Abstract
Background
Chronic granulomatous disease is a rare immunodeficiency complicated by dysregulated inflammation and granulomatous complications of the gastrointestinal tract. The management of chronic granulomatous disease colitis presents the dilemma of an immunocompromised host requiring immunosuppressive therapy which can potentiate fatal infections.
Objective
To identify the types of gastrointestinal surgery performed in patients and determine the role of surgery in the management of refractory colitis.
Design and Settings
A retrospective single institution chart review was performed.
Patients
Of 268 patients with chronic granulomatous disease treated at the National Institutes of Health between 1985 and 2011, 98 (37%) were identified as having colitis; 27 (10%) had a history of gastrointestinal luminal surgery.
Main outcome measures
Patient characteristics, type of gastrointestinal surgery and clinical outcomes were documented.
Results
A total of 62 gastrointestinal luminal surgeries were performed in 27 patients with chronic granulomatous disease and colitis. All 27 had a history of perineal disease requiring intervention. Four (15%) had additional surgery performed for reasons other than colitis. Otherwise, 12 (44%) had surgery limited to the perineum, 2 (7%) had a segmental resection and 13 (48%) underwent fecal diversion with ileostomy or colostomy. Despite local procedures, 7 (58%) patients in the perineal only group remained symptomatic. Both patients with a segmental resection had persistent perineal disease and 1 had a recurrent colovesicular fistula. Of the 13 ostomy patients, 11 initially received a diverting ostomy. Eight (73%) of these ultimately required additional procedures for refractory disease and 4 (36%) developed peristomal pyoderma gangrenosum. Four patients who underwent proctocolectomy with end ileostomy, either initially (2) or as a definitive procedure (2), experienced resolution of colitis and perineal disease.
Limitations
This study is limited by its retrospective design, small sample size and highly selected patient population.
Conclusions
Proctocolectomy with end ileostomy may offer a definitive treatment in a patient with refractory chronic granulomatous disease colitis given current therapeutic limitations.
Keywords: chronic granulomatous disease, colitis, surgical management
Introduction
Chronic granulomatous disease (CGD) is a rare congenital immunodeficiency affecting approximately 1 in every 200,000 persons. It has X-linked (65%) and autosomal recessive modes of inheritance, resulting in heterogeneous phenotype and clinical presentations.1 CGD is caused by mutations affecting the NAPDH oxidase system, which results in inability to produce superoxide and hydrogen peroxide radicals required to kill certain bacteria and fungi.2–3 As a result, patients suffer from severe bacterial and fungal infections as well as excessive inflammation.3
This abnormal inflammatory response can lead to tissue granuloma formation and development of chronic inflammatory disease even in the absence of active infection.1 While involvement of any hollow viscera is possible, the gastrointestinal (GI) tract is the most common site and is affected in up to 50% of patients.2, 4–8 Patients can present with inflammation anywhere from the mouth to the anus characterized by ulcers, abscesses, fistulae and strictures leading to obstruction.9–11 An inflammatory granulomatous colitis (CGD colitis) resembling inflammatory bowel disease (IBD) is reported in 15–44%1–2, 5, 8, 10, 12–15 of patients, with a greater propensity in X-linked inherited disease.1–2 Additionally, perineal involvement, such as fistula-in-ano and abscess formation can occur in up to 100% of patients with CGD colitis.3, 7–8, 10
The exact pathophysiology of CGD associated colitis is not well understood. It is hypothesized that chronic antigenic stimulation from organisms not readily killed by defective CGD phagocytes leads to granuloma formation and bowel wall thickening.16–17 In the absence of infection, the inability of phagocytes to degrade chemotactic factors may result in excessive inflammation.12, 18 Despite clinical similarities, the mechanism of inflammation, profile of inflammatory mediators and histologic features differ between IBD and CGD.6, 19–20
The management of CGD colitis has not been clearly defined in the literature. A dilemma exists between the immunocompromised host and the immunosuppressive agents conventionally used to control excessive inflammation which may potentiate fatal infections.21 We sought to determine the types of GI luminal surgeries in patients with CGD and concurrent colitis, and to examine the role of surgery in the management of refractory CGD colitis.
Materials and Methods
A retrospective review identified 268 patients with CGD treated on various research protocols at the National Institutes of Health (NIH) from 1985 to January 2011. Of these patients, 98 (37%) had documented colitis. Twenty-seven (10% of all CGD patients, 28% of patients with colitis) had a history of both CGD colitis and GI luminal surgery either at the NIH or at a referral institution. A retrospective chart review was performed on this cohort of patients.
Patient medical records were evaluated for patient characteristics, GI involvement, previous medical therapy, type of GI luminal surgery and clinical outcome after surgical intervention. For the purpose of this review, GI involvement was defined as abdominal pain (persistent and unrelated to other causes), diarrhea (persistent and without an infectious etiology), constipation, obstipation, perineal disease or CGD colitis as documented on colonoscopy or pathologic examination. All other GI involvement from proven causes, such as gastroenteritis or infectious colitis were excluded.
Only GI luminal surgeries which pertained to the patient’s history of CGD were included. GI luminal surgery was defined as surgery involving any part of the continuous gastrointestinal luminal tract including the esophagus, stomach, small intestine, appendix, colon, rectum or anus. Any surgery performed on the biliary tract, liver or pancreas was excluded. Additionally, we excluded exploratory laparotomy and lysis of adhesions for bowel obstruction. Finally, perineal disease was defined as documented abscess, fistula, fissure or anal stenosis. Procedures for perineal disease were counted as a single surgical intervention, as these procedures can be performed in the emergency department, operating room or endoscopy suite, therefore preventing an accurate tally of the actual number of procedures performed. We excluded patients with rectal pain or perineal erythema without specific clinical or endoscopic diagnosis, as diarrhea is a common manifestation in this population and could lead to these findings.
All patients signed an institutional review board approved consent form for tissue procurement and participation in subsequent protocols. All patients had the diagnosis of CGD confirmed either by nitroblue tetrazolium reduction or dihydrorhodamine oxidation. The specific gene defect was determined by immunoblotting or sequencing or both. When available, data prior to the initial NIH visit were also collected.
Results
Patient characteristics
Twenty-seven patients with CGD and related colitis had a history of GI luminal surgery (Table 1). An additional 4 patients without colitis were identified and had undergone 5 GI luminal surgeries for other reasons including 2 appendectomies and 1 each of nissen procedure, gastric pull-up, colectomy for volvulus and small bowel resection for CGD diagnosis during infancy.
TABLE 1.
Characteristics of patients with CGD and GI manifestations requiring surgery
| Value | n | % |
|---|---|---|
| Total no. of patients in database | 268 | |
| No. of patients with GI manifestations | 98 | 37 |
| No. of patients with GI luminal surgery | 31 | 12 |
| No. of patients with colitis + GI luminal surgery | 27 | 10 |
| Sex | ||
| Male | 25 | 93 |
| Female | 2 | 7 |
| Ethnicity | ||
| White | 25 | 93 |
| Hispanic | 1 | 4 |
| African American | 1 | 4 |
| Family history | 25 | 93 |
| X-linked | 20 | 80 |
| Autosomal recessive | 4 | 16 |
| Lionized carrier | 1 | 4 |
| Previous treatment | ||
| Antibiotics | 27 | 100 |
| Antifungals | 16 | 59 |
| Steroids | 19 | 70 |
| 5-ASA (Asacol, Pentasa, mesalamine) | 14 | 52 |
| Sulfasalazine | 1 | 4 |
| Promotility agents | 1 | 4 |
| Antisecretory agents | 2 | 7 |
| Immunomodulator | ||
| IFN-γ | 15 | 56 |
| 6-MP | 10 | 37 |
| Remicade | 6 | 22 |
| Imuran | 3 | 11 |
| Adalimumab | 3 | 11 |
CGD = chronic granulomatous disease; 5-ASA = 5-aminosalicylic acid; IFN-γ = interferon-γ; 6-MP = 6-mercaptopurine.
Of the 27 surgical patients with colitis, 25 (93%) were male and 25 (93%) had a family history of CGD, with 21 (96%) exhibiting the more common X-linked inheritance. All patients had received prior systemic medical treatment before undergoing surgical intervention. The most common treatments were antibiotics (100%), steroids (73%), IFN gamma (64%) and antifungal therapy (45%). Additional agents included 5-ASA, sulfasalazine, pro-motility agents, anti-secretory agents and other immunomodulator regimens including 6MP, remicade, imuran and adalimumab. In all, 7% received 1 previous treatment regimen, 18% received 2 different regimens and 75% received 3 or more regimens.
The length of follow-up for patients varied from 1 to 265 months (average 101 months), with 2 patients lost to follow-up after a single assessment. For both patients lost to follow-up, clinical outcomes were assessed since surgical intervention had occurred 5 and 228 months previously.
Surgical interventions and outcomes
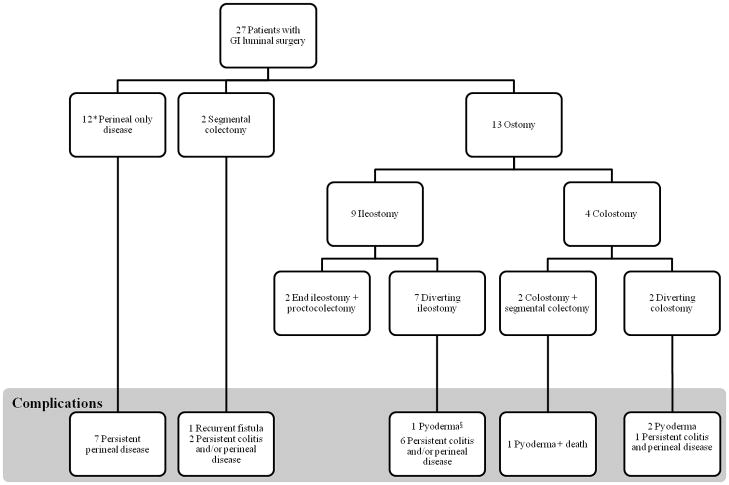
A total of 62 GI luminal surgeries were performed in 27 patients (Table 2). Four (14%) had additional procedures for reasons other than management of colitis. These included small bowel resection for obstruction secondary to CGD mycosis and inflammation during infancy (2), colectomy for iatrogenic bowel perforation during drainage of an intrahepatic abscess and concomitant colon resection to treat a mesenteric abscess. All other procedures were performed for the management of CGD colitis. Of the 27 patients, 12 (44%) had surgery limited only to the perineum, 2 (7%) had a history of segmental resection without an ostomy, and 13 (48%) underwent fecal diversion with creation of an ileostomy or colostomy for management of perineal disease or severe colitis refractory to medical management (Figure 1).
TABLE 2.
Types of initial GI luminal surgery in 27 patients with CGD and colitis
| Value | n | % |
|---|---|---|
| No. of patients | 27 | 10 |
| Total no. of GI luminal surgeriesa | 62 | |
| No. of patients with perineal diseaseb | 27 | 100 |
| No. of patients with additional surgery unrelated to CGD colitis | 4 | 15 |
| Mycotic intestinal obstruction | 2 | |
| Iatrogenic bowel perforation | 1 | |
| Mesenteric abscess | 1 | |
| No. of patients with perineal only surgery | 12 | 44 |
| Anal stenosis | 4 | |
| Abscess, fistula, or fissure | 7 | |
| Both stenosis and fissure | 1 | |
| No. of patients with segmental resection | 2 | 7 |
| Low anterior resection (CVF) | 1 | |
| Colectomy | 1 | |
| No. of patients with fecal diversion | 13 | 48 |
| Ileostomy | 9 | |
| Without bowel resection | 7 | |
| With bowel resection | 2 | |
| Colostomy | 4 | |
| Without bowel resection | 2 | |
| With bowel resection | 2 |
CVF = colovesicular fistula; CGD = chronic granulomatous disease.
Many patients required multiple surgeries for persistent symptoms in the management of CGD colitis.
All 27 patients required at least 1 intervention for perineal disease.
Figure 1. Schematic of refractory disease in 27 patients with CGD colitis and GI luminal surgery.
*1 patient lost to follow-up.
§Pyoderma; peristomal pyoderma gangrenosum.
Twelve patients with surgery limited only to the perineum underwent procedures including incision and drainage of abscess, seton placement for complicated fistula or rectal dilation for management of a stricture or fissure. Four (33%) of the 12 patients had resolution of perineal pathology after initial intervention. This included 2 patients with successful rectal dilatation and 2 patients with successful healing of perineal fistula. The remaining 7 (58%) patients experienced refractory disease following surgery. Two patients had persistent perineal fistula, 2 had recurrent anal stricture, 1 had both fistula and stricture and 2 had persistent fissures. One patient did not have adequate follow-up.
For the 2 patients who had resection without an ostomy, 1 underwent a low anterior resection for a colovesicular fistula and another a segmental colectomy for colitis. Both of these patients continued to experience active perineal disease after resection as well as recurrence of the colovesicular fistula in the patient described.
The remaining 13 patients had fecal diversion as part of their operative strategy including 9 (69%) with an ileostomy and 4 (31%) with a colostomy. Two (22%) of the 9 patients had an end ileostomy combined with a total proctocolectomy at the initial procedure. Both experienced complete resolution of perineal disease after colectomy and did not require further intervention. The remaining 7 (78%) patients had a diverting ileostomy with only 1 (14%) experiencing improvement of colitis and perineal disease at last follow-up. The other 6 (86%) patients have all experienced refractory symptoms requiring additional intervention such as segmental colonic resection, multiple perineal procedures, conversion of ileostomy to colostomy and/or eventual total proctocolectomy. In addition, 1 patient developed peristomal pyoderma gangrenosum. Despite alternative management strategies, every patient has remained symptomatic at last follow-up with the exception of the patient who eventually underwent a total proctocolectomy with end ileostomy. This patient subsequently had resolution of colitis and perineal disease.
There were 4 patients who initially received a colostomy for the management of active CGD colitis. Three (75%) of these patients developed peristomal pyoderma gangrenosum and 2 (50%) ultimately required additional surgical procedures. One patient had an initial diverting colostomy converted to an ileostomy due to the development of peristomal fistula and pyoderma gangrenosum. Severe perineal disease requiring frequent anal dilatations resulted in an iatrogenic rectal perforation managed with a diverting colostomy. Ultimately, this patient received an abdominoperineal resection with end ileostomy for refractory colitis and anal stenosis. Resolution of all disease has occurred after resection. The second patient initially underwent a Hartman’s procedure and subsequently developed pyoderma gangrenosum around the colostomy site necessitating revision. Escalated immunosuppressive therapy for treatment of pyoderma precipitated confounding CMV colitis and development of fulminant colitis, septic shock and death despite emergent subtotal colectomy.
Of note, all 27 patients with CGD and colitis had a history of perineal inflammatory disease consisting of fistula, abscess, fissure or stenosis requiring intervention. Although perineal procedures were often multiple and performed over several years, each was counted as a single procedure for the purpose of this analysis. Sixteen (59%) patients required dilation for severe anal stenosis. Six (38%) of these 16 patients required multiple dilatations to prevent colonic obstruction.
Discussion
CGD is a rare immunodeficiency resulting in recurrent infections, chronic inflammation and granulomatous complications which occur mostly in the GI tract. Inflammatory GI involvement resembling Crohn’s colitis has been reported.1–2, 5, 8, 10, 14–15 Yet, therapies successful in the treatment of Crohn’s have had varying results when utilized for the management of CGD colitis.22–24 In this investigation, 27 patients with GI luminal surgery had refractory disease which failed medical management prior to requiring surgical intervention. This group, representing nearly one-third of all patients with CGD colitis, are a unique cohort requiring alternative management strategies.
From this analysis, it is apparent that CGD colitis can result in a chronic disease process. While we found that perineal manifestations can be temporized with local procedures, most patients remained symptomatic and were unlikely to experience resolution of a fistula with either fecal diversion or drainage. In the group of patients with perineal only disease, 7 (58%) of the 12 patients remain symptomatic at last follow-up despite intervention (Figure 1). Similarly, of the 15 patients with a segmental resection or ostomy, only 4 (27%) experienced resolution of colitis and perineal disease, 2 of which initially underwent proctocolectomy and end ileostomy.
Interestingly, all 27 patients who had surgery for CGD colitis had a history of perineal inflammatory disease necessitating intervention. Previous literature has suggested that CGD colitis increases in likelihood from the proximal to distal colon.25 Therefore, perineal disease may provide a first indication of colitis which could ultimately require surgery.
Fecal diversion was frequently used to temporize inflammation and control infection. While morbidity after diverting loop ileostomy was low, 6 (86%) of 7 patients required numerous additional surgeries without resolution of disease. Additionally, 3 (75%) of 4 patients with colostomies developed peristomal pyoderma gangrenosum and 2 (50%) experienced further complications necessitating intervention. Despite this, the use of fecal diversion to temporarily reduce inflammation and infection is well accepted for the management of colitis, irrespective of the etiology. We found that fecal diversion with an ileostomy was superior to colostomy for acute management of CGD colitis. Yet, it is paramount to recognize that fecal diversion alone does not offer definitive treatment. In all, use of a diverting ostomy led to an additional surgical procedure in 8 (73%) out of 11 patients (Figure 1).
Four (15%) of the 27 patients received a total proctocolectomy with end ileostomy or abdominoperineal resection as an initial (2) or definitive strategy (2). Every single one of these patients experienced complete resolution of CGD colitis and perineal disease after resection. While based on limited experience, these findings suggest that refractory CGD colitis may be successfully managed with proctocolectomy and end ileostomy. This is an important observation since escalating immunosuppressive therapy can precipitate fatal complications, as seen in the patient who developed CMV colitis and death.15
Reluctance to perform proctocolectomy and end ileostomy in CGD patients may exist. However, in the setting of uncontrolled disease precipitating severe colitis and perineal sequelae, quality of life following proctocolectomy and end ileostomy with resolution of disease is markedly improved. The effect of proctocolectomy and end ileostomy on other CGD related inflammatory and infectious manifestations is currently under investigation.
Finally, no patients in our review had a history of CGD enteritis which required surgical intervention. While the abnormal inflammatory response in CGD can precipitate tissue granuloma formation in any part of the GI tract, it is unusual in the small bowel and when present rarely necessitates intervention.25 In fact, we found that patients with end ileostomy fared better overall, suggesting that total proctocolectomy combined with ileoanal reservoir might serve as an additional surgical option in these patients, and is an interest in future investigations.
Potential limitations of this study include the retrospective nature of data analysis and patient selection bias. Patients referred to the NIH to receive treatment under an approved protocol are a highly selected group who by nature of referral may experience increased disease severity. Additionally, while this is the largest review in the literature, there is inherent bias associated with a small patient sample size. Finally, because a significant number of surgical procedures were performed at outside institutions, specific characteristics related to operative strategy, perioperative outcomes and postoperative morbidity could not be delineated.
In summary, patients with active CGD colitis should be monitored closely for the presence of perineal manifestations. Conversely, patients with perineal disease should be evaluated for sub-clinical CGD colitis. Perineal disease should be initially managed with local procedures such as seton drainage or anal dilation, while simultaneously optimizing medical therapy. In our experience, perineal fistula associated with CGD colitis eventually recurred if left undrained, thereby challenging the practice of electively removing a properly placed seton. In the setting of significant inflammation or persistent infection, a temporary diverting loop ileostomy can offer an alternative strategy to immediate surgical resection. Ultimately, the decision to proceed to proctocolectomy and end ileostomy should be based on the presence or absence of extensive strictures, the ability to control complications, and the patient’s quality of life and preference.
Acknowledgments
Source of Funding: Intramural Research Programs of the National Cancer Institute, National Institute of Allergy and Infectious Diseases, Clinical Center and National Institute of Diabetes and Digestive and Kidney Diseases.
The authors thank John I. Gallin, M.D. for his dedicated efforts to develop a center for chronic granulomatous disease at the National Cancer Institute. Without his continued support and expertise, the ability to work with this unique patient population would not be possible.
Footnotes
Conflicts of Interest: None declared.
The abstract of this manuscript was presented as a poster presentation at The American Society of Colon and Rectal Surgeons, San Antonio, TX, June 2 to 6, 2012.
Authorship contribution:
- MMD: Manuscript conception and design, acquisition of data, analysis and interpretation of data, critical drafting and revision of the manuscript with final approval of the version to be published.
- NK: Acquisition of data, analysis and interpretation of data, revision of the manuscript and final approval of the version to be published.
- SMI: Acquisition of data, analysis and interpretation of data, critical drafting and revision of the manuscript with final approval of the version to be published.
- SKK: Acquisition of data, analysis and interpretation of data, critical drafting and revision of the manuscript with final approval of the version to be published.
- HLM: Manuscript conception, acquisition of data, critical drafting and revision of the manuscript and final approval of the version to be published.
- SMH: Manuscript conception, acquisition of data, critical drafting and revision of the manuscript and final approval of the version to be published.
- gMSH: Manuscript conception, acquisition of data, critical drafting and revision of the manuscript and final approval of the version to be published.
- TH: Manuscript conception and design, analysis and interpretation of data, critical drafting and revision of the manuscript with final approval of the version to be published.
- RMS: Manuscript conception and design, analysis and interpretation of data, critical drafting and revision of the manuscript with final approval of the version to be published.
References
- 1.Winkelstein JA, Marino MC, Johnston RB, Jr, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Marciano BE, Rosenzweig SD, Kleiner DE, et al. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114:462–468. doi: 10.1542/peds.114.2.462. [DOI] [PubMed] [Google Scholar]
- 3.Segal BH, Veys P, Malech H, Cowan MJ. Chronic granulomatous disease: lessons from a rare disorder. Biol Blood Marrow Transplant. 2011;17:S123–131. doi: 10.1016/j.bbmt.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer A, Segal AW, Seger R, Weening RS. The management of chronic granulomatous disease. Eur J Pediatr. 1993;152:896–899. doi: 10.1007/BF01957525. [DOI] [PubMed] [Google Scholar]
- 5.Ament ME, Ochs HD. Gastrointestinal manifestations of chronic granulomatous disease. N Engl J Med. 1973;288:382–387. doi: 10.1056/NEJM197302222880802. [DOI] [PubMed] [Google Scholar]
- 6.Schappi MG, Klein NJ, Lindley KJ, et al. The nature of colitis in chronic granulomatous disease. J Pediatr Gastroenterol Nutr. 2003;36:623–631. doi: 10.1097/00005176-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Mulholland MW, Delaney JP, Simmons RL. Gastrointestinal complications of chronic granulomatous disease: surgical implications. Surgery. 1983;94:569–575. [PubMed] [Google Scholar]
- 8.Marks DJ, Miyagi K, Rahman FZ, Novelli M, Bloom SL, Segal AW. Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn’s disease. Am J Gastroenterol. 2009;104:117–124. doi: 10.1038/ajg.2008.72. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz JF, Aronow E, Rausen AR, Aiges H, Silverberg M, Daum F. Progressive esophageal dysfunction in chronic granulomatous disease. J Pediatr Gastroenterol Nutr. 1982;1:145–149. doi: 10.1097/00005176-198201010-00024. [DOI] [PubMed] [Google Scholar]
- 10.Barton LL, Moussa SL, Villar RG, Hulett RL. Gastrointestinal complications of chronic granulomatous disease: case report and literature review. Clin Pediatr (Phila) 1998;37:231–236. doi: 10.1177/000992289803700403. [DOI] [PubMed] [Google Scholar]
- 11.Gopal L, Forbes J, Uzel G, Holland SM, Heller T. Gastrointestinal fistulae in chronic granulomatous disease. Am J Gastroenterol. 2009;104:2112–2113. doi: 10.1038/ajg.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seger RA. Modern management of chronic granulomatous disease. Br J Haematol. 2008;140:255–266. doi: 10.1111/j.1365-2141.2007.06880.x. [DOI] [PubMed] [Google Scholar]
- 13.Seger RA. Advances in the diagnosis and treatment of chronic granulomatous disease. Curr Opin Hematol. 2010 doi: 10.1097/MOH.0b013e32834115e7. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs D, Wright VM, Shaw DG, Raafat F, Walker-Smith JA. Chronic granulomatous disease mimicking Crohn’s disease. J Pediatr Gastroenterol Nutr. 1985;4:498–501. doi: 10.1097/00005176-198506000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Arimura Y, Goto A, Yamashita K, et al. Intractable colitis associated with chronic granulomatous disease. J Med Microbiol. 2006;55:1587–1590. doi: 10.1099/jmm.0.46722-0. [DOI] [PubMed] [Google Scholar]
- 16.Huang A, Abbasakoor F, Vaizey CJ. Gastrointestinal manifestations of chronic granulomatous disease. Colorectal Dis. 2006;8:637–644. doi: 10.1111/j.1463-1318.2006.01030.x. [DOI] [PubMed] [Google Scholar]
- 17.Rosh JR, Tang HB, Mayer L, Groisman G, Abraham SK, Prince A. Treatment of intractable gastrointestinal manifestations of chronic granulomatous disease with cyclosporine. J Pediatr. 1995;126:143–145. doi: 10.1016/s0022-3476(95)70519-8. [DOI] [PubMed] [Google Scholar]
- 18.Hamasaki T, Sakano T, Kobayashi M, Sakura N, Ueda K, Usui T. Leukotriene B4 metabolism in neutrophils of patients with chronic granulomatous disease: phorbol myristate acetate decreases endogenous leukotriene B4 via NADPH oxidase-dependent mechanism. Eur J Clin Invest. 1989;19:404–411. doi: 10.1111/j.1365-2362.1989.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 19.Schappi MG, Smith VV, Goldblatt D, Lindley KJ, Milla PJ. Colitis in chronic granulomatous disease. Arch Dis Child. 2001;84:147–151. doi: 10.1136/adc.84.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitomi H, Mikami T, Takahashi H, et al. Colitis in chronic granulomatous disease resembling Crohn’s disease: comparative analysis of CD68-positive cells between two disease entities. Dig Dis Sci. 1999;44:452–456. doi: 10.1023/a:1026643609944. [DOI] [PubMed] [Google Scholar]
- 21.Uzel G, Orange JS, Poliak N, Marciano BE, Heller T, Holland SM. Complications of tumor necrosis factor-alpha blockade in chronic granulomatous disease-related colitis. Clin Infect Dis. 2010;51:1429–1434. doi: 10.1086/657308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. The International Chronic Granulomatous Disease Cooperative Study Group. N Engl J Med. 1991;324:509–516. doi: 10.1056/NEJM199102213240801. [DOI] [PubMed] [Google Scholar]
- 23.Margolis DM, Melnick DA, Alling DW, Gallin JI. Trimethoprim-sulfamethoxazole prophylaxis in the management of chronic granulomatous disease. J Infect Dis. 1990;162:723–726. doi: 10.1093/infdis/162.3.723. [DOI] [PubMed] [Google Scholar]
- 24.Gallin JI, Alling DW, Malech HL, et al. Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med. 2003;348:2416–2422. doi: 10.1056/NEJMoa021931. [DOI] [PubMed] [Google Scholar]
- 25.Khangura SKKN, Quezado M, Zhao X, Marciano B, Simpson J, Yao M, Khuns DB, Gallin JI, Malech HL, Holland SM, Heller T. American College of Gastroenterology. Washington, DC: 2011. Endoscopic Features of Gastrointestinal Disease in Chronic Granulomatous Disease: A Distinct Entity. [Google Scholar]