Abstract
K-Ras is frequently mutated in human cancers. Mutant (mt) K-Ras can stimulate both oncogenic transformation and apoptosis through activation of extracellular signal-regulated kinase (ERK) and AKT pathways and the MST2 pathway, respectively. The biological outcome is determined by the balance and cross talk between these pathways. In colorectal cancer (CRC), a K-Ras mutation is negatively correlated with MST2 expression, as mt K-Ras can induce apoptosis by activating the MST2 pathway. However, wild-type (wt) K-Ras can prevent the activation of the MST2 pathway upon growth factor stimulation and enable transformation by mt K-Ras in CRC cells that express MST2. Here we have investigated the mechanism by which wt and mt K-Ras differentially regulate the MST2 pathway and MST2-dependent apoptosis. The ability of K-Ras to activate MST2 and MST2-dependent apoptosis is determined by the differential activation kinetics of mt K-Ras and wt K-Ras. Chronic activation of K-Ras by mutation or overexpression of Ras exchange factors results in the activation of MST2 and LATS1, increased MST2-LATS1 complex formation, and apoptosis. In contrast, transient K-Ras activation upon epidermal growth factor (EGF) stimulation prevents the formation of the MST2-LATS1 complex in an AKT-dependent manner. Our data suggest that the close relationship between Ras prosurvival and proapoptotic signaling is coordinated via the differential regulation of the MST2-LATS1 interaction by transient and chronic stimuli.
INTRODUCTION
The Ras family of small GTPases comprises three isoforms: H-Ras, N-Ras, and K-Ras (1). Mutations of these proteins, in particular K-Ras, are present in more than 30% of cancers, making Ras mutations one of the most frequent events in cancer (2). Upon growth factor stimulation, Ras proteins cycle between an inactive state bound to GDP and an active state bound to GTP. Ras activation is induced by guanidine exchange factors (GEFs), and inactivation is catalyzed by GTPase activating proteins (GAPs), which enhance the intrinsic ability of Ras to hydrolyze GTP (1). Activated Ras proteins bind to several effector proteins that mediate a range of different biological processes, such as proliferation, differentiation, and apoptosis. The best-characterized Ras effectors are Raf-1, phosphoinositide-3 kinase (PI3K), and RalGDS (3). Raf-1 and PI3K pathways play a central role in the regulation of prosurvival and proliferation signals, and aberrant activation of these pathways is observed in most transformed cells (4, 5). These pathways interact at different levels through various positive and negative feedback loops upstream and downstream of Ras. Hence, the cross talk between these important pathways plays a central role in cell fate decisions (6).
Recently, the tumor suppressor RASSF1A was recognized as a Ras effector, specifically of K-Ras (7, 8). The expression of RASSF1A is frequently suppressed in cancer due to gene silencing by promoter methylation (9, 10). RASSF1A is a member of the Ras-associated family of proteins, which comprises 10 genes, each featuring several splice variants. RASSF1A function is further regulated by phosphorylation (11) and is involved in the control of cell cycle progression, microtubule stability, and apoptosis (10). How RASSF1A regulates most of its biological effects is not well understood, as it lacks catalytic activity. However, its role in apoptosis is better studied. RASSF1A can trigger apoptosis through at least two pathways. One involves binding of RASSF1A to the Bax binding protein MOAP-1/MAP-1 adaptor, which induces a conformational change that activates the proapoptotic function of Bax (12, 13). The other mechanism relies on the stimulation of the MST/Hippo pathway (14, 15), which is emerging as a central regulator of organ size, cell polarization, and apoptosis (16, 17). RASSF1A dissociates MST2 from the inhibitory complex with Raf-1 and stimulates MST2 kinase activity as well as binding to its substrate LATS1 (18, 19). Activated LATS1 can phosphorylate different effectors, including YAP1. LATS1 phosphorylates YAP1 on several residues (20), which have different functions. The phosphorylation of S127 plays a role in cell growth and size control and inactivates the transcriptional function of YAP1 by promoting its retention in the cytosol and degradation (20, 21). However, RASSF1A stimulation causes LATS1 to phosphorylate YAP1 on a different residue(s), which is yet to be identified, enabling YAP1 to translocate to the nucleus and bind p73 (18). The YAP1/p73 complex activates the transcription of several proapoptotic genes (18, 22). In addition, we have recently demonstrated that mutant (mt) K-Ras regulates the MST2-LATS1 pathway through RASSF1A in colorectal cancer (CRC) cells (7). In this case apoptosis is due to LATS1 binding to and sequestering the ubiquitin ligase Mdm2 from p53, which results in the stabilization and activation of p53 and subsequent apoptosis (7). Thus, depending on the mode of upstream activation the MST2-LAST1 pathway can utilize different downstream effectors. Another intricacy of this pathway, at least in mammalian cells, is its differential regulation by mt versus wild-type (wt) K-Ras. Whereas mt K-Ras stimulates apoptotic signaling through this pathway, wt K-Ras can inhibit it (7).
