Abstract
BACKGROUND
The incidence of rectal cancer in the United States in young patients is considered to be low. Under-estimating this incidence may result in a failure to diagnose younger patients with rectal cancer in a timely manner.
METHODS
The authors conducted a retrospective cohort study using Surveillance, Epidemiology, and End Results (SEER) cancer registry data. A total of 7661 patients with colon, rectal, and rectosigmoid cancer who were diagnosed at age <40 years were identified between 1973 and 2005. The change in incidence over time for colon and rectal/rectosigmoid cancer was calculated and the annual percent change for anatomic subsites of colorectal cancer compared.
RESULTS
SEER data demonstrated an increase in the incidence of rectal cancer without any increase in colon cancer (annual percent change of 2.6% vs −0.2%). The difference was statistically significant and extended to rectosigmoid cancer, but not cancer of the sigmoid colon or descending colon (annual percent change of 2.2% vs 0.4% and −2.8%, respectively). Joinpoint analysis of the slope of the curve of rectal and rectosigmoid cancer incidence identified the beginning of the increase to be 1984. All races and both sexes demonstrated similar statistically significant increases in the incidence of rectal and rectosigmoid cancer.
CONCLUSIONS
The incidence of rectal and rectosigmoid cancer appears to be increasing in patients aged <40 years. Patients presenting with rectal bleeding or other alarming signs or symptoms should be evaluated with this finding in mind.
Keywords: adult, colonic neoplasms/epidemiology, humans, incidence, rectal neoplasms/epidemiology, Surveillance, Epidemiology, End Results (SEER), program/statistics and numerical data, United States/epidemiology
Colorectal cancer (CRC) is the second most common cause of cancer-related death in the United States. Improved adherence to CRC screening guidelines and the subsequent prevention of polyp progression to cancer has led to a recent decline in the incidence and mortality from CRC. Approximately one-third of patients with CRC have their cancer limited to the rectum.1
Although CRC is often thought of as a disease of the elderly, recent studies have reported a rise in the incidence of CRC in younger populations.2,3 Risk factors for this include a genetic predisposition to colon cancer or diet and lifestyle changes in the current era leading to a rise in the incidence of cancers in young patients.4-9
The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute is a source of information regarding cancer incidence and survival in the United States. SEER collects and publishes cancer incidence and survival data from population-based cancer registries covering approximately 26% of the US population. The age-adjusted incidence of rectal cancer as per the SEER database from 1975 through 2005 was 16.1 per 100,000. Although there is no clear age cutoff defined, the majority of studies classified patients diagnosed with cancer at age <40 years as young.
Several studies have indicated a rise in colon cancer in younger patients; however, to the best of our knowledge, few have specifically addressed the incidence of cancer involving the rectum. An observed increase in the presentation of rectal cancer in patients aged <40 years at our institution (New York-Presbyterian Hospital/Weill Cornell Medical College Center) led us to analyze the trends in rectal cancer incidence in the SEER database. This study was designed to assess the change in the incidence of rectal cancer over time using the SEER database and evaluate the need for a modification in screening guidelines to include certain patients aged <40 years.
MATERIALS AND METHODS
The SEER database was queried for cases defined as “rectum,” “rectosigmoid junction,” “sigmoid colon,” “descending colon,” and “colon excluding rectum” between the years of 1973 and 2005. Only cases in patients aged <40 years were used for analysis. All cases were sorted by year of diagnosis, sex, and race. Cases per 100,000 were calculated using SEER*Stat software (http://seer.cancer.gov/seerstat/index.html) and age-adjusted to the 2000 US standard population. The annual percent change (AnPC) for each anatomic location from 1973 through 2005 was estimated using the weighted least squares method in the SEER*Stat software, version 6.4.4 (Silver Spring, MD). The 95% confidence intervals (95% CIs) for the AnPC were calculated to assess the precision of the obtained estimates using the Tiwari modification. Additional join-point analysis was performed using Joinpoint 3.3.1 (http://srab.cancer.gov/joinpoint; Silver Spring, MD).
