10.1016/j.tem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Temmerman L, et al. C. elegans homologs of insect clock proteins: a tale of many stories. Ann N Y Acad Sci. 2011;1220:137–148. doi: 10.1111/j.1749-6632.2010.05927.x. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee D, et al. Developmental timing in C. elegans is regulated by kin-20 and tim-1, homologs of core circadian clock genes. Dev Cell. 2005;8:287–295. doi: 10.1016/j.devcel.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 42.van der Linden AM, et al. Genome-wide analysis of light- and temperature-entrained circadian transcripts in Caenorhabditis elegans. PloS Biol. 2010;8:e1000503. doi: 10.1371/journal.pbio.1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasegawa K, et al. Caenorhabditis elegans opens up new insights into circadian clock mechanisms. Chronobiol Int. 2005;22:1–19. doi: 10.1081/cbi-200038149. [DOI] [PubMed] [Google Scholar]
- 44.Tennessen JM, et al. Novel heterochronic functions of the Caenorhabditis elegans period-related protein LIN-42. Dev Biol. 2006;289:30–43. doi: 10.1016/j.ydbio.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 45.Simonetta SH, et al. Timing of locomotor activity circadian rhythms in Caenorhabditis elegans. PLoS ONE. 2009;4:e7571. doi: 10.1371/journal.pone.0007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saigusa T, et al. Circadian behavioural rhythm in Caenorhabditis elegans. Curr Biol. 2002;12:R46–47. doi: 10.1016/s0960-9822(01)00669-8. [DOI] [PubMed] [Google Scholar]
- 47.Simonetta SH, et al. Circadian stress tolerance in adult Caenorhabditis elegans. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:821–828. doi: 10.1007/s00359-008-0353-z. [DOI] [PubMed] [Google Scholar]
- 48.Kippert F, et al. Caenorhabditis elegans has a circadian clock. Curr Biol. 2002;12:R47–49. doi: 10.1016/s0960-9822(01)00670-4. [DOI] [PubMed] [Google Scholar]
- 49.Romanowski A, et al. Circadian variation in Pseudomonas fluorescens (CHA0)-mediated paralysis of Caenorhabditis elegans. Microb Pathog. 2011;50:23–30. doi: 10.1016/j.micpath.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Migliori ML, et al. Circadian rhythms in metabolic variables in Caenorhabditis elegans. Physiol Behav. 2011;103:315–320. doi: 10.1016/j.physbeh.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 51.Duvall LB, Taghert PH. The circadian neuropeptide PDF signals preferentially through a specific adenylate cyclase isoform AC3 in M pacemakers of Drosophila. PloS Biol. 2012;10:e1001337. doi: 10.1371/journal.pbio.1001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loh DH, et al. Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs. J Biol Rhythms. 2011;26:200–209. doi: 10.1177/0748730411401740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janssen T, et al. Discovery and characterization of a conserved pigment dispersing factor-like neuropeptide pathway in Caenorhabditis elegans. J Neurochem. 2009;111:228–241. doi: 10.1111/j.1471-4159.2009.06323.x. [DOI] [PubMed] [Google Scholar]
- 54.Migliori ML, et al. Daily variation in melatonin synthesis and arylalkylamine N-acetyltransferase activity in the nematode Caenorhabditis elegans. J Pineal Res. 2012;53:38–46. doi: 10.1111/j.1600-079X.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 55.Hardin PE. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv Genet. 2011;74:141–173. doi: 10.1016/B978-0-12-387690-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abruzzi KC, et al. Drosophila CLOCK target gene characterization: implications for circadian tissue-specific gene expression. Genes Dev. 2011;25:2374–2386. doi: 10.1101/gad.178079.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taghert PH, Shafer OT. Mechanisms of clock output in the Drosophila circadian pacemaker system. J Biol Rhythms. 2006;21:445–457. doi: 10.1177/0748730406293910. [DOI] [PubMed] [Google Scholar]
- 58.Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beaver LM, Klichko VI, Chow ES, Kotwica-Rolinska J, Williamson M, Orr WC, Radyuk SN, Giebultowicz JM. Circadian regulation of glutathione levels and biosynthesis in Drosophila melanogaster. PLoS ONE. 2012;7:e50454. doi: 10.1371/journal.pone.0050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mazzoccoli G, et al. REV-ERBalpha and the clock gene machinery in mouse peripheral tissues: a possible role as a synchronizing hinge. J Biol Regul Homeost Agents. 2012;26:265–276. [PubMed] [Google Scholar]
