Abstract
The RAD51 paralogs XRCC3 and RAD51C have been implicated in homologous recombination (HR) and DNA damage responses. However, the molecular mechanism(s) by which these paralogs regulate HR and DNA damage signaling remains obscure. Here, we show that an SQ motif serine 225 in XRCC3 is phosphorylated by ATR kinase in an ATM signaling pathway. We find that RAD51C but not XRCC2 is essential for XRCC3 phosphorylation, and this modification follows end resection and is specific to S and G2 phases. XRCC3 phosphorylation is required for chromatin loading of RAD51 and HR-mediated repair of double-strand breaks (DSBs). Notably, in response to DSBs, XRCC3 participates in the intra-S-phase checkpoint following its phosphorylation and in the G2/M checkpoint independently of its phosphorylation. Strikingly, we find that XRCC3 distinctly regulates recovery of stalled and collapsed replication forks such that phosphorylation is required for the HR-mediated recovery of collapsed replication forks but is dispensable for the restart of stalled replication forks. Together, these findings suggest that XRCC3 is a new player in the ATM/ATR-induced DNA damage responses to control checkpoint and HR-mediated repair.
INTRODUCTION
The genome of every living organism is susceptible to various types of DNA damage. Cells have evolved with DNA damage surveillance mechanisms to activate cell cycle checkpoints and DNA repair processes to preserve the integrity of the genome (1–6). The DNA damage response (DDR) pathways are primarily regulated by ataxia telangiectasia mutated (ATM) and ATM- and Rad3-related (ATR) kinases, which are members of the phosphatidylinositol 3-kinase-like kinase (PIKK) family. ATM is recruited to double-strand breaks (DSBs) in part by the MRN (MRE11-RAD50-NBS1) complex, whereas ATR—with its regulator ATRIP (ATR-interacting protein)—senses replication protein A (RPA)-coated single-stranded DNA (ssDNA) that is generated from stalled replication forks or from the processing of DSBs (7–10). When activated, ATM and ATR phosphorylate various substrates at their serine and/or threonine residues within the conserved S/TQ motif, thereby inducing DDR to pause cell cycle progression and activate DNA repair processes (11).
The RAD51 recombinase plays a central role in the homologous recombination (HR)-mediated repair of DSBs, daughter-strand gaps (DSGs), and interstrand cross-links (ICLs) (10, 12). Mammals possess five RAD51 paralogs—RAD51B, RAD51C, RAD51D, XRCC2, and XRCC3—which have been implicated in RAD51-mediated HR. RAD51 paralog-deficient cells exhibit sensitivity to DNA damage, spontaneous chromosomal aberrations, reduced RAD51 nuclear focus formation, and decreased HR (12, 13). These paralogs act in the BRCA1/2 pathway to control HR (14, 15). The RAD51 paralogs differentially regulate both short- and long-tract gene conversions and have been implicated in the late stages of HR for resolving recombination intermediates (16, 17). In addition to HR, RAD51 paralogs have been implicated in chromosome segregation and in the prevention of aberrant mitosis and aneuploidy (18, 19). RAD51D plays a role in telomere maintenance (20), and XRCC3 promotes t-loop deletions at human telomeres (21–23). Recent studies indicate that RAD51 paralogs participate in DNA damage signaling, in the Fanconi anemia pathway of ICL repair, and in the prevention of tumorigenesis (12, 24–27). RAD51C binding partner XRCC3 serine 225 is an SQ motif residue and has been implicated as the ATM/ATR target substrate (28). Here, we report that XRCC3 S225 is phosphorylated in response to ionizing radiation (IR)-induced DNA damage via an ATM/ATR-mediated pathway and that this activation is essential for implementing the intra-S-phase checkpoint and HR-mediated DSB repair. Our analysis with respect to the G2/M checkpoint revealed a previously unknown function of XRCC3 in regulating this checkpoint. Interestingly, this function is independent of XRCC3 phosphorylation. Notably, we find that XRCC3 phosphorylation is dispensable for the rescue of stalled replication forks but is crucial for the recovery of collapsed replication forks.
MATERIALS AND METHODS
Cell lines, cell culture, and transfections.
HEK293T, HeLa, U2OS, U2OS SCR24, MCF7, skin fibroblast IBR3, A-T, and ATR Seckel cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37°C in humidified air containing 5% CO2. All plasmid transfections for stable and transient expression were performed using a Bio-Rad Gene Pulsar X cell (250 V and 950 μF). Small interfering RNA (siRNA) transfections were performed using Lipofectamine (Invitrogen) in accordance with the manufacturer's guidelines.
DNA constructs.
Design and generation of the HR/sister chromatid recombination (SCR) reporter and I-SceI expression vectors were reported previously (29). Hemagglutinin (HA)-tagged human XRCC3 (hXRCC3) wild-type (WT) and mutant constructs were generated using PCR-based mutagenesis and cloned into the pcDNA3β vector. hXRCC3, hRAD51C, and hXRCC2 short hairpin RNA (shRNA) constructs were generated using reported sequences (24) and cloned into the pRS shRNA vector.
DNA damage induction.
For IR treatment, the cells were trypsinized, resuspended in phosphate-buffered saline (PBS), and irradiated on ice using a 137Cs source at the indicated dose (dose rate, ∼3 Gy/min) for the indicated times. For drug treatment, the cells were incubated with various DNA-damaging agents and inhibitors at the indicated concentrations and times. Mock treatment was similar to the experimental conditions except that the cells were incubated in the respective solvent.
Cell synchronization and cell cycle analysis.
HeLa cells were synchronized at the G2/M phase by the addition of nocodazole. Exponentially growing HeLa cells were incubated in 150 ng/ml nocodazole for 14 h. The floating mitotic cells were then collected, washed with fresh medium, and then replated. The cells were collected after 1 h (predominantly M phase), 4 h (predominantly G1 phase), and 12 h (predominantly S/G2 phase). HeLa cells were synchronized in the G1/S phase by the addition of aphidicolin. Logarithmic-phase HeLa cells were treated with 1 μg/ml aphidicolin for 18 h. After 18 h, the cells were washed and released from the G1/S boundary in fresh medium. The cells were collected and processed for analyzing the cell cycle stages. Collected single-cell suspensions were fixed overnight with 70% ethanol in PBS at −20°C. After centrifugation, the cells were incubated with 0.10 mg/ml RNase A (Fermentas) in PBS at 42°C for 4 h and then incubated for 10 min with 50 μg/ml propidium iodide (PI) in the dark. A total of 1 × 104 cells were analyzed with a Canto flow cytometer (BD Biosciences). Aggregates were gated out, and percentages of cells with 2N and 4N DNA content were calculated using FACSDiva version 6.1.1 software (BD Biosciences).
Immunoprecipitation, Western blotting, and antibodies.
Cells were harvested and lysed in radioimmunoprecipitation assay (RIPA) buffer (without SDS) supplemented with a complete protease and phosphatase inhibitor cocktail (Roche). For the immunoprecipitation assays, cell lysates were incubated with an anti-HA polyclonal antibody (Santa Cruz) using protein A beads. The proteins were resolved on a 10% SDS-PAGE gel and transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore). The membranes were blocked using 3% bovine serum albumin (BSA) in TBST (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and incubated with primary antibody for 1 h. The primary antibodies against the HA epitope, RAD51C, RAD51, XRCC3, XRCC2, CHK1P (S345), CHK2P (T68), H2A, KU70, β-actin, and α-tubulin that were used for Western blot analysis were purchased from Santa Cruz. Antiphosphoserine and antiphosphothreonine antibodies were obtained from Invitrogen; the anti-MRE11, anti-NBS1, anti-CDK1P (Y15), anti-CDK1, and anti-γ-H2AX antibodies were obtained from BD Biosciences; and the rabbit polyclonal XRCC3 S225-phosphospecific antibody was custom generated and affinity purified by Imgenex India. The membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies, developed by chemiluminescence, and imaged using Chemidoc (Fujifilm LAS 3000).
Immunofluorescence.
Exponentially growing HeLa cells were seeded onto coverslips and then treated (or mock treated) with the indicated DNA-damaging agent. After treatment, the cells were washed with PBS and fixed in 4% formaldehyde. The cells were then permeabilized with 0.5% Triton X-100 for 5 min and blocked in 3% BSA for 30 min. The cells were incubated with the indicated primary antibodies and fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Sigma) for 1 h each at room temperature and then stained with PI–4′,6-diamidino-2-phenylindole (DAPI) before mounting onto slides. Cells containing ≥8 foci were scored as being positive for foci. Cells were acquired using a Carl Zeiss confocal microscope, and images were processed using Zeiss LSM image browser software.
Cell survival assay.
Cells (200) were seeded onto 6-well plates in duplicate and then treated with ionizing radiation or various DNA-damaging agents at the indicated dose or concentration. Treated cells were recovered from the genotoxic agents and then grown for 8 to 10 days before being stained with methylene blue. Colonies containing ∼50 cells were counted as one cell. Percent growth was calculated as treated cells/untreated cells × 100.
HR assays.