RESULTS
A total of 5125 patients with a diagnosis of “colon excluding rectum” cancer (hereafter referred to as colon cancer) and 1922 patients with cancer of the rectum were identified in the SEER database as being aged <40 years. Approximately 52% of the patients were male, and the greatest percentage (52%) were ages 35 to 39 years. Approximately 75% of the patients were white. Approximately 70% of tumors were classified as adenocarcinoma, and the most predominant grade was moderately differentiated (reported in 41% of patients) (Table 1).
Table 1.
Patient Characteristics
| Patient Characteristics | No. (%) | |
|---|---|---|
| Sex | Male | 3999 (52) |
| Female | 3662 (48) | |
| Age, y | <20 | 168 (2.2) |
| 20-24 | 395 (5.2) | |
| 25-29 | 980 (13) | |
| 30-34 | 2127 (28) | |
| 35-39 | 3991 (52) | |
| Race | American Indian/Alaskan Native | 76 (1.0) |
| Asian or Pacific Islander | 720 (9.4) | |
| Black | 1095 (14) | |
| White | 5714 (75) | |
| Other/unknown | 56 (0.7) | |
| Site | Rectum | 1922 (25) |
| Rectosigmoid | 614 (8.0) | |
| Sigmoid colon | 1429 (19) | |
| Descending colon | 428 (5.6) | |
| Colon excluding rectum | 5125 (67) | |
| Histology | Adenocarcinoma | 5345 (70) |
| Adenocarcinoma in adenomatous polyposis coli | 64 (0.8) | |
| Cystadenocarcinoma/mucinous adenocarcinoma | 996 (13) | |
| Signet ring cell carcinoma | 198 (2.6) | |
| Squamous cell carcinoma | 51 (0.7) | |
| Carcinoma, NOS or other | 177 (2.3) | |
| Carcinoid/neuroendocrine | 763 (10) | |
| Sarcoma | 35 (0.5) | |
| Grade | Unknown/other | 32 (0.4) |
| Well differentiated | 707 (9.2) | |
| Moderately differentiated | 3138 (41) | |
| Poorly differentiated | 1500 (20) | |
| Undifferentiated | 144 (1.9) | |
| Unknown | 2172 (28) | |
NOS indicates not otherwise specified.
The incidence of colon cancer during the years of the study was 1.11 per 100,000 (95% CI, 1.08-1.14). During the same period, the incidence of rectal cancer was 0.42 per 100,000 (95% CI, 0.40-0.44). When the trend over time was examined, the incidence of rectal cancer increased significantly, whereas the incidence of colon cancer remained unchanged. To quantify these differences, the AnPC was calculated for both groups. The AnPC for rectal cancer was 2.6% (95% CI, 1.9-3.3%; P <.0001) compared with a nonsignificant AnPC for colon cancer of −0.2% (95% CI, −0.6% to 0.3%; P =.50) (Fig. 1).
Figure 1.
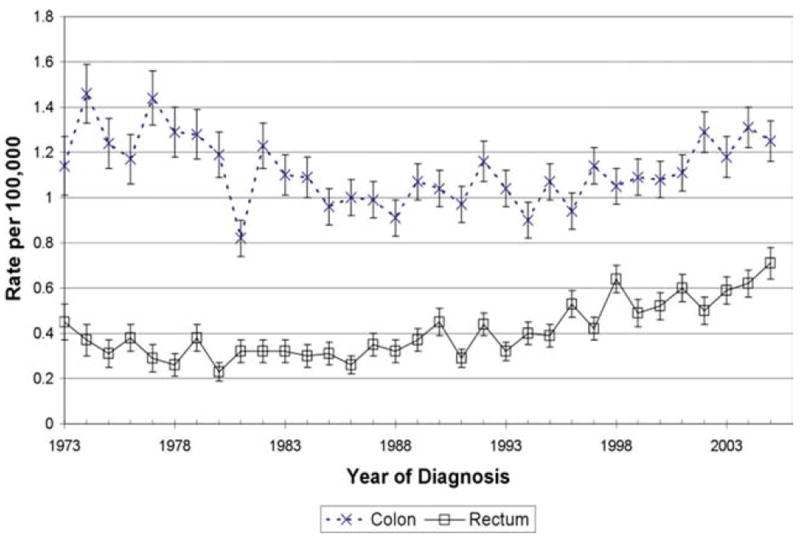
Surveillance, Epidemiology, and End Results (SEER) database incidence of rectal cancer and colon cancer is shown.