Here, we have investigated the mechanistic basis for the differential regulation of the MST2 pathway by wt and mt K-Ras. We show that the different activation dynamics of mt K-Ras and RASSF1A are responsible for the differential effects of mt K-Ras and wt K-Ras on the MST2 pathway. We also present evidence that in response to growth factor stimulation RASSF1A specifically interacts with K-Ras but not H- or N-Ras. Furthermore, our data indicate that mt and wt K-Ras differ in their abilities to activate AKT and that AKT activation is central for inhibiting the MST2-LATS1 pathway.
MATERIALS AND METHODS
Constructs and siRNA.
Constructs encoding pCEFL-HA-H-Ras, -K-Ras, -N-Ras, -K-RasV12, -H-RasV12, and -N-RasV12, pCEFL-Flag-GRF2, -K-RasV12, -H-RasV12, and -N-RasV12, and pCEFL-AU5-SOS1 and pGEX-4T-RBD have been described before (24, 25). HA-RASSF1A and Flag-RASSF1A constructs have been described before (8). Myc-K-RasV12 was cloned in the pEF6 plasmid. Small interfering RNAs (siRNAs) against MST2, RASSF1A, LATS1, wt K-Ras, YAP1, and p73 have been described and validated before (7, 18).
Cell culture.
Cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Subconfluent cells were transfected with Lipofectamine 2000 (Invitrogen) by following the manufacturer's instructions.
Immunoprecipitation and immunoblotting.
Immunoprecipitations were performed as described before (18). Briefly, cells were lysed in 20 mM HEPES, pH 7.5, 150 mM NaCl, 1% NP-40, 2 mM NaF, 10 mM β-glycerophosphate, 2 mM Na4P2O4, and protease and phosphatase inhibitors. After incubation at 4°C for 2 h, immunoprecipitates were washed 3 times with lysis buffer containing 0.5% NP-40, separated by SDS-PAGE, and analyzed by Western blotting.
Antibodies and reagents.
All antibodies were from commercial sources: mouse monoclonal antihemagglutinin (anti-HA) (Santa Cruz), anti-HA–horseradish peroxidase (anti-HA-HRP) 3F10 (Roche), rabbit polyclonal anti-MST2 (Epitomics), goat polyclonal anti-MST2 (C-19; Santa Cruz), mouse monoclonal anti-C-Raf (BD Transduction Laboratories), goat polyclonal anti-LATS1 (n-18 and g-16; Santa Cruz), rabbit polyclonal anti-YAP1 (Santa Cruz), mouse monoclonal anti-RASSF1A (eBioscience), rabbit polyclonal anti-RASSF1 (Santa Cruz), anti-p73 monoclonal antibody (MAb) (Ab4) (Neomarkers), anti-H-Ras mouse monoclonal, anti-K-Ras mouse monoclonal and anti-N-Ras rabbit polyclonal (Santa Cruz), mouse monoclonal ppErk and rabbit polyclonal Erk (Sigma), rabbit polyclonal phospho-YAP S127 (New England Biosciences), mouse monoclonal Myc tag (Upstate), mouse monoclonal anti-AU5 (Covance), rabbit polyclonal AKT, p-S308-AKT, p-S473-AKT, and p-AKT-substrate cell signaling, rabbit polyclonal p-T180-MST2 cell signaling (New England BioLabs), mouse monoclonal glycogen synthase kinase 3B (GSK3B; Santa Cruz), and rabbit monoclonal phospho-GSK3α/β (Ser21/9) (Cell Signaling) antibodies. LY294002, Akt inhibitor IV, and epidermal growth factor (EGF) are from Calbiochem.
MST2 in gel kinase activity.
