Abstract
Background
Cognitive deficits contribute strongly to functional disability in schizophrenia. The cost of identifying and testing candidate procognitive agents is substantial. Conceivably, candidate drugs might be first identified by positive effects on cognitive domains in sensitive subgroups of healthy subjects. Here, we examined whether the MATRICS Consensus Cognitive Battery (MCCB) detected procognitive drug effects in subgroups of healthy individuals.
Methods
The effects of 20 mg amphetamine (AMPH) on MCCB performance were tested in a double-blind, placebo-controlled crossover study of 60 healthy adults. AMPH effects were compared in subgroups of subjects characterized by low vs. high placebo MCCB scores, and by extreme values on personality subscales associated with schizophrenia-relevant biomarkers.
Results
AMPH produced autonomic and subjective effects, but did not significantly change MCCB composite scores or individual domain scores across the inclusive sample of 60 subjects. AMPH-induced MCCB changes were significantly (inversely) related to placebo MCCB performance: among individuals with lower placebo scores, AMPH enhanced performance, while among individuals with higher placebo scores, it impaired performance. A potential impact of regression to the mean was assessed, and could not be ruled out. Both placebo MCCB performance and AMPH effects on MCCB scores were significantly related to personality domains associated with schizophrenia-linked genetic- and/or neurophysiological substrates.
Conclusions
Among healthy adults, AMPH effects on MCCB performance were detected only among specific subgroups, and in specific cognitive domains. Strategies that utilize drug-induced changes in MCCB performance in healthy subjects to screen for candidate procognitive drugs should consider the use of “enriched” subgroups with specific neurocognitive or personality characteristics.
Keywords: amphetamine, cognition, schizophrenia, MCCB, MATRICS, neurocognition
Introduction
Cognitive deficits in schizophrenia (SZ) are a primary determinant of functional disability (Bowie et al. 2008; Cervellione et al. 2007; Gold et al. 2002; Green et al. 2000; Heinrichs et al. 2010; Heinrichs and Zakzanis 1998; Keefe and Harvey 2012; McClure et al. 2007; Williams et al. 2008). The MATRICS Consensus Cognitive Battery (MCCB) was developed to evaluate neurocognition in trials of procognitive drugs and cognitive remediation programs (Kern et al. 2008; Nuechterlein et al. 2008). The MCCB provides measures of cognitive change in repeated testing designs as well as a cognitive reference point for non-intervention studies, and is accepted by the FDA as a primary endpoint for clinical trails targeting cognition in SZ.
The MCCB includes ten tests that assess seven cognitive domains: speed of processing (SP), attention/vigilance (A/V), working memory (verbal and nonverbal) (WM), verbal learning (VerL), visual learning (VisL), reasoning and problem solving (R/PS), and social cognition (SC), and provides a composite score of these domains. In multi-site trials, the MCCB has demonstrated sensitivity to cognitive deficits in all domains, excellent test-retest and inter-site reliability and high correlations with measures of functional capacity (Buchanan et al. 2011; Keefe et al. 2011). Despite this sensitivity, many early trials using the MCCB have failed to identify strong procognitive drug effects (Goff et al. 2007; Harvey et al. 2011; Javitt et al. 2012; Marx et al. 2009). Other studies utilizing the MCCB are in progress with a number of other new compounds, as well as atypical antipsychotics (http://clinicaltrials.gov/ct2/results?term=matrics).
There are substantial challenges in the design and implementation of trials to identify procognitive drug effects in SZ patients, ranging from subject recruitment and retention, to complex medication regimens and interactions, to ethical issues related to the use of placebo (PBO) controls (Barch 2010; Barch and Carter 2008; Correll et al. 2011; Emanuel and Miller 2001; Kemp et al. 2010; Patel 2003; Touwen and Engberts 2012). One productive strategy in studies of the clinical neuroscience of SZ has been to investigate cognitive and neurobiological phenomena in unaffected healthy subjects characterized by personality or other domains that are conceptually or biologically related to psychosis (Koycheve et al. 2012; Kumari et al. 2004; Laurent et al. 2002; Smith 1992; Swerdlow et al. 2003b). Indeed, drug challenges in healthy subjects and specific subgroups have identified neurocognitive and neurophysiological changes suggestive of procognitive drug properties (e.g. Barch and Carter 2005; Swerdlow et al. 2009; Talledo et al. 2009). Conceivably, drugs that enhance specific cognitive functions in healthy subjects characterized by psychosis-linked phenotypes may be promising candidates for enhancing cognition in SZ. This study was designed to determine whether the MCCB detects procognitive drug effects in subgroups of healthy individuals distinguished by their levels of neurocognitive performance.
Amphetamine (AMPH) was selected as a “test” procognitive agent based on our understanding of its biological mechanisms and its well-established neurocognitive and neurophysiological profile. AMPH improves cognition in SZ patients as well as in healthy volunteers (Barch and Carter 2005, Pietrzak et al. 2010), and AMPH and other dopamine agonists may be particularly effective in enhancing neurocognition in healthy subjects with low basal performance levels (Kimberg et al. 1997; Kimberg and D’Esposito 2003; Mattay et al. 2003; Mattay et al. 2000; Mehta et al. 2001; Swerdlow 2011), and in individuals carrying certain genetic biomarkers or related phenotypes (Ersche et al. 2011; Fleming et al. 1995; Giakoumaki et al. 2008; Hutchison et al. 1999; Kumari et al. 1999; Mattay et al. 2003; Roussos et al. 2009; White et al. 2006). Here, we assessed the effects of a single “challenge” dose of 20 mg AMPH on MCCB performance in a double-blind, PBO-controlled crossover design in 60 clinically healthy adults.
Methods
Subject Recruitment and Testing
Methods were similar to those described in recent reports (e.g. Talledo et al. 2009), approved by the UCSD Human Subjects Institutional Review Board and supported by the NIMH. Sixty R-handed adults (M:F = 37:23; mean age = 24.2, SD = 4.9, range = 18–35; Table 1) completed an initial telephone contact and 3 laboratory visits. Phone screening procedures were identical to those in previous reports (Swerdlow et al. 2003; 2003a). After passing the phone interview (assessing current and past medical and psychiatric history, medication and recreational drug use, and family history of psychosis), subjects came to the laboratory (for women, within 72 h of menses onset).
Table 1.
Subject Characteristics (n=60; M:F = 37:23)
| Characteristic | Mean (SD) |
|---|---|
| Age (y) | 24.2 (4.9) |
| Weight (kg) | 77.1 (15.0) |
| Education (y) | 14.5 (1.6) |
| Caffeine (mg/d) | 64.1 (89.8) |
| TPQ: Novelty seeking | 14.8 (4.5) |
| SSS: Disinhibition | 4.2 (2.8) |
| EPQ: Total score | 21.4 (4.5) |
| Smoking (n) | |
|---|---|
| ≥ 20 cigarettes/week | 1 |
| ≤ 5 cigarettes/week | 2 |
TPQ: Tridimensional Personality Questionnaire, SSS: Sensation Seeking Scale, EPQ: Eysenck Personality Questionnaire
During the screening visit, subjects were informed of the potential risks and benefits of the study, read and signed a study consent, underwent a screening medical interview, a modified Structured Clinical Interview for DSM-IV-Non-Patient (SCID-NP), physical examination and electrocardiogram to rule out exclusionary conditions, and urine toxicology test (exclusion for any drug); women underwent a urine-based pregnancy test. Audiometry confirmed hearing threshold < 40 dB(A) at 1000 Hz. Because subjects were also to be tested in measures of acoustic startle for a larger, ongoing study of AMPH effects on this measure, a screening session was used to confirm reliable startle reflex magnitude. The total number of subjects excluded based on the screening visit, and the basis for these exclusions, are found in Table 2.