Homologous recombination (HR) assays were performed using 30 μg of I-SceI plasmid as described previously (17). U2OS SCR24 cells were transfected with either an shRNA construct or various XRCC3 cDNAs (WT or mutant). Twenty-four hours after transfection, the cells were then transfected with I-SceI plasmid. Forty-eight hours later, the cells were harvested and analyzed for green fluorescent protein (GFP) by fluorescence-activated cell sorting (FACS) analysis. In each experiment, the percentage of green (GFP+) cells (either I-SceI induced or uninduced) was measured in triplicate samples, and I-SceI-transfected values were corrected for transfection efficiency (∼65%). The spontaneous GFP+ frequency was subtracted from this value to obtain the I-SceI-induced GFP+ frequency.
RDS assay.
Radioresistant DNA synthesis (RDS) was assayed as described previously (30, 31). Briefly, cells were treated with the indicated dose of IR or left untreated. Thirty minutes later, cells were pulsed with [3H]thymidine by incubation for 30 min. The incorporation of [3H]thymidine was measured in a liquid scintillation counter.
Cell cycle checkpoint assays.
For intra-S-phase checkpoint analysis, bromodeoxyuridine (BrdU) incorporation was performed as described previously (26). In brief, the indicated cells in the logarithmic phase of growth were irradiated with the indicated IR dose, incubated for 1 h, and then pulse-labeled with 50 μM BrdU for 20 min. The cells were harvested, washed twice with PBS, and fixed in 70% ethanol for at least 2 h. After fixing, the samples were brought to room temperature. The DNA was then denatured with 2 N HCl in PBS containing 0.5% Triton X-100 for 30 min and then neutralized by serial PBS washes. Subsequently, the cells were washed with blocking buffer (PBS containing 0.5% Triton X-100 and 0.5% BSA) and incubated with an anti-BrdU mouse monoclonal antibody (BD Biosciences) for 2 h at room temperature. The cells were then washed with blocking buffer and incubated with FITC-conjugated anti-mouse secondary antibody (Sigma) for 1 h. After washing with PBS, the samples were incubated with RNase A and PI and then analyzed by FACS analysis. The aggregates and apoptotic cells were gated out, and cell cycle analysis was performed using FACSDiva version 6.1.1 software (BD Biosciences). For the BrdU incorporation experiments, the cells were treated with camptothecin (CPT) (1 μM CPT for 2 h) and hydroxyurea (HU) (1 mM for 2.5 or 24 h) followed by pulse-labeling with 50 μM BrdU for 20 min at the indicated time points and processed as described above.
The G2/M checkpoint assay was performed by detecting phosphorylated histone H3. The cells were harvested 1 h after irradiation, washed with PBS, and fixed in suspension form by the addition of 5 ml of 70% ethanol at −20°C for 12 h. After fixation, the cells were washed with PBS, permeabilized by the addition of 1 ml 0.25% Triton X-100 in PBS, and incubated on ice for 15 min. After centrifugation, the cell pellet was suspended in 100 μl of PBS containing 0.5% bovine serum albumin (BSA) and 2 μg of an antibody that specifically recognizes the phosphorylated form of histone H3 (Santa Cruz) and incubated for 4 h at room temperature. The cells were then rinsed with PBS containing 0.5% BSA and incubated with FITC-conjugated anti-mouse secondary antibody (Sigma). After a 30-min incubation at room temperature in the dark, the cells were washed again, resuspended in 50 μg/ml PI (Sigma) and 100 μg/ml RNase A (Sigma) in PBS, and incubated at room temperature for 15 min. Cellular fluorescence was measured using a FACSCanto flow cytometer (BD Biosciences), and the data were analyzed using FACSDiva version 6.1.1 software (BD Biosciences).
Cell fractionation and chromatin extraction.
Cytoplasmic and nuclear extracts were obtained as described previously (32). Briefly, after removal of the medium, cells were harvested by washing and trypsinization and collected in 1.5-ml tubes. Cell pellets were resuspended in 10 mM HEPES-KOH (pH 7.5), 50 mM NaCl, 0.5 M sucrose, 0.5% Triton X-100, 0.1 mM EDTA, 1 mM dithiothreitol (DTT), and protease inhibitors. After 15 min of incubation on ice, samples were centrifuged at 1,500 × g for 7 min. Supernatants (cytosolic fraction) were transferred to fresh tubes and stored on ice. Pellets were washed with 10 mM HEPES-NaOH (pH 7.5), 0.1 mM EDTA, 1 mM DTT, and protease inhibitors, after which they were resuspended in buffer A (10 mM HEPES-KOH [pH 7.5], 500 mM NaCl, 0.5% Nonidet P-40, 0.1 mM EDTA, 1 mM DTT, and protease inhibitors) and vortexed for 15 min at 4°C. Later, samples were centrifuged for 10 min at 10,000 × g, and supernatants (nuclear fraction) were transferred to fresh tubes. Fractions were quantified using the standard Bradford method, and 50 μg of protein was used for further analysis.
Chromatin extraction was performed as described previously (33). In brief, U2OS cells (∼5 × 106) were treated with 2 mM HU for 2.5 or 24 h. The HU-containing medium was removed, and the cells were recovered for 60 min and then cross-linked in 1% paraformaldehyde for 20 min. The cytosolic protein fraction was removed by incubation in hypotonic buffer (10 mM HEPES, pH 7, 50 mM NaCl, 0.3 M sucrose, 0.5% Triton X-100, supplemented with protease inhibitor; Roche) for 15 min on ice and centrifuged at 1,500 × g for 5 min. The soluble nuclear fraction was removed by incubation with nuclear buffer (10 mM HEPES, pH 7, 200 mM NaCl, 1 mM EDTA, 0.5% NP-40, and protease inhibitor cocktail) for 10 min on ice and then centrifuged at 13,000 rpm for 2 min. The pellets were resuspended in lysis buffer (10 mM HEPES, pH 7, 500 mM NaCl, 1 mM EDTA, 1% NP-40, and protease inhibitor cocktail), sonicated at low amplitude, and centrifuged for 1 min at 13,000 rpm; the supernatant was then transferred to a new tube. Total chromatin protein was quantified using the standard Bradford method, and 50 μg of protein was used to detect RAD51 on the chromatin.
Comet assay.
HU-induced DSBs were measured by neutral comet assay as described previously (26). In brief, U2OS cells were seeded in 12-well plates and treated with HU for 2.5 or 24 h and then recovered in fresh medium for 1 h prior to harvesting. Harvested cells (20,000) were mixed with 500 μl of 1% low-melting-point agarose (Sigma) and spread on agarose-precoated frosted microscopic glass slides. The slides were placed in lysis solution (30 mm EDTA, 0.5% SDS, pH 8.3) and then incubated at 42°C for 5 h. Following lysis, the slides were washed with 90 mM Tris, 90 mM boric acid, 2 mM EDTA, and electrophoresed in the same buffer at 0.66 V/cm for 20 min. DNA was stained with 2 μg/ml PI for analysis. Images of individual cells were acquired using a fluorescence microscope (Olympus model IX71).
RESULTS
hXRCC3 is phosphorylated at S225 in S/G2 phase following DSB induction.
Human XRCC3 (hXRCC3) S225 has been implicated as a phosphorylation target of ATM/ATR (28). This residue is conserved among primates (Fig. 1A) but not in other species (see Fig. S1A in the supplemental material). An analysis of HA-tagged XRCC3 in HeLa cells after induction of various types of DNA damage revealed that hXRCC3 is phosphorylated on serine residues but not on threonine residues (Fig. 1B). We generated a polyclonal antibody (anti-pS225 XRCC3) against a synthetic polypeptide (XRCC3 residues 221 to 233). In a dot blot assay, the purified anti-pS225 XRCC3 antibody reacted specifically with the immunizing phosphopeptide but not with the nonphosphopeptide (see Fig. S1B), while crude serum recognized both polypeptides (see Fig. S1B). To confirm the specificity of this antibody to XRCC3, we knocked down endogenous XRCC3 in HeLa cells (Fig. 1C; see also Fig. S1C) and analyzed phosphorylated XRCC3. XRCC3-depleted cells had reduced S225-phosphorylated XRCC3 (Fig. 1C and D). Notably, treating cells with λ-phosphatase (λ-PPase) eliminated the XRCC3 pS225 signal (see Fig. S1D). Moreover, the pS225 XRCC3 antibody was immunodepleted when the immunoblots were incubated with the immunizing phosphopeptide (see Fig. S1E). Transiently expressing phosphorylation-site mutant proteins abrogated XRCC3 phosphorylation upon IR-induced DNA damage, confirming S225-specific phosphorylation (Fig. 1E; see also Fig. S2A in the supplemental material). These data confirm the specific phosphorylation of XRCC3 at S225 in response to IR-induced DSBs.
Fig 1.