MST2 kinase activity was measured as before (19). Briefly, cell lysates were divided in half and MST2 was immunoprecipitated from both fractions as described above. For each sample one immunoprecipitate aliquot was Western blotted for MST2 as a loading control for the experiment, while the other immunoprecipitate aliquot was subjected to an in-gel kinase assay. For this purpose, immunoprecipitates were resolved on an SDS-PAGE gel containing myelin basic protein. The gel was washed 3 times to remove SDS with 20% propanol, 50 mM Tris, pH 8.0, and equilibrated with kinase buffer (40 mM HEPES, pH 8.0, 10 mM MgCl2, 0.5 mM EGTA, and 50 mM 25 mCi ATP) and [γ-32P]ATP and then incubated in kinase buffer with 25 mCi [γ-32P]ATP for 2 h. After several washes with 5% trichloroacetic acid (TCA) and 1% sodium pyrophosphate, the gel was dried and exposed to X-ray film.
Ras pulldown activation assay.
Ras activation was measured as described previously (25). Briefly, cells were lysed using MLB buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% NP-40, 1% Na deoxycholate, 10% glycerol, 10 mM MgCl2, and protease and phosphatase inhibitors). Active (i.e., GTP-loaded) Ras was affinity purified using a recombinant GST-Raf Ras binding domain (RBD) (amino acids 1 to 149) protein and detected by immunoblotting with anti-K-Ras antibody. Three experiments were quantified using ImageJ.
Apoptosis assays.
Apoptosis levels were measured by assessing DNA fragmentation using PI staining by fluorescence-activated cell sorter (FACS) as described before (19). The graphs show the quantitation of cells with fragmented (i.e., sub-G1) DNA content from at least 3 independent experiments. Error bars represent standard deviations.
RESULTS
RASSF1A selectively interacts with K-Ras in a growth factor-regulated manner.
We recently have demonstrated that K-Ras binds RASSF1A in a GTP-dependent manner and that K-Ras can upregulate MST2 signaling through RASSF1A (7). This effect seemed to be specific for K-Ras, since H-Ras and N-Ras failed to activate MST2 signaling. Moreover, while oncogenic mt K-RasV12 stimulated MST2 kinase activity and binding to its substrate LATS1, oncogenic mt H-RasV12 or mt N-RasV12 exerted an inhibitory effect upon MST2 activation. These observations indicated that Ras isoforms differentially regulate the MST2 pathway. This differential effect of Ras isoforms may be due to direct regulation of RASSF1A signaling by the three isoforms or by indirect regulation of the MST2 pathway through cross talk with other signaling pathways regulated by N- and H-Ras. A possible interaction of Ras with RASSF1A proteins has been described in the literature (26). However, the reports were contradictory. For instance, it has been reported that RASSF1A binds preferentially to H-Ras (27, 28) or K-Ras (10). These discrepancies likely arise from the use of overexpression systems, different assays, or different cell types. Therefore, in order to clarify this issue, we reevaluated this issue using physiological growth factor stimulation in two experimental systems, i.e., MCF7 breast carcinoma and HeLa cervical carcinoma cells. In these cells we have a wealth of data on the biochemical and biological consequences of MST2 signaling, in particular the induction of apoptosis (18). HeLa is one of the few immortalized cell lines that retain RASSF1A expression, while MCF7 cells lack endogenous RASSF1A expression.
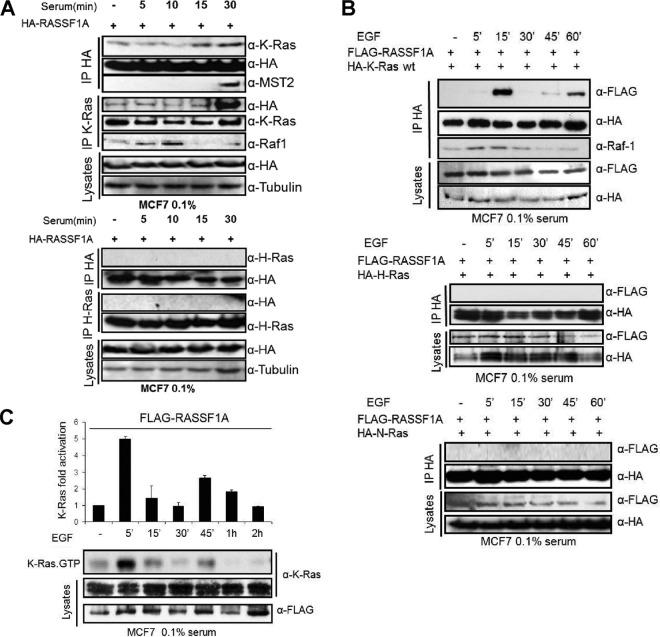