Table 2.
Excluded subjects (n=73)
| Startle magnitude < 50 units1 | 35 |
| Drug history/positive toxicology2 | 12 |
| “No show”/uncooperative | 12 |
| Affective disorder history | 3 |
| General medical problem | 6 |
| Other | 5 |
1.22 μV/unit
positive urine toxicology, n=5; substance dependence or abuse by SCID, n=7
Subjects completed several questionnaires based on reported relationships between specific scale scores and dopaminergic function and/or AMPH sensitivity, including: 1) the Tridimensional Personality Questionnaire (TPQ) (Cloninger 1987; Novelty Seeking Subscale (NS)); 2) the Sensation Seeking Scale (SSS) (Zuckerman et al. 1972; Disinhibition Subscale (DIS)); and 3) the Eysenck Personality Questionnaire (EPQ) (Eysenck and Eysenck 1994; Total Score) (Table 1) (e.g. Fleming et al. 1995; Ebstein et al. 1996; Benjamin et al. 1996; Hutchison et al. 1999; Kumari et al. 2004; Hamidovic et al. 2009). Subjects who passed screening criteria were tested 5–9 d later, and retested 26–30 d after their first test (i.e. for women, at the corresponding date of their next menstrual cycle). This schedule was designed to ensure, to the degree possible, that testing with AMPH and PBO occurred under relatively comparable hormonal states for women. Testing was double-blind, and drug order was randomized.
On test days, subjects arrived at 0830, ate a standardized breakfast, repeated audiometry, urine toxicology (and pregnancy testing in women), and d-amphetamine (20 mg) or PBO was administered at 0900. Heart rate and blood pressure were determined (sitting position, brachial cuff), and subjects completed symptom rating scales at intervals that avoided test interruptions, the first one occurring before pill ingestion. Autonomic measures and visual analog scale (VAS) scores of symptoms across the post-pill intervals were thus anchored by a pre-pill baseline value. VAS scores were designed to assess general somatic and psychological symptoms and level of consciousness (Bond and Lader 1974; Bunney et al. 1999; Norris 1971). Subjects made a single, vertical mark representing their current state along a 100 mm line (0 mm representing “not true” and 100 mm represents “true”). Ratings assessed several states: “happy”, “queasy”, “dizzy”, “drowsy” and perceptual sensitivity. Details of these scales are found in Swerdlow et al. (2002), and included prompts such as “Normal sounds seem unusually intense or loud”.
During the 90 min after pill ingestion, subjects completed startle testing as part of a larger, ongoing study (Swerdlow et al. 2003; Talledo et al. 2009) to be reported separately. One hundred and ten min after pill ingestion (at a time known to correspond to bioactivity of this dose of AMPH, based on autonomic and subjective measures of arousal (Swerdlow et al. 2001 at a time known to correspond to bioactivity of this dose of AMPH, based on autonomic and subjective measures of arousal (Swerdlow et al. 2003; Talledo et al. 2009)), subjects completed the MCCB. As described above, the MCCB measures key cognitive domains relevant to cognitive deficits in SZ. Upon completion of the MCCB, autonomic and subjective VAS ratings were continued until 430 min post-pill. On test day two, subjects also provided blood for future genetic studies. Subjects were paid on completion of each visit. The study sample was considered complete after the 60th subject was tested; this planned “mid-point” analysis is part of a larger anticipated sample of 120 subjects for whom genetic information will also be analyzed.
Data Analysis
MCCB T Scores, autonomic measures, and personality scores were treated as continuous measures and analyzed with repeated measure analyses of variance (ANOVAs) with appropriate post-hoc comparisons. VAS scores were not normally distributed and were analyzed via non-parametric statistics. Based on known effects of age, sex and PBO neurocognitive performance (henceforth, “baseline”) on either MCCB performance, AMPH sensitivity, or both (e.g. Mattay et al. 2000; Kern et al. 2008; Riccardi et al. 2011), these variables were typically used as either categorical grouping factors (including groupings based on a median or quartile split) or covariates in primary or exploratory analyses. To avoid potential confounding effects of sex differences in neurocognitive function, median and quartile splits were created by ranking variables for male (n=37) and female (n=23) subjects separately. Both ANOVAs and simple regressions were used to explore the relationship between specific personality indices, MCCB performance and AMPH sensitivity. Alpha for planned comparisons and empirical findings were set at 0.05 and 0.01 respectively.
Analyses progressed in a systematic fashion. We first compared our sample’s baseline performance with published MCCB patterns, examining sample characteristics, and the effects of age, sex, years of education, and personality measures on MCCB performance. We next assessed the fidelity of our intervention (drug) by examining evidence of bioactivity (autonomic response, VAS of subjective experience), and the fidelity of our measurements, by examining test-retest reliability. With this assurance of bioactivity and measurement fidelity, we then examined the effect of AMPH on MCCB performance using a difference score (AMPH performance minus PBO performance), starting with the most global metric: the MCCB composite score. Based on previous findings, the two a priori factors included in this analysis were: 1) sex, and 2) baseline performance. We then proceeded to a finer-grain analysis of AMPH’s effects on individual MCCB domains, using the same analytic strategy (i.e. difference score and factors). Lastly, we explored correlations between MCCB effects of AMPH, and 1) measures of AMPH bioactivity, and 2) personality scale scores associated with dopaminergic function and/or AMPH sensitivity.
Some patterns detected in our results were consistent with a regression to the mean (RTM), i.e. based on chance, high-scorers in one condition (e.g., AMPH) were more likely to score lower in the other condition (e.g., PBO), and vice versa (Barnett et al. 2005). We thus assessed whether baseline (PBO)-dependent effects of AMPH on MCCB performance were attributable to RTM using permutation tests. For each of the 7 MCCB domains, data were first residualized based on a linear mixed effects model (LME) with each domain in turn as the dependent variable and treatment (AMPH vs. PBO) and test number as independent variables along with their interaction. For each residualized measure, high scoring subjects were selected based on surpassing the median of residualized scores. For each of these subjects, the difference between their scores in the AMPH and PBO conditions was computed. The mean differences between conditions for high AMPH scorers was then computed. To determine if this mean difference was greater or smaller than expected by usual RTM, we performed a permutation test, randomly permuting the treatment label (AMPH or PBO) within each subject and computing the mean difference for high AMPH scorers for each permutation; the permutations give the distribution of differences for high scorers under the null hypothesis that the difference is unrelated to treatment assignment. The same permutation test procedure was repeated for AMPH low scores, and PBO high and low scorers.
