Abstract
Fyn is a tyrosine kinase with multiple roles in a variety of cellular processes. Here we report that Fyn is a new kinase involved in adipocyte differentiation. Elevated Fyn protein is detected specifically in the adipocytes of obese mice. Moreover, Fyn expression increases progressively in 3T3-L1 cells during in vitro adipogenesis, which correlates with its kinase activity. Inhibition of Fyn by either genetic or pharmacological manipulation restrains the 3T3-L1 preadipocytes from fully differentiating into mature adipocytes. Mechanistically, Fyn regulates the activity of the adipogenic transcription factor signal transducer and activator of transcription 5a (STAT5a) through enhancing its interaction with the GTPase phosphoinositide 3-kinase enhancer A (PIKE-A). The STAT5a activity is therefore reduced in Fyn- or PIKE-ablated adipose tissues, leading to diminished expression of adipogenic markers and adipocyte differentiation. Our data thus demonstrate a novel functional interaction between Fyn, PIKE-A, and STAT5a in mediating adipogenesis.
INTRODUCTION
New adipocytes are generated from differentiation of mesenchymal stem cell-derived preadipocytes via a process called adipogenesis, which is controlled by a variety of hormones/cytokines (1). Prolactin (PRL) is one of these factors, but the intrinsic mechanism of the hormone-induced adipogenesis has not been well established (2). It is suggested that PRL stimulates adipocyte differentiation by activating signal transducer and activator of transcription 5a (STAT5a), a transcription factor involved in numerous physiological functions via inducing the transcription of target genes (3, 4). In 3T3-F442A preadipocytes, activated STAT5a initiates the expression of the master adipogenic transcription factors peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα to mediate differentiation (5). Moreover, overexpression of STAT5a in NIH 3T3 cells is sufficient to confer adipogenesis activity to the nonprecursor cells, whereas deletion of the STAT5a gene in mice results in a significant reduction of fat pad size (6, 7). Stewart et al. recently demonstrated that STAT5a-overexpressed Swiss 3T3 cells are able to develop into a functional fat pad in athymic mouse, a finding which further supports the imperative role of this transcription factor in adipocyte development (8). Thus, STAT5a is one of the central factors controlling the progression of adipogenesis. Nevertheless, how STAT5a activity is regulated during adipocyte differentiation remains a mystery.
Fyn, a tyrosine-specific kinase that belongs to the Src kinase family (SKF) (9), is known as a PRL downstream effector that regulates cell proliferation and ion channel activity (10, 11). The observation of a lean phenotype in Fyn knockout (Fyn−/−) mice links the kinase to obesity development (12). Indeed, Fyn is involved in energy expenditure by increasing fatty acid oxidation (12). It has been reported that Fyn phosphorylates LKB1 on tyrosine 261 and 365, which causes its nuclear translocation and inhibits the subsequent activation of AMP-activated protein kinase (AMPK), the key cellular regulator of energy metabolism (13). Thus, the activity of AMPK in both muscle and white adipose tissue (WAT) is increased in Fyn−/− mice (12). Nevertheless, scattered pieces of information also show that Fyn may play a role in adipogenesis in addition to regulating energy expenditure. A recent study performed in pig indicates that a substantial level of Fyn mRNA can be detected in WAT (14). Using a comparative functional genomics approach, it has also been reported that the adipose Fyn expression is increased in obese rats (15). These data suggest that Fyn may take part in controlling adipocyte functions, but its specific role in adipogenesis has never been investigated.
Phosphoinositide 3-kinase enhancer A (PIKE-A) is a GTPase that is amplified in a variety of cancer cells to potentiate the Akt kinase activity (16, 17). It has a broad expression profile in that the mRNA of PIKE-A can be found in brain, liver, muscle, lung, spleen, prostate, thymus, and intestine (18–20). However, the function of PIKE-A in these tissues remains largely unknown. Recently, we reported that PIKE-A has a substantial expression in mouse WAT which is elevated in genetically or diet-induced obese mice (21). Moreover, adipocyte differentiation is impaired in PIKE knockout (PIKE−/−) mouse embryonic fibroblasts (MEF) (21). PIKE-A is also found to be controlled by PRL during mammary gland development. In response to PRL stimulation, PIKE-A serves as an adaptor that mediates the interaction between phosphorylated STAT5a and PRLR. This receptor-associated PIKE-A/STAT5a complex is disrupted by JAK2 phosphorylation on STAT5a (22). Given the tight relationship of STAT5a activation and adipogenesis, PIKE-A may be an unappreciated adipogenic factor through modulating the STAT5a activity in adipocytes.
In this report, we present evidence that both Fyn and PIKE-A are novel factors to promote adipogenesis. We also show that Fyn can be activated by the pleiotropic hormone PRL in adipocytes. Activation of Fyn triggers the association between PIKE-A and STAT5a, which is a prerequisite for PRL-mediated STAT5a activation and adipogenesis. Therefore, the interaction between Fyn, PIKE-A, and STAT5a forms a new functional network in mediating adipogenesis.
MATERIALS AND METHODS
Animals and reagents.
3T3-L1 cells were obtained from ATCC. PP3 cells were purchased from EMD Chemicals. All other chemicals were purchased from Sigma-Aldrich. Control small interfering RNA (siRNA) and siFyn were purchased from Dharmacon (Thermo Fisher Scientific Inc.). Human Fyn plasmid (PRK5-Fyn) was obtained from Addgene. Control adenovirus and adenovirus overexpressing PIKE-A were prepared as previously reported (23). Cell culture materials were purchased from Invitrogen. Antihemagglutinin (anti-HA)-horseradish peroxidase (HRP), anti-green fluorescent protein (anti-GFP), and glutathione transferase (GST)-HRP antibodies were obtained from Sigma-Aldrich. Anti-Fyn, anti-STAT5a, and antitubulin antibodies were purchased from Santa Cruz Biotechnology. Anti-phospho-JAK2 Y1007/1008, anti-JAk2, and anti-phospho-STAT5 Y964 were purchased from Cell Signaling Inc. Recombinant mouse PRL was obtained from the National Hormone and Peptide Program (NIH). Fyn knockout mice were obtained from the Jackson Laboratory, and PIKE knockout mice were developed in our laboratory as reported previously (21, 22). All animal experiments were performed according to the care of experimental animal guidelines from Emory University.
Cell cultures, transfection, electroporation, and adenovirus infection.
3T3-L1 preadipocytes were maintained in Dulbecco's modified Eagle medium (DMEM) with 10% calf serum (CS), 50 U/ml penicillin, and 50 μg/ml streptomycin. After differentiation, the CS was replaced by fetal bovine serum (FBS). SiRNA transfection in 3T3-L1 cells was performed using DharmaFECT (Thermo Fisher Scientific Inc.) as instructed. For adenovirus infection, the virus (1 × 106 PFU) was added to the 3T3-L1 preadipocytes 24 h before isobutylmethylxanthine-dexamethasone-insulin (MDI) induction. PIKE−/− and Fyn−/− MEF were isolated from an E13.5 embryo using trypsin digestion as previously described (21). The cells were maintained in DMEM with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin. Electroporation of 3T3-L1 or MEF cells (50 μg DNA/2 × 106 cells) was performed at 0.95 kV, 200 Ω, and 25 μF using the Gene Pulser II system (Bio-Rad) according to the manufacturer's instructions.
Adipose tissue fractionation.
Inguinal WAT was isolated using sterile techniques from chow-fed, high-fat-diet (HFD)-fed (40% kcal, 20 weeks), or obese (ob/ob) male mice. Weighted tissues were minced and added to DMEM–F-12 in a ratio of 1 g/3 ml. Type I collagenase (Worthington Chemicals) was added at a concentration of 280 U/ml. The collagenase solution was incubated at 37°C for 40 min with gentle shaking. By centrifugation at 500 × g for 5 min, the floating adipocytes were separated from the stromal vascular fraction (SVF) pellet. The cells were then washed with phosphate-buffered saline (PBS) buffer twice and lysed. Cellular debris was removed by centrifugation, and protein concentrations were determined using a Bio-Rad protein assay kit (Bio-Rad). Equal amounts of protein were subjected to SDS-PAGE. The protein yields of the adipocyte and SVF fractions were about 0.6 mg and 1 mg, respectively.
Adipocyte differentiation assay.
MEF or 3T3-L1 cells were grown in DMEM with 10% CS. Two days after 100% confluence, the cells were induced to differentiate into adipocyte by a change in medium to DMEM containing a standard induction cocktail of 10% FBS, 0.5 mM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone, and 1.7 μM insulin. After 48 h, this medium was replaced with DMEM supplemented with 10% FBS, 1.7 μM insulin, and 1 μM ciglitazone for 48 h. The cells were then cultured in DMEM with 10% FBS until assayed. Lipid accumulation was examined by oil red O staining, followed by extraction of the absorbed dye using 100% isopropanol and measurement at 500 nm as reported previously (21).
Immunoprecipitation and Western blot.
Tissue or cell extracts were prepared by homogenization in lysis buffer as reported previously (24). Cell debris was removed by centrifugation, and the supernatant (cleared cell lysate) was collected. Immunoprecipitation using antibodies as indicated was performed as reported previously (24). Western blot results were visualized using Pierce ECL Western blotting substrate (Thermo Scientific). The immunoblots shown were representative results from experiments that have been performed twice. The blot images were densitometrically scanned and quantified by the computer program ImageJ (NIH).
In vitro kinase assay.