Therefore, we tested whether H-Ras and K-Ras can bind to RASSF1A upon serum stimulation of MCF7 cells transfected with a HA-RASSF1A expression vector (Fig. 1A). Endogenous K-Ras coimmunoprecipitated with exogenously expressed RASSF1A, and RASSF1A coimmunoprecipitated with endogenous K-Ras. Serum increased this interaction and also induced the coprecipitation of MST2, suggesting that RASSF1A can bind to K-Ras and MST2 in a growth factor-induced manner. Both the RASSF1A and MST2 interaction with K-Ras increased steadily over a time course of 45 min of serum stimulation (see Fig. S1 in the supplemental material). K-Ras also coimmunoprecipitated with endogenous Raf-1 in a serum-stimulated fashion, but this interaction preceded the interaction of K-Ras with MST2 showing that K-Ras engages the Raf-1 and MST2 pathways with different kinetics. In contrast, in the same type of experiment we did not observe any interaction of RASSF1A with endogenous H-Ras or N-Ras (Fig. 1A and data not shown), indicating that K-Ras is the only member of the Ras family that can bind to RASSF1A. It is possible that this observation was due to different levels of expression of the Ras isoforms in MCF7 cells or to the different affinity of the Ras isoform-specific antibodies. In addition, binding of RASSF family members to different Ras isoforms has been reported but has been investigated in depth only for RASSF5 (29). RASSF1A was previously reported to associate with K-Ras proteins using heterodimerization with RASSF5 as an intermediate (8). Using purified protein fragments the isolated Ras association domains of both RASSF5 and RASSF1A also were shown to directly bind to recombinant H-Ras protein in vitro (29, 30). In transfection experiments RASSF1A coimmunoprecipitated with activated K-Ras (28), and one study reported as unpublished results that RASSF1A binds better to K-Ras than H-Ras (10). Our own data showing that mt K-Ras, but not mt H-Ras or mt N-Ras, can activate MST2 (7) suggested that RASSF1A binding may be selective for Ras isoforms. However, it is problematic to draw definitive conclusions from the results of the previous experiments as they are difficult to compare, having been performed in very different experimental settings. Therefore, we directly compared the capacities of H-, K-, and N-Ras isoforms to associate with RASSF1A under the same experimental conditions (Fig. 1B). For this purpose we coexpressed HA-tagged-Ras family members with Flag-RASSF1A and stimulated the cells with EGF, which is a known regulator of Ras and MST2 (23, 31, 32). Only K-Ras coprecipitated with RASSF1A, confirming that K-Ras selectively associates with RASSF1A (Fig. 1B). This result is further supported by the observation that K-RasV12 coimmunoprecipitated with RASSF1A, whereas no mt H-RasV12 or N-RasV12 could coimmunoprecipitate with RASSF1A (see Fig. S2 in the supplemental material).
Fig 1.
(A) MCF7 cells were transfected with 1 μg HA-RASSF1A. After 16 h in 0.1% serum, cells were treated for the indicated time by readding serum to a final concentration of 10%. Cell extracts were split in two and immunoprecipitated with anti-HA, anti-K-Ras (upper panel), or anti-H-Ras (lower panel) and Western blotted with the indicated antibodies. (B) MCF7 cells were cotransfected with FLAG-RASSF1A and HA-K-, HA-H-or N-Ras wt. After 16 h of starvation (0.1% serum), the cells were treated with EGF (100 ng/ml) for the indicated times. HA immunoprecipitates were subjected to Western blotting with the indicated antibodies. (C) Endogenous K-Ras GTP levels were measured in EGF (100 ng/ml)-stimulated MCF7 cells transfected with Flag-RASSF1A. Error bars show standard deviations.
Interestingly, EGF regulated the interaction of K-Ras with RASSF1A in a bimodal way, showing a transient peak at 15 min and a second peak at the 45- and 60-min time points. This binding pattern followed the activation kinetics of K-Ras in MCF7 cells transfected with RASSF1A and stimulated with EGF, where K-Ras activation occurred biphasically with a first peak at 5 min and a second smaller peak commencing after 45 min of stimulation (Fig. 1C). Interestingly, the first peak coincided with K-Ras binding to Raf-1, while Raf-1 binding was back to basal levels at the second peak of K-Ras activation These results show that EGF induces the formation of K-Ras-RASSF1A and K-Ras-Raf-1 complexes in a GTP-dependent but kinetically different manner. They further suggest that K-Ras may coordinate different biological effects resulting from the differential activation of the Raf-1 versus the RASSF1A pathway as specified by the K-Ras activation kinetics.