Results
Subject Demographics
As seen in Table 1, the subjects were generally college-aged, well- educated, non-smokers and moderate caffeine consumers.
Baseline MCCB
Measures of baseline MCCB performance - T Scores of each of the 7 MCCB domains after PBO - are seen in Figure 1A; values ranged from 47.68 (range 13–69) in SC to 59.7 (range 34–66) in VisL. Baseline performance was consistent with previously reported MCCB patterns related to age (Figure 1B) (e.g. main effect of age (median split): F=12.01, df 1,58, p=0.001; Composite T Score vs. age, R = −0.52, p<0.0001) and sex (Figure 1C)(Kern et al. 2008). ANOVA of domain T scores with sex as a between-subject factor revealed no significant effect of sex (F<1) and a significant effect of domain (F=17.23, df 6,348, p<0.0001), and a significant interaction of sex x domain (F=2.15, df 6, 348, p<0.05). Post-hoc comparisons across the 7 domains detected no significant main effect of sex in any single domain. MCCB performance was not significantly related to years of education in this sample (R = 0.10), likely reflecting a restrictive “ceiling effect”. Expected significant correlations among the different MCCB domains were also confirmed for SP vs. WM (R = 0.64, p<0.0001), and VerL vs. VisL (R = 0.57, p<0.0001). The full 7-domain correlation matrix is found in Supplemental Table 1.
Fig 1.
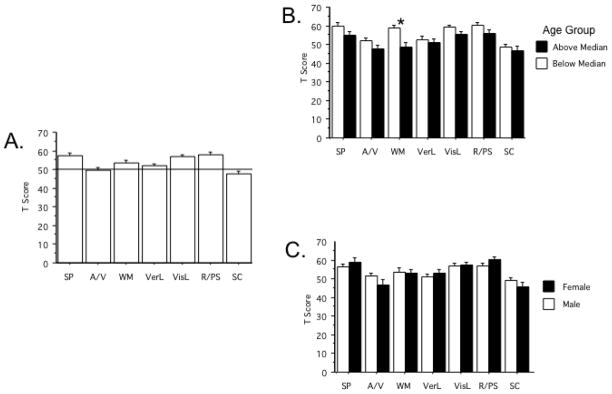
Baseline MCCB performance. A Mean T-Scores in each of the 7 MCCB domains after PBO ingestion). B Mean T-Scores of subjects below and above median age. (*) significant main effect of age by ANOVA (Below median age > Above median age). C Mean T-Score values of subjects divided by sex.
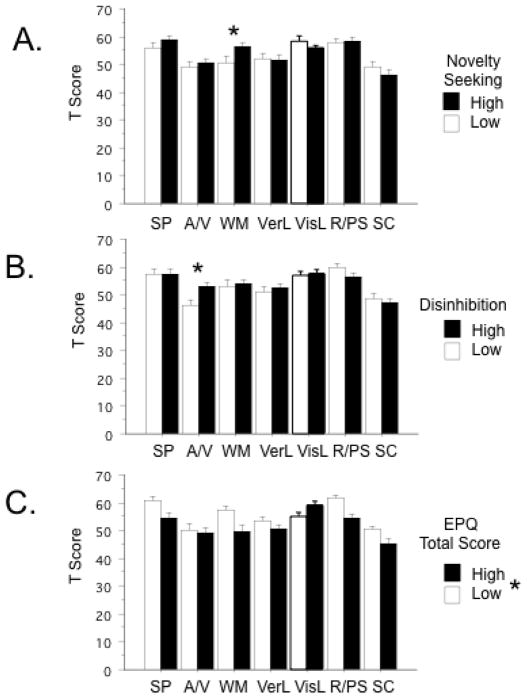
Personality Measures
The impact of TPQ NS, SSS DIS and EPQ Total scores on MCCB performance are seen in Figure 2. Primary analyses focused on these scales based on specific reported relationships to AMPH sensitivity or dopaminergic neural mechanisms (e.g. Fleming et al. 1995; Ebstein et al. 1996; Benjamin et al. 1996; Hutchison et al. 1999; Kumari et al. 2004; Hamidovic et al. 2009). Performance on individual MCCB domains appeared to be impacted by elevated NS (elevated WM scores) and DIS scores (elevated A/V scores), while MCCB scores were more globally impaired among individuals with elevated EPQ Total scores. Significant negative correlations were detected between EPQ Total Scores and T Scores on domains of WM (R = −0.31; p<0.02), R/PS (R = −0.38; p<0.003) and SC (R = −0.38; p<0.003).
Fig 2.
T-Scores in each of the 7 MCCB domains as a function of median split in personality measures of A. TPQ Novelty Seeking (*) significant difference in WM, p<0.05, after significant domain x median split interaction by ANOVA; B. SSS Disinhibition; and C. EPQ Total score. (*) significant main effect of EPQ score by ANOVA (Low score > High score).
MCCB Test-Retest reliability
Independent of drug condition, test performance for subjects across test days was highly correlated (Composite T Score: R = 0.92, p<0.0001; T Scores for 7 individual domains, R’s = 0.482 – 0.749, with all p < 0.0001). Also independent of drug, MCCB scores increased significantly with retesting: ANOVA revealed a significant main effect of test day (F=61.05, df 1,59, p<0.0001), and a significant domain x test day interaction (F=2.38, df 6,354, p<0.03). Post-hoc comparisons revealed significant test day effects for domains of SP (p<0.015), A/V (p<0.04), VerL (p<0.0001) and VisL (p<0.0001). Effect sizes for practice effects on performance in all MCCB domains are found in the Supplemental materials, as a function of low vs. high baseline performance.
Bioactivity
Measured immediately upon MCCB completion, heart rate, diastolic and systolic blood pressure were all significantly elevated by AMPH (F’s = 21.08, 17.04 and 55.50; p’s < 0.0001, respectively (Supplemental Figure 1)); at this time point, more subjects reported greater reductions in drowsiness and perceived sound intensity, and a near-significant increase in the ability to focus attention with AMPH vs. PBO (paired sign tests, p<0.036, 0.029 and 0.06, respectively). In contrast, this dose of AMPH had no significant effect on perceived queasiness, happiness or dizziness (all NS).
AMPH effects on MCCB performance
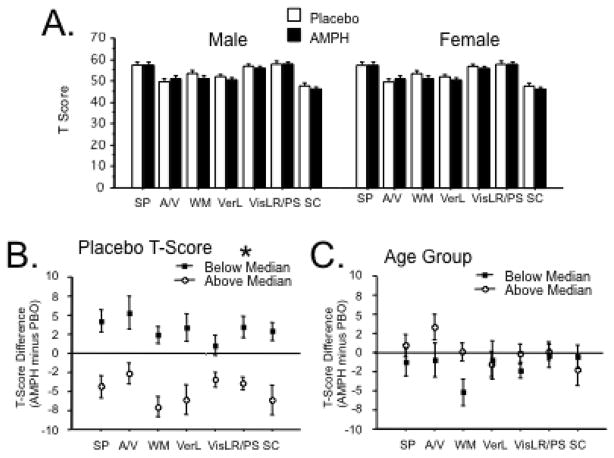
A simple ANOVA of the MCCB Composite Score using AMPH dose as a within-subject factor revealed no significant main effect of AMPH (F<1). Thus, in the inclusive study sample, AMPH had no significant effects on overall MCCB performance.
Sex and Baseline Performance
Two variables reported to impact AMPH effects on a variety of measures, including neurocognition, are sex (cf. Becker et al. 2001; Riccardi et al. 2011) and baseline performance (Mattay et al. 2000). ANOVA of MCCB Composite Score using sex and low vs. high baseline MCCB performance (based on a median split of PBO MCCB values) revealed no significant effect of sex (F<1) or AMPH dose (F<1), or sex x AMPH dose interaction (F<1). The median produced the expected significant effect of baseline MCCB performance (F=66.54, df 1,56, p<0.0001), and a significant interaction of AMPH dose x baseline MCCB performance (F=4.73, df 1,56, p<0.035). This interaction reflected the tendency for AMPH to increase MCCB composite scores among subjects with low baseline performance, and to decrease MCCB composite scores among subjects with high baseline performance. This relationship was supported statistically by a significant negative correlation between the magnitude of the AMPH effect on MCCB performance (calculated as a difference score: AMPH minus PBO) and baseline MCCB composite score (R = −0.43, p<0.001).
Regression to the Mean
Conceivably, “rate-dependent” effect of AMPH might be due to a “regression to the mean” (RTM), i.e. a likelihood that, if two populations differ by random chance in the PBO (baseline) test, subjects scoring low in the PBO test are likely to score higher in the AMPH test, and vice versa. The RTM hypothesis is a challenging one to “falsify” in a within-subject, cross-over design (Barnett et al. 2005; Nesselroade et al. 1980), but can be explored via other properties of the data. We first explored the RTM hypothesis using two known “non-random” sources of low MCCB Composite Score in this sample: higher age, and higher EPQ score. We next tested whether the distribution of AMPH effect scores among subjects scoring above or below the median of each drug condition differed from permuted null distributions.
RTM, Age and EPQ score
We first examined whether the effect of AMPH on MCCB Composite scores (i.e. “AMPH minus PBO” difference score) was related to known sources of low MCCB scores, independent of any selection for extreme values. We fit linear regressions with AMPH difference score as the dependent variable and either age or EPQ Total score as independent variables, controlling for mean MCCB Composite score (averaged across the two test days). There was a trend toward higher age predicting higher differential scores (coef = 0.025, se = 0.014, p = 0.086), and higher EPQ scores significantly predicted lower differential scores (coef = −0.036, se = 0.015, p = 0.020). Thus, independent of baseline performance, older subjects tended to exhibit a greater effect of AMPH on MCCB performance, while subjects with elevated EPQ scores exhibited a significantly diminished effect of AMPH on MCCB performance.
Permuted null distributions
We next computed the null distribution of differences in cognitive outcomes between conditions (PBO vs. AMPH) for AMPH high scorers for all 7 MCCB domains using permutation tests. The mean difference of cognitive scores was contained within the 95% confidence intervals of the null permutation distributions for all 7 domains, indicating no evidence that the mean “AMPH minus PBO” difference in AMPH high scorers differed from that predicted by an RTM effect. We repeated the analysis for AMPH low scorers (below the median of scores in the AMPH condition) and on PBO high and low scorers. As with AMPH high scorers, all results were within the bounds of the 95% permutation null distributions, indicating no significant departures from a model based on RTM.
AMPH effects on Individual MCCB domains
Analyses next proceeded with the 7 MCCB domains. ANOVA of domain T Scores with AMPH dose and domain as within-subject factors and sex as a between-subject factor revealed no significant main effects of AMPH dose (F=1.56, df 1,58, ns) or sex (F<1), and no interaction of AMPH dose x sex (F<1). There was a significant effect of domain (F=26.35, df 6,348, p<0.0001), but no other significant 2- or 3-way interactions (Figure 3A).
Fig 3.
Effect of drug (PBO vs. 20 mg AMPH) on the 7 MCCB domains. A All subjects, divided by sex. B T Score Difference (AMPH minus PBO) divided by median split of PBO T Scores; (*) significant main effect of PBO T score by ANOVA. C T Score Difference (AMPH minus PBO) divided by median split of age. SP: Speed of Processing, A/V: Attention/Vigilance, WM: Working Memory (verbal and nonverbal), VerL: Verbal Learning, VisL: Visual Learning, R/PS: Reasoning and Problem Solving, SC: Social Cognition.
Based on findings of significant interactions of baseline MCCB performance and age on AMPH effects on the Composite MCCB Score, we next assessed the impact of these variables on AMPH effects within individual MCCB domains (Figures 3B,C; Table 3). ANOVAs revealed significant main effects of baseline score (median split) for each domain on the AMPH difference score for that domain (Figure 3B), with AMPH-induced decreases in subjects with baseline scores above the median, and AMPH-induced increases in subjects with baseline scores below the median. Using a more extreme criterion of 1 SD above or below the PBO mean, AMPH significantly decreased performance among high baseline subjects in MCCB domains of SP (p<0.002), WM (p<0.025), VerL (p<0.002), Vis (p<0.01) and SC (p<0.006), and significantly increased performance among low PBO scorers in MCCB domains of A/V (p<0.03), VerL (p<0.03) and R/P (p<0.01). Effect sizes for AMPH effects on performance in all MCCB domains are shown in Supplemental Table 2, as a function of low vs. high baseline performance. Assessing effects of age on AMPH sensitivity, ANOVA with age (above vs. below median) as a grouping factor detected significant effects only on WM (p = 0.01; Figure 3C).
Table 3.
Correlations (R) between age & baseline MCCB performance vs. AMPH effect (difference scores)
| SP | A/V | WM | VerL | VisL | R/PS | SC | |
|---|---|---|---|---|---|---|---|
| Age | 0.24 | 0.29* | 0.41** | 0.09 | 0.12 | 0.13 | 0 |
| Baseline MCCB | −0.46*** | −0.54**** | −0.57**** | −0.66**** | −0.36** | −0.51**** | −0.60**** |
p < 0.03;
p < 0.005;
p < 0.0005;
p < 0.0001;
SP: Speed of Processing, A/V: Attention/Vigilance, WM: Working Memory (verbal and nonverbal), VerL: Verbal Learning, VisL: Visual Learning, R/PS: Reasoning and Problem Solving, SC: Social Cognition
As with the Composite MCCB score, ANCOVAs with AMPH effect on domain scores as the dependent variable and EPQ score as a covariate suggested substantial shared variance with EPQ score (i.e. a loss of significant effect of baseline MCCB performance) for all domains except WM and SP. For WM, the main effect of baseline MCCB performance remained statistically significant (p<0.02); conversely, the significant relationship between WM and baseline MCCB performance was lost when age was entered as a covariate. For SP, the main effect of baseline MCCB performance remained significant with covariates of both EPQ score and age (p’s < 0.007 and 0.002, respectively); both covariates interacted significantly with baseline MCCB performance (p’s < 0.04 and 0.008, respectively).
While AMPH did not exhibit significant main effects on any individual MCCB domain across the inclusive study sample, subjects exhibiting AMPH-induced increases (or decreases) on one MCCB domain were more likely to exhibit similar AMPH-induced changes on other domains. AMPH difference scores were significantly correlated between domains in 5 out of 21 possible pair-wise comparisons (p’s < 0.02 – 0.0002), with trend level correlations in 3 additional pairings (p < 0.10 - 0.05). In total, of the 21 possible comparisons (R), 20 were positive, and one was −0.10; this distribution exceeds a chance probability (Sign test: p<0.0001). The full correlation matrix of AMPH difference scores across MCCB domains is found in Supplemental Table 3.
AMPH bioactivity and MCCB effects
Simple regressions revealed no strong correlations between AMPH effects on autonomic measures and its effects on MCCB Composite or individual domain scores: of the 24 correlations, only 2 achieved nominal alpha values (social cognition vs. change in heart rate (R = 0.26, p<0.05) and vs. change in diastolic blood pressure (R = −0.32, p<0.015)), neither of which survived correction for multiple comparisons. Because VAS scores for change in drowsiness were not normally distributed, Spearman Rank Correlations were used to assess the relationship between this measure of “central” AMPH bioactivity and AMPH effects on MCCB scores. Significant correlations for reduced drowsiness and increased MCCB scores were detected for MCCB Composite Score (R’s = 0.28, p<0.04), SP (r’s = 0.31, p<0.02), VerL (R’s = 0.30, p<0.02) and R/PS (R’s = 0.30, p<0.025), but not A/V, WM, VisL or SC (R’s = 0.00 – 0.15). No consistent pattern emerged when subject weight (as a proxy for dose in mg/kg) was included as a predictor of AMPH effects.
Personality measures and AMPH sensitivity
As noted above, baseline performance in individual MCCB domains was impacted significantly by elevated Novelty Seeking (NS), Disinhibition (DIS) and EPQ Total scores. Simple regressions also revealed significant correlations between these personality measures and AMPH effects on MCCB performance within specific domains: NS score and DIS scores vs. AMPH effects on SP (R = 0.32 and 0.31, respectively; p < 0.015 and < 0.02, respectively); and EPQ Total score vs. VisL (R= −0.29, p<0.025). Correlations of personality measures and AMPH effects on individual MCCB domains among individuals with low vs. high baseline performance scores are found in Supplemental Table 4.
Discussion
Evolving therapeutic strategies for SZ are requiring new approaches to experimental designs. Efforts to “augment” antipsychotic therapies with procognitive agents have largely proven unsuccessful, and trials have now instead begun to focus on the ability of drugs to enhance the therapeutic impact of cognitive therapies, by targeting specific cognitive abilities that are engaged or otherwise required by those therapies (Swerdlow 2011). Importantly, the process of matching candidate drugs and cognitive interventions, and testing them for additive or synergistic therapeutic impact in patients is extremely time- and resource-intensive, given the 12 – 26 weeks required for many cognitive interventions, and the logistical complexities of already medicated and severely ill patients with heterogeneous symptom profiles and a high propensity for study attrition (cf. Rosenheck et al. 2011). Predictive information garnered from preclinical studies in healthy populations could thus have a very significant impact on the efficiency and viability of this discovery process (http://www.nimh.nih.gov/about/director/2012/experimental-medicine.shtml). Here, we report the effects of the known pro-cognitive agent and psychostimulant, AMPH, on MCCB performance in 60 healthy adults; this study was undertaken as part of a larger investigation of dopamine agonist effects on psychophysiological and neurocognitive measures and their relationship to genotype.
AMPH generated evidence of bioactivity, including autonomic activation, and subjective reductions in drowsiness and self-reported increases in attentional capacity. In our past reports, this dose of AMPH also resulted in significant changes in prepulse inhibition (PPI) of the startle reflex; a preliminary inspection confirmed significant AMPH-induced increases in PPI among subjects in the present study (data to be reported as part of a larger sample (n=120)).
Despite this evidence of robust autonomic and CNS effects of AMPH, its impact on MCCB performance was subtle -- detected only among specific subgroups, and on specific MCCB domains. This is perhaps not surprising based on the overall high level of MCCB performance within this highly educated, clinically healthy sample. Nor does the overall insensitivity of the MCCB to AMPH effects suggest that the MCCB does not detect meaningful drug-induced improvements in neurocognition in all healthy individuals: ceiling effects in this inclusive sample of high functioning individuals might be expected to obviate any but the most robust AMPH-mediated cognitive enhancement. Because the goal of this preclinical strategy is to identify drugs that might enhance cognition in cognitively impaired SZ patients – rather than in an inclusive sample of high functioning healthy subjects - it was our a priori design to focus on drug effects among subgroups of subjects who are conceptually linked to SZ, either through their relatively poor MCCB performance, or through psychological or other markers associated with SZ. We previously used this strategy in psychophysiological measures of several drug effects (Swerdlow et al. 2006, 2009; Talledo et al. 2009). One might argue that neurocognitive performance in a sample of healthy adults should be expected to be insensitive to improvement, because “healthy” neurocognition has been optimized by evolutionary forces (Hills & Hertwig 2011). An alternative strategy to “taking all comers” would be to screen subjects for a single characteristic, e.g. exclude all but the lowest MCCB scorers; this strategy would not be optimal for examining multiple contributors to AMPH MCCB sensitivity, e.g. personality scale scores or age, it would limit performance range and thereby hinder the detection of correlations among performance-predicting variables, and it also would preclude detection of potential deleterious effects of AMPH on performance in higher scoring subgroups.
Among individuals with relatively poor baseline MCCB performance, AMPH increased MCCB scores; this was true to varying degrees, depending on the threshold defining “poor performance”: e.g. lowest 50% or 25%, or 1 SD below the sample mean. For comparison, values 1 SD below the present sample mean for T Scores in the 7 MCCB domains were 46.82, 39.17, 41.95, 42.41, 52.02, 49.01 and 37.17 for SP, A/V, WM, VerL, VisL, R/PS and SC, respectively. These values roughly compare with means reported for a somewhat older sample of SZ outpatients for some domains (e.g. A/V, SC), but remain elevated compared to other SZ domain scores (e.g. VisL, R/PS) (Kern et al. 2011). As is perhaps best illustrated in Figure 4B, it is clear that across all MCCB domains, individuals who scored in the lower part of the present distribution at baseline tended to have their scores elevated under AMPH conditions. How to interpret this effect, and particularly the potential role of “regression to the mean” in this apparent AMPH-induced cognitive improvement, is less straightforward.
On the one hand, we demonstrated rate-dependent effects of AMPH in this same sample, that are not easily attributable to an RTM explanation. Specifically, we determined that in older subjects, AMPH tended to have a greater effect on MCCB performance, while in subjects with elevated EPQ scores, AMPH has a significantly diminished effect of MCCB performance. Because these analyses were not based on extreme values of baseline MCCB performance, these relationships cannot be explained on the basis of a RTM. On the other hand, using permutation tests, we found no significant differences of observed rate-dependent effects of AMPH from those predicted by RTM. More definitive assessments on whether observed effects constitute RTM or actual AMPH effects can be determined from other study designs, such as those using three or more time points (Nesselroade et al, 1980). For example, under usual psychometric assumptions of measurement error independence, if a third measurement of MCCB performance were obtained, it would then be possible to determine if any drug effect detected across the previous two measurements were “real” (i.e., whether low baseline performers performed significantly better in the AMPH condition).
While there is not absolute clarity to the basis for the current observation of AMPH-enhanced MCCB performance among “low baseline” subjects, our findings are consistent with previous reports of dopamine agonist effects on neurocognition. In both SZ patients and healthy comparison subjects, AMPH has been shown to improve cognition (Barch and Carter 2005; Pietrzak et al. 2010; Rapoport et al. 1980). Of most relevance to our present findings, Mattay et al. (2000) reported that AMPH enhanced WM among healthy subjects with low baseline WM performance, but worsened performance among subjects who had high baseline WM scores. This same pattern of baseline-dependent drug effects was reported by Kimberg et al. (1997) with the D2 agonist, bromocriptine. Other reports have identified an “inverted-U” relationship between baseline performance and AMPH or other DA agonist effects on forebrain-mediated psychophysiological measures such as prepulse inhibition of startle (Bitsios et al. 2005; Talledo et al. 2009) and antisaccade latency (Allman et al. 2010). Mechanisms responsible for the baseline-dependent impact of DA agonists on neurocognitive and other forebrain-mediated processes are a focus of investigation, and may involve differential levels of DA catabolism effected the COMT Val158-Met polymorphism (Hamidovic et al. 2010; Mattay et al. 2003).
We examined other variables that might moderate MCCB performance and/or AMPH effects on neurocognition: sex, personality measures and bioactivity. The MCCB did not detect significant sex differences in any domain, nor were there sex differences in AMPH sensitivity on these measures. By contrast, personality measures – NS, DIS and EPQ Total Score - were found to significantly moderate both baseline MCCB scores and AMPH effects on MCCB performance. Previous studies reported distinct cognitive performance and drug sensitivities among groups of normals differing in several of these same personality dimensions (Fleming et al. 1995; Hutchison et al. 1999; Corr & Kumari 2000; Koychev et al. 2012; Kumari et al. 2004; Roussos et al. 2009; Soubelet & Salthouse 2011); to our knowledge, this is the first report of the relationship between personality dimensions and MCCB performance in healthy individuals. Fleming et al. (1995) reported that among healthy women, AMPH disrupted verbal memory performance in high NS individuals, but enhanced performance in low NS subjects; interestingly, in our present sample, higher NS scores predicted MCCB-enhancing effects of AMPH, for composite MCCB performance, and particularly for specific MCCB domains. Hutchison et al. (1999) reported that DIS significantly moderated the subjective effects of AMPH, with higher scores predicting greater AMPH-induced subjective stimulation and elation; in our present sample, higher DIS scores were associated with greater AMPH-induced increases in speed of processing (SP) domain of the MCCB. We previously reported significant differences in AMPH effects on sensorimotor gating of the startle reflex among healthy women distinguished by low vs. high NS or DIS scores. Mechanisms mediating the relationship between personality dimensions and AMPH sensitivity on subjective, cognitive or neurophysiological measures have been explicated at the levels of endocrine reactivity (White et al. 2006), differential neural circuit effects (Kumari et al. 2004) and genetic polymorphisms (Roussos et al. 2009).
To the degree that AMPH does enhance MCCB performance in a particular subgroup of healthy subjects, this effect might reflect either primary drug actions (e.g. stimulation of dopamine release in prefrontal areas regulating attention) or secondary drug actions (e.g. reducing drowsiness to permit better test performance). While no measures of primary drug effects on brain chemistry were used, it was possible to detect significant correlations between positive AMPH effects on MCCB performance and reduced drowsiness. These correlations do not preclude the possibility that both changes in performance and drowsiness might both be correlated with one or more primary drug effects, such as prefrontal dopamine release. At the least, it appears that reduced drowsiness might be one parsimonious mechanism by which AMPH enhances MCCB performance in this sample.
There are rational reasons why AMPH might raise concerns as a drug to augment the therapeutic impact of cognitive therapies in SZ patients, even though AMPH has been shown to enhance neurocognition in antipsychotic-medicated SZ patients (Daniel et al. 1991, Pietrzak et al. 2010) and is not associated with an exacerbation of psychosis in these patients who received a single challenge dose (Barch and Carter 2005; Goldberg et al. 1991). Nonetheless, in the absence of antipsychotic medications, there are at least theoretical reasons to believe that AMPH might carry a risk of triggering psychotic symptoms in SZ patients. Given the pragmatic difficulties of ensuring antipsychotic adherence in SZ patients, it is viewed by some as problematic to prescribe a drug (AMPH) that might trigger activate psychosis in the absence of antipsychotics. Other psychostimulants, such as modafinil, are being investigated for their potential pro-cognitive effects (Bobo et al. 2011; Kane et al. 2010; Scoriels et al. 2012), and might also prove to enhance the therapeutic impact of cognitive interventions.
The present study tested a strategy – the use of healthy populations to predict the potential for drug-enhanced neurocognition in SZ patients. The findings suggest that, in a sample of clinically healthy, young adults, the MCCB is only modestly sensitive to procognitive effects of a dose of AMPH that shows evidence of both bioactivity and central activation, and which is known to enhance SZ neurocognition in other studies. However, the present findings also suggest that by identifying a priori subgroups of interest, it is possible to detect significant positive cognitive effects of AMPH; conceivably, drug effects in these subgroups may be most relevant to their potential activity in SZ patients. Our larger study, for which the present sample is a planned “first wave”, may identify specific genetic predictors of such drug effects. Until definitive biomarkers are available, the use of healthy populations to predict “pro-cognitive therapy” candidate drugs may be enhanced by designs that minimize potential “RTM” effects, and that focus on population subgroups characterized by low baseline levels of neurocognitive performance or extreme values of specific SZ-linked personality indices.
Supplementary Material
Autonomic measures after MCCB completion. HR: Heart rate, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure. (*) significant main effect of drug (AMPH > PBO).
Acknowledgments
NRS is supported by R01-MH059803, R34-MH093453, R01-MH094320 and the VISN22 Mental Illness Research, Education & Clinical Center (MIRECC); HHC is supported by T32-MH018399 and the APF/Merck Early Academic Career Award. Outstanding administrative support was provided by Ms. Maria Bongiovanni. Excellent technical support was provided by Mr. Justin Kei.
References
- Barch DM. Pharmacological strategies for enhancing cognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:43–96. doi: 10.1007/7854_2010_39. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS. Measurement issues in the use of cognitive neuroscience tasks in drug development for impaired cognition in schizophrenia: a report of the second consensus building conference of the CNTRICS initiative. Schizophr Bull. 2008;34:613–618. doi: 10.1093/schbul/sbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res. 2005;77:43–58. doi: 10.1016/j.schres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Population and familial association between the D4 dopamine receptor gene and measures of Novelty Seeking. Nat Genet. 1996;12:81–84. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- Bobo WV, Woodward ND, Sim MY, Jayathilake K, Meltzer HY. The effect of adjunctive armodafinil on cognitive performance and psychopathology in antipsychotic-treated patients with schizophrenia/schizoaffective disorder: a randomized, double-blind, placebo-controlled trial. Schizophr Res. 2011;130:106–113. doi: 10.1016/j.schres.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63:505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Keefe RSE, Umbricht D, Green MF, Laughren T, Marder SR. The FDA-NIMH-MATRICS guidelines for clinical trial design of cognitive-enhancing drugs: what do we know 5 years later? Schizophr Bull. 2011;37:1209–1217. doi: 10.1093/schbul/sbq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney WE, Hetrick WP, Bunney BG, Patterson JV, Jin Y, Potkin SG, Sandman CA. Structured Interview for Assessing Perceptual Anomalies (SIAPA) Schizophr Bull. 1999;25:577–592. doi: 10.1093/oxfordjournals.schbul.a033402. [DOI] [PubMed] [Google Scholar]
- Cervellione KL, Burdick KE, Cottone JG, Rhinewine JP, Kumra S. Neurocognitive deficits in adolescents with schizophrenia: longitudinal stability and predictive utility for short-term functional outcome. J Am Acad Child Adolesc Psychiatry. 2007;46:867–878. doi: 10.1097/chi.0b013e318054678d. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Correll CU, Kishimoto T, Kane JM. Randomized controlled trials in schizophrenia: opportunities, limitations, and trial design alternatives. Dialogues Clin Neurosci. 2011;13:155–172. doi: 10.31887/DCNS.2011.13.2/ccorrell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Berman KF, Kleinman JE. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci. 1991;11:1907–1917. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Bennett ER, Nemanov L, Katz M, Belmaker RH. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of Novelty Seeking. Nat Genet. 1996;12:78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Emanuel EJ, Miller FG. The ethics of placebo-controlled trials--a middle ground. N Engl J Med. 2001;345:915–919. doi: 10.1056/NEJM200109203451211. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Lucas M, Domenici E, Robbins TW, Bullmore ET. Peripheral biomarkers of cognitive response to dopamine receptor agonist treatment. Psychopharmacology. 2011;214:779–89. doi: 10.1007/s00213-010-2087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck H, Eysenck S. Manual of the Eysenck Personality Questionnaire; comprising the EPQ-revised (EPQ-R) & EPQ-R Short Scale. Edits; San Diego, CA: 1994. [Google Scholar]
- Fleming K, Bigelow LB, Weinberger DR, Goldberg TE. Neuropsychological effects of amphetamine may correlate with personality characteristics. Psychopharmacol Bull. 1995;31:357–362. [PubMed] [Google Scholar]
- Giakoumaki SG, Roussos P, Bitsios P. Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism. Neuropsychopharmacology. 2008;33:3058–3068. doi: 10.1038/npp.2008.82. [DOI] [PubMed] [Google Scholar]
- Goff DC, Keefe R, Citrome L, et al. Lamotrigine as add-on therapy in schizophrenia: results of 2 placebo-controlled trials. J Clin psychopharmacol. 2007;27:582–589. doi: 10.1097/jcp.0b013e31815abf34. [DOI] [PubMed] [Google Scholar]
- Gold JM, Goldberg RW, McNary SW, Dixon LB, Lehman AF. Cognitive correlates of job tenure among patients with severe mental illness. Am J Psychiatry. 2002;159:1395–1402. doi: 10.1176/appi.ajp.159.8.1395. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Bigelow LB, Weinberger DR, Daniel DG, Kleinman JE. Cognitive and behavioral effects of the coadministration of dextroamphetamine and haloperidol in schizophrenia. Am J Psychiatry. 1991;148:78–84. doi: 10.1176/ajp.148.1.78. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Palmer AA, de Wit H. Catechol-O-methyltransferase val158met genotype modulates sustained attention in both the drug-free state and in response to amphetamine. Psychiatr Genet. 2010;20:85–92. doi: 10.1097/YPG.0b013e32833a1f3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Ogasa M, Cucchiaro J, Loebel A, Keefe RS. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. ziprasidone. Schizophr Res. 2011;127:188–194. doi: 10.1016/j.schres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Ammari N, Miles AA, McDermid Vaz S. Cognitive performance and functional competence as predictors of community independence in schizophrenia. Schizophr Bull. 2010;36:381–387. doi: 10.1093/schbul/sbn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hills T, Hertwig R. Why aren’t we smarter already: Evolutionary Trade-Offs and Cognitive Enhancements. Current Directions in Psychological Science. 2011;20:373–377. [Google Scholar]
- Hutchison KE, Wood MD, Swift R. Personality factors moderate subjective and psychophysiological responses to d-amphetamine in humans. Exp Clin Psychopharmacol. 1999;7:493–501. doi: 10.1037//1064-1297.7.4.493. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF, Lieberman J, Goff DC, Csernansky JG, McEvoy JP, Jarskog F, Seidman LJ, Gold JM, Kimhy D, Nolan KS, Barch DS, Ball MP, Robinson J, Marder SR. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res. 2012;136:25–31. doi: 10.1016/j.schres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Kane JM, D’Souza DC, Patkar AA, Youakim JM, Tiller JM, Yang R, Keefe RS. Armodafinil as adjunctive therapy in adults with cognitive deficits associated with schizophrenia: a 4-week, double-blind, placebo-controlled study. J Clin Psychiatry. 2010;71:1475–1481. doi: 10.4088/JCP.09m05950gry. [DOI] [PubMed] [Google Scholar]
- Keefe R, Harvey P. Handbook of Experimental Pharmacology: Novel Anti-Schizophrenia Treatments. 2012. Cognitive Impairment in Schizophrenia. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Fox KH, Harvey PD, Cuccchiaro J, Siu C, Loebel A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr Res. 2011;125:161–168. doi: 10.1016/j.schres.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Kemp AS, Schooler NR, Kalali AH, Alphs L, Anand R, Awad G, Davidson M, Dubé S, Ereshefsky L, Gharabawi G, Leon AC, Lepine JP, Potkin SG, Vermeulen A. What is causing the reduced drug-placebo difference in recent schizophrenia clinical trials and what can be done about it? Schizophr Bull. 2010;36:504–509. doi: 10.1093/schbul/sbn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’Esposito M. Cognitive effects of the dopamine receptor agonist pergolide. Neuropsychologia. 2003;41:1020–1027. doi: 10.1016/s0028-3932(02)00317-2. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- Koychev I, McMullen K, Lees J, Dadhiwala R, Grayson L, Perry C, Schmechtig A, Walters J, Craig KJ, Dawson GR, Dourish CT, Ettinger U, Wilkinson L, Williams S, Deakin JF, Barkus E. A validation of cognitive biomarkers for the early identification of cognitive enhancing agents in schizotypy: a three-center double-blind placebo-controlled study. Eur Neuropsychopharmacol. 2012;22(7):469–81. doi: 10.1016/j.euroneuro.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kumari V, Cotter PA, Mulligan OF, Checkley SA, Gray NS, Hemsley DR, Thornton JC, Corr PJ, Toone BK, Gray JA. Effects of d-amphetamine and haloperidol on latent inhibition in healthy male volunteers. J Psychopharmacol. 1999;13:398–405. doi: 10.1177/026988119901300411. [DOI] [PubMed] [Google Scholar]