Human XRCC3 (hXRCC3) is phosphorylated at S225 following DSB induction in S/G2 phase. (A) Sequence alignment of XRCC3 with the ATM/ATR target SQ motif S225. (B) HeLa cells were used to analyze phosphorylation, and—where indicated—DNA damage was induced with IR (10 or 20 Gy), UVC (60 J/m2), or CPT (5 μM). The cells were transfected with pCDNA3-HA-XRCC3, and cell extracts were prepared 1 h postdamage and immunoprecipitated using HA antibody. Following immunoprecipitation (IP), the proteins were immunoblotted with antiphosphoserine, antiphosphothreonine, and anti-HA antibodies. (C and D) The pXRCC3 antibody recognizes phosphorylated S225 specifically during DSB formation. Immunoblotting (C) and immunofluorescence (D) were performed with or without depleting hXRCC3 using pXRCC3 antibody. Bars, 10 μm. (E) HeLa cells were transfected with wild-type (WT) XRCC3 or the S225A or S225D mutant; 24 h after transfection, the cells were irradiated and XRCC3 S225 phosphorylation was analyzed. (F) CPT-induced XRCC3 phosphorylation in the presence or absence of aphidicolin (APH) to halt DNA replication. (G and H) HeLa cells were synchronized with nocodazole, and at the indicated stages of the cell cycle, cells were irradiated with 10 Gy. Extracts were prepared 1 h postirradiation and analyzed for XRCC3 S225 phosphorylation. Asy, asynchronous cells.
As with IR treatment, exposure of cells to different concentration of H2O2 for 10 min induced DSB-specific XRCC3 phosphorylation (see Fig. S2B in the supplemental material). To confirm DSB-specific phosphorylation of XRCC3, cells were preincubated with aphidicolin (5 or 10 μM) and subsequently treated with camptothecin (CPT). Aphidicolin treatment reduced XRCC3 phosphorylation compared to that in cells that were treated with CPT alone (Fig. 1F). Next, we investigated the cell cycle-dependent phosphorylation of XRCC3 and its potential function in the DNA damage response. HeLa cells were synchronized in the G2/M phase, released into various cell cycle phases, irradiated, and then analyzed for XRCC3 phosphorylation. Interestingly, XRCC3 phosphorylation was predominantly observed in the S and G2 phases of the cell cycle (Fig. 1G and H). Similarly, when cells were arrested at the G1/S boundary using aphidicolin, released, and then irradiated at various time points (see Fig. S2C), XRCC3 phosphorylation was prominently observed in the S and G2 phases (see Fig. S2D). The level of phosphorylation increased with increasing doses of irradiation (see Fig. S2E), suggesting that XRCC3 phosphorylation is an integral part of the DNA damage response. An examination of the kinetics of XRCC3 phosphorylation revealed readily detectable phosphorylation within 1 h of 10-Gy IR, and the phosphorylation signal decreased by 2.5 h post-IR (see Fig. S2F and G).
RAD51C but not XRCC2 is essential for XRCC3 phosphorylation.
XRCC3 and RAD51C are known to form a complex (CX3), and this association stabilizes both proteins (34). Depletion of RAD51C in human cells compromised the stability and phosphorylation of XRCC3 (Fig. 2A). Consistent with previous studies (24, 26), RAD51C depletion reduced CHK2 activation—but not CHK1 activation—in response to IR damage (Fig. 2A). To confirm that RAD51C association is a prerequisite for XRCC3 phosphorylation, we overexpressed the pathological RAD51C mutants L138F and G125V and analyzed XRCC3 phosphorylation. Interestingly, expression of these mutants reduced XRCC3 phosphorylation (Fig. 2B) and disrupted the formation of discrete XRCC3 nuclear foci (see Fig. S2A in the supplemental material), as well as DNA damage-induced chromatin loading of XRCC3 (Fig. 2C), suggesting that the interaction of RAD51C with XRCC3 is essential for loading of XRCC3 at the damaged sites and XRCC3 activation. Because RAD51C can also form a complex with RAD51B, RAD51D, and XRCC2 (the BCDX2 complex) (12), we asked whether depletion of any protein other than RAD51C in this complex would affect XRCC3 phosphorylation. Interestingly, we found that depleting XRCC2 had no effect on either XRCC3 phosphorylation at S225 or CHK2 activation via T68 phosphorylation (Fig. 2D), indicating that the BCDX2 complex is not required for XRCC3 activation.
Fig 2.
RAD51C-XRCC3 (CX3) complex is essential for XRCC3 phosphorylation but not RAD51BCD-XRCC2 (BCDX2) complex. (A) Control or RAD51C-depleted HeLa cells were analyzed for XRCC3 phosphorylation after IR damage. (B) Cells were transfected with the WT RAD51C and indicated RAD51C mutants, and 24 h later, cells were irradiated and examined for XRCC3 phosphorylation. (C) Analyses of chromatin-bound XRCC3 in HeLa cells expressing WT RAD51C and its mutants. H2A was used as a chromatin marker. Twenty-four hours posttransfection, cells were treated with 10 Gy of IR, and 1 h later, the chromatin fraction was prepared and analyzed using anti-XRCC3. (D) Effect of XRCC2 depletion on IR-induced XRCC3 phosphorylation.
hXRCC3 S225 phosphorylation is mediated by ATR via an ATM-dependent signaling pathway.
To identify the kinase responsible for the DNA damage-induced phosphorylation of XRCC3 at S225, we used inhibitors specific to DNA-dependent protein kinase catalytic subunit (DNAPKcs) (LY294002) and ATM (KU55933) and then measured XRCC3 phosphorylation. Pretreatment of cells with dimethyl sulfoxide (DMSO), 10 μM LY294002, or 10 μM KU55933 had no effect on XRCC3 levels. Inhibiting ATM with KU55933 blocked XRCC3 phosphorylation (Fig. 3A and C); in contrast, inhibiting DNAPKcs with LY294002 had no effect on XRCC3 phosphorylation (Fig. 3A). At lower concentrations, wortmannin inhibits both ATM and DNAPKcs but not ATR. We therefore examined XRCC3 phosphorylation using wortmannin at a lower concentration in parallel with the ATM-specific inhibitor and found that wortmannin inhibited XRCC3 phosphorylation (Fig. 3B). These data clearly indicate that damage-induced XRCC3 S225 phosphorylation is ATM dependent. To confirm this finding, XRCC3 phosphorylation was analyzed either in A-T cells or in IBR3 cells expressing a kinase-dead ATM (ATM-KD). We found that XRCC3 phosphorylation was normal in wild-type IBR3 cells, whereas no phosphorylation was observed in either A-T cells or IBR3 cells expressing ATM-KD (Fig. 3D).
Fig 3.
The ATM kinase signaling pathway and end resection are required for ATR-mediated XRCC3 phosphorylation. (A and B) IR-induced XRCC3 phosphorylation was examined in HEK293T cells after incubation with DMSO, wortmannin (20 μM), LY294002 (10 μM), or KU55933 (10 μM) for 1 h prior to IR treatment. (C) IR-induced XRCC3 phosphorylation in HeLa cells with and without ATM-specific inhibitor (KU55933). (D) Analysis of XRCC3 phosphorylation in WT IBR3 cells, AT cells, and IBR3 cells expressing kinase-dead ATM (ATM-KD). (E) The effect of MRE11 inhibitor mirin on IR-induced XRCC3 phosphorylation. (F) Analysis of XRCC3 phosphorylation in MRE11- and NBS1-depleted cells. (G) Analysis of end resection in HeLa cells following CPT treatment. End resection was monitored by staining for BrdU under neutral conditions. (H) The kinetics of CPT-induced XRCC3 phosphorylation in HeLa cells. (I) Analysis of XRCC3 phosphorylation in IBR3, A-T, and ATR-Seckel cells. (J and K) The interaction between ATR and XRCC3 in HeLa cells. (L) UV-induced activation of XRCC3 by the ATM/ATR signaling pathway.
In response to IR-induced DSBs, ATM and MRE11 activate each other, and activated MRE11 initiates end resection. Because XRCC3 phosphorylation was restricted primarily to the S/G2 phase, we tested whether end resection is a prerequisite for the recruitment and phosphorylation of XRCC3 at the site of DSBs. Inhibiting end resection by using the MRE11-specific inhibitor mirin prevented the localization of XRCC3 and RAD51C to the DSB sites (see Fig. S4A and B in the supplemental material). Consistent with this finding, XRCC3 phosphorylation decreased with increasing doses of mirin (Fig. 3E). Similarly, depleting either MRE11 or NBS1 reduced XRCC3 phosphorylation (Fig. 3F). To confirm the end resection dependence of XRCC3 phosphorylation, we performed an end resection assay using BrdU labeling under native conditions following CPT treatment (Fig. 3G). BrdU incorporation was evident within 30 min of CPT treatment (Fig. 3G). However, inhibiting MRE11 abolished end resection as monitored by BrdU staining (see Fig. S3B in the supplemental material). The timing of CPT-induced phosphorylation of XRCC3 was parallel with the timing of end resection (Fig. 3H). A few reports showed that ATR is activated in response to IR-induced DNA damage in an ATM-dependent manner and that this activation is required for DDR (35, 36). Interestingly, IR-induced XRCC3 phosphorylation was abolished in ATR-deficient Seckel cells (Fig. 3I), suggesting that ATR-mediated XRCC3 phosphorylation is dependent on ATM. To gain insight into ATR-mediated XRCC3 phosphorylation, we examined ATR-XRCC3 interactions. Coimmunoprecipitation (co-IP) experiments revealed that ATR and XRCC3 interact endogenously, and this interaction was enhanced in response to IR-induced damage (Fig. 3J and K). Immunofluorescence experiments showed partial colocalization of ATR with XRCC3 (see Fig. S5A in the supplemental material), confirming this interaction. To demonstrate the DSB-specific activation of XRCC3 by ATR, we examined UV-induced XRCC3 phosphorylation. Interestingly, XRCC3 phosphorylation was correlated with CHK2 activation during DSB formation following 30 min of UV exposure. However, ATR-mediated CHK1 phosphorylation was measured as early as 10 min following UV treatment (Fig. 3L). These data clearly indicate that ATR mediates the late activation of XRCC3 following DSB accumulation.