EGF suppresses the proapoptotic MST2 pathway downstream of RASSF1A.
In light of the above data and our previous work showing that activation of the EGF receptor can protect against MST2-induced apoptosis (7), we studied how EGF stimulation regulates the MST2 pathway in HeLa cells. These cells express endogenous RASSF1A and allowed us to investigate endogenous protein complexes (Fig. 2A). In unstimulated cells, MST2 kinase activity was low, and MST2 coimmunoprecipitated with Raf-1 and LATS1 but not with RASSF1A. EGF stimulation rapidly disrupted the association between MST2 and LATS1 and subsequently induced binding of MST2 to RASSF1A and Raf-1. Concomitant with these changes in association, MST2 kinase activity was first reduced and then enhanced by EGF. We have previously shown that MST2 bound to Raf-1 is inhibited whereas MST2 bound to RASSF1A is activated (7). As Raf-1 and RASSF1A compete for MST2 binding, the MST2-Raf-1 and MST2-RASSF1A complexes are mutually exclusive but coexist as separate complexes (7), Thus, although EGF increases the sequestration of MST2 into the inhibitory complex with Raf-1, it also enhances the formation of the activating MST2-RASSF1A complex resulting in an overall increase in MST2 kinase activity at later time points (Fig. 2A). These observations suggested an intricate coordination of the kinetics of MST2 activity by EGF.
Fig 2.
(A) Serum-starved HeLa cells were treated with EGF for the indicated times. MST2 immunoprecipitates were subjected to an in-gel kinase assay (MST2 KA) or Western blotted with the indicated antibodies. LATS1 immunoprecipitates were blotted with the indicated antibodies. Numbers indicate the fold kinase activation obtained by dividing kinase activity by amount of MST2 immunoprecipitated. (B) HeLa cells were treated with LY294002 (10 μM) or Akt inhibitor (10 μM) for 45 min and stimulated with 10 nM EGF for a further 10 min. Cell lysates (10 μg) were analyzed by Western blotting using antibodies against phospho- and total proteins as indicated. (C) HeLa cells were transfected with empty vector (−) or Flag-K-RasN17 (+). After serum starvation the cells were treated with 10 nM EGF for 5 or 45 min. The lysates were split in half and immunoprecipitated with MST2 or LATS1 antibodies. The immunoprecipitates were blotted with the indicated antibodies. (D) HeLa cells were transfected with 1 μg of wt FLAG-MST2 (left panels) or the double mutant FLAG-MST2 T117/384A (right panels) and HA-K-RasN17 where indicated. After 16 h in 0.1% serum the cells were treated with 10 nM EGF for 45 min. Cell lysates were split in half and immunoprecipitated with anti-MST2 or anti-LATS1 antibodies. The immunoprecipitates and cell extracts were Western blotted with the indicated antibodies. (E) HeLa cells were transfected with empty vector or H-RasN17, K-RasN17, or N-RasN17 and then stimulated with 10 nM EGF for 10 min. Cell lysates (10 μg) were analyzed by Western blotting using antibodies against phospho- and total proteins as indicated. (F) Serum-starved MCF7 cells transfected with HA-RASSF1A or empty vector were treated with 10 nM EGF for 45 min. LATS1 immunoprecipitates were blotted for MST2 and YAP1 coprecipitation.