Fyn kinase was immunoprecipitated from 3T3-L1 cells using anti-Fyn antibody and protein A/G agarose (Santa Cruz Biotechnology). The agarose was then washed extensively with lysis buffer, and the kinase activity in phosphorylating poly(Glu-Tyr) was determined using a colorimetric tyrosine kinase assay kit (Millipore) as instructed. In the Fyn phosphorylation assay, recombinant active Fyn (Millipore; 0.5 μg/reaction) was incubated with the protein A/G agarose containing immunoprecipitated proteins in the reaction buffer (25 mM Tris [pH 7.0] and 100 mM MnCl2) containing 10 μCi 32P-γ-ATP as described previously (25). The reaction mixture was resolved in SDS and detected using autoradiography.
Real-time reverse transcription-PCR (RT-PCR).
Total RNA was prepared by using TRIzol isolation reagent (Invitrogen). First-strand cDNA was synthesized from 5 μg total RNA using Superscript III reverse transcriptase (Invitrogen) and oligo(dT)17 as the primer with recommended procedures. Amplifications of Pref-1, aP2, PPARγ, and C/EBPα were performed using the following primers: mPref1-F (5′-GACCCACCCTGTGACCCC-3′), mPref1-R (5′-CAGGCAGCTCGTGCACCCC-3′), maP2-F (5′-CAAAATGTGTGATGCCTTTGTG-3′), maP2-R (5′-CTCTTCCTTTGGCTCATGCC-3′), mPPARγ2-F (5′-ATGCTGTTATGGGTGAAACT-3′), mPPARγ2-R (5′-CTTGGAGCTTCAGGTCATATTTGTA-3′), mC/EBPα-F (5′-ATCCCAGAGGGACTGGAGTT-3′), and mC/EBPα-R (5′-AAGTCTTAGCCGGAGGAAGC-3′). Expression of PIKE-A was determined using the primers 5′-ACAGGATCAGTGCATCATCTC-3′ (forward) and 5′-AGTAGCTACAGCGCTTCATG-3′ (reverse). Expression of Fyn was determined using the primers 5′-ACCTCCATCCCGAACTACAAC-3′ (forward) and 5′-CGCCACAAACAGTGTCACTC-3′ (reverse). Actin fragment was also amplified as an internal standard (26–28) using the primers 5′-AACCGTGAAAAGATGACCCAGAT-3′ (forward) and 5′-CACAGCCTGGATGGCTACGT-3′ (reverse). Real-time PCR was performed using Power SYBR green master mix (Applied Biosystems).
Statistical analysis.
Results are expressed as means and standard errors of the mean (SEM) and were considered significant when P was <0.05. Statistical analysis of the data was performed using either Student's t test or a one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison test using the computer program GraphPad Prism (GraphPad Software).
RESULTS
Adipogenesis regulates Fyn expression.
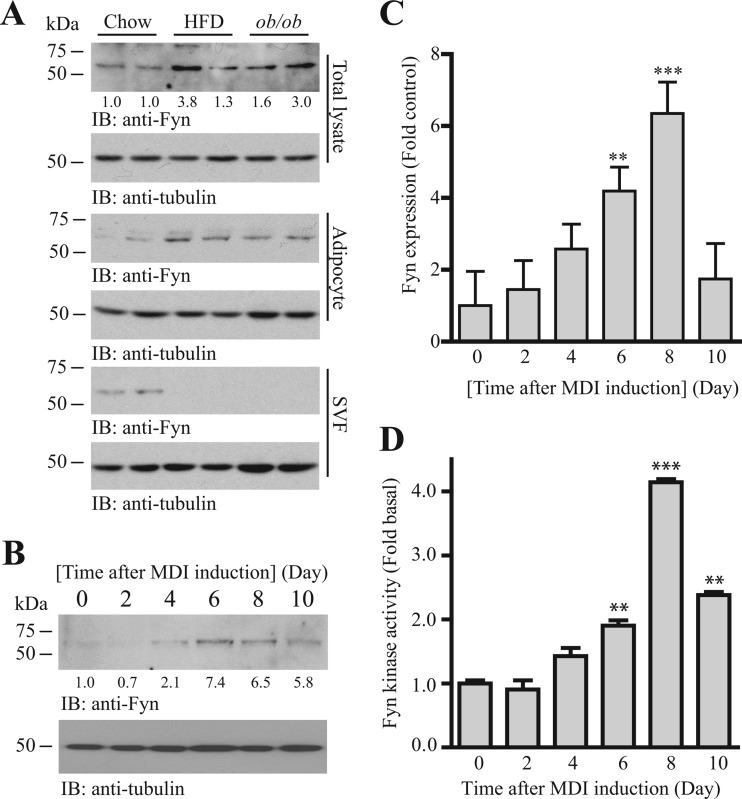
To test if Fyn is involved in adipogenesis, we first compared the expressions of Fyn in the adipose tissues of lean and obese mice. Higher Fyn expression was detected in the inguinal WAT of the HFD (40% kcal, 20 weeks)-fed and obese (ob/ob) mice than in the chow-fed mice (Fig. 1A, 1st panel). Separation of the adipose tissue into adipocytes and stromal vascular fractions (SVF) further indicated a specific increase of Fyn expression solely in the adipocytes (Fig. 1A, 3rd panel). Interestingly, the amount of Fyn protein in SVF was greatly diminished in the obese mice (Fig. 1A, 5th panel). The elevated Fyn expression during adipocyte development was further demonstrated in vitro using a 3T3-L1 differentiation assay. The amount of Fyn protein increased progressively in isobutylmethylxanthine-dexamethasone-insulin (MDI)-induced 3T3-L1 cells, with the largest amount of Fyn protein found at day 6 after induction (Fig. 1B). The accumulation of Fyn protein was possibly a result of enhanced transcription, as the Fyn mRNA was also elevated progressively during 3T3-L1 cell differentiation (Fig. 1C). Fitting with the mRNA expression pattern (Fig. 1C), the highest enzymatic activity of Fyn occurred 8 days after MDI induction (Fig. 1D). This elevated Fyn activity was simply an outcome of increased expression, as the normalized Fyn activity (i.e., total Fyn activity/Fyn expression) did not increase significantly during adipogenesis (data not shown).
Fig 1.
Fyn is a novel regulator of adipogenesis. (A) The amount of Fyn kinase increases in the adipocytes of obese adipose tissue. Immunoblots were performed using cell lysates prepared from intact inguinal WAT (total lysate), isolated adipocytes (adipocytes), and stromal vascular fractions (SVF). Relative Fyn expression values after normalization with the corresponding tubulin levels are also shown at the bottom of the 1st panel. (B) The amount of Fyn protein increases during adipogenesis. Cell lysates from 3T3-L1 cells were prepared at different differentiation statuses and used in Western blot analysis. Relative Fyn expression values after normalization with the corresponding tubulin levels are also shown at the bottom of the 1st panel. (C) Increase of Fyn transcription in differentiating 3T3-L1 cells. Total RNA was collected from 3T3-L1 cells at different time intervals after MDI induction. Real-time RT-PCR was then conducted to evaluate the expression of Fyn. Results are SEM of triplicate readings using cDNAs from three independent differentiation experiments, normalized with the corresponding β-actin expression and expressed relative to the undifferentiated cells (**, P < 0.01; ***, P < 0.001; versus day 0; one-way ANOVA; n = 3). (D) Fyn kinase activity increases during adipogenesis. Cell lysates of 3T3-L1 cells were collected at various time intervals after MDI induction. The Fyn kinase was then immunoprecipitated, and the enzymatic activity to phosphorylate poly(Glu-Tyr) was measured by an in vitro kinase assay (**, P < 0.001; ***, P < 0.001; versus day 0; one-way ANOVA; n = 3).
Manipulation of Fyn activity or expression controls adipogenesis.
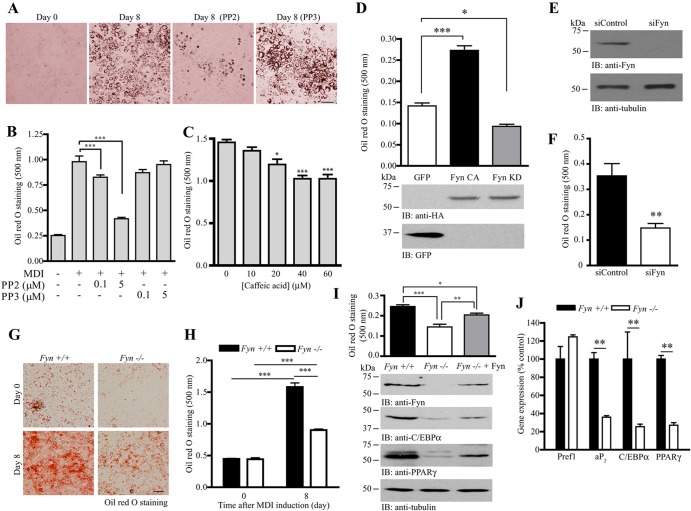
If Fyn is an important kinase in controlling adipocyte differentiation, suppressing its activity or expression may result in impaired adipogenesis. Because no Fyn-specific inhibitor was commercially available, we thus tested if inhibiting Fyn by the pan-SFK inhibitor protein phosphatase 2 (PP2) would reduce adipocyte maturation in 3T3-L1 cells. As expected, pharmacological inhibition of SFK activity using PP2 during MDI induction reduced the number of mature adipocytes formed (Fig. 2A). Spectrophotometric analysis of the stained oil red O showed that PP2 reduced lipid accumulation in a dose-dependent manner (Fig. 2B). In contrast, the presence of an inactive PP2 analogue, PP3, has no effect in inhibiting MDI-induced adipogenesis. Treating the differentiating 3T3-L1 cells with another nonspecific Fyn inhibitor, caffeic acid (29), also significantly reduced lipid accumulation in a dose-dependent manner (Fig. 2C). We further tested the specific role of Fyn in adipogenesis by expressing hemagglutinin (HA)-tagged constitutively active Fyn (Fyn CA) or kinase-dead Fyn (Fyn KD) plasmid in 3T3-L1 preadipocytes before MDI induction. Overexpression of Fyn CA significantly increased the adipogenesis compared to that with the control (green fluorescent protein [GFP]-transfected) cells. On the other hand, inhibition of Fyn kinase by Fyn KD overexpression decreased the amount of lipid accumulation (Fig. 2D). To further demonstrate the important role of Fyn in adipogenesis, we depleted Fyn in 3T3-L1 preadipocytes using specific siRNA against Fyn (Fig. 2E). Lipid accumulation was impaired in the siFyn-transfected cells after MDI induction (Fig. 2F). Similarly, the amount of lipid in MDI-induced Fyn−/− MEF was substantially smaller than the amount in wild-type (WT) MEF (Fig. 2G and H). Nevertheless, overexpression of Fyn in the Fyn−/− MEF rescued its adipogenic ability, which was indicated by the increased oil red O staining and expression of mature adipocyte markers (C/EBPα and PPARγ) (Fig. 2I). Expressions of these key adipocyte markers (aP2, C/EBPα, and PPARγ) were also significantly reduced in the inguinal WAT of Fyn−/− mice (Fig. 2J), suggesting that the Fyn−/− adipose tissue may contain fewer mature adipocytes. In contrast, the expressions of the preadipocyte marker Pref1 were comparable for the wild-type and Fyn−/− WAT.
Fig 2.
Manipulation of Fyn controls adipogenesis. (A) Inhibition of SFK suppresses adipogenesis. Adipocyte differentiation was induced in 3T3-L1 cells in the presence of DMSO, PP2 (5 μM), or PP3 (5 μM). After 8 days, the cells were fixed and stained with oil red O. Bar, 100 μm. (B) Quantification of stained oil red O in differentiated 3T3-L1 adipocytes treated with different concentrations of PP2 or PP3 (***, P < 0.001; one-way ANOVA; n = 3). (C) Caffeic acid inhibits adipocyte differentiation. Various concentrations of caffeic acid were added to the 3T3-L1 preadipocytes during MDI induction. Eight days after induction, the cells were fixed, stained with oil red O, extracted with isopropanol, and spectrophotometrically quantified (*, P < 0.05; ***, P < 0.001; versus the control; one-way ANOVA; n = 3). (D) Overexpression of Fyn enhances 3T3-L1 differentiation. HA-tagged Fyn CA, Fyn KD, or control vector (GFP) was transfected into the 3T3-L1 preadipocytes. After transfection, the cells were subjected to MDI induction. Eight days after induction, the cells were stained with oil red O and quantified (top panel) (*, P < 0.05; ***, P < 0.001; one-way ANOVA; n = 3). Expressions of the Fyn mutants (middle panel) and GFP (bottom panel) were also verified. (E) Depletion of Fyn in 3T3-L1 cells. 3T3-L1 preadipocytes were transfected with control siRNA (siControl) or siRNA against Fyn (siFyn). After 72 h, expressions of Fyn (upper panel) and tubulin (lower panel) were verified by immunoblotting. (F) Fyn depletion inhibits 3T3-L1 differentiation. Seventy-two hours after siRNA transfection, 3T3-L1 cells were stimulated with the MDI cocktail. Eight days after induction, the cells were fixed, stained with oil red O, and quantified (top panel) (**, P < 0.01; Student's t test; n = 3). (G) Defective adipocyte differentiation in Fyn−/− MEF. MDI inductions were performed in confluent MEF isolated from wild-type or Fyn−/− embryos (E13.5). Eight days after induction, the cells were fixed and stained with oil red O. Bar, 50 μm. (H) Quantification of stained oil red O in differentiated MEF shown in panel G (***, P < 0.001; Student's t test; n = 3). (I) Rescue of adipogenesis in Fyn−/− MEF by overexpressing Fyn. DNA plasmid (pRK5-Fyn) was delivered into Fyn−/− MEF by electroporation. Seventy-two hours after electroporation, the cells were stimulated with MDI cocktail. Eight days after induction, the cells were fixed, stained with oil red O, and quantified (1st panel) (*, P < 0.05; **, P < 0.01; ***, P < 0.001; one-way ANOVA; n = 4). Expressions of Fyn (2nd panel), C/EBPα (3rd panel), PPARγ (4th panel), and tubulin (5th panel) were also examined. (J) Reduced expression of adipogenic markers in Fyn−/− inguinal WAT. Total RNA was isolated from wild-type and Fyn−/− (3-month-old female) WAT and used to perform real-time RT-PCR (**, P < 0.01; Student's t test; n = 3 mice).
Fyn regulates JAK2-mediated STAT5 phosphorylation in adipose tissue.
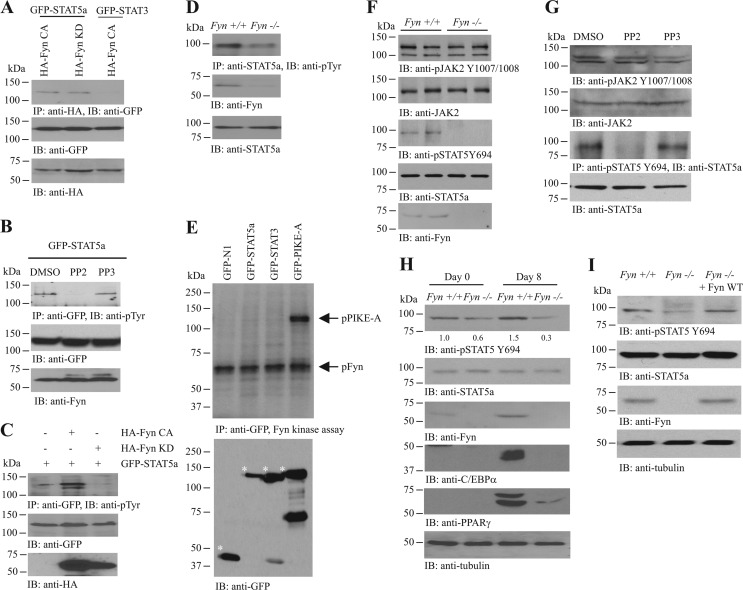
STAT5a is an important transcription factor in triggering adipogenesis (5, 6, 30). To test if STAT5a is a binding target of Fyn, we cotransfected various Fyn mutants and STAT proteins into HEK293 cells and examined their interactions. Both Fyn CA and Fyn KD interacted with STAT5a (Fig. 3A), indicating that the kinase activity is dispensable for their association. The Fyn/STAT5a interaction was specific, as Fyn did not associate with STAT3a (Fig. 3A). This result thus prompted us to hypothesize that Fyn may regulate STAT5a activity through altering its phosphorylation status. In differentiated 3T3-L1 adipocytes, inhibiting SFK by PP2 diminished STAT5a phosphorylation (Fig. 3B). In addition, overexpressing Fyn KD in differentiated 3T3-L1 cells decreased the total tyrosine phosphorylation of STAT5a, whereas the presence of Fyn CA increased STAT5a phosphorylation (Fig. 3C). Total tyrosine phosphorylation of STAT5a was also reduced in the inguinal WAT of Fyn−/− mice (Fig. 3D). These results suggest that Fyn may be a physiological kinase of STAT5a. However, recombinant Fyn kinase did not phosphorylate STAT5a in the in vitro kinase assay but robustly phosphorylated PIKE-A, a result which is in good agreement with our previous finding that Fyn phosphorylates PIKE-A on tyrosine 682 and 774 (31) (Fig. 3E). Thus, Fyn may modulate STAT5a phosphorylation indirectly through other signaling molecules/kinases. Notably, STAT5a Y694 phosphorylation was reduced in Fyn−/− WAT (Fig. 3F, 3rd panel) and PP2-treated 3T3-L1 cells (Fig. 3G, 3rd panel). We did not observe any change in JAK2 activity (as revealed by Y1007/1008 phosphorylation) in Fyn−/− WAT (Fig. 3F, 1st panel) or PP2-treated 3T3-L1 cells (Fig. 3G, 1st panel), a result which fits with the previous finding in the bone marrow-derived pro-B-cell line BaF-3 that SFK inhibitors did not affect the basal or PRL-stimulated JAK2 phosphorylation (10). STAT5a Y694 phosphorylation was also reduced in Fyn−/− MEF (Fig. 3H, 1st panel). However, the impaired STAT5a phosphorylation in MDI-treated Fyn−/− MEF was able to be rescued when Fyn was overexpressed in these cells (Fig. 3I). Thus, our data support the idea that Fyn regulates STAT5a activation in adipose tissues.
Fig 3.
Fyn enhances JAK2-mediated STAT5 phosphorylation. (A) Fyn interacts with STAT5a. Various GFP-tagged STAT constructs were cotransfected with HA-tagged Fyn mutants into HEK293 cells. Fyn proteins were immunoprecipitated, and the associated STAT proteins were analyzed by Western blotting (top panel). Expressions of GFP-STATs (middle panel) and HA-Fyn proteins (bottom panel) were also examined. (B) Inhibition of Fyn reduces STAT5a phosphorylation. GFP-STAT5a was transfected into differentiated 3T3-L1 cells, followed by DMSO, PP2 (5 μM), or PP3 (5 μM) treatment (24 h). The GFP-STAT5a proteins were then immunoprecipitated, and the total tyrosine phosphorylation was examined using antiphosphotyrosine (PY20) antibody (top panel). Expressions of the transfected GFP-STAT5a (middle panel) and endogenous Fyn (bottom panel) are also shown. (C) Overexpression of Fyn increases STAT5a total phosphorylation. Differentiated 3T3-L1 cells were transfected with a different combination of GFP-STAT5a, HA-Fyn CA, or HA-Fyn KD. GFP-STAT5a was then immunoprecipitated, and the total tyrosine phosphorylation of the protein was detected using PY20 antibody (top panel). Expressions of the transfected GFP-STAT5a (middle panel) and HA-Fyn (bottom panel) were also verified. (D) Total STAT5a phosphorylation is diminished in the inguinal WAT of Fyn−/− mice. STAT5a proteins in wild-type and Fyn−/− WAT were immunoprecipitated, and the total tyrosine phosphorylation of the protein was detected using PY20 antibody (top panel). Expressions of Fyn (middle panel) and STAT5a (bottom panel) were also verified. (E) Fyn does not phosphorylate STAT5a. Cell lysates from HEK293 cells overexpressing various GFP-tagged constructs were collected, and the GFP-tagged proteins were immunoprecipitated. The proteins were then incubated with recombinant Fyn kinase in the presence of 32P-γ-ATP and resolved in SDS-PAGE, and the protein phosphorylation was detected by autoradiography (upper panel). Expression of various GFP-tagged proteins (asterisked) was also examined (lower panel). (F) Reduced STAT5 Y694 phosphorylation in the inguinal WAT of Fyn−/− mice. Cell lysates were prepared from wild-type and Fyn−/− (3-month-old female) WAT, and the autophosphorylation of JAK2 (1st panel) and JAK2-mediated STAT5 phosphorylations (3rd panel) were examined. Expressions of JAK2 (2nd panel), STAT5a (4th panel), and Fyn (5th panel) were also examined. (G) Inhibition of SFK kinase activity diminishes JAK2-mediated STAT5a phosphorylation. Differentiated 3T3-L1 cells were treated with DMSO, PP2 (1 μM), or PP3 (1 μM) for 24 h. The STAT5a was then immunoprecipitated, and its phosphorylation by JAK2 was measured using anti-phospho-STAT5 Y694 antibody (3rd panel). Phosphorylation of JAK2 in the presence of SFK inhibitor was also examined (1st panel). Expressions of the endogenous JAK2 (2nd panel) and STAT5a (4th panel) were also examined. (H) STAT5a Y694 phosphorylation is reduced in differentiated Fyn−/− MEF. MDI inductions were performed in confluent MEF isolated from wild-type and Fyn−/− embryos (E13.5). Eight days after induction, cell lysates were collected and the STAT5a was immunoprecipitated. JAK2-induced phosphorylation of the precipitated STAT5a was examined using anti-phospho-STAT5 Y694 antibody (1st panel). Expressions of the endogenous STAT5a (2nd panel), Fyn (3rd panel), C/EBPα (5th panel), PPARγ (6th panel), and tubulin (7th panel) are also shown. The relative STAT5 Y694 phosphorylation values after normalization with the corresponding STAT5a levels are shown at the bottom of the 1st panel. (I) Enhanced STAT5 phosphorylation in Fyn-overexpressed Fyn−/− MEF. DNA plasmid (pRK5-Fyn) was delivered into Fyn−/− MEF by electroporation. Seventy-two hours after electroporation, the cells were stimulated with MDI cocktail. Eight days after induction, the cells were collected to examine STAT5 Y694 phosphorylation (1st panel). Expressions of STAT5a (2nd panel), Fyn (3rd panel), and tubulin (4th panel) were also determined.
PIKE-A is implicated in adipogenesis.
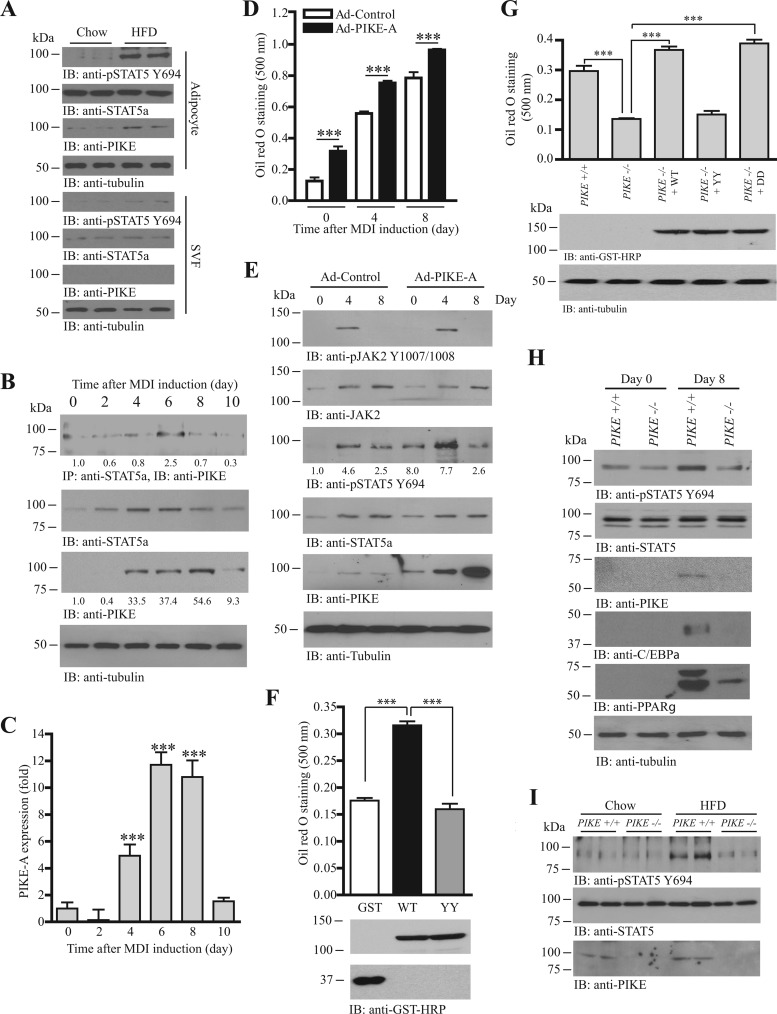
PIKE expression increases in the WAT of obese mice and PIKE−/− mice are obesity resistant with reduced adipose mass (21), suggesting that PIKE-A may be involved in adipogenesis. To further demonstrate that the increased PIKE expression is adipocyte specific, we separated the adipocyte and SVF from the adipose tissue collected from chow- or HFD-fed mice. The amount of PIKE-A was greatly enhanced in adipocytes but not SVF of the obese adipose tissue (Fig. 4A, 3rd and 7th panels). Similarly, STAT5a phosphorylation was elevated mainly in the adipocytes (Fig. 4A, 1st and 5th panels). The amount of PIKE-A in 3T3-L1 cells also increased when 3T3-L1 cells were committing adipocyte differentiation, with the maximal amount of PIKE-A found 8 days after MDI induction (Fig. 4B, 3rd panel). Real-time RT-PCR revealed a similar pattern of PIKE-A expression; a significant increase of PIKE-A transcription occurred 4 days after MDI induction, and it maximized at 6 to 8 days and returned to near the basal level at 10 days (Fig. 4C). The PIKE-A/STAT5a association in differentiating 3T3-L1 cells peaked at the time (day 6) (Fig. 4B, 1st panel) when the amount of STAT5a (Fig. 4B, 2nd panel) and Fyn protein (Fig. 1B) was the highest, indirectly supporting the idea that Fyn activity is critical for their association. We next asked if increasing PIKE-A expression enhances adipogenesis. We infected 3T3-L1 preadipocytes with adenovirus overexpressing PIKE-A, followed by MDI induction. As expected, lipid accumulation was significantly increased in PIKE-A-overexpressed cells before and after MDI induction (Fig. 4D). STAT5a Y694 phosphorylation was also elevated in the differentiating (day 0 and day 4) but not differentiated Ad-PIKE-A cells (Fig. 4E, 3rd panel). In contrast, activation of JAK2 was not altered by PIKE overexpression (Fig. 4E, 1st panel). Interestingly, mutation of Fyn phosphorylation sites (PIKE-A YY) compromised the adipogenic effect of PIKE-A, as overexpressing PIKE-A YY in 3T3-L1 preadipocytes did not enhance cell differentiation, suggesting that the phosphorylation by Fyn is essential for PIKE-A to promote adipogenesis (Fig. 4F). Rescue experiments performed in PIKE−/− MEF further proved that the adipogenic activity of PIKE-A is regulated by Fyn phosphorylation. While adipogenesis in PIKE−/− MEF was significantly impaired, overexpressing PIKE-A WT or Fyn phosphorylation-mimicked mutant (PIKE-A DD) in these cells restored their adipogenic ability (Fig. 4G). In contrast, PIKE−/− MEF were not able to differentiate into adipocytes when the Fyn phosphorylation-defective PIKE-A construct (PIKE-A YY) was overexpressed (Fig. 4G). Since PIKE-A is critical for JAK2-mediated STAT5a phosphorylation in mammary epithelial cells (22), we thus monitored if STAT5a phosphorylation was also altered in PIKE−/− MEF. As shown in Fig. 4H, STAT5a Y694 phosphorylation was increased in wild-type MEF after MDI induction, whereas no such phosphorylation increase was detected in PIKE−/− MEF, a finding which resembled the observation in Fyn−/− MEF (Fig. 3H, 1st panel). Consequently, STAT5a activation was impaired in the MDI-induced PIKE−/− MEF, as expressions of the STAT5a-controlled adipogenic markers (C/EBPα and PPARγ) were reduced (Fig. 4H, 4th and 5th panels). We also found that STAT5a Y694 phosphorylation was diminished in the inguinal WAT of PIKE−/− mice after HFD feeding (Fig. 4I, 1st panel). Consequently, the STAT5a-mediated aP2, C/EBPα, and PPARγ expressions were diminished (21). Taken together, our results suggest that PIKE-A promotes adipogenesis through regulating STAT5a phosphorylation.
Fig 4.
PIKE-A enhances adipogenesis by promoting the JAK2-mediated STAT5a phosphorylation. (A) Increased PIKE-A expression and STAT5 phosphorylation in the adipocytes of HFD-fed mice. Immunoblots were performed using isolated adipocytes (adipocytes) and stromal vascular fractions (SVF) from chow- or HFD-fed mice. (B) PIKE-A/STAT5a association is upregulated during adipogenesis. Lysates of 3T3-L1 cells were prepared at different time points after MDI induction. Endogenous STAT5a was then immunoprecipitated, and the associated PIKE-A was examined (1st panel). Expressions of STAT5a (2nd panel), PIKE-A (3rd panel), and tubulin (4th panel) are also shown. The relative amount of coprecipitated PIKE-A at various time intervals after normalization with the expression level of STAT5a is indicated at the bottom of the 1st panel. Relative PIKE-A expression values after normalization with the corresponding tubulin levels are also shown at the bottom of the 4th panel. (C) PIKE-A expression is increased during adipogenesis. Total RNA was collected from 3T3-L1 cells at different time intervals after MDI induction. Real-time RT-PCR was then conducted to evaluate the expression of PIKE-A. Results are SEM of triplicate readings using cDNAs from three independent differentiation experiments and are expressed as fold inductions after normalization with the corresponding β-actin expression (***, P < 0.001; versus day 0; one-way ANOVA; n = 3). (D) Overexpression of PIKE-A in 3T3-L1 cells enhances adipogenesis. 3T3-L1 preadipocytes were infected with control adenovirus (Ad-Control) or adenovirus overexpressing PIKE-A (Ad-PIKE-A) for 48 h. The cells were then subjected to MDI induction. Lipid accumulation at different time intervals after MDI induction was quantified by oil red O staining (***, P < 0.001; Student's t test; n = 3). (E) PIKE-A overexpression enhances JAK2-mediated STAT5a phosphorylation in differentiating 3T3-L1 cells. 3T3-L1 preadipocytes were infected with control adenovirus (Ad-Control) or adenovirus overexpressing PIKE-A (Ad-PIKE-A) for 48 h. The cells were then subjected to MDI induction. Cell lysates were collected at different time points, and the phosphorylations of JAK2 on Y1007/1008 (1st panel) and STAT5a on Y694 (3rd panel) were examined. Expressions of JAK2 (2nd panel), STAT5a (4th panel), PIKE-A (5th panel), and tubulin (6th panel) are also shown. The relative values of STAT5 Y694 phosphorylation after normalization with the STAT5a expression are shown at the bottom of the 3rd panel. (F) Mutation of Fyn phosphorylation sites on PIKE-A abolishes its adipogenic activity. Control (GFP), mGST-PIKE-A WT, or mGST-PIKE-A YY plasmid was delivered into 3T3-L1 preadipocytes using electroporation. The cells were then subjected to MDI induction. After 8 days, lipid accumulation was evaluated by oil red O staining (top panel) (***, P < 0.001; one-way ANOVA; n = 3). Expressions of the transfected PIKE-A (middle panel) and GFP (bottom panel) were also verified. (G) Rescue of adipogenesis in PIKE−/− MEF. Various mGST-tagged PIKE-A plasmids [PIKE-A WT, PIKE-A Y682,774F (YY), and PIKE-A Y682,774D (DD)] were delivered into PIKE−/− MEF using electroporation. Seventy-two hours after the gene delivery, the cells were stimulated with MDI cocktail. Eight days after induction, the cells were fixed, stained with oil red O, and quantified (top panel) (***, P < 0.001; one-way ANOVA; n = 4). Expressions of the transfected plasmids (middle panel) and endogenous tubulin (bottom panel) were also determined. (H) JAK2-mediated STAT5a phosphorylation is reduced in differentiated PIKE−/− MEF. MDI inductions were performed in confluent MEF isolated from wild-type and PIKE−/− embryos (E13.5). Eight days after induction, cell lysates were collected and the JAK2-induced phosphorylation of STAT5 was examined using anti-phospho-STAT5 Y694 antibody (1st panel). Expressions of the endogenous STAT5a (2nd panel), PIKE-A (3rd panel), C/EBPα (4th panel), PPARγ (5th panel), and tubulin (6th panel) are also shown. (I) Reduced STAT5 Y694 phosphorylation in PIKE−/− WAT after HFD feeding. Cell lysates were prepared from the inguinal WAT of wild-type and PIKE−/− (6-month-old female) mice after chow or HFD feeding for 14 weeks, and the phospho-STAT5 Y649 (top panel) was examined. Expressions of the endogenous STAT5a (middle panel) and PIKE-A (bottom panel) are also examined.
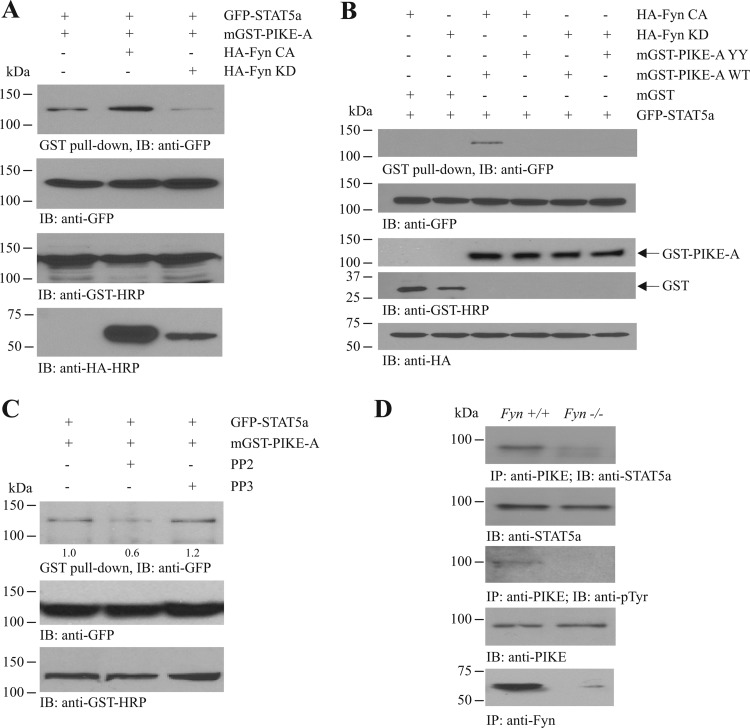
Fyn regulates the PIKE-A/STAT5a association.
We have previously reported that PIKE-A is a novel regulator of STAT5a by coupling the transcription factor to PRLR for JAK2-mediated activation (22). Given that PIKE-A is also a substrate of Fyn (31), we thus hypothesized that Fyn may regulate the JAK2-induced STAT5a phosphorylation through modulating the PIKE-A/STAT5a interaction. To test this hypothesis, we cotransfected GFP-tagged STAT5a (GFP-STAT5a), mammalian GST-tagged PIKE-A (mGST-PIKE-A), and various HA-tagged Fyn mutants (HA-Fyn CA and KD) into HEK293 cells and monitored the PIKE-A/STAT5a interaction. The association between PIKE-A and STAT5a was readily detected in HEK293 cells without Fyn transfection (Fig. 5A, 1st lane). When Fyn CA was overexpressed, the PIKE-A/STAT5a interaction was enhanced (Fig. 5A, 2nd lane). On the other hand, the association between STAT5a and PIKE-A decreased when Fyn KD was overexpressed (Fig. 5A, 3rd lane). This result suggests that Fyn positively regulates the PIKE-A/STAT5a binding. We also examined the effect of PIKE-A phosphorylation by Fyn on its association with STAT5a. While robust PIKE-A/STAT5a interaction was detected in HEK293 cells expressing mGST-PIKE-A WT and GFP-STAT5a in the presence of HA-Fyn CA, no STAT5a interaction with PIKE-A was found when both Fyn phosphorylation sites (Y682 and Y774) were mutated (mGST-PIKE-A YY) (Fig. 5B, lanes 3 and 4). As a negative control, Fyn KD was not found to provoke PIKE-A/STAT5a association, regardless of the use of PIKE-A WT or YY (Fig. 5B, lanes 5 and 6), suggesting that the integrity of Fyn kinase activity and the PIKE-A phosphorylation sites is critical for PIKE-A/STAT5a association. Moreover, inhibiting Fyn kinase activity by PP2, but not PP3, suppressed the PIKE-A/STAT5a association in HEK293 cells (Fig. 5C). To further test whether Fyn is critical for regulating PIKE-A/STAT5a binding, we examined their interaction in Fyn−/− WAT. As shown in the 1st panel of Fig. 5D, the association between PIKE-A and STAT5a was evident in wild-type WAT, whereas their interaction was greatly diminished in Fyn−/− WAT. Total tyrosine phosphorylation of PIKE-A was also greatly diminished in the Fyn−/− tissue (Fig. 5D, 3rd panel).
Fig 5.
Fyn regulates PIKE-A/STAT5a interaction. (A) Fyn activation enhances PIKE-A/STAT5a association. HEK293 cells were transfected with different combinations of plasmids as indicated. Transfected PIKE-A was precipitated using GST pulldown, and the associated STAT5a was detected using anti-GFP antibody (1st panel). Expressions of the transfected STAT5a (2nd panel), PIKE-A (3rd panel), and Fyn (4th panel) are also shown. (B) Mutation of Fyn phosphorylation sites in PIKE-A abolishes its binding with STAT5a. HEK293 cells were transfected with different combinations of plasmids. The association between PIKE-A and STAT5a was examined using GST pulldown (1st panel). Expressions of the transfected STAT5a (2nd panel), PIKE-A (3rd panel), GST (4th panel), and Fyn (5th panel) were also verified. (C) Inhibition of Fyn diminishes the PIKE-A/STAT5a association. HEK293 cells were cotransfected with GFP-STAT5a and mGST-PIKE-A. The transfected cells were then treated with PP2 (1 μM) or PP3 (1 μM) for 24 h. The interaction between PIKE-A and STAT5a was then examined using immunoprecipitation (top panel). Expressions of the transfected STAT5a (middle panel) and PIKE-A (bottom panel) were also verified. Relative values of the coprecipitated STAT5a after normalization with the expression of mGST-PIKE-A are indicated at the bottom of top panel. (D) Reduced PIKE-A/STAT5a interaction in Fyn−/− WAT. Endogenous PIKE-A in the inguinal WAT of wild-type and Fyn−/− (3-month-old) mice was immunoprecipitated using antibody against the C terminus of PIKE-A, and the associated STAT5a was examined (1st panel). Total tyrosine phosphorylation of the immunoprecipitated PIKE-A was also determined (3rd panel). Expressions of the endogenous STAT5a (2nd panel), PIKE-A (4th panel), and Fyn (5th panel) are also shown.
PRL-induced STAT5a activation in adipocytes is regulated by Fyn and PIKE-A.
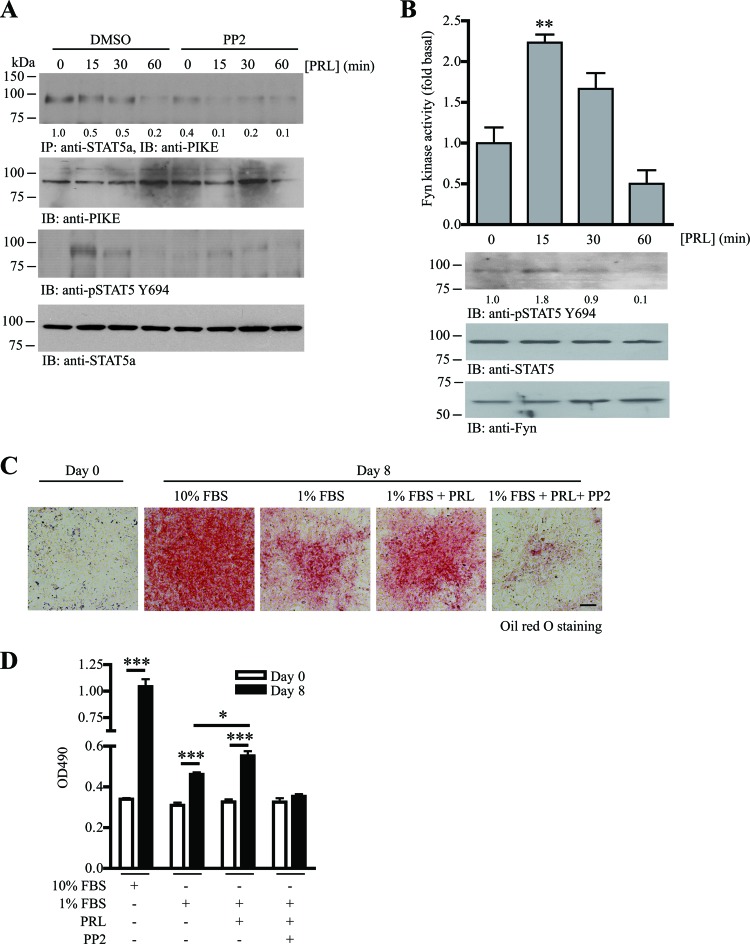
Since PIKE-A/STAT5a association is regulated by Fyn (Fig. 5) and PRL (22), we tested if Fyn is critical for PRL-regulated PIKE-A/STAT5a tethering. In differentiated 3T3-L1 cells, PIKE-A constitutively associated with STAT5a (Fig. 6A, 1st panel). After PRL stimulation for 60 min, the binding between PIKE-A and STAT5a diminished, a finding which agreed with our observation in PRL-stimulated HC11 cells (22). This reduced binding was possibly caused by the nuclear translocation of STAT5a after PRL stimulation, whereas PIKE-A remained associated with the cytoplasmic PRLR (22). In contrast, the association of PIKE-A and STAT5a was reduced when Fyn was inhibited by PP2 and remained low during the PRL stimulation (Fig. 6A, 1st panel). The STAT5a Y694 phosphorylation induced by PRL was also diminished (Fig. 6A, 3rd panel). These data suggest that Fyn is a downstream effector of PRL to induce adipogenesis. To test this hypothesis, we examined if PRL can activate Fyn in adipocytes. As expected, PRL increased the adipose Fyn kinase activity after 15 min of stimulation. Fyn kinase activity returned to the basal level after 1 h (Fig. 6B). Differentiated (day 8) 3T3-L1 cells were used in this assay because the highest expression of PRLR was found in differentiated adipocytes (32). To further demonstrate that Fyn is a downstream effector of PRL signaling during adipocyte differentiation, we tested if blocking Fyn activity can inhibit PRL-induced adipogenesis. During adipocyte differentiation, the presence of sufficient fetal bovine serum (FBS) is essential (30). Since FBS contains numerous growth factors that may overwhelm the effect of additional PRL in inducing adipocyte differentiation, we therefore reduced the amount of FBS from 10% to 1% when we induced 3T3-L1 differentiation in this experiment (Fig. 6C). As shown in Fig. 6D, lipid accumulation in 3T3-L1 cells after MDI induction was greatly reduced when 1% FBS was used. However, the lipid accumulation in 1% FBS-stimulated cells was still significantly higher than that in the undifferentiated cells, suggesting that 3T3-L1 cells are still able to differentiate even though the FBS is as low as 1% (Fig. 6D). Addition of PRL to the induction medium, containing 1% FBS, further increased the amount of lipid accumulation significantly, indicating that PRL plays a positive role in promoting 3T3-L1 adipogenesis. The presence of PP2 abolished both ordinary and PRL-induced adipocyte differentiation (Fig. 6D), consistent with the finding that SFK is a downstream effector of PRL and is able to induce adipogenesis.
Fig 6.
PRL-induced PIKE-A/STAT5a tethering is mediated by Fyn activation. (A) PRL-stimulated STAT5a and PIKE-A phosphorylations are obstructed by SFK inhibitor. Fyn kinase activity in differentiated 3T3-L1 cells was inhibited by PP2 (1 μM) for 1 h. The cells were then stimulated with PRL (2 μg/ml) for various time intervals. The association of PIKE-A/STAT5a was examined using immunoprecipitation (1st panel). Phosphorylation on STAT5 Y649 was also examined (3rd panel). Expressions of the endogenous PIKE-A (2nd panel) and STAT5a (4th panel) are also shown. The relative amount of coprecipitated PIKE-A at various time intervals after normalization with the expression level of STAT5a is indicated at the bottom of the 1st panel. (B) PRL activates Fyn in adipocytes. Eight days after MDI induction, 3T3-L1 cells (serum starved for 24 h) were stimulated with PRL (2 μg/ml) for various time intervals. Cell lysates were then prepared, and Fyn was immunoprecipitated to measure its kinase activity on poly(Glu-Tyr) phosphorylation (1st panel) (**, P < 0.01; one-way ANOVA; n = 3). Phosphorylation of STAT5a on Y649 was detected as a positive control of PRL stimulation (2nd panel). Expressions of STAT5a (3rd panel) and Fyn (4th panel) are also shown. The relative values of STAT5 Y694 phosphorylation after normalization with the STAT5a expression are indicated at the bottom of the 2nd panel. (C) PRL-induced adipogenesis requires the activity of SFK. 3T3-L1 cells were subjected to MDI induction in the presence of 10% FBS, 1% FBS, 1% FBS with 1 μg/ml PRL, or 1% FBS with 1 μg/ml PRL and 1 μM PP2. Eight days after MDI induction, the cells were then fixed and stained with oil red O. Bar, 100 μm. (D) Quantification of the stained oil red O in the differentiated 3T3-L1 cells under various treatments shown in panel C (*, P < 0.05; ***, P < 0.001; versus day 0; Student's t test; n = 3).
DISCUSSION
Here we report a new signaling cascade that promotes adipogenesis. We show that Fyn phosphorylates PIKE-A, thereby increasing its association with STAT5a, and triggers JAK2-mediated STAT5a activation and gene transcriptions. Depletion of Fyn or PIKE through genetic ablation or pharmacological inhibition impedes adipogenesis both in vitro and in vivo. On the other hand, increasing the expression of Fyn or PIKE enhances adipocyte differentiation in 3T3-L1 cells. Moreover, we have proposed that the Fyn/PIKE-A/STAT5a pathway can be activated by the adipogenic hormone PRL. Thus, Fyn is a new adipogenic kinase that functions by regulating the PIKE-A/STAT5a interaction. However, Fyn is not the sole SFK member involved in adipogenesis. Sun et al. have reported that Src kinase controlled adipocyte differentiation in 3T3-L1 cells via mediating insulin/c-Cbl signaling (33), suggesting that different SFK members may participate in the process through multiple pathways.
Fyn is a tyrosine kinase involved in numerous functions, including immune cell activation, brain development, neuronal transmission, differentiation and myelination, cell migration, cell proliferation, and fertilization (24, 34 –38). Bastie et al. reported that Fyn−/− mice have less adipose mass and elevated fatty oxidation in muscle and fat tissues (12). They also found that the decrease of adipose mass in Fyn−/− mice was due to a reduction of adipocyte cell size but not cell number (12). Using a cell size-based estimation method (39), however, we found a contradictory result that the total number of adipocytes in the inguinal WAT of our Fyn−/− mice is smaller than that in their wild-type control (our unpublished data). Since Bastie et al. did not provide the total amount of adipocytes in Fyn−/− inguinal WAT or any details of the method used to determine the adipocyte numbers in their study, we were unable to compare this discrepant observation with their result. Nevertheless, our finding of reduced adipogenic marker expression in Fyn−/− WAT aligns with our observation (Fig. 2J). Moreover, adipogenesis is inhibited when the 3T3-L1 preadipocytes are challenged with SFK kinase inhibitors (Fig. 2B and C). Fyn-depleted 3T3-L1 preadipocytes or Fyn−/− MEF also fail to differentiate into mature adipocytes (Fig. 2F and H), a finding which provides strong evidence of the importance of Fyn in the process. Additional support of this notion is observed by inhibiting Fyn through overexpression of a Fyn mutant with no kinase activity, which suppresses the adipocyte differentiation (Fig. 2D). Indeed, Fyn expression and activity are increased during adipocyte differentiation, possibly by PRL or other unidentified adipogenic factors (Fig. 1B and D and 6B). Thus, our data support the conclusion that Fyn is important for adipocyte differentiation both in vitro and in vivo.
STAT proteins participate in adipogenesis in a member-specific manner; STAT1, -3, and -5 are all markedly elevated during 3T3-L1 differentiation (40). Functionally, STAT1 is a regulator of those genes involved in insulin sensitivity, whereas STAT5a and -b contribute to the lipid accumulation (41). Studies in 3T3-L1 cells further suggest that STAT5a, but not STAT5b, induces adipogenesis in nonprecursor cells, although both STAT5a−/− and STAT5b−/− mice display reduced adipose mass (7, 42). Nevertheless, the nontranscriptional regulatory mechanism of these important transcription factors during adipogenesis has not been systematically studied. We show in this report that Fyn is a new regulator of STAT5a activation during adipogenesis. STAT5a activation (as indicated by Y694 phosphorylation) in adipocytes is decreased if Fyn kinase is silenced in both basal and PRL-stimulated conditions (Fig. 3F to I and 6A). Interestingly, Fyn mediates STAT5a phosphorylation indirectly, as Fyn cannot phosphorylate STAT5a but instead is able to complex with STAT5a (Fig. 3A and E). Thus, the regulatory process on STAT5a phosphorylation must involve an additional protein. Our previous report that PIKE-A interacts with STAT5a to mediate its activation after PRL stimulation in mammary epithelial cells (22) and that Fyn is a kinase of PIKE-A, which modulates its association with other proteins (21), prompted us to suspect that PIKE-A may be the missing link between Fyn and STAT5a. Indeed, phosphorylation of PIKE-A by Fyn is critical for its ability to bind to STAT5a, since either inhibiting Fyn kinase activity or mutating the Fyn phosphorylation sites in PIKE-A diminishes the PIKE-A association with STAT5a and STAT5a activation (Fig. 5). As a result, ablating Fyn or its downstream effector PIKE-A in both MEF and WAT causes a reduction of STAT5a phosphorylation (Fig. 3F and H and 4H).
What is the extracellular factor that stimulates Fyn activation during adipogenesis? Adipocyte differentiation is regulated by numerous hormones, cytokines, or growth factors, including transforming growth factor (TGF), growth hormone (GH), insulin-like growth factor (IGF), insulin, etc. (43). Accumulating evidence also suggests that PRL is implicated in adipogenesis. Freemark et al. reported that PRLR knockout mice have reduced fat deposition, but no such change in adipose mass was detected by a subsequent study (44, 45). Given that in vivo adipogenesis is affected by numerous factors, including genetic background, hormone status, age, and the type of food intake, it is reasonable to find that the relationship between PRL and adiposity in intact animals is not conclusive. However, it is in no doubt that PRLR expression is upregulated in adipocytes differentiated from murine bone marrow stromal cells or human preadipocytes (32, 46, 47). Moreover, ectopic expression of PRLR provokes adipogenic conversion of nonprecursor fibroblasts, and addition of PRL can replace the differentiation-promoting effects of FBS in 3T3-L1 cells (2). Furthermore, chronic hyperprolactinemia is associated with body weight gain and obesity in humans, which is ameliorated by pharmacological suppression of PRL secretion (48, 49). Nevertheless, the molecular mechanism of PRL-induced adipocyte differentiation is not clear. Our data suggest that Fyn is involved in the PRL-induced STAT5a activation through PIKE-A. Indeed, studies by others have demonstrated that Fyn directly associates with all isoforms of PRLR and that Fyn, but not other SFK members, can be activated by PRL (25, 50). It has also been reported that Fyn is important for PRL-induced cell proliferation (10). However, the PRL-induced Fyn activation may not be involved in the clonal expansion of 3T3-L1 cells because proliferation is not affected in Fyn−/− MEF or 3T3-L1 preadipocytes treated with PP2 (data not shown). Moreover, expression and kinase activity of Fyn increased only when the cell proliferation of preadipocytes had already ceased (Fig. 1B to D). Presumably, the PRL-provoked Fyn/PIKE-A/STAT5a cascade participates in the terminal differentiation of adipocytes when all the proteins have the highest expression.
Collectively, we have identified a new function of Fyn in promoting adipogenesis and elucidated the underpinning mechanism. Since malfunction of new adipose tissue formation is critical in obesity (adipocyte hyperplasia) or lipodystrophy development (degenerative conditions) (51, 52), the identification of Fyn as an adipogenic factor may provide a novel intervention target to prevent obesity/lipodystrophy and their complications.
ACKNOWLEDGMENTS
We are grateful to N. C. Reich (Stony Brook University, NY) for the GFP-STAT5a plasmid and C. Schindler (Columbia University, NY) for the GFP-STAT3 plasmid. We also thank Obiamaka Okianyo (Emory University, Atlanta, GA) for her critical reading of the manuscript.
This work is supported by grant R01-NS045627 from the NIH to K. Ye and the URC grant 00016337 from Emory University to C. B. Chan.
Footnotes
Published ahead of print 25 February 2013
REFERENCES
- 1. Gesta S, Tseng YH, Kahn CR. 2007. Developmental origin of fat: tracking obesity to its source. Cell 131: 242–256 [DOI] [PubMed] [Google Scholar]
- 2. Nanbu-Wakao R, Fujitani Y, Masuho Y, Muramatu M, Wakao H. 2000. Prolactin enhances CCAAT enhancer-binding protein-beta (C/EBP beta) and peroxisome proliferator-activated receptor gamma (PPAR gamma) messenger RNA expression and stimulates adipogenic conversion of NIH-3T3 cells. Mol. Endocrinol. 14: 307–316 [DOI] [PubMed] [Google Scholar]
- 3. White UA, Stephens JM. 2010. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell. Endocrinol. 318: 10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hennighausen L, Robinson GW. 2008. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 22: 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shang CA, Waters MJ. 2003. Constitutively active signal transducer and activator of transcription 5 can replace the requirement for growth hormone in adipogenesis of 3T3-F442A preadipocytes. Mol. Endocrinol. 17: 2494–2508 [DOI] [PubMed] [Google Scholar]
- 6. Floyd ZE, Stephens JM. 2003. STAT5A promotes adipogenesis in nonprecursor cells and associates with the glucocorticoid receptor during adipocyte differentiation. Diabetes 52: 308–314 [DOI] [PubMed] [Google Scholar]
- 7. Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. 1997. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11: 179–186 [DOI] [PubMed] [Google Scholar]
- 8. Stewart WC, Pearcy LA, Floyd ZE, Stephens JM. 2011. STAT5A expression in Swiss 3T3 cells promotes adipogenesis in vivo in an athymic mice model system. Obesity (Silver Spring) 19: 1731–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kefalas P, Brown TR, Brickell PM. 1995. Signalling by the p60c-src family of protein-tyrosine kinases. Int. J. Biochem. Cell Biol. 27: 551–563 [DOI] [PubMed] [Google Scholar]
- 10. Fresno Vara JA, Caceres MA, Silva A, Martin-Perez J. 2001. Src family kinases are required for prolactin induction of cell proliferation. Mol. Biol. Cell 12: 2171–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Coppenolle F, Skryma R, Ouadid-Ahidouch H, Slomianny C, Roudbaraki M, Delcourt P, Dewailly E, Humez S, Crepin A, Gourdou I, Djiane J, Bonnal JL, Mauroy B, Prevarskaya N. 2004. Prolactin stimulates cell proliferation through a long form of prolactin receptor and K+ channel activation. Biochem. J. 377: 569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bastie CC, Zong H, Xu J, Busa B, Judex S, Kurland IJ, Pessin JE. 2007. Integrative metabolic regulation of peripheral tissue fatty acid oxidation by the SRC kinase family member Fyn. Cell Metab. 5: 371–381 [DOI] [PubMed] [Google Scholar]
- 13. Yamada E, Pessin JE, Kurland IJ, Schwartz GJ, Bastie CC. 2010. Fyn-dependent regulation of energy expenditure and body weight is mediated by tyrosine phosphorylation of LKB1. Cell Metab. 11: 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long H, Bock HH, Lei T, Chai X, Yuan J, Herz J, Frotscher M, Yang Z. 2011. Identification of alternatively spliced Dab1 and Fyn isoforms in pig. BMC Neurosci. 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li S, Zhang HY, Hu CC, Lawrence F, Gallagher KE, Surapaneni A, Estrem ST, Calley JN, Varga G, Dow ER, Chen Y. 2008. Assessment of diet-induced obese rats as an obesity model by comparative functional genomics. Obesity (Silver Spring) 16: 811–818 [DOI] [PubMed] [Google Scholar]
- 16. Ahn JY, Hu Y, Kroll TG, Allard P, Ye K. 2004. PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc. Natl. Acad. Sci. U. S. A. 101: 6993–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai Y, Wang J, Li R, Ayala G, Ittmann M, Liu M. 2009. GGAP2/PIKE-a directly activates both the Akt and nuclear factor-κB pathways and promotes prostate cancer progression. Cancer Res. 69: 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elkahloun AG, Krizman DB, Wang Z, Hofmann TA, Roe B, Meltzer PS. 1997. Transcript mapping in a 46-kb sequenced region at the core of 12q13.3 amplification in human cancers. Genomics 42: 295–301 [DOI] [PubMed] [Google Scholar]
- 19. Nagase T, Seki N, Ishikawa K, Tanaka A, Nomura N. 1996. Prediction of the coding sequences of unidentified human genes. V. The coding sequences of 40 new genes (KIAA0161-KIAA0200) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res. 3: 17–24 [DOI] [PubMed] [Google Scholar]
- 20. Nie Z, Fei J, Premont RT, Randazzo PA. 2005. The Arf GAPs AGAP1 and AGAP2 distinguish between the adaptor protein complexes AP-1 and AP-3. J. Cell Sci. 118: 3555–3566 [DOI] [PubMed] [Google Scholar]
- 21. Chan CB, Liu X, Jung DY, Jun JY, Luo HR, Kim JK, Ye K. 2010. Deficiency of phosphoinositide 3-kinase enhancer protects mice from diet-induced obesity and insulin resistance. Diabetes 59: 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan CB, Liu X, Ensslin MA, Dillehay DL, Ormandy CJ, Sohn P, Serra R, Ye K. 2010. PIKE-A is required for prolactin-mediated STAT5a activation in mammary gland development. EMBO J. 29: 956–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan CB, Liu X, He K, Qi Q, Jung DY, Kim JK, Ye K. 2011. The association of phosphoinositide 3-kinase enhancer A with hepatic insulin receptor enhances its kinase activity. EMBO Rep. 12: 847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. 1999. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J. Cell Biol. 145: 1209–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kline JB, Roehrs H, Clevenger CV. 1999. Functional characterization of the intermediate isoform of the human prolactin receptor. J. Biol. Chem. 274: 35461–35468 [DOI] [PubMed] [Google Scholar]
- 26. Abella A, Dubus P, Malumbres M, Rane SG, Kiyokawa H, Sicard A, Vignon F, Langin D, Barbacid M, Fajas L. 2005. Cdk4 promotes adipogenesis through PPARγ activation. Cell Metab. 2: 239–249 [DOI] [PubMed] [Google Scholar]
- 27. Kim WK, Jung H, Kim DH, Kim EY, Chung JW, Cho YS, Park SG, Park BC, Ko Y, Bae KH, Lee SC. 2009. Regulation of adipogenic differentiation by LAR tyrosine phosphatase in human mesenchymal stem cells and 3T3-L1 preadipocytes. J. Cell Sci. 122: 4160–4167 [DOI] [PubMed] [Google Scholar]
- 28. Wang J, Lazar MA. 2008. Bifunctional role of Rev.-erbα in adipocyte differentiation. Mol. Cell. Biol. 28: 2213–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kang NJ, Lee KW, Shin BJ, Jung SK, Hwang MK, Bode AM, Heo YS, Lee HJ, Dong Z. 2009. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 30: 321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stewart WC, Baugh JE, Jr, Floyd ZE, Stephens JM. 2004. STAT 5 activators can replace the requirement of FBS in the adipogenesis of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 324: 355–359 [DOI] [PubMed] [Google Scholar]
- 31. Tang X, Feng Y, Ye K. 2007. Src-family tyrosine kinase fyn phosphorylates phosphatidylinositol 3-kinase enhancer-activating Akt, preventing its apoptotic cleavage and promoting cell survival. Cell Death Differ. 14: 368–377 [DOI] [PubMed] [Google Scholar]
- 32. Fleenor D, Arumugam R, Freemark M. 2006. Growth hormone and prolactin receptors in adipogenesis: STAT-5 activation, suppressors of cytokine signaling, and regulation of insulin-like growth factor I. Horm. Res. 66: 101–110 [DOI] [PubMed] [Google Scholar]
- 33. Sun Y, Ma YC, Huang J, Chen KY, McGarrigle DK, Huang XY. 2005. Requirement of SRC-family tyrosine kinases in fat accumulation. Biochemistry 44: 14455–14462 [DOI] [PubMed] [Google Scholar]
- 34. Cooke MP, Abraham KM, Forbush KA, Perlmutter RM. 1991. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn). Cell 65: 281–291 [DOI] [PubMed] [Google Scholar]
- 35. Morse WR, Whitesides JG, III, LaMantia AS, Maness PF. 1998. p59fyn and pp60c-src modulate axonal guidance in the developing mouse olfactory pathway. J. Neurobiol. 36: 53–63 [DOI] [PubMed] [Google Scholar]
- 36. Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. 1992. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science 258: 1903–1910 [DOI] [PubMed] [Google Scholar]
- 37. Yadav V, Denning MF. 2011. Fyn is induced by Ras/PI3K/Akt signaling and is required for enhanced invasion/migration. Mol. Carcinog. 50: 346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu W, Kinsey WH. 2000. Fertilization triggers activation of Fyn kinase in the zebrafish egg. Int. J. Dev. Biol. 44: 837–841 [PubMed] [Google Scholar]
- 39. Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. 2008. Dynamics of fat cell turnover in humans. Nature 453: 783–787 [DOI] [PubMed] [Google Scholar]
- 40. Stephens JM, Morrison RF, Pilch PF. 1996. The expression and regulation of STATs during 3T3-L1 adipocyte differentiation. J. Biol. Chem. 271: 10441–10444 [DOI] [PubMed] [Google Scholar]
- 41. Stephens JM, Morrison RF, Wu Z, Farmer SR. 1999. PPARγ ligand-dependent induction of STAT1, STAT5A, and STAT5B during adipogenesis. Biochem. Biophys. Res. Commun. 262: 216–222 [DOI] [PubMed] [Google Scholar]
- 42. Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. 1997. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc. Natl. Acad. Sci. U. S. A. 94: 7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gregoire FM, Smas CM, Sul HS. 1998. Understanding adipocyte differentiation. Physiol. Rev. 78: 783–809 [DOI] [PubMed] [Google Scholar]
- 44. Freemark M, Fleenor D, Driscoll P, Binart N, Kelly P. 2001. Body weight and fat deposition in prolactin receptor-deficient mice. Endocrinology 142: 532–537 [DOI] [PubMed] [Google Scholar]
- 45. LaPensee CR, Horseman ND, Tso P, Brandebourg TD, Hugo ER, Ben-Jonathan N. 2006. The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology 147: 4638–4645 [DOI] [PubMed] [Google Scholar]
- 46. McAveney KM, Gimble JM, Yu-Lee L. 1996. Prolactin receptor expression during adipocyte differentiation of bone marrow stroma. Endocrinology 137: 5723–5726 [DOI] [PubMed] [Google Scholar]
- 47. Harp JB, Franklin D, Vanderpuije AA, Gimble JM. 2001. Differential expression of signal transducers and activators of transcription during human adipogenesis. Biochem. Biophys. Res. Commun. 281: 907–912 [DOI] [PubMed] [Google Scholar]
- 48. Doknic M, Pekic S, Zarkovic M, Medic-Stojanoska M, Dieguez C, Casanueva F, Popovic V. 2002. Dopaminergic tone and obesity: an insight from prolactinomas treated with bromocriptine. Eur. J. Endocrinol. 147: 77–84 [DOI] [PubMed] [Google Scholar]
- 49. Greenman Y, Tordjman K, Stern N. 1998. Increased body weight associated with prolactin secreting pituitary adenomas: weight loss with normalization of prolactin levels. Clin. Endocrinol. (Oxf.) 48: 547–553 [DOI] [PubMed] [Google Scholar]
- 50. Clevenger CV, Medaglia MV. 1994. The protein tyrosine kinase P59fyn is associated with prolactin (PRL) receptor and is activated by PRL stimulation of T-lymphocytes. Mol. Endocrinol. 8: 674–681 [DOI] [PubMed] [Google Scholar]
- 51. Agarwal AK, Garg A. 2006. Genetic disorders of adipose tissue development, differentiation, and death. Annu. Rev. Genomics Hum. Genet. 7: 175–199 [DOI] [PubMed] [Google Scholar]
- 52. Camp HS, Ren D, Leff T. 2002. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol. Med. 8: 442–447 [DOI] [PubMed] [Google Scholar]