XRCC3 activation is essential for the recruitment of RAD51 to the sites of DNA lesions.
XRCC3-deficient vertebrate cells and XRCC3-depleted human cells had reduced RAD51 nuclear focus formation and decreased HR following DNA damage. XRCC3 is found in the nucleus prior to DSB damage and forms chromatin foci following DSB induction (see Fig. S1C in the supplemental material). Moreover, RAD51 loading and/or focus formation at damaged sites occurs after the DNA ends have been resected and processed by HR. RPA is recruited to the resected ends; therefore, to study whether end resection proceeds normally in XRCC3-depleted cells, we examined RPA focus formation following IR damage in both XRCC3-depleted and control cells. We found that RPA focus formations were similar in the two groups (Fig. 4A and B). Because XRCC3 phosphorylation is restricted primarily to S phase following DSB induction, we investigated the role of XRCC3 phosphorylation in recruiting RAD51, the key player in HR. XRCC3-depleted HeLa cells had an ∼3-fold reduction in IR-induced RAD51 focus formation compared to control cells (Fig. 4C and D). Interestingly, expressing the XRCC3 S225A mutant caused an ∼2-fold reduction in RAD51 focus formation, whereas expressing the S225D mutant reversed the defective phenotype to levels similar to those of cells transfected with vector or WT XRCC3 (Fig. 4D). Ectopic expression of phosphomutants showed approximately endogenous levels of XRCC3 protein measured by both FACS and immunoblotting (see Fig. S6A and B in the supplemental material). To rule out the possibility that defective RAD51 focus formation in S225A-expressing cells was due to perturbations in the cell cycle, we analyzed the cell cycle profiles of cells expressing various XRCC3 mutants or controls and found that the cell cycle profiles were highly similar among these groups (see Fig. S5B in the supplemental material).
Fig 4.
XRCC3 colocalizes with RAD51 in repair foci, and XRCC3 phosphorylation is crucial for the recruitment of RAD51 onto the chromatin in response to DSBs. (A) IR-induced RPA foci in HeLa cells expressing either XRCC3 shRNA or a control vector. Bar, 10 μm. (B) Quantitative analysis of the RPA-positive cells in the indicated cell lines following IR damage. Cells containing ≥8 RPA foci were considered to be positive for RPA. (C) IR-induced RAD51 foci in HeLa cells expressing either XRCC3 shRNA or a control vector. (D) Quantitative analysis of RAD51-positive cells in the indicated cell lines. Cells containing ≥8 RAD51 foci were considered to be positive for RAD51. (E) Colocalization of RAD51 with XRCC3 after IR damage. HeLa cells stably expressing HA-tagged XRCC3 were irradiated, and 2 h later, immunofluorescence analysis was carried out to stain endogenous RAD51 and HA-XRCC3. Bar, 10 μm. (F) Effect of RAD51C and XRCC3 depletion on cytosolic and nuclear fractions of RAD51 in the presence or absence of IR damage. HeLa cells were irradiated with 10 Gy, and 1 h postdamage, cytoplasmic and nuclear fractions were prepared to analyze the RAD51 levels using anti-RAD51. α-Tubulin and lamin A served as markers for cytoplasmic and nuclear fractions, respectively. (G) Analysis of XRCC3 phosphorylation on chromatin loading of RAD51. HeLa cells expressing WT and S225A XRCC3 were either left untreated or treated with 2.5 and 5 Gy of IR; later, they were harvested to prepare chromatin fractions. H2A was used as a loading control for chromatin while α-tubulin and lamin A served as markers for cytoplasmic and nuclear fractions, respectively.
Next, we asked the question whether XRCC3 and RAD51 colocalize after IR damage. Staining with HA antibody showed that upon IR damage, induced XRCC3 nuclear foci colocalized with RAD51 (Fig. 4E). This result highlights the function of XRCC3 in the RAD51 pathway of DSB repair. RAD51C but not XRCC3 contains a putative nuclear localization signal (NLS) at its C terminus, and it may carry RAD51 from the cytoplasm to the nucleus upon damage. We examined the effect of XRCC3 depletion on redistribution of RAD51 upon IR damage. Figure S5D in the supplemental material shows the purity of cytosolic and nuclear fractionation. Depleting RAD51C or XRCC3 in HeLa cells led to compromised entry of RAD51 in the nucleus (Fig. 4F), which may be due to reduced RAD51C stability (see Fig. S5C). Parallel examination of cells expressing XRCC3 S225A did not change RAD51 distribution in the nucleus (data not shown). To test whether XRCC3 phosphorylation affects chromatin loading of RAD51, we carried out a modified chromatin fractionation protocol (see Fig. S5E) and examined RAD51 loading in the presence or absence of IR damage. Interestingly, cells expressing the XRCC3 S225A phosphomutant showed compromised chromatin loading of RAD51 upon IR damage (Fig. 4G) while the nuclear and cytosolic fractions of RAD51 were largely unchanged (Fig. 4G).
XRCC3 phosphorylation is required for cellular resistance to IR-induced DNA damage and DSB repair by HR.
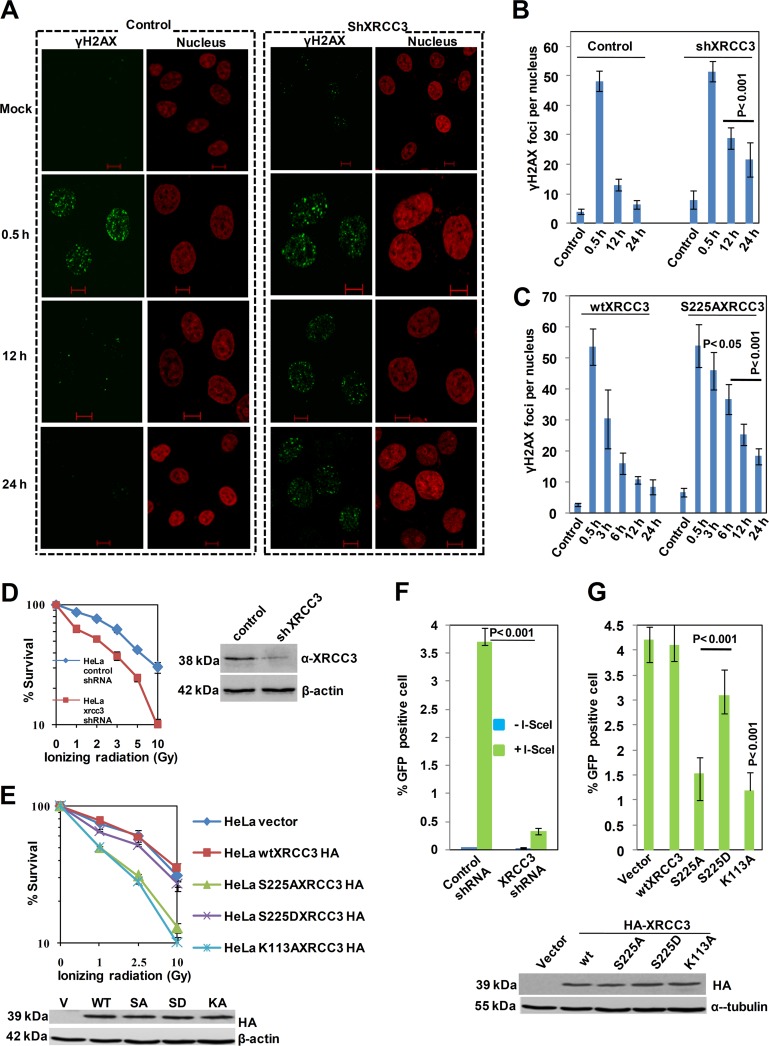
XRCC3 has been implicated in DSB repair; however, the underlying mechanism(s) remains elusive. To determine whether XRCC3 phosphorylation regulates DSB repair, we depleted XRCC3 in HeLa cells and measured γ-H2AX foci as a marker for DSB formation and repair. Thirty minutes after IR treatment, the number of γ-H2AX foci per nucleus was marginally higher in XRCC3-depleted cells than in control cells. Increases of ∼2- and 3-fold for γ-H2AX were observed at 12 and 24 h, respectively, in XRCC3-depleted cells over control cells (Fig. 5A and B), indicating the unrepaired DSBs in XRCC3-depleted cells. Immunoblotting experiments yielded similar results (see Fig. S6C in the supplemental material). Notably, expressing the XRCC3 S225A mutant significantly increased the number of γ-H2AX foci at 6 h compared to cells transfected with WT XRCC3. At later time points, the cells expressing the S225A mutant had twice as many γ-H2AX foci as did control cells (Fig. 5C), suggesting that cells expressing the phosphorylation-deficient XRCC3 mutant protein have defective DSB repair. These results were confirmed by immunoblotting experiments (see Fig. S6D). Next, we examined whether XRCC3 phosphorylation is essential for cellular resistance to IR damage. XRCC3-depleted cells had increased sensitivity to IR compared to that of control cells (Fig. 5D). Interestingly, expressing the S225A mutant caused a marked reduction in cell survival compared to that of WT cells (Fig. 5E), whereas cells expressing the S225D mutant were resistant to IR (Fig. 5E), suggesting a direct role of XRCC3 phosphorylation in DSB repair. As expected, cells expressing the XRCC3 K113A Walker motif mutant had increased sensitivity to IR compared to that of control cells (Fig. 5E). The decreased survival of cells expressing either XRCC3 S225A or K113A was not due to instability of the mutant proteins (Fig. 5E).