When the incidence rates for cancers of other anatomic sites were examined, an increase was also found in rectosigmoid junction cancer (hereafter referred to as “rectosigmoid cancer”). A total of 614 cases of rectosigmoid cancer diagnosed in patients aged <40 years were found during the study period, resulting in an incidence of 0.13 per 100,000 (95% CI, 0.12-0.14) and an increasing AnPC of 2.2% (95% CI, 1.2-3.1%; P <.0001). During the same study period, there was no significant increase noted in the incidence of cancer of either the sigmoid or descending colon. The AnPC of sigmoid cancer was a nonsignificant 0.4% (95% CI, −0.3% to 1.2%; P = .30) and cancer of the descending colon had a significantly decreasing AnPC of −1.8% (95% CI, −2.8% to −0.7%; P <.0001) (Table 2).
Table 2.
Annual Percent Change by Disease Site
| Site | AnPC, % | 95% CI | P |
|---|---|---|---|
| Rectum | +2.6 | +1.9 to +3.3 | <.0001 |
| Rectosigmoid | +2.2 | +1.2 to +3.1 | <.0001 |
| Sigmoid | +0.4 | −0.3 to +1.2 | .30 |
| Descending colon | −1.8 | −2.8 to −0.7 | <.0001 |
| Colon excluding rectum | −0.2 | −0.6 to +0.3 | .50 |
AnPC indicates annual percent change; 95% CI, 95% confidence interval.
Because data regarding the incidence of rectal and rectosigmoid cancer were not statistically different and the SEER database does not define the distinction between the 2 sites anatomically, the rectal and rectosigmoid cancer groups were pooled together for further analysis. This was done to increase the statistical power of subsequent analyses, and for the sake of simplicity.
Joinpoint analysis was then performed to identify the year that the increase in incidence began in the patient population with cancer of the rectum and rectosigmoid junction. A single joinpoint was found at 1984. The AnPC for years 1973 through 1984 was calculated to be a nonsignificant −1.8% (95% CI, −5.3% to 1.7%). The AnPC in Segment 2, between 1984 and 2005, was calculated to be 3.8% (95% CI, 2.8-4.8%), which was statistically significant from 0 (Fig. 2).
Figure 2.
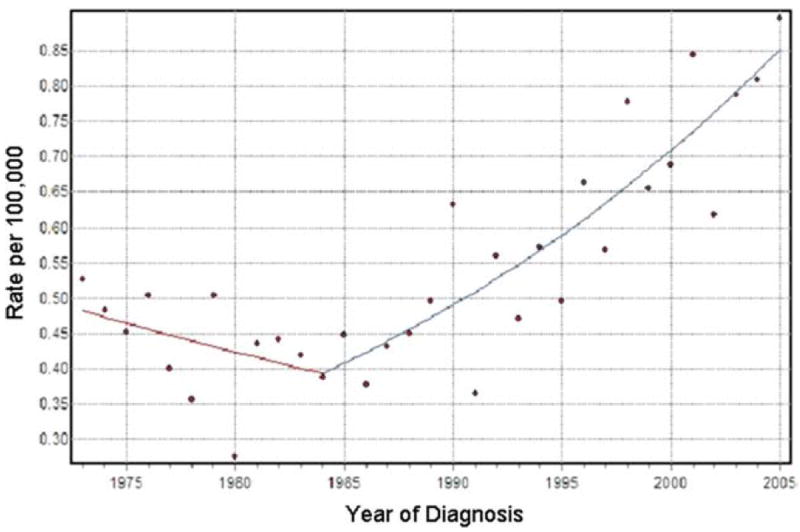
Joinpoint analysis of rectal and rectosigmoid cancer is shown (slope of segment 1 = −1.8; slope of segment 2 = 3.8).
