Abstract
Purpose
Acetaminophen (APAP) protein adducts are a biomarker of APAP metabolism, reflecting oxidation of APAP and generation of the reactive metabolite N-acetyl-p-benzoquinone imine. High levels of adducts correspond to liver toxicity in patients with APAP related acute liver failure. Adduct formation following low dose exposure to APAP has not been well studied. APAP protein adducts were measured in blood samples collected from fasted subjects that participated in a cross-over study of APAP (80 mg/kg) comparing extended release (ER) and immediate release (IR) formulations.
Methods
Adducts were quantified in all post-dose blood samples using a validated HPLC-EC assay.
Results
Comparison of pharmacokinetic parameters for adducts did not reveal significant differences between the ER and IR formulations, with one exception. Formation rates for adducts were faster for the IR than the ER formulation (0.420 ± 0.157 vs. 0.203 ± 0.080 1/hr), respectively. The Cmax of adducts for IR and ER were 0.108 (±0.020) and 0.100 (±0.028) nmol/mL serum, respectively, and were two orders of magnitude lower than adduct levels previously reported in adults with acute liver failure secondary to APAP.
Conclusions
APAP protein adducts are rapidly formed following non-toxic ingestion of APAP at levels significantly lower than those associated with acute liver failure.
Keywords: biomarker, acetaminophen, hepatotoxicity, adducts, metabolism, glutathione
Introduction
Acetaminophen (APAP) is the most widely used drug for the treatment of pain and fever around the world. While the drug is generally regarded to be safe when used in doses recommended by the manufacturer, large doses of APAP lead to fulminant hepatotoxicity [1]. In the United States and in many European countries, APAP is recognized as a major cause of acute liver failure [1]. The drug is widely available in hundreds of over-the-counter cough and cold remedies and as single ingredient preparations. In addition, APAP-opioid combination products are widely used for the treatment of moderate pain.
Following therapeutic exposure, APAP is primarily metabolized by glucuronidation and sulfation reactions in the liver and a small proportion of the drug undergoes oxidation. With supratherapeutic APAP doses, metabolism shifts and an increased proportion of the parent drug undergoes oxidation via the cytochrome P450 system. Greater amounts of the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) are formed and NAPQI binds to cysteine groups on proteins, forming APAP protein adducts. In previous work, we have shown that high levels of APAP protein adducts are present in the blood of patients that have consumed large doses of APAP leading to acute liver failure (ALF) [2]. In addition, we found that APAP protein adducts have a long elimination half-life that markedly exceeds that of the parent compound, APAP, permitting detection of APAP toxicity long after the parent compound has cleared the blood [3]. For example, the mean elimination half-life of APAP protein adducts for adults with APAP-induced ALF was 41.3 ± 8.3 hours (hr). By comparison, the mean elimination half-life for APAP in adults with APAP-induced ALF varied from 5.4 to 18.4 hr, depending on the severity of the liver injury and the presence of encephalopathy [4]. In milder forms of APAP toxicity, levels of adducts are inversely related to the lag time of starting treatment with the antidote, N-acetylcysteine [5].
Following proteolytic digestion of human blood samples, adducts can be detected through a highly specific and sensitive assay using high performance liquid chromatography with electrochemical detection (HPLC-EC) [2, 6]. Previous studies have shown that high levels of adducts are present in the blood samples of patients with APAP-induced ALF and are absent in patients with other causes of ALF [2].
The issue of whether or not adducts are formed following lower dose exposure to APAP is also of interest. Previously, it was generally accepted that APAP protein adducts were only formed during severe APAP toxicity, and then only after depletion of hepatic glutathione [7]. The sensitivity and precision of the HPLC-EC method of determination of adducts has extended our capability to specifically detect adducts after low doses of APAP. In a small study, we previously reported low levels of adducts in patients following therapeutic exposure to multiple doses of APAP [8]. To date, the rate of adduct formation and magnitude of adduct levels have not been examined following single dose exposure to APAP. Thus, the following study examined adduct profiles in the blood samples of healthy adults that participated in a study of single dose APAP in a cross-over design comparing IR and ER formulations.
Methods
Study population
Banked serum samples obtained from nine adults that participated in a recently published study to examine the pharmacokinetics of APAP were utilized for analysis of APAP protein adducts [9]. The previously published study received institutional review board approval and was an open label, non-blinded, cross-over design in nine healthy adults, ages 18 to 65 years. Exclusion criteria for enrollment were pregnancy, pre-existing liver disease, chronic alcohol consumption of greater than 20 grams per day, chronic illness, regular use of any medication, body weight greater than 100 kg, use of an APAP containing product in previous seven days, and abnormal liver function tests. Analysis of adducts in banked samples was approved by the institutional review boards of the participating institutions.
Study design
Baseline serum bilirubin, alanine aminotransferase (AST) and alanine aminotransferase (ALT) were performed one week prior to the study and three days following the completion of each arm of the study. The subjects underwent an 8 hr fast the evening prior to the administration of APAP. Using a “simulated overdose design”, APAP (approximately 80 mg/kg) was administered by mouth either as Panadol Extend® (665 mg tablets, 69% slow release and 31% immediate release, hereafter referred to as EX) or Panadol immediate-release APAP (500 mg tablets, hereafter referred to as IR) [9]. Blood samples (5 mL) were obtained immediately prior to APAP and at 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 hr post-ingestion. All subjects completed the study protocol and blood samples were also obtained at 16 and 24 hr post-ingestion in six of the nine patients. One week after completion of the first arm of the study, study subjects completed the second arm of the study.
Analytical method
Serum samples were frozen at -80 degrees for batched analysis of APAP protein adducts using a modification of the previously reported HPLC-EC assay for APAP-adducts [3, 6]. Assay modifications included centrifugal gel-filtration, high efficiency proteolytic digestion, and increased sample injection volumes, resulting in improved sensitivity and efficiency of the assay. The lower limit of quantitation for the assay was defined as 0.03 μM. The laboratory technician was blinded to the sample group.
Clinical Data
Patient data collected from the study subjects included patient age (yr), gender, weight (kg), APAP dose (mg/kg), and base-line and post-APAP administration AST and ALT levels.
Pharmacokinetic analysis
Model-independent pharmacokinetic analyses were used to assess the disposition profiles of APAP, APAP metabolites and APAP protein adducts. Serum concentration vs. time data were curve fit using a peeling algorithm to generate initial polyexponential parameter estimates. Final estimates of the terminal elimination rate constant (λz) were determined from an iterative, nonlinear least squares regression algorithm. Individual Cmax and Tmax were obtained by direct examination of the serum concentration versus time profiles. The area under the plasma concentration versus time curve during the sampling period (AUC0-n) was calculated using the mixed log-linear method (i.e. trapezoidal between values where Cn < Cn+1 and logarithmic when Cn > Cn+1). Extrapolation of the AUC to infinity (AUC0-∞) was achieved by the summation of AUC0-n + Cn/λz, where Cn is the predicted plasma concentration calculated from the curve fit and λz is the apparent terminal elimination rate constant.
For participants in whom a sufficient number of post-peak serum concentrations were available, model-dependent pharmacokinetic analyses were used to arrive at a formation rate constant for each of the APAP metabolites and the adduct. Model-dependent rate constants were calculated from final polyexponential parameter estimates after application of the Akaike Information and Schwartz criteria and examination of the coefficients of variation for the polyexponential parameters estimated from a given model. All pharmacokinetic analyses were performed with Kinetica version 5.0 (ThermeElectron, Philadelphia, PA).
Statistical analysis
Pharmacokinetic data for the study were examined using standard descriptive statistics. Relationships between the pharmacokinetics of APAP, APAP conjugates, and APAP protein adducts were examined using both linear and nonlinear least squares regression. Evaluation of the data for possible formulation dependence in adduct formation was undertaken using univariate analysis of variance. The comparison of pharmacokinetic parameters between formulations was performed using a paired t-test. Statistical analyses were performed using SPSS version 12 (SPSS, Inc., Chicago, IL) and the significance limit accepted for all analyses was α=0.05.