Fig 5.
XRCC3 phosphorylation is essential for cellular resistance to IR-induced DNA damage and DSB repair by HR. (A) HeLa cells expressing XRCC3 shRNA or a control vector were subjected to IR damage for the indicated times and then analyzed using immunofluorescence with the γ-H2AX antibody. Bars, 10 μm. (B and C) Quantitative analysis of γ-H2AX foci per nucleus. The bar graph represents means ± standard deviations from three independent experiments. P values in panel B represent the significance for each time point between control and depleted cells. For panel C, the significance was determined for each time point for WT compared with S225A. (D and E) Colony survival of the XRCC3-depleted HeLa cells and cells expressing WT XRCC3 and various indicated XRCC3 mutants following treatment with the indicated IR doses. The immunoblots show similar protein levels in each transfected cell line. (F) Quantification of I-SceI-induced GFP+ cells in the XRCC3-depleted and control cells. (G) I-SceI-induced HR frequency in U2OS cells expressing WT XRCC3 and indicated XRCC3 mutants. The expression of various HA-tagged XRCC3 constructs was measured by immunoblotting; α-tubulin served as a loading control. Bar graphs in all the experiments represent means ± standard deviations from three independent experiments.
To test whether XRCC3 phosphorylation controls HR, we analyzed HR events in previously characterized U2OS cells containing HR reporter (see Fig. S6E in the supplemental material) (17). Consistent with our previous study (16), XRCC3-depleted cells exhibited an ∼10-fold reduction in the I-SceI-induced GFP-positive cells (Fig. 5F; see also Fig. S6F). Cells expressing either XRCC3 S225A or the ATPase-deficient XRCC3 K113A mutant had a >3-fold reduction in HR efficiency (Fig. 5G), suggesting that XRCC3 has phosphorylation- and ATPase-dependent HR functions. The decrease in HR efficiency in cells expressing the XRCC3 S225A and K113A mutants was not due to instability of these proteins (Fig. 5G). To understand the distinction between phosphorylation and ATPase function, we examined XRCC3 K113A-expressing HeLa cells and found that they showed IR-induced phosphorylation (see Fig. S7A in the supplemental material). Although the XRCC3 K113A mutant was stable in the absence of damage, it was partially unstable after IR damage (Fig. 5E; see also Fig. S7A). Immunoprecipitation using HA antibody also confirmed the phosphorylation of the XRCC3 K113A mutant (see Fig. S7B). However, XRCC3 K113A displayed a partial reduction in RAD51 focus formation compared to WT XRCC3, but a further reduction in RAD51 focus formation was evident with cells expressing the XRCC3 S225A mutant (see Fig. S7C and D).
XRCC3 phosphorylation is essential for the intra-S-phase checkpoint but is dispensable for G2/M checkpoint function.
We previously demonstrated that RAD51C, the binding partner of XRCC3, is required for the intra-S-phase checkpoint and HR-mediated DNA repair (26). Here, we examined radioresistant DNA synthesis (RDS), a measure of intra-S-phase checkpoint defects in XRCC3-depleted cells. Interestingly, DNA synthesis, measured by [3H]thymidine incorporation, was inhibited in HeLa cells when subjected to increasing doses of IR (from 2.5 Gy to 10 Gy), and depletion of XRCC3 attenuated this inhibition (Fig. 6A). The RDS phenotype in XRCC3-depleted cells was further validated by BrdU incorporation assay (Fig. 6C), suggesting intra-S-phase checkpoint regulation by XRCC3. Interestingly, this function of XRCC3 is controlled by phosphorylation at S225, indicating a role for XRCC3 in the early response to DNA damage in the S phase (Fig. 6B and D). ATM activates CHK2 by its phosphorylation at T68, and this activation is essential for intra-S-phase checkpoint regulation. Similar to RAD51C, depleting XRCC3 abolished IR (5 Gy)-induced CHK2 activation (Fig. 6E; see also Fig. S8A and B in the supplemental material). However, at a higher dose of IR (20 Gy), XRCC3 depletion did not abolish CHK2 activation (see Fig. S8A and B). Although XRCC3 depletion affected S-phase-specific activation of CHK2 at low doses of IR, the G1-phase-specific activation of CHK2 was unaffected (see Fig. S8C). However, although CHK2 activation was dependent on RAD51C and XRCC3 at the early time point after a low dose of IR (5 Gy), the later activation appears to be independent of these proteins (see Fig. S8D). Notably, expression of XRCC3 S225A—which had impaired XRCC3 function in S phase—reduced CHK2 activation (Fig. 6E), which suggests that XRCC3 phosphorylation regulates—at least in part—ATM-mediated CHK2 activation. However, this activation is to a certain extent independent of XRCC3 phosphorylation, which requires end resection and ATR. Regulation of the intra-S-phase checkpoint by XRCC3 phosphorylation was further confirmed by CPT-induced DSBs. We found that although DNA synthesis was nearly undetectable in both WT- and S225A-expressing cells at 0 h, by 3 and 5 h, DNA synthesis had resumed in both cell types; this synthesis was restricted to early to mid-S phase in WT cells, whereas S225A-expressing cells progressed toward late S phase (Fig. 6F). After 10 h, the S225A-expressing cells progressed into and accumulated in the G2 phase, whereas the WT-expressing cells remained in the G1-to-S phase (Fig. 6F), suggesting a clear role of XRCC3 S225 phosphorylation in the intra-S-phase checkpoint as an early player in the DNA damage response.
Fig 6.
XRCC3 phosphorylation regulates the intra-S-phase checkpoint but is dispensable for the G2/M checkpoint. (A) HeLa cells transfected with control shRNA or shXRCC3 were irradiated with increasing doses of IR, and DNA synthesis was measured by analyzing incorporation of [3H]thymidine. (B) Quantitative analysis of [3H]thymidine incorporation in HeLa cells transfected with the indicated XRCC3 plasmids after 10 Gy of IR. (C) Depleting XRCC3 in HeLa cells fails to suppress DNA synthesis in the presence of difference doses of IR damage as analyzed by BrdU incorporation. (D) Quantification of BrdU incorporation (normalized to mock-treated cells) upon IR-induced DSB in the indicated cells. (E) Analysis of CHK2 phosphorylation in the indicated HeLa cell lines. (F) Analysis of DNA synthesis following CPT treatment in cells expressing WT or S225A XRCC3. (G) Depleting XRCC3 in HeLa cells fails to suppress mitotic entry in the presence of IR damage. (H and I) Quantification of H3-Ser∼P-10-positive cells in the indicated cell lines at various time intervals after IR. The bar graph represents means ± standard deviations from three independent experiments.
In addition to the intra-S-phase checkpoint, the G2/M checkpoint is important for protecting cells from DNA damage. We therefore investigated the role of XRCC3 in G2/M checkpoint function. We found that a significant number of XRCC3-depleted cells progressed into M phase compared to control cells 2 and 5 h after IR damage, indicating a defect in the G2/M checkpoint (Fig. 6G and H). Strikingly, cells expressing XRCC3 S225A arrested normally at the G2/M boundary, indicating that XRCC3 is required for maintaining an IR-induced G2/M checkpoint, although this function of XRCCC3 is independent of phosphorylation at S225 (Fig. 6I). We were puzzled by the fact that G2/M checkpoint regulation by XRCC3 was independent of its phosphorylation. Since ATM regulates G2/M checkpoint, we compared the dominant negative effect of XRCC3 S225A with WT ATM and the kinase-dead mutant of ATM (ATM-KD) in the G2/M checkpoint regulation. Cells expressing WT ATM arrested at the G2/M boundary in response to IR damage while ATM-KD failed to arrest at G2/M. Parallel examination showed that the XRCC3 S225A mutant was functional in the G2/M checkpoint (see Fig. S9A in the supplemental material).
XRCC3 activation is critical for RAD51-mediated recovery of collapsed replication forks but is dispensable for the rescue of stalled forks.
Prolonged stalling of replication forks results in the generation of DSBs in S phase and requires HR-mediated restart (33, 37). We used the replication inhibitor hydroxyurea (HU) to induce replication fork stalling. Extended treatment (i.e., 12 to 24 h) of cells with HU induced XRCC3 phosphorylation (Fig. 7A). Similarly, prolonged exposure (15 h) of cells to mitomycin C (MMC), which generates DSBs, also triggered XRCC3 phosphorylation (see Fig. S10A and B in the supplemental material). HU- and MMC-dependent XRCC3 phosphorylation was required for cellular resistance to these genotoxic agents (Fig. 8C; see also Fig. S10C). Next, we investigated the role of XRCC3 in the recovery of stalled or collapsed replication forks by treating cells with HU for 2.5 or 24 h and analyzed XRCC3 phosphorylation after 1 h of recovery. As expected, prolonged exposure of cells to HU (24 h) resulted in the generation of DSBs, which in turn caused XRCC3 phosphorylation and γ-H2AX formation; in contrast, short exposure (2.5 h) of cells to HU failed to significantly increase XRCC3 phosphorylation or γ-H2AX levels (Fig. 7B). Consistent with these results, microscopy studies also revealed that XRCC3 becomes phosphorylated only after cells are treated with HU for 24 h and are then allowed to recover for 1 h (Fig. 7C). RAD51 and XRCC3 have been shown to promote replication restart after an HU pulse (33). RAD51 promotes the restart of stalled replication forks after a short pulse of HU treatment but does not form foci. However, distinct RAD51 foci were visible only after prolonged exposure to HU (Fig. 7D), which correlated with both the formation of γ-H2AX foci (Fig. 7E) and neutral comet assays, in which comet tails that represent DSBs were visible only after 24 h of HU treatment (Fig. 7F). Interestingly, we found that RAD51 was more abundant in the chromatin fraction after both brief (2.5-h) and prolonged (24-h) treatment with HU than in untreated control cells (Fig. 7G). Notably, a short (2.5-h) HU pulse caused less RAD51 recruitment to the chromatin in the XRCC3-depleted cells than in control cells (Fig. 7H), suggesting a direct role for XRCC3 in RAD51 loading to the sites of stalled replication forks. Next, we examined the role of XRCC3 phosphorylation and ATPase activity in RAD51 recruitment and movement along replication forks. Strikingly, we found that the XRCC3 S225A mutant behaved similarly to WT, whereas the K113A ATPase mutant caused a severe defect in RAD51 loading onto chromatin (Fig. 7J), confirming that XRCC3-mediated rescue of stalled replication forks is independent of phosphorylation at S225 but requires ATPase activity. Similar experiments were performed using a prolonged exposure to HU (24 h) and confirmed that XRCC3 is indeed required for RAD51- and HR-mediated recovery of collapsed replication forks (Fig. 7I). Interestingly, expressing the S225A and ATPase mutant (K113A) reduced the RAD51 recruitment to the replication forks (Fig. 7K).
Fig 7.
XRCC3 is required for loading RAD51 at stalled and collapsed replication forks. (A) Kinetics of HU-induced XRCC3 phosphorylation in HeLa cells. (B and C) U2OS cells were either untreated or treated with 1 mM HU for 2.5 or 24 h and recovered for 1 h in fresh medium, and XRCC3 phosphorylation and γ-H2AX were analyzed. Bars in panel C, 10 μm. (D) Quantification of HU-induced RAD51 foci in U2OS cells after 1 mM HU treatment for 2.5 or 24 h. (E) Quantification of HU-induced γ-H2AX foci. For panels D and E, bar graphs represent means ± standard deviations from two independent experiments. (F) Representative neutral comet images of U2OS cells that were treated with HU for the indicated times before recovering for 1 h. (G) Chromatin-bound RAD51 at stalled replication forks in U2OS cells. (H and I) Analysis of chromatin-bound RAD51 in control and XRCC3 shRNA-transfected cells after a short or long HU pulse. (J and K) Chromatin loading of RAD51 in XRCC3 S225A-, K113A-, and WT-transfected U2OS cells after a short or long HU pulse. (L) Analysis of RAD51 chromatin loading in U2OS cells treated with the ATM inhibitor KU55933.
Fig 8.
XRCC3 phosphorylation is dispensable for the rescue of RAD51-mediated stalled replication forks but is essential for the HR-mediated recovery of collapsed replication forks. (A) U2OS cells were transfected with WT, S225A, or K113A XRCC3. Twenty-four hours after transfection, the cells were either left untreated or treated with 1 mM HU for 2.5 or 24 h. The cells were then recovered in fresh medium at the indicated time points and pulsed with 50 μM BrdU for 20 min. The cells were then harvested, fixed, and stained, and BrdU+ cells were measured to indicate DNA synthesis at various stages of the S phase by FACS analysis. (B) U2OS cells transfected with WT or S225A XRCC3 were treated with 1 mM HU for 24 h and recovered at the indicated time points before harvesting to analyze Cdk1 Y15 phosphorylation. (C) Cell survival of the indicated HeLa cell lines after treatment with 0.5 and 1 mM HU. The bar graph represents means ± standard deviations from three independent experiments. P values were determined comparing S225A and K113A with respect to the WT. (D) Model of XRCC3's function in the repair of DSBs, checkpoint signaling, and replication fork recovery (see the text for discussion).
To further study whether ATM-dependent phosphorylation of XRCC3 is required for RAD51-mediated recovery of collapsed replication forks, we performed chromatin fractionation after short and long exposures of HU in the presence of the ATM-specific inhibitor KU55933. We found that the cells that were treated for 24 h—but not the cells treated for 2.5 h—had reduced RAD51 levels (Fig. 7L).
To investigate these observations further, we measured ongoing replication using BrdU incorporation assays in cells expressing WT, S225A, or K113A XRCC3. We detected BrdU-positive cells after both short and long exposures to HU and at various recovery time points. We found that DNA replication was inhibited with both short and long HU exposures at 0 h of recovery (Fig. 8A). When the recovery time was extended after the 2.5-h HU treatment, WT- and S225A-expressing cells resumed normal DNA replication by 6 h; in contrast, DNA replication failed to restart in the K113A-expressing cells (Fig. 8A). Strikingly, when cells recovered following a 24-h HU treatment, WT-expressing cells showed a delay in restart at 3 h but displayed normal DNA replication at 8 h; at 3 h, the S225A-expressing cells resumed DNA replication faster than did the WT-expressing cells and progressed into the late S and G2 phase within 8 h after HU treatment (Fig. 8A). DNA replication failed to restart in the K113A-expressing cells, and these cells showed no signs of DNA synthesis even after 8 h of recovery. Resumption of DNA synthesis was faster in S225A-expressing cells than in WT-expressing cells after 24 h of HU treatment. Recovery of S225A-expressing cells was due to failure in the checkpoint arrest, which is evident by activated cyclin-CDK1 in these cells (Fig. 8B). Moreover, preventing XRCC3 phosphorylation and ATPase activity abrogated cellular resistance to HU after 24 h of HU exposure and recovery (Fig. 8C). To further confirm the defective recovery of stalled replication forks in the S225A- and K113A-expressing cells, we treated WT-, S225A-, and K113A-expressing cells with HU for 24 h and recovered the cells in fresh medium containing nocodazole to arrest the cells in mitosis. We found that WT-expressing cells recovered from replication stress and accumulated in the G2/M phase between 12 and 18 h of HU treatment (see Fig. S9B in the supplemental material), whereas the S225A-expressing cells progressed faster after recovery (at 3 h) but had a delayed accumulation in G2/M in the later hours of recovery (see Fig. S9B). K113A-expressing cells were severely defective in their ability to recover from replication stress and accumulated in the S-to-G2 phase even after 18 h of recovery (see Fig. S9B).
DISCUSSION
Here, we show that XRCC3 S225 is phosphorylated in the S and G2 phases of the cell cycle and that this modification is transient in nature. Our results suggest that the upstream kinase ATM is required to activate XRCC3, but XRCC3 phosphorylation occurs via ATR following DSB formation. Our data indicate that XRCC3 activation occurs downstream of single-stranded DNA (ssDNA) generation and RPA focus formation (Fig. 8D). S-phase-specific and end resection-dependent XRCC3 phosphorylations suggest that ATR is involved primarily in XRCC3 activation (Fig. 8D). Interestingly, XRCC3 phosphorylation was dependent on RAD51C, and disruption of the BCDX2 complex did not affect XRCC3 phosphorylation. It is likely that the recruitment of RAD51C to the sites of DNA lesions can promote XRCC3 phosphorylation and activate the DNA damage response pathway(s) in the S and G2 phases. Consistent with this hypothesis, the formation of RAD51C foci has been shown to be dependent on ATM in response to IR damage and occurs before the formation of RAD51 foci (24). Conceivably, the CX3 complex may have evolved to mediate ATM-regulated DNA damage responses through the phosphorylation of XRCC3.
We show that XRCC3 phosphorylation is required for repairing DSBs in a timely manner. RAD51 foci are prominent in the S and G2 phases, and their formation is dependent on ATM, ATR, RAD51C, and XRCC3 (12, 24, 38). It is likely that the CX3 complex acts as a mediator in DNA damage signaling to coordinate with HR. The transient activation of XRCC3 may be required for the timely initiation of HR at damaged sites to perform legitimate repair (Fig. 8D). Although BRCA2 directly binds to RAD51 and mediates RAD51 loading onto the sites of DNA lesions (39), mechanistically how XRCC3 phosphorylation influences RAD51-mediated HR is unclear. It is likely that BRCA2 may directly participate in RAD51 recruitment and XRCC3 may stabilize the RAD51 filament which is in part mediated by phosphorylation. Consistent with our previous study, the XRCC3 ATPase-deficient (K113A) mutant showed impaired HR. However, this mutant was phosphorylation competent in response to DSBs, suggesting that in addition to XRCC3 activation, its ATPase function is essential for the completion of HR.
Interestingly, as with ATM (40, 41), XRCC3-deficient cells exhibited RDS and impaired CHK2 activation, suggesting that XRCC3 activation regulates the intra-S-phase checkpoint in an ATM- and CHK2-dependent pathway. However, G1-phase-specific activation of CHK2 was independent of XRCC3 and its activation. Notably, early activation of CHK2 in S/G2 phase was downstream of XRCC3 recruitment as well as its phosphorylation at the sites of DSBs. NBS1 also has been shown to be involved in the early activation of CHK2 in response to IR (42). It is likely that NBS1-dependent CHK2 phosphorylation is mediated through XRCC3 activation. CDK1 regulates HR initiation by end resection and also ensures the completion of HR by targeting BRCA2 in the late S/G2 phase (43). The activation of XRCC3 is dependent on CDK1-mediated end resection, and XRCC3 activation may in turn regulate CDK1 through CHK2 to slow S-phase progression. Inhibiting CDK1 would keep BRCA2 active and complete RAD51-mediated HR. It is likely that XRCC3 phosphorylation is a key event in the coordination of checkpoint activation and repair processes (Fig. 8D). We also identified a previously unknown function of XRCC3 in the G2/M checkpoint, although S225 phosphorylation was not essential for this function. It is likely that XRCC3 phosphorylation is not required for the activation of the G2/M checkpoint. However, XRCC3 may function to maintain the G2/M checkpoint in response to IR-induced DNA damage.
We show that XRCC3 activation is dependent on the formation of DSBs. The daughter-strand gaps (DSGs) that are generated as a result of a short HU pulse failed to trigger XRCC3 phosphorylation, whereas prolonged exposure to HU generated DSBs and resulted in XRCC3 activation. Here, we show that XRCC3 depletion reduces RAD51 loading and results in defective replication restart. Interestingly, replication resumed with short-pulse HU treatment, and RAD51 loading was also unaffected when DSGs were formed in cells expressing the XRCC3 S225A mutant, suggesting that XRCC3 activation is dispensable for the recovery of stalled replication forks. However, when DSBs were generated with a prolonged exposure to HU, XRCC3 S225A-expressing cells displayed reduced RAD51 loading and impaired replication fork recovery, indicating that XRCC3 activation is crucial in the recovery of collapsed replication forks. Interestingly, the XRCC3 Walker motif was essential for the rescue of both stalled and collapsed replication forks. The fact that the XRCC3 S225A mutant was able to rescue the stalled replication forks but not the XRCC3 ATPase mutant suggests that the XRCC3 S225A mutant possesses ATPase function. Recently, BRCA2 and FANCD2 were shown to stabilize replication forks (44, 45), and these two proteins are not required for the restart of replication forks (46, 47). It is likely that XRCC3 is essential for the restart of replication forks. RAD51 plays an important role in the HR-mediated restart of replication forks and has also been implicated in fork reversal (33) (Fig. 8D). The resumption of replication through reversed forks may involve the formation of Holliday junctions (HJs). The BLM-TOP3 complex is known to dissolve these structures, allowing replication to resume (48). BLM and XRCC3 have epistatic interactions, and XRCC3 is upstream of BLM (49); this suggests that XRCC3 facilitates RAD51 loading to catalyze fork reversal (thereby generating HJ structures) and that BLM dissolves HJs to promote replication restart (Fig. 8D).
Notably, RAD51 paralogs have been recently shown to participate in BRCA1/2-mediated HR (14, 15). However, BRCA2 has been implicated in replication fork stabilization but not its restart (44, 45). XRCC3 also has been implicated in the restart of stalled/collapsed replication forks (33). Strikingly, our results revealed phosphorylation-independent functions of XRCC3 in the recovery of stalled replication forks. However, there is no indication that RAD51C has a similar function. XRCC3 may facilitate RAD51-mediated replication restart independently of BRCA proteins, which is crucial for both genome stability and tumor suppression. Interestingly, mutations in the RAD51 paralogs XRCC2 and RAD51D have been identified recently in patients with familial breast cancer (50, 51). Thus, screening for XRCC3 mutations in the unidentified familial breast cancer samples will be highly valuable.
This study is the first to show that XRCC3 is a novel phosphorylation target of ATM/ATR kinase and participates in DNA damage responses to control the intra-S-phase checkpoint and HR-mediated DSB repair as well as the rescue of collapsed replication forks. Our detailed analyses suggest that XRCC3 distinctly regulates DNA damage signaling and repair via phosphorylation and ATP hydrolysis. Importantly, we also identified a phosphorylation-independent function of XRCC3 in both the G2/M checkpoint and the restart of stalled replication forks.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yosef Shiloh, Sandeep Burma, and K. Muniyappa for their critical comments on the manuscript. We thank Michael Kastan, Penny Jeggo, Yosef Shiloh, Titia deLange, Karlene Cimprich, K. Muniyappa, Anjali Karande, and Sathees Raghavan for providing various constructs and reagents. We also thank Meenakshi Sen and Deepti Bapat of the IISc confocal microscopy facility and Omana Joy, Pooja Pai, and Kavya Ananthswamy from the IISc FACS facility and the IISc animal facility for their help.
Funding support from the Department of Science and Technology and the Council of Scientific and Industrial Research (CSIR) is greatly acknowledged. K.S. is supported by a fellowship from CSIR and a Bristol-Myers Squibb fellowship from the United Kingdom.
There are no conflicts of interest to report.
Footnotes
Published ahead of print 25 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01521-12.