We then analyzed the combined rectal and rectosigmoid cancer patients by race, and found the incidence to be increasing in each racial group. A total of 1858 patients were identified as white, with an incidence of 0.51 per 100,000 (95% CI, 0.48-0.53). The AnPC for this population was 2.46% (95% CI, 1.67-3.26%; P < .0001). When black patients were analyzed, 347 patients were identified, with an incidence of 0.67 per 100,000 (95% CI, 0.60-0.74) and an AnPC of 1.92% (95% CI, 0.59-3.28%; P = .01). The remaining patients were characterized as “other” with respect to race in the SEER database. This group included 311 patients with an incidence of 0.72 per 100,000 (95% CI, 0.64-0.80) and an AnPC of 2.16% (95% CI, 0.96-3.37%; P < 0.0001) (Fig. 3).
Figure 3.
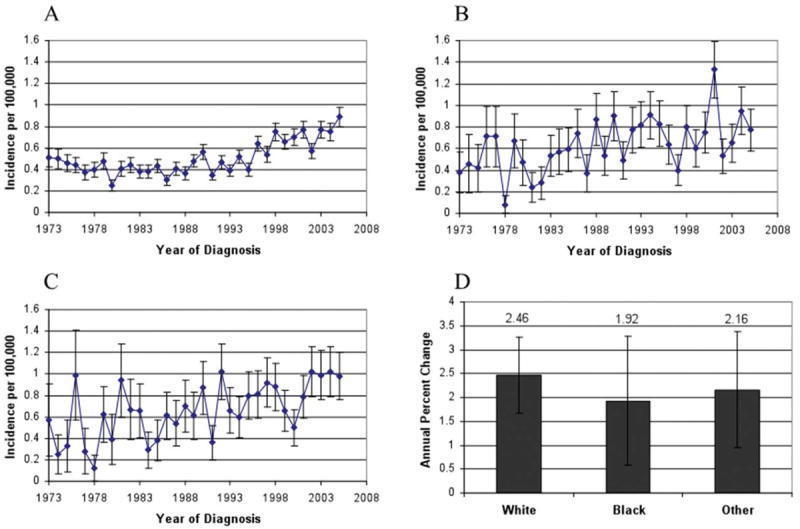
Rectal and rectosigmoid cancer incidence is shown by race. (A) Incidence in whites is shown. (B) Incidence in blacks is shown. (C) Incidence in other racial groups is shown. (D) Annual percent change is shown by race.
When we analyzed the rectal and rectosigmoid cancer group by sex, we found the incidence increasing in both males and females, without any significant difference noted in the rate of change. The 54% of patients who were male were found to have an incidence of 0.59 per 100,000 (95% CI, 0.56-0.62) with an AnPC of 2.5% (95% CI, 1.6-3.4%; P < .0001). Females demonstrated an incidence of 0.51 per 100,000 (95% CI, 0.48-0.54) with an AnPC of 2.5% (95% CI, 1.8-3.2%; P < .0001) (Fig. 4).
Figure 4.
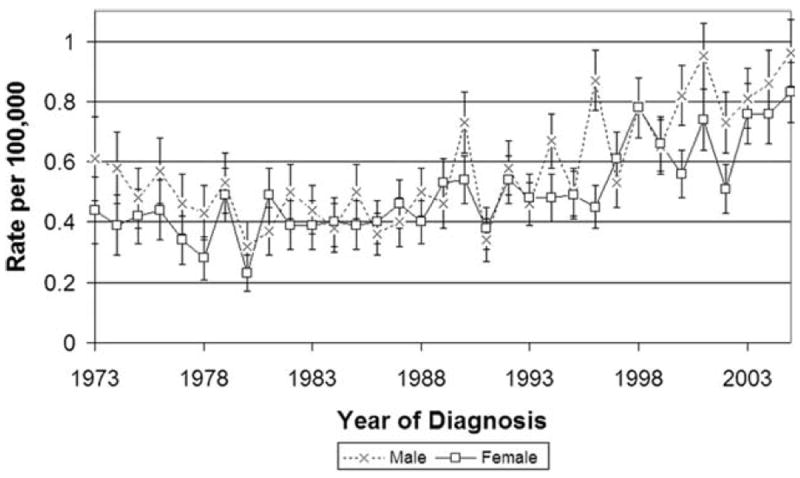
Rectal and rectosigmoid cancer incidence is shown by sex.