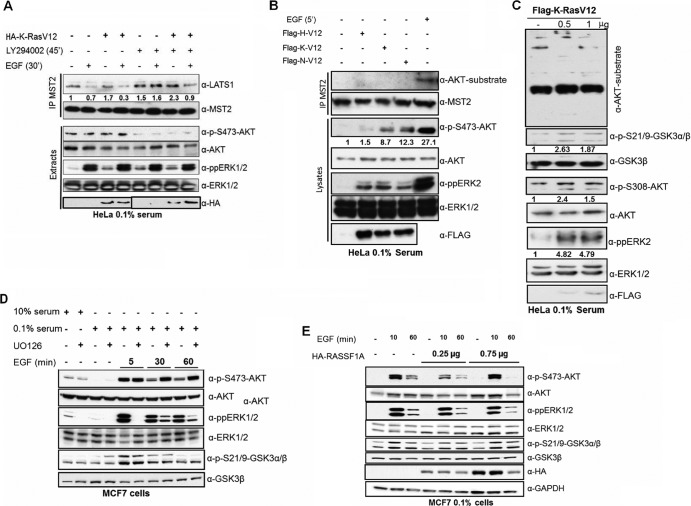
Therefore, we analyzed other EGF effector pathways that are known to impinge on the regulation of MST2. AKT can phosphorylate MST2 on Thr117 and Thr384, in a PI3K-dependent manner, inhibiting MST2 kinase activity by preventing its autophosphorylation on Thr180 and enhancing its binding to Raf-1 (33). Hence, we analyzed EGF-mediated AKT activation and MST2 phosphorylation in more detail. For this purpose we used an antibody specific for AKT substrate phosphorylation motifs that allowed us to monitor the AKT-directed phosphorylation of MST2 (33). In parallel, we measured MST2 activation by performing in-gel kinase assays or using an anti-T180-MST2 specific antibody, which detects an autophosphorylation event that is essential for MST2 activation (34). While EGF activated AKT rapidly, coinciding with the disruption of the MST2-LATS1 complex, MST2 kinase activation occurred later when AKT activity was declining (Fig. 2A). Chemical inhibitors of AKT (AKT I) or its upstream activator PI3K (LY294002) completely reverted the EGF-mediated suppression of MST2 autophosphorylation and therefore its activation (Fig. 2B). These results indicate that the high level of AKT activity triggered by EGF early after stimulation promotes sequestration of MST2 into the inactive complex with Raf-1, while the decline of AKT activity at later time points permits MST2 activity to rise. Interestingly, the increase in MST2 kinase activity occurs at a time when MST2 is dissociated from its substrate LATS1, suggesting that it cannot translate into downstream effects mediated by LATS1. Consequently, EGF activates MST2 but interferes with its proapoptotic functions by disrupting MST2 binding to its substrate LATS1. In contrast, mt K-Ras enhances both MST2 kinase activity and binding to LATS1, leading to an increase in apoptosis (23). These results suggest that K-Ras promotes proapoptotic signaling through the MST2 pathway when constitutively activated by mutation but interferes when transiently activated by physiological growth factors. We currently do not know the mechanistic basis for this differential effect of EGF and mt K-Ras, but this finding is consistent with our previous results showing that EGF activation of wt K-Ras interferes with MST2-mediated apoptosis triggered by mt K-Ras (7).
To confirm that the effect of EGF on the MST2-LATS1 interaction is mediated by K-Ras, we used the dominant inhibitory mutant K-RasN17 (Fig. 2C). This mutant selectively inhibits the activation of endogenous K-Ras with minor effects on H-Ras and N-Ras activation (25). Expression of K-RasN17, but not of H- and N-RasN17 (see Fig. S3 in the supplemental material), prevented the decrease of the MST2-LATS1 interaction caused by EGF, supporting the hypothesis that the inhibitory effect of EGF on MST2 signaling involves the K-Ras-dependent uncoupling of MST2 from LATS1. K-RasN17 also impaired the activation of AKT by EGF as well as the phosphorylation of MST2 by AKT (Fig. 2C). As previously shown, this phosphorylation inhibits MST2 (33), suggesting that K-Ras can inhibit MST2 via activation of AKT. We confirmed this hypothesis by expressing the FLAG-tagged MST2 (T117/384AA) double mutant. Contrary to what happened to FLAG-tagged MST2 wt, the interaction of this mutant with LATS1 was not affected by EGF stimulation, confirming that AKT mediates this effect (Fig. 2D).
In order to compare the role of different Ras isoforms in the activation of AKT upon EGF stimulation, we used dominant inhibitory mutants of each Ras isoform. H-RasN17 is able to inhibit the activation of all three Ras isoforms, while K-RasN17 and N-Ras specifically inhibit the activation of their cognate wild-type proteins and to a lesser degree the activation of H-Ras, allowing us to discriminate which Ras isoforms are mediating the activation of their different effectors (25). We expressed the three Ras dominant inhibitory mutants and treated the cells with EGF for 10 min (Fig. 2E). EGF activation of AKT was severely reduced by the expression of K-RasN17 (∼60% reduction) and H-RasN17 (∼43% reduction) while N-RasN17 (∼26% reduction) was less effective, indicating that K-Ras is the main Ras isoform mediating AKT activation upon EGF stimulation.