REFERENCES
- 1. Ciccia A, Elledge SJ. 2010. The DNA damage response: making it safe to play with knives. Mol. Cell 40: 179– 204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackson SP, Bartek J. 2009. The DNA-damage response in human biology and disease. Nature 461: 1071– 1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kastan MB, Bartek J. 2004. Cell-cycle checkpoints and cancer. Nature 432: 316– 323 [DOI] [PubMed] [Google Scholar]
- 4. Lavin MF. 2008. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 9: 759– 769 [DOI] [PubMed] [Google Scholar]
- 5. O'Driscoll M, Jeggo PA. 2006. The role of double-strand break repair—insights from human genetics. Nat. Rev. Genet. 7: 45– 54 [DOI] [PubMed] [Google Scholar]
- 6. Shiloh Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3: 155– 168 [DOI] [PubMed] [Google Scholar]
- 7. Cimprich KA, Cortez D. 2008. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9: 616– 627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flynn RL, Zou L. 2011. ATR: a master conductor of cellular responses to DNA replication stress. Trends Biochem. Sci. 36: 133– 140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lavin MF. 2007. ATM and the Mre11 complex combine to recognize and signal DNA double-strand breaks. Oncogene 26: 7749– 7758 [DOI] [PubMed] [Google Scholar]
- 10. Thompson LH. 2012. Recognition, signaling, and repair of DNA double-strand breaks produced by ionizing radiation in mammalian cells: the molecular choreography. Mutat. Res. 751: 158– 246 [DOI] [PubMed] [Google Scholar]
- 11. Traven A, Heierhorst J. 2005. SQ/TQ cluster domains: concentrated ATM/ATR kinase phosphorylation site regions in DNA-damage-response proteins. Bioessays 27: 397– 407 [DOI] [PubMed] [Google Scholar]
- 12. Somyajit K, Subramanya S, Nagaraju G. 2010. RAD51C: a novel cancer susceptibility gene is linked to Fanconi anemia and breast cancer. Carcinogenesis 31: 2031– 2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suwaki N, Klare K, Tarsounas M. 2011. RAD51 paralogs: roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin. Cell Dev. Biol. 22: 898– 905 [DOI] [PubMed] [Google Scholar]
- 14. Chun J, Buechelmaier ES, Powell SN. 2013. Rad51 paralog complexes BCDX2 and CX3 act at different stages in the BRCA1-BRCA2-dependent homologous recombination pathway. Mol. Cell. Biol. 33: 387– 395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qing Y, Yamazoe M, Hirota K, Dejsuphong D, Sakai W, Yamamoto KN, Bishop DK, Wu X, Takeda S. 2011. The epistatic relationship between BRCA2 and the other RAD51 mediators in homologous recombination. PLoS Genet. 7: e1002148 doi:10.1371/journal.pgen.1002148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagaraju G, Hartlerode A, Kwok A, Chandramouly G, Scully R. 2009. XRCC2 and XRCC3 regulate the balance between short- and long-tract gene conversions between sister chromatids. Mol. Cell. Biol. 29:4283– 4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagaraju G, Odate S, Xie A, Scully R. 2006. Differential regulation of short- and long-tract gene conversion between sister chromatids by Rad51C. Mol. Cell. Biol. 26: 8075– 8086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Griffin CS, Simpson PJ, Wilson CR, Thacker J. 2000. Mammalian recombination-repair genes XRCC2 and XRCC3 promote correct chromosome segregation. Nat. Cell Biol. 2: 757– 761 [DOI] [PubMed] [Google Scholar]
- 19. Rodrigue A, Coulombe Y, Jacquet K, Gagne JP, Roques C, Gobeil S, Poirier G, Masson JY. 29 October 2012. The RAD51 paralogs ensure cellular protection against mitotic defects and aneuploidy. J. Cell Sci. [Epub ahead of print.] doi:10.1242/jcs.114595 [DOI] [PubMed] [Google Scholar]
- 20. Tarsounas M, Munoz P, Claas A, Smiraldo PG, Pittman DL, Blasco MA, West SC. 2004. Telomere maintenance requires the RAD51D recombination/repair protein. Cell 117: 337– 347 [DOI] [PubMed] [Google Scholar]
- 21. Compton SA, Choi JH, Cesare AJ, Ozgur S, Griffith JD. 2007. Xrcc3 and Nbs1 are required for the production of extrachromosomal telomeric circles in human alternative lengthening of telomere cells. Cancer Res. 67: 1513– 1519 [DOI] [PubMed] [Google Scholar]
- 22. Oganesian L, Karlseder J. 2011. Mammalian 5′ C-rich telomeric overhangs are a mark of recombination-dependent telomere maintenance. Mol. Cell 42: 224– 236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang RC, Smogorzewska A, de Lange T. 2004. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119: 355– 368 [DOI] [PubMed] [Google Scholar]
- 24. Badie S, Liao C, Thanasoula M, Barber P, Hill MA, Tarsounas M. 2009. RAD51C facilitates checkpoint signaling by promoting CHK2 phosphorylation. J. Cell Biol. 185: 587– 600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, Freund M, Lichtner P, Hartmann L, Schaal H, Ramser J, Honisch E, Kubisch C, Wichmann HE, Kast K, Deissler H, Engel C, Muller-Myhsok B, Neveling K, Kiechle M, Mathew CG, Schindler D, Schmutzler RK, Hanenberg H. 2010. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat. Genet. 42: 410– 414 [DOI] [PubMed] [Google Scholar]
- 26. Somyajit K, Subramanya S, Nagaraju G. 2012. Distinct roles of FANCO/RAD51C protein in DNA damage signaling and repair: implications for Fanconi anemia and breast cancer susceptibility. J. Biol. Chem. 287: 3366– 3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vaz F, Hanenberg H, Schuster B, Barker K, Wiek C, Erven V, Neveling K, Endt D, Kesterton I, Autore F, Fraternali F, Freund M, Hartmann L, Grimwade D, Roberts RG, Schaal H, Mohammed S, Rahman N, Schindler D, Mathew CG. 2010. Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat. Genet. 42: 406– 409 [DOI] [PubMed] [Google Scholar]
- 28. Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, III, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. 2007. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316: 1160– 1166 [DOI] [PubMed] [Google Scholar]
- 29. Puget N, Knowlton M, Scully R. 2005. Molecular analysis of sister chromatid recombination in mammalian cells. DNA Repair (Amst.) 4: 149– 161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lou Z, Minter-Dykhouse K, Wu X, Chen J. 2003. MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature 421: 957– 961 [DOI] [PubMed] [Google Scholar]
- 31. Wu L, Luo K, Lou Z, Chen J. 2008. MDC1 regulates intra-S-phase checkpoint by targeting NBS1 to DNA double-strand breaks. Proc. Natl. Acad. Sci. U. S. A. 105: 11200– 11205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gildemeister OS, Sage JM, Knight KL. 2009. Cellular redistribution of Rad51 in response to DNA damage: novel role for Rad51C. J. Biol. Chem. 284: 31945– 31952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. 2010. Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol. Cell 37: 492– 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lio YC, Schild D, Brenneman MA, Redpath JL, Chen DJ. 2004. Human Rad51C deficiency destabilizes XRCC3, impairs recombination, and radiosensitizes S/G2-phase cells. J. Biol. Chem. 279: 42313– 42320 [DOI] [PubMed] [Google Scholar]
- 35. Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. 2006. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 8: 37– 45 [DOI] [PubMed] [Google Scholar]
- 36. Myers JS, Cortez D. 2006. Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J. Biol. Chem. 281: 9346– 9350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petermann E, Helleday T. 2010. Pathways of mammalian replication fork restart. Nat. Rev. Mol. Cell Biol. 11: 683– 687 [DOI] [PubMed] [Google Scholar]
- 38. Yuan SS, Chang HL, Lee EY. 2003. Ionizing radiation-induced Rad51 nuclear focus formation is cell cycle-regulated and defective in both ATM(-/-) and c-Abl(-/-) cells. Mutat. Res. 525: 85– 92 [DOI] [PubMed] [Google Scholar]
- 39. Jensen RB, Carreira A, Kowalczykowski SC. 2010. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 467: 678– 683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410: 842– 847 [DOI] [PubMed] [Google Scholar]
- 41. Xu B, Kim S, Kastan MB. 2001. Involvement of BRCA1 in S-phase and G(2)-phase checkpoints after ionizing irradiation. Mol. Cell. Biol. 21: 3445– 345011313470 [Google Scholar]
- 42. Buscemi G, Savio C, Zannini L, Micciche F, Masnada D, Nakanishi M, Tauchi H, Komatsu K, Mizutani S, Khanna K, Chen P, Concannon P, Chessa L, Delia D. 2001. Chk2 activation dependence on Nbs1 after DNA damage. Mol. Cell. Biol. 21: 5214– 5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Esashi F, Christ N, Gannon J, Liu Y, Hunt T, Jasin M, West SC. 2005. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 434: 598– 604 [DOI] [PubMed] [Google Scholar]
- 44. Schlacher K, Christ N, Siaud N, Egashira A, Wu H, Jasin M. 2011. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 145: 529– 542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schlacher K, Wu H, Jasin M. 2012. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell 22: 106– 116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moldovan GL, D'Andrea AD. 2012. To the rescue: the Fanconi anemia genome stability pathway salvages replication forks. Cancer Cell 22: 5– 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ying S, Hamdy FC, Helleday T. 2012. Mre11-dependent degradation of stalled DNA replication forks is prevented by BRCA2 and PARP1. Cancer Res. 72: 2814– 2821 [DOI] [PubMed] [Google Scholar]
- 48. Bussen W, Raynard S, Busygina V, Singh AK, Sung P. 2007. Holliday junction processing activity of the BLM-Topo IIIalpha-BLAP75 complex. J. Biol. Chem. 282: 31484– 31492 [DOI] [PubMed] [Google Scholar]
- 49. Otsuki M, Seki M, Inoue E, Yoshimura A, Kato G, Yamanouchi S, Kawabe Y, Tada S, Shinohara A, Komura J, Ono T, Takeda S, Ishii Y, Enomoto T. 2007. Functional interactions between BLM and XRCC3 in the cell. J. Cell Biol. 179: 53– 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Loveday C, Turnbull C, Ramsay E, Hughes D, Ruark E, Frankum JR, Bowden G, Kalmyrzaev B, Warren-Perry M, Snape K, Adlard JW, Barwell J, Berg J, Brady AF, Brewer C, Brice G, Chapman C, Cook J, Davidson R, Donaldson A, Douglas F, Greenhalgh L, Henderson A, Izatt L, Kumar A, Lalloo F, Miedzybrodzka Z, Morrison PJ, Paterson J, Porteous M, Rogers MT, Shanley S, Walker L, Eccles D, Evans DG, Renwick A, Seal S, Lord CJ, Ashworth A, Reis-Filho JS, Antoniou AC, Rahman N. 2011. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat. Genet. 43: 879– 882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park DJ, Lesueur F, Nguyen-Dumont T, Pertesi M, Odefrey F, Hammet F, Neuhausen SL, John EM, Andrulis IL, Terry MB, Daly M, Buys S, Le Calvez-Kelm F, Lonie A, Pope BJ, Tsimiklis H, Voegele C, Hilbers FM, Hoogerbrugge N, Barroso A, Osorio A, Giles GG, Devilee P, Benitez J, Hopper JL, Tavtigian SV, Goldgar DE, Southey MC. 2012. Rare mutations in XRCC2 increase the risk of breast cancer. Am. J. Hum. Genet. 90: 734– 739 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.