DISCUSSION
Based on a trend initially observed within our institution, the results of the current study demonstrate an increase in the incidence of rectal and rectosigmoid cancer in young patients (defined as those aged <40 years) in the United States. This rise was observed independent of sex or race. However, it was not observed when the rates of colon cancer were examined in the same population.
Although an increase in rectal cancer in younger patients has been reported previously, the magnitude of this increase has not previously been demonstrated. O’Connell et al demonstrated an increase in rectal cancers using the SEER database data from 1973 through 1999 in patients ages 20 to 40 years.3 However, the current study reported on the 6 most recent years for which data were available, analyzed the significance of race and sex, and included the joinpoint analysis, which allowed for the identification of the true slope of the current increase. Two other reports of increasing rectal cancer in younger patients included patients ages 40 to 49 years, and therefore are more likely to be biased by screening.11,12 A recent study of 1854 patients ages 40 to 55 years found that 11.6% had a family history that warranted screening before age 50 years.13
One reason for the selection of our study population was that it is a group that is not impacted by screening. The most recent colorectal screening guidelines developed by the American College of Gastroenterology recommend screening to begin at age 50 years for the average-risk population or age 45 years for African Americans.14 The only groups who are recommended to be screened earlier and more frequently are those with a positive family history of colorectal cancer (who are to be screened 10 years earlier than the age at diagnosis of the youngest affected relative) and patients with familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (HNPCC).14
It is unlikely that the increase in the incidence in rectal and rectosigmoid cancer that we have observed is related to patients with FAP or HNPCC. Patients with a histology identified as adenocarcinoma in adenomatous polyposis coli comprised only 0.83% of the total population in the current study. In addition, although these patients develop CRC early, patients with HNPCC are known to have a greater proportion of right-sided colon lesions, with fewer cancers diagnosed in the rectum.15 FAP patients are also not known to develop a preponderance of cancers in the rectum.15 Because we did not observe the incidence of colon cancer rising, this is not a satisfactory explanation.
Another possible explanation for the increase in the incidence of rectal and rectosigmoid cancer that we observed is the increased availability of colonoscopy for diagnosis. Although colonoscopy was first performed in 1969, it was not widely available for approximately another 10 years. In addition, colon cancer and colonoscopic screening were widely discussed in the news media in 1985 in relation to the colon cancer diagnosis of President Ronald Reagan.16 However, the incidence of rectal cancer continued to rise, with the slope of increase unchanged, long after colonoscopy was widely available, which suggests that this does not explain the change we are reporting. In addition, sigmoidoscopy was available long before colonoscopy was popularized; therefore, with the increasing use of colonoscopy, one would predict an increase in colon cancers relative to rectal cancers.17 Yet this is not what we observed.
Many etiologic factors have been identified that increase the risk of colorectal cancer.18 Although others have implicated lifestyle factors in the increased incidence we are reporting, a thorough review of the literature did not reveal any cause of rectal or rectosigmoid cancer independent of colon cancer. A study by Birkenkamp-Demtroder et al using microarray analysis demonstrated a small number of differences in the genetic expression of right-sided colon cancers compared with sigmoid and rectal cancers. Immunohistochemistry confirmed an increase in cyclooxygenase 2 and a decrease in cytokeratin 20 in left-sided cancers.19 However, the clinical implications of this study are not yet clear.
A recent pathologic study compared tissue samples from patients aged > and <40 years with colorectal cancer.20 Approximately 58% of the patients aged <40 years were diagnosed with rectal cancer. Increased lymphovascular and venous invasion were noted in the tumors of younger patients, with an increased infiltrative growth pattern. In addition, there was an increase of α-methyl-acyl-CoA racemase expression noted, and several micro-RNAs were found to be more highly expressed. The authors postulated that post-translational regulation of RNA may be important in the development of colorectal cancer in younger patients.