These data suggested that EGF-activated wt K-Ras should have an inhibitory effect on the activation of the MST2 proapoptotic signaling pathway initiated by RASSF1A. In order to test this hypothesis we assessed how EGF affected the effects of RASSF1A expression on downstream signaling events. As previously reported, RASSF1A causes the release of YAP1 from LATS1 and binding of YAP1 to p73, which together activate expression of the proapoptotic BH3 domain protein Puma (18). The decrease of LATS1-YAP1 interaction caused by RASSF1A was rescued by treatment with EGF (Fig. 2F). These findings reveal opposite effects of EGF and stimulation of the Fas death receptor, which elevated MST2 kinase activity and interaction with its substrate LATS1 and promoted YAP1-p73 binding (7). This is likely due to the activation of AKT antiapoptotic signal mediated by EGF-activated K-Ras.
Oncogenic K-Ras activates the MST2 pathway proapoptotic signal in HeLa cells.
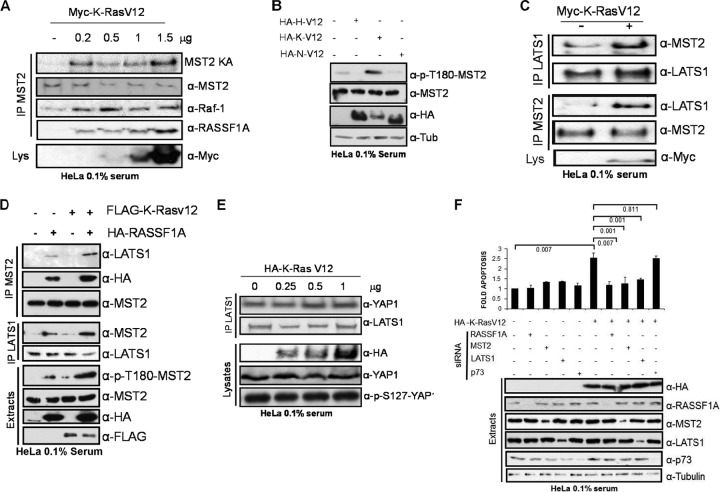
In CRC cells oncogenic mt K-Ras promotes apoptosis by activating the MST2 pathway, and this requires EGF receptor activity and a wt K-Ras allele (7). The above data suggest that in HeLa cells wt K-Ras can suppress MST2 pathway activation by activating AKT and disrupting the interaction between MST2 and LATS1. These findings prompted us to perform a detailed study of how oncogenic mt K-Ras affects the MST2 pathway in HeLa cells. Overexpression of increasing amounts of mt K-RasV12 activated MST2 kinase activity and increased MST2-RASSF1A and Raf-1-MST2 interactions in a dose-dependent manner (Fig. 3A). In contrast, expression of oncogenic mt H- or N-RasV12 mutants did not enhance MST2 kinase activity but reduced MST2 activation (Fig. 3B; see Fig. S4 in the supplemental material). This effect is probably due to an indirect effect of this mutant on the regulation of the MST2 pathway since only wt and mt-K-Ras, not H- or N-Ras, can bind RASSF1A in these cells (Fig. 1; see Fig. S2 in the supplemental material). Overall, these results showed that among the different oncogenic Ras isoforms the MST2 pathway is specifically activated by mt K-Ras. Therefore, these observations are similar to what we observed when wt K-Ras was activated by physiological stimuli, such as EGF (Fig. 1). In order to track down the differences between wt and mt K-Ras signaling, we investigated downstream MST2 signaling. K-RasV12 but not H-RasV12 or N-RasV12 increased the binding of MST2 to LATS1 (Fig. 3C; see Fig. S4B). In contrast, EGF disrupted the MST2-LATS1 complex (Fig. 2). In MCF7 cells, which do not express endogenous RASSF1A, both MST2 kinase activity and LATS1 binding were dramatically enhanced by the coexpression of RASSF1A and K-RasV12 (Fig. 3D; see Fig. S5 in the supplemental material), indicating that K-RasV12 cooperates with RASSF1A to activate LATS1.
Fig 3.