Results
Nine subjects (eight males; one female) completed the research protocol. The mean subject weight and age were 73.1 (±7.7) kg and 34 (±7.1) years, respectively. The mean dose of IR and ER APAP administered to the study subjects was comparable (5778 ± 565 mg vs. 5763 ± 665 mg, respectively; p=not significant). Weight normalized doses were also comparable between the two study arms (79.1 ± 2.2 mg/kg vs. 78.8 ± 2.6 mg/kg). No differences were detected in AST and ALT from baseline and post APAP dosing (Figure 1).
Figure 1.
Summary data for ALT and AST for study subjects for baseline and post-APAP dosing. Data are presented as median (line within box), 25th to 75th percentile (boundary of box), and 10 and 90th percentile (whiskers). No differences were present between ALT and AST at baseline compared to post-APAP dosing.
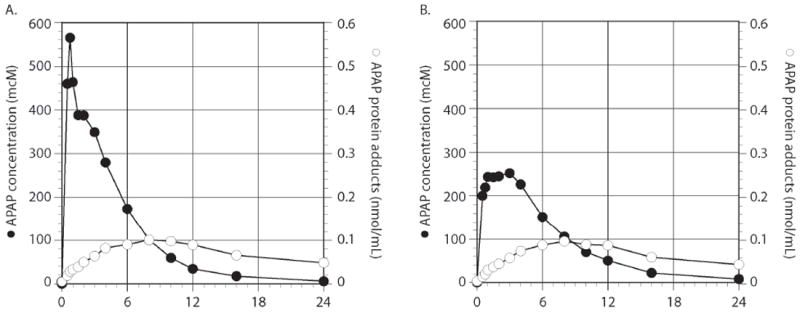
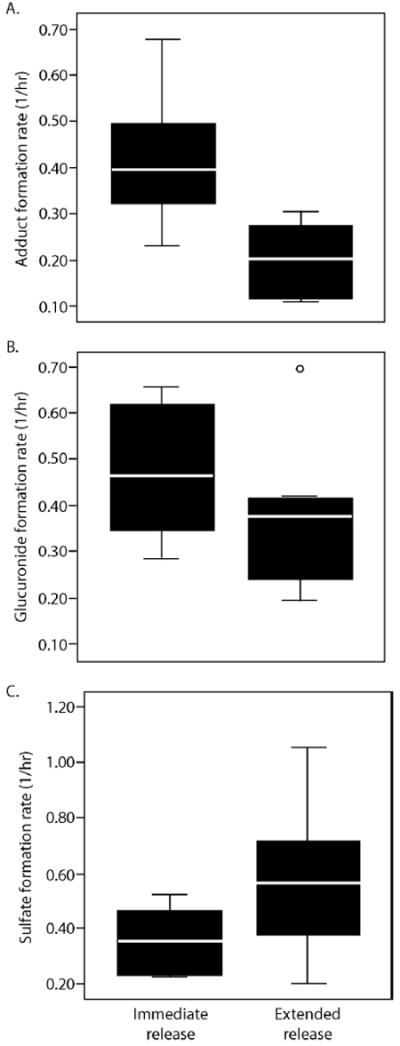
Summary data for adduct pharmacokinetic parameters is presented in Table 1 and summary composite plots for APAP and APAP protein adducts are presented in Figure 2. As reported in the original study [9], APAP concentrations were higher and occurred earlier with the IR formulation compared to the ER formulation. The serum concentration profiles for APAP protein adducts were more protracted than that of APAP, peaked much later, and did not return to baseline over the time course of the study (Figure 2). Despite significant differences in the release of APAP into blood between the two formulations (p<0.05), no significant differences were detected in the Tmax, Cmax, or AUC values of adducts (Table 1). No correlation was observed between APAP exposure and the level of adduct formation as would be expected in a study of very narrow dose range (ie., 74-84 mg/kg). The formation rates for APAP protein adducts were faster for the IR than the ER formulation (Table 1; Figure 3A). Although formation rates for the glucuronide and sulfate metabolites appeared to be influenced to some extent by formulation, these differences were not statistically significant (Figure 3B; 3C).
Table 1.
Summary of Pharmacokinetic Parameters (Mean ± SD) for APAP Protein Adducts following administration of Immediate and Extended Release formulations.
| Immediate Release | Extended Release | |
|---|---|---|
| Tmax (hr) | 8.4 ± 1.9 | 8.6 ± 3.4 |
| Cmax (μM) | 0.108 ± 0.020 | 0.100 ± 0.028 |
| AUC0-12 (μM*hr) | 0.95 ± 0.17 | 0.85 ± 0.21 |
| Half life (hr) | 23.4 ± 21.7a | 21.0 ± 14.0b |
| MRT (hr) | 36.8 ± 30.9a | 33.3 ± 20.1b |
| formation rate (1/hr)c | 0.420 ± 0.157b | 0.203 ± 0.080b |
n=7 subjects;
n=6 subjects;
p=0.035
Figure 2.
Summary profile for APAP (●) and APAP protein adducts (○) for immediate release APAP and extended release APAP.
Figure 3.
Box plots of formation rates for (A) APAP protein adducts, (B) APAP glucuronide, and (C) APAP sulfate for immediate release APAP and extended release APAP. The solid bold lines indicate the median of the data and the boxes indicated the 25th to 75th percentiles. The whisker lines indicated the minimum and maximum values of the data. ○ denotes outliers.
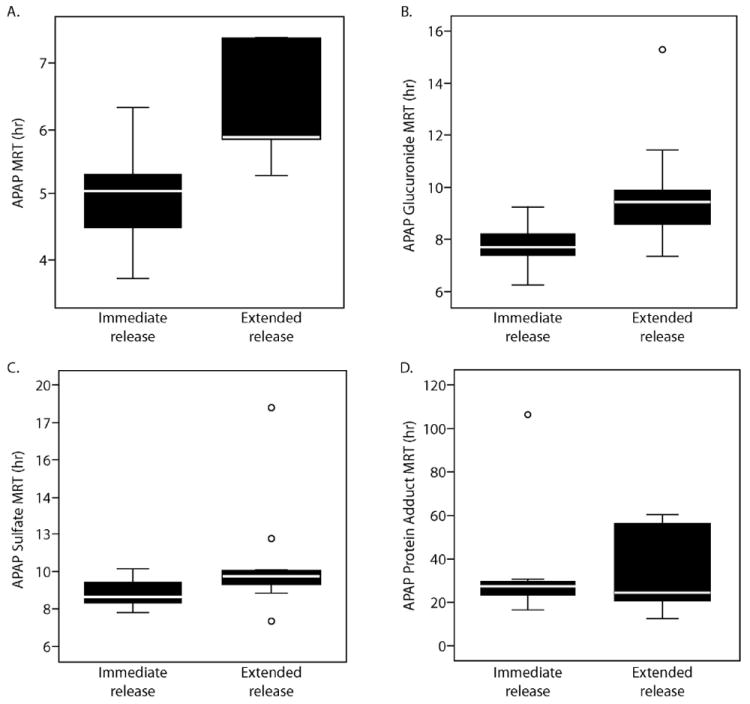
Analysis of total body exposure for APAP adducts (i.e. AUC) was somewhat limited because of the prolonged elimination half-life (Table 1; Figure 2) and the limited number of post-peak samples obtained in some of the subjects (i.e. only 6 of 9 subjects had the 16 and 24 hr samples obtained). Thus, determination of AUC0-12 for the two formulations was performed. No relationship between AUC0-12 for either APAP or adducts was noted as a function of dose (data not shown). Comparison of mean residence time (MRT) between formulations indicated that the MRT of APAP and the glucuronide were shorter following administration of the IR formulation compared to the ER formulation (5.0 ± 0.8 vs. 6.3 ± 0.8 hr, p=0.004; 7.8 ± 0.9 vs. 9.9 ± 2.3 hr, p=0.01, respectively) (Figure 4). No differences in MRT for APAP sulfate or APAP protein adducts were observed between the formulations.
Figure 4.

Box plots of mean residence time (MRT) for (A) APAP, (B) APAP glucuronide, (C) APAP sulfate, and (D) APAP protein adducts for immediate release APAP and extended release APAP. The solid bold lines indicate the median of the data and the boxes indicated the 25th to 75th percentiles. The whisker lines indicated the minimum and maximum values of the data. ○ denotes outliers.
Discussion
APAP toxicity is responsible for approximately half of all cases of ALF in the United States [1] and is a major cause of ALF in Great Britain and other countries in Europe [10]. We previously found that measurement of APAP protein adducts by HPLC-EC accurately distinguished between well-characterized cases of APAP-related ALF and known other causes of ALF [2]. It is noteworthy that the elimination half-life of APAP protein adducts is long (Table 1) and exceeds that of the parent compound [3]. Patients with APAP-related ALF typically present for medical evaluation in the later stages of toxicity and diagnosis of the etiology of the ALF may be challenging due to difficulties in obtaining accurate histories and the relatively rapid clearance of APAP in the systemic circulation. Thus, measurement of APAP protein adducts in patients with ALF of unknown or questionable etiology may be of diagnostic value. In two previous studies, we detected high levels of APAP protein adducts in 18 and 19% of patients with ALF of unknown etiology [2, 11]. The levels of adducts in patients with APAP-related ALF were greater than 1.0 nmol/mL. Criteria for the diagnosis of APAP-related ALF were ALT values > 1000 IU/L, encephalopathy, and coagulopathy [2, 11].
An important component of biomarker validation is characterizing the dynamic range of the biomarker within various patient populations. The HPLC-EC assay utilized in the present study provides a measurement of the total concentration of protease-cleaved APAP protein adducts in peripheral blood. We previously reported that adducts can be detected at low levels in patients receiving multiple therapeutic doses of APAP in the clinical setting [8]. The availability of samples from a single dose study comparing two formulations of APAP allowed us to continue this line of research and to compare the formation and decay characteristics of APAP and APAP protein adducts within a single study. The data demonstrate that adducts are formed following single dose (“lower dose”) exposure to APAP, albeit at levels approximately two orders of magnitude lower than observed in APAP-induced ALF. The dose of APAP administered to study subjects was approximately 20% greater than the standard daily dose of APAP previously recommended by the manufacturer (4 grams) and approximately half the dose known to be toxic. Due to safety concerns associated with frequent use of APAP, the manufacturers of APAP recently announced the lowering of the daily dose from 4 grams to 3 grams.
The mean Cmax for adducts (0.10 nmol/mL adducts) in the present study was comparable between the two formulations of APAP (Figure 2), suggesting that the total APAP dose, rather than the APAP absorption profile, influences the extent of adduct formation. Importantly, the value for Cmax observed in the present study is considerably lower than peak levels of adducts previously observed in patients with APAP-induced ALF. In the ALF study, patient samples were collected at a mean time of 73 hours after large overdoses of APAP and the mean “peak” levels were 10.85 (±9.26) nmol/mL adducts [3]. Thus, levels of adducts following low dose APAP administration in healthy adults are approximately two orders of magnitude below those measured in the acute stages of APAP-related ALF.
Finally, differences were noted between the ER and IR formulations for the formation rate of APAP protein adducts. While the formation rate for adducts may have been secondary to differences in absorption profiles between the two formulations, this finding also speaks to the precision and sensitivity of the HPLC-EC assay.
The significance of low levels of APAP protein adducts in the blood samples of subjects receiving doses of APAP considered to be non-toxic is unclear. The traditional understanding of covalent modification in APAP toxicity is based on time course studies conducted in the mouse model of APAP toxicity. Early studies showed that APAP protein adducts were formed following the depletion of hepatic glutathione and saturation of conjugation pathways [12, 13]. However, these early studies were based on methods for detection of APAP protein adducts that were less sensitive and precise than the HPLC-EC methodology used in the current study. The finding of low levels of adducts in peripheral blood in human subjects receiving low doses of APAP is consistent with the concept of localized depletion of hepatic glutathione and subsequent adduct formation in centrilobular hepatocytes that metabolize APAP as it enters the liver through the portal vein. Measurement of liver glutathione, the main source of glutathione in humans, is not possible due to the invasive nature of the required methodology. Unfortunately, peripheral measurements of glutathione do not accurately reflect liver levels of this intracellular defense mechanism for oxidative stress. In contrast to earlier assays used in previously conducted studies [7, 14], the HPLC-EC assay is capable of detecting adducts at a lower magnitudes of APAP exposure, prior to the onset of overt hepatocyte injury, and below the sensitivity threshold of hepatic transaminase assays available in clinical laboratories. Thus, the data presented herein demonstrate the capability, precision and sensitivity of the HPLC-EC assay for determining APAP exposure and complement previous reports on APAP protein adducts [2, 3, 5, 8], by providing new data on anticipated levels of adducts in volunteer subjects following APAP doses considered to be sub-toxic exposures.
Acknowledgments
This work was supported in part by a research grant from the National Institute for Diabetes and Digestive and Kidney Diseases (DK81406). Drs. James and Roberts have a pending patent application for a point-of-care test for the measurement of acetaminophen protein adducts. Dr. James also receives salary support from the UAMS Translational Research Institute (grant UL1TR000039) through the NIH National Center for Research Resources and National Center for Advancing Translational Sciences.
References
- 1.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 2.Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 3.James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, Lee WM. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37:1779–1784. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiodt FV, Ott P, Christensen E, Bondesen S. The value of plasma acetaminophen half-life in antidote-treated acetaminophen overdosage. Clin Pharmacol Ther. 2002;71:221–225. doi: 10.1067/mcp.2002.121857. [DOI] [PubMed] [Google Scholar]
- 5.James LP, Capparelli EV, Simpson PM, Letzig L, Roberts D, Hinson JA, Kearns GL, Blumer JL, Sullivan JE. Acetaminophen-associated hepatic injury: evaluation of acetaminophen protein adducts in children and adolescents with acetaminophen overdose. Clin Pharmacol Ther. 2008;84:684–690. doi: 10.1038/clpt.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high- performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H. Acetaminophen-induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther. 1974;16:676–684. doi: 10.1002/cpt1974164676. [DOI] [PubMed] [Google Scholar]
- 8.Heard KJ, Green JL, James LP, Judge BS, Zolot L, Rhyee S, Dart RC. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol. 2012;11:20. doi: 10.1186/1471-230X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiew A, Day P, Salonikas C, Naidoo D, Graudins A, Thomas R. The comparative pharmacokinetics of modified-release paracetamol and immediate-release paracetamol in a simulated overdose model. Emerg Med J Austral. 2010;22:548–555. doi: 10.1111/j.1742-6723.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 10.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 11.Khandelwal NJL, Sanders C, Larson AM, Lee WM The Acute Liver Failure Study Group. Unrecognized acetaminophen toxicity as a cause of ‘indeterminate’ acute liver failure. Hepatology. 2011;53:567–576. doi: 10.1002/hep.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- 13.Roberts DW, Pumford NR, Potter DW, Benson RW, Hinson JA. A sensitive immunochemical assay for acetaminophen-protein adducts. J Pharmacol Exp Ther. 1987;241:527–533. [PubMed] [Google Scholar]
- 14.Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, Hinson JA. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol. 1991;138:359–371. [PMC free article] [PubMed] [Google Scholar]