- 61.Vinciguerra M, et al. SirT1 in muscle physiology and disease: lessons from mouse models. Dis Model Mech. 2010;3:298–303. doi: 10.1242/dmm.004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamia KA, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng X, Sehgal A. AKT and TOR signaling set the pace of the circadian pacemaker. Curr Biol. 2010;20:1203–1208. doi: 10.1016/j.cub.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao R, et al. Circadian regulation of mammalian target of rapamycin signaling in the mouse suprachiasmatic nucleus. Neuroscience. 2011;181:79–88. doi: 10.1016/j.neuroscience.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seay DJ, Thummel CS. The circadian clock, light, and cryptochrome regulate feeding and metabolism in Drosophila. J Biol Rhythms. 2011;26:497–506. doi: 10.1177/0748730411420080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corbalan-Tutau D, et al. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiol Behav. 2012 doi: 10.1016/j.physbeh.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Bahr I, et al. Evidence of the receptor-mediated influence of melatonin on pancreatic glucagon secretion via the Galphaq protein-coupled and PI3K signaling pathways. J Pineal Res. 2012;53:390–398. doi: 10.1111/j.1600-079X.2012.01009.x. [DOI] [PubMed] [Google Scholar]
- 69.Obayashi K, et al. Exposure to Light at Night, Nocturnal Urinary Melatonin Excretion, and Obesity/Dyslipidemia in the Elderly: A Cross-Sectional Analysis of the HEIJO-KYO Study. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-2874. [DOI] [PubMed] [Google Scholar]
- 70.Muhlbauer E, et al. Melatonin influences insulin secretion primarily via MT(1) receptors in rat insulinoma cells (INS-1) and mouse pancreatic islets. J Pineal Res. 2012;52:446–459. doi: 10.1111/j.1600-079X.2012.00959.x. [DOI] [PubMed] [Google Scholar]
- 71.Muhlbauer E, et al. Melatonin inhibits insulin secretion in rat insulinoma beta-cells (INS-1) heterologously expressing the human melatonin receptor isoform MT2. J Pineal Res. 2011;51:361–372. doi: 10.1111/j.1600-079X.2011.00898.x. [DOI] [PubMed] [Google Scholar]
- 72.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delezie J, Challet E. Interactions between metabolism and circadian clocks: reciprocal disturbances. Ann N Y Acad Sci. 2011;1243:30–46. doi: 10.1111/j.1749-6632.2011.06246.x. [DOI] [PubMed] [Google Scholar]
- 74.Froy O. The circadian clock and metabolism. Clin Sci (Lond) 2011;120:65–72. doi: 10.1042/CS20100327. [DOI] [PubMed] [Google Scholar]
- 75.Boden G, et al. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes. 1999;48:2182–2188. doi: 10.2337/diabetes.48.11.2182. [DOI] [PubMed] [Google Scholar]
- 76.Szosland D. Shift work and metabolic syndrome, diabetes mellitus and ischaemic heart disease. Int J Occup Med Environ Health. 2010;23:287–291. doi: 10.2478/v10001-010-0032-5. [DOI] [PubMed] [Google Scholar]
- 77.Pan A, et al. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scheer FA, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zanquetta MM, et al. Body weight, metabolism and clock genes. Diabetol Metab Syndr. 2010;2:53. doi: 10.1186/1758-5996-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scott EM, et al. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 81.Hatori M, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fuse Y, et al. Differential roles of breakfast only (one meal per day) and a bigger breakfast with a small dinner (two meals per day) in mice fed a high-fat diet with regard to induced obesity and lipid metabolism. J Circadian Rhythms. 2012;10:4. doi: 10.1186/1740-3391-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sherman H, et al. Timed high-fat diet resets circadian metabolism and prevents obesity. Faseb J. 2012;26:3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 84.Kuroda H, et al. Meal frequency patterns determine the phase of mouse peripheral circadian clocks. Sci Rep. 2012;2:711. doi: 10.1038/srep00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou KI, et al. Longevity and stress in Caenorhabditis elegans. Aging (Albany NY) 2011;3:733–753. doi: 10.18632/aging.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rakshit K, et al. Effects of aging on the molecular circadian oscillations in Drosophila. Chronobiol Int. 2012;29:5–14. doi: 10.3109/07420528.2011.635237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13:325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhdanova IV, et al. Aging of intrinsic circadian rhythms and sleep in a diurnal nonhuman primate, Macaca mulatta. J Biol Rhythms. 2011;26:149–159. doi: 10.1177/0748730410395849. [DOI] [PubMed] [Google Scholar]
- 89.Weinert D, Waterhouse J. The circadian rhythm of core temperature: effects of physical activity and aging. Physiol Behav. 2007;90:246–256. doi: 10.1016/j.physbeh.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 90.Sellix MT, et al. Aging Differentially Affects the Re-entrainment Response of Central and Peripheral Circadian Oscillators. J Neurosci. 2012;32:16193–16202. doi: 10.1523/JNEUROSCI.3559-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farajnia S, et al. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J Neurosci. 2012;32:5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weinert H, et al. Impaired expression of the mPer2 circadian clock gene in the suprachiasmatic nuclei of aging mice. Chronobiol Int. 2001;18:559–565. doi: 10.1081/cbi-100103976. [DOI] [PubMed] [Google Scholar]
- 93.Wyse CA, Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010;1337:21–31. doi: 10.1016/j.brainres.2010.03.113. [DOI] [PubMed] [Google Scholar]
- 94.von Gall C, Weaver DR. Loss of responsiveness to melatonin in the aging mouse suprachiasmatic nucleus. Neurobiol Aging. 2008;29:464–470. doi: 10.1016/j.neurobiolaging.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 95.Jagota A, Kalyani D. Effect of melatonin on age induced changes in daily serotonin rhythms in suprachiasmatic nucleus of male Wistar rat. Biogerontology. 2010;11:299–308. doi: 10.1007/s10522-009-9248-9. [DOI] [PubMed] [Google Scholar]
- 96.Yamazaki S, et al. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo W, et al. Old flies have a robust central oscillator but weaker behavioral rhythms that can be improved by genetic and environmental manipulations. Aging Cell. 2012;11:428–438. doi: 10.1111/j.1474-9726.2012.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dubrovsky YV, et al. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2010;2:936–944. doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kondratov RV, et al. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Antoch MP, Kondratov RV. Circadian proteins and genotoxic stress response. Circ Res. 2010;106:68–78. doi: 10.1161/CIRCRESAHA.109.207076. [DOI] [PubMed] [Google Scholar]
- 101.Wang TA, et al. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science. 2012;337:839–842. doi: 10.1126/science.1222826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krishnan N, et al. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging (Albany NY) 2009;1:937–948. doi: 10.18632/aging.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krishnan N, et al. Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol Dis. 2012;45:1129–1135. doi: 10.1016/j.nbd.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kondratova AA, et al. Circadian clock proteins control adaptation to novel environment and memory formation. Aging (Albany NY) 2010;2:285–297. doi: 10.18632/aging.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galano A, et al. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 106.Duffy JF, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hardeland R. Melatonin in aging and disease -multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012;3:194–225. [PMC free article] [PubMed] [Google Scholar]
- 108.Bondy SC, et al. Melatonin alters age-related changes in transcription factors and kinase activation. Neurochem Res. 2010;35:2035–2042. doi: 10.1007/s11064-010-0206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 110.Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- 111.Xu K, et al. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 2008;8:289–300. doi: 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chatterjee A, et al. Regulation of gustatory physiology and appetitive behavior by the Drosophila circadian clock. Curr Biol. 2010;20:300–309. doi: 10.1016/j.cub.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu K, et al. The circadian clock interacts with metabolic physiology to influence reproductive fitness. Cell Metab. 2011;13:639–654. doi: 10.1016/j.cmet.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beaver LM, et al. Circadian clock regulates response to pesticides in Drosophila via conserved Pdp1 pathway. Toxicol Sci. 2010;115:513–520. doi: 10.1093/toxsci/kfq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gachon F, Firsov D. The role of circadian timing system on drug metabolism and detoxification. Expert Opin Drug Metab Toxicol. 2011;7:147–158. doi: 10.1517/17425255.2011.544251. [DOI] [PubMed] [Google Scholar]
- 116.Froy O, Miskin R. Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging (Albany NY) 2010;2:7–27. doi: 10.18632/aging.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mendoza J, et al. High-fat feeding alters the clock synchronization to light. J Physiol. 2008;586:5901–5910. doi: 10.1113/jphysiol.2008.159566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mendoza J, et al. Setting the main circadian clock of a diurnal mammal by hypocaloric feeding. J Physiol. 2012;590:3155–3168. doi: 10.1113/jphysiol.2012.230300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goodrick CL, et al. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 120.Gutman R, et al. Long-lived mice exhibit 24 h locomotor circadian rhythms at young and old age. Exp Gerontol. 2011;46:606–609. doi: 10.1016/j.exger.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 121.Garaulet M, Madrid JA. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv Drug Deliv Rev. 2010;62:967–978. doi: 10.1016/j.addr.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 122.Kasukawa T, et al. Human blood metabolite timetable indicates internal body time. Proc Natl Acad Sci U S A. 2012;109:15036–15041. doi: 10.1073/pnas.1207768109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]