Although these pathologic studies have demonstrated some novel differences in protein and microRNA expression in patient populations similar to that in which we observed an increase in rectal cancer, to the best of our knowledge no clear etiology has been elucidated to date. Further work may include an attempt at the identification of a specific geographic region or ethnic group that is particularly strongly affected. In addition, there may be an interaction between environmental factors and the changed genetics of a particular population in the context of the shifting demographics within the United States.
Several important limitations of this study exist. First, the accuracy of coding in the SEER database cannot be independently verified. Second, information regarding family history or the clinical diagnosis of FAP or HNPCC is not captured in the database. This would be helpful in ruling out this population a source of our increased incidence. Third, there is no standardized definition of subsites such as “rectum” or “rectosigmoid junction”; the interpretation is left up to the individual physician. Finally, there have been significant changes in the geographic regions participating in the SEER registry since its inception, with significant expansions within the last 10 years. This allows the registry to more accurately reflect the diversity of the United States population as a whole; however, it complicates the longitudinal analysis of the data.
The importance of the timely diagnosis of younger patients with rectal cancer is demonstrated by several studies examining outcomes in these patients. Younger patients tend to present with more advanced disease, and their overall survival has been reported to be inferior to that of older patients.21-26 This may be because of a delay in diagnosis. The common presenting signs and symptoms of colorectal cancer are rectal bleeding, a change in bowel habits, anemia, weight loss, and diarrhea.27 In a younger patient, any 1 of these symptoms may not trigger the patient’s physician to include malignancy in the differential diagnosis. The most common presenting symptom, rectal bleeding, often may be attributed to the exceedingly common clinical diagnosis of hemorrhoids.27,28 Therefore, endoscopic examination may be deferred. This bias in younger patients may explain why the mean time from the onset of symptoms to diagnosis has been reported to be approximately 7 months in studies of colorectal cancer occurring in patients aged <40 years.26,29
In the current study, we demonstrated that young patients are developing rectal and rectosigmoid cancer at an increasing rate. Although these rates are not high enough to warrant a change in current screening guidelines, we suggest strong consideration of the endoscopic evaluation of young patients presenting with rectal bleeding or other common signs or symptoms of rectal or rectosigmoid cancer. Although colonoscopy may not be warranted, we suggest that flexible sigmoidoscopy, at a minimum, should be performed in this setting to rule out rectal or rectosigmoid cancer.
Acknowledgments
We thank Ira M. Jacobson, MD, for insightful comments on this article.
CONFLICT OF INTEREST DISCLOSURES
Dr. Paul Christos was partially supported by the following grant: Clinical Translational Science Center (CTSC) UL1-RR024996.
References
- 1.Jemal A, Siegal R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Pignone M, Rich M, Teutch SM, et al. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell JB, Maggard M, Liu J, et al. Rates of colon and rectal cancer are increasing in young adults. Am Surg. 2003;69:866–872. [PubMed] [Google Scholar]
- 4.Peters R, Garabrant DH, Yu MC, et al. A case-control study of occupational and dietary factors in colorectal cancer in young men by subsite. Cancer Res. 1989;49:5459–5468. [PubMed] [Google Scholar]
- 5.Byers T, Nestle M, McTiernan A, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2002;52:92–119. doi: 10.3322/canjclin.52.2.92. [DOI] [PubMed] [Google Scholar]
- 6.Wynder EL, Reddy BS. The epidemiology of cancer of the large bowel. Am J Dig Dis. 1975;19:443–448. doi: 10.1007/BF01076220. [DOI] [PubMed] [Google Scholar]
- 7.Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28:3–13. doi: 10.1002/1097-0142(197107)28:1<3::aid-cncr2820280104>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 9.Hall NR, Finan PJ, Ward B, Turner G, Bishop DT. Genetic susceptibility to colorectal cancer in patients under 45 years of age. Br J Surg. 1994;81:1485–1489. doi: 10.1002/bjs.1800811029. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–559. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 11.Cress RD, Morris C, Ellison GL, Goodman MT. Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992-2001. Cancer. 2006;107(suppl 5):1142–1152. doi: 10.1002/cncr.22011. [DOI] [PubMed] [Google Scholar]
- 12.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1695–1698. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher RH, Lobb R, Bauer MR, et al. Screening patients with a family history of colorectal cancer. J Gen Intern Med. 2007;22:508–513. doi: 10.1007/s11606-007-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009. Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. corrected. [DOI] [PubMed] [Google Scholar]
- 15.Lynch PM. Prevention of colorectal cancer in high-risk populations: the increasing role for endoscopy and chemoprevention in FAP and HNPCC. Digestion. 2007;76:68–76. doi: 10.1159/000108395. [DOI] [PubMed] [Google Scholar]
- 16.Wolff WI. Colonoscopy: history and development. Am J Gastroenterol. 1989;84:1017–1025. [PubMed] [Google Scholar]
- 17.Overholt BF. Clinical experience with the fibersigmoidoscope. Gastrointest Endosc. 1968;15:27–30. [PubMed] [Google Scholar]
- 18.Martinez ME. Primary prevention of colorectal cancer: lifestyle, nutrition, exercise. Recent Results Cancer Res. 2005;166:177–211. doi: 10.1007/3-540-26980-0_13. [DOI] [PubMed] [Google Scholar]
- 19.Birkenkamp-Demtroder K, Olesen SH, Sorensen FB, et al. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut. 2005;54:374–384. doi: 10.1136/gut.2003.036848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yantiss RK, Goodarzi M, Zhou XK, et al. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am J Surg Pathol. 2009;33:572–582. doi: 10.1097/PAS.0b013e31818afd6b. [DOI] [PubMed] [Google Scholar]
- 21.Endreseth BH, Romundstad P, Myrvold HE, Hestvik UE, Bjerkeset T, Wibe A Norwegian Rectal Cancer Group. Rectal cancer in the young patient. Dis Colon Rectum. 2006;49:993–1001. doi: 10.1007/s10350-006-0558-6. [DOI] [PubMed] [Google Scholar]
- 22.Mcgoy GF, Parkes TG. Colorectal carcinoma in young patients. A 20-year retrospective review. J R Coll Surg Edinb. 1984;29:129–133. [PubMed] [Google Scholar]
- 23.D’Onofrio GM, Tan EG. Is colorectal carcinoma in the young a more deadly disease? Aust N Z J Surg. 1985;55:537–540. doi: 10.1111/j.1445-2197.1985.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 24.Palmer ML, Herrera L, Petrelli NJ. Colorectal adenocarcinoma in patients less than 40 years of age. Dis Colon Rectum. 1991;34:343–345. doi: 10.1007/BF02050596. [DOI] [PubMed] [Google Scholar]
- 25.Marble K, Banerjee S, Greenwald L. Colorectal carcinoma in young patients. J Surg Oncol. 1992;51:179–182. doi: 10.1002/jso.2930510311. [DOI] [PubMed] [Google Scholar]
- 26.Minardi AJ, Sittig KM, Zibari GB, McDonald JC. Colorectal cancer in the young patient. Am Surg. 1998;64:849–853. [PubMed] [Google Scholar]
- 27.Ford AC, Vedhuyzen van Zanten SJO, Rodgers CC, Talley NJ, Vakil NB, Moayyedi P. Diagnostic utility of alarm features for colorectal cancer: systematic review and meta-analysis. Gut. 2008;57:1545–1553. doi: 10.1136/gut.2008.159723. [DOI] [PubMed] [Google Scholar]
- 28.Alonso-Coello P, Mills E, Heels-Ansdell D, et al. Fiber for the treatment of hemorrhoids complications: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:181–188. doi: 10.1111/j.1572-0241.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 29.Parramore JB, Wei JP, Yeh KA. Colorectal cancer in patients under forty: presentation and outcome. Am Surg. 1998;64:563–568. [PubMed] [Google Scholar]