- Kumari V, ffytche DH, Williams SCR, Gray JA. Personality predicts brain responses to cognitive demands. J Neurosci. 2004;24:10636–10641. doi: 10.1523/JNEUROSCI.3206-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent A, Gilvarry C, Russell A, Murray R. Personality dimensions and neuropsychological performance in first-degree relatives of patients with schizophrenia and affective psychosis. Schizophr Res. 2002;55:239–248. doi: 10.1016/s0920-9964(01)00280-8. [DOI] [PubMed] [Google Scholar]
- Marx CE, Keefe RSE, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL, Shampine LJ. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology. 2009;34:1885–1903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, Berman KF, Goldberg TE, Weinberger DR. Effects of dextroamphetamine on cognitive performance and cortical activation. NeuroImage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure MM, Bowie CR, Patterson TL, Heaton RK, Weaver C, Anderson H, Harvey PD. Correlations of functional capacity and neuropsychological performance in older patients with schizophrenia: evidence for specificity of relationships? Schizophr Res. 2007;89:330–338. doi: 10.1016/j.schres.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology. 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- Nesselroade JR, Stigler SM, Baltes PB. Regression Toward the Mean and the Study of Change. Psychol Bull. 1980;88:622–637. [Google Scholar]
- Norris H. The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology. 1971;10:181–191. doi: 10.1016/0028-3908(71)90039-6. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Patel MX. Challenges in recruitment of research participants. Adv Psychiatr Treat. 2003;9:229–238. [Google Scholar]
- Pietrzak RH, Snyder PJ, Maruff P. Use of an acute challenge with d-amphetamine to model cognitive improvement in chronic schizophrenia. Hum psychopharmacol. 2010;25:353–358. doi: 10.1002/hup.1118. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Snyder PJ, Maruff P. Amphetamine-related improvement in executive function in patients with chronic schizophrenia is modulated by practice effects. Schizophr Res. 2010;124(1–3):176–82. doi: 10.1016/j.schres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine. Its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Park S, Anderson S, Doop M, Ansari MS, Schmidt D, Baldwin R. Sex differences in the relationship of regional dopamine release to affect and cognitive function in striatal and extrastriatal regions using positron emission tomography and [8F]fallypride. Synapse. 2011;65:99–102. doi: 10.1002/syn.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheck RA, Krystal JH, Lew R, Barnett PG, Thwin SS, Fiore L, Valley D, Huang GD, Neal C, Vertrees JE, Liang MH the CSP 555 Research Group. Challenges in the design and conduct of controlled clinical effectiveness trials in schizophrenia. Clinical Trials. 2011;8:196–204. doi: 10.1177/1740774510392931. [DOI] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Bitsios P. Tolcapone effects on gating, working memory, and mood interact with the synonymous catechol-O-methyltransferase rs4818c/g polymorphism. Biol Psychiatry. 2009;66:997–1004. doi: 10.1016/j.biopsych.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Scoriels L, Barnett JH, Soma PK, Sahakian BJ, Jones PB. Effects of modafinil on cognitive functions in first episode psychosis. Psychopharmacology. 2012;220:249–258. doi: 10.1007/s00213-011-2472-4. [DOI] [PubMed] [Google Scholar]
- Smith AP. Effects of time of day, introversion and neuroticism on selectivity in memory and attention. Percept Mot Skills. 1992;74:851–860. doi: 10.2466/pms.1992.74.3.851. [DOI] [PubMed] [Google Scholar]
- Soubelet A, Salthouse TA. Personality–cognition relations across adulthood. Dev Psychol. 2011;47:303–310. doi: 10.1037/a0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR. Are we studying and treating schizophrenia correctly? Schizophr Res. 2011;130:1–10. doi: 10.1016/j.schres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Eastvold A, Karban B, Ploum Y, Stephany N, Geyer MA, Cadenhead K, Auerbach PP. Dopamine agonist effects on startle and sensorimotor gating in normal male subjects: time course studies. Psychopharmacology. 2002;161:189–201. doi: 10.1007/s00213-002-1040-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Shoemaker J, Auerbach PP. Amphetamine effects on prepulse inhibition across-species: replication and parametric extension. Neuropsychopharmacology. 2003a;28:640–650. doi: 10.1038/sj.npp.1300086. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Talledo J, Sutherland AN, Nagy D, Shoemaker JM. Antipsychotic effects on prepulse inhibition in normal ‘low gating’ humans and rats. Neuropsychopharmacology. 2006;31:2011–2021. doi: 10.1038/sj.npp.1301043. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, van Bergeijk DP, Bergsma F, Weber E, Talledo J. The effects of memantine on prepulse inhibition. Neuropsychopharmacology. 2009;34:1854–1864. doi: 10.1038/npp.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Wasserman LC, Talledo JA, Casas R, Bruins P, Stephany NL. Prestimulus modification of the startle reflex: relationship to personality and physiological markers of dopamine function. Biol Psychology. 2003b;62:17–26. doi: 10.1016/s0301-0511(02)00090-x. [DOI] [PubMed] [Google Scholar]
- Talledo JA, Sutherland Owens AN, Schortinghuis T, Swerdlow NR. Amphetamine effects on startle gating in normal women and female rats. Psychopharmacology. 2009;204:165–75. doi: 10.1007/s00213-008-1446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touwen DP, Engberts DP. Those famous red pills-Deliberations and hesitations. Ethics of placebo use in therapeutic and research settings. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.03.005. [Epub ahead of print, April 23] [DOI] [PubMed] [Google Scholar]
- White TL, Lott DC, de Wit H. Personality and the subjective effects of acute amphetamine in healthy volunteers. Neuropsychopharmacology. 2006;31:1064–1074. doi: 10.1038/sj.npp.1300939. [DOI] [PubMed] [Google Scholar]
- Williams LM, Whitford TJ, Flynn G, Wong W, Liddell BJ, Silverstein S, Galletly C, Harris AW, Gordon E. General and social cognition in first episode schizophrenia: identification of separable factors and prediction of functional outcome using the IntegNeuro test battery. Schizophr Res. 2008;99:182–191. doi: 10.1016/j.schres.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Bone RN, Neary R, Mangelsdorff D, Brustman B. What is the sensation seeker? Personality trait and experience correlates of the Sensation-Seeking Scales. J Consult Clin Psychol. 1972;39:308–321. doi: 10.1037/h0033398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Autonomic measures after MCCB completion. HR: Heart rate, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure. (*) significant main effect of drug (AMPH > PBO).