(A) HeLa cells were transfected with increasing amounts of Myc-K-RasV12. MST2 was immunoprecipitated and blotted for LATS1, Raf-1, and RASSF1A coprecipitation. An in-gel kinase assay was performed for MST2 immunoprecipitates (MST2 KA). (B) HeLa cells were transfected with 0.5 μg of HA-H-RasV12, HA-K-RasV12, or HA-N-RasV12 as indicated. After 16 h in 0.1% serum the cell lysates were Western blotted with the indicated antibodies. (C) LATS1 and MST2 coimmunoprecipitation in HeLa cells transfected with Myc-K-RasV12. (D) MCF7 cells were cotransfected with HA-RASSF1A and FLAG-K-RasV12. MST2 and LATS1 immunoprecipitates were assayed for coprecipitation. Total lysates were Western blotted with the indicated antibodies. (E) HeLa cells were transfected with increasing amounts of HA-K-RasV12. After 20 h of serum starvation, cells were lysed and LATS1 immunoprecipitates were blotted for YAP1 coprecipitation. p73 protein levels and S127 phosphorylation were monitored in the extracts using specific antibodies. (F) HeLa cells were transfected with HA-K-RasV12 expression plasmid and 50 ng/ml siRNAs against RASFF1A, MST2, LATS1, and p73 as indicated. A nontargeting siRNA pool was used as a control. Protein expression was monitored by Western blotting. Cells were assayed for apoptosis by measuring DNA fragmentation. Error bars show standard deviations (n = 3).
Further downstream, K-RasV12 did not affect the association of LATS1 with YAP1 or YAP1 phosphorylation on S127 (Fig. 3E), which is thought to be a critical phosphorylation site for enabling the oncogenic potential of YAP1. This observation also indicated that mt K-Ras activation of apoptosis through the MST2 pathway was not mediated by YAP1-p73, as we had previously observed when the MST2 pathway was activated by Fas or overexpression of RASSF1A (18). Therefore, we tested whether mt K-Ras-induced apoptosis of HeLa cells was dependent on the other components of the MST2 pathway (Fig. 3F; see Fig. S6 in the supplemental material). siRNA-mediated downregulation of RASSF1A, MST2, and LATS1, but not of YAP1 and p73, rescued mt K-Ras-induced apoptosis, confirming that mt K-Ras-induced apoptosis in HeLa is dependent on the kinase core of the pathway but uses an effector different from p73.
Differential activation of AKT by mt K-Ras and wt K-Ras explains their distinct regulation of the MST pathway.
These results confirmed the fundamental differences in the signaling properties of wt versus mt K-Ras. While mitogen-activated wt K-Ras inhibits the MST2 pathway, mt K-Ras activates it. This functional divergence centers on the regulation of MST2-LATS1 and LATS1-YAP1 binding. Both the formation of MST2-LATS1 and disruption of LATS1-YAP1 complexes are critical for the induction of apoptosis by RASSF1A (18). EGF disrupts MST2-LATS1 complexes, whereas mt K-Ras promotes MST2-LATS1 binding. In addition, EGF, but not mt K-Ras, antagonizes the RASSF1A-induced decrease of LATS1-YAP1 binding.
We have previously shown that AKT can inactivate MST2 by direct phosphorylation (33) and that the antiapoptotic effect of wt K-Ras in CRC cells requires the activation of AKT signaling (7). Now we observe that upon EGF stimulation K-Ras-dependent activation of AKT inhibits MST2 activation (Fig. 2D). Our previous findings also indicated that in CRC the inhibition of MST2 by AKT was dependent on the presence of wt K-Ras, as mt K-Ras was not able to exert this inhibition when HCT116 cells were depleted of wt K-Ras. We observed the same paradoxical interaction between wt K-Ras and mt K-Ras in HeLa cells (Fig. 4A), where the mt K-Ras induction of MST-LAST1 interaction was rescued by EGF stimulation. This regulation of the MST2 pathway is mediated by AKT, as PI3K inhibition increases the MST2-LAST1 interactions and rescues MST2 inhibition caused by EGF (see Fig. S7 in the supplemental material). This made us wonder whether mt K-Ras and EGF-activated wt K-Ras could differentially regulate AKT activation. To test this hypothesis, we transfected all three oncogenic mt Ras isoforms into HeLa cells and compared their effects on AKT activation with EGF (Fig. 4B). The expression of mt Ras isoforms resulted in a modest activation of AKT, with N-RasV12 being the strongest activator, H-RasV12 the weakest, and K-RasV12 intermediate. In contrast, EGF treatment caused a much higher activation of AKT than any of the oncogenic RasV12 proteins (∼3-fold > mt K-Ras). Similarly, we observed that EGF activated ERK1/2 more strongly than mt RasV12, although in this case H-, K- and N-RasV12 activated ERK at similar levels. Importantly, the different abilities of EGF and mt RasV12 proteins to activate AKT translated into a marked disparity of MST2 phosphorylation. Only EGF stimulated the phosphorylation of MST2 on AKT consensus sites, while RasV12 proteins were ineffective. The different levels of AKT and extracellular signal-regulated kinase 1 and 2 (ERK1/2) activation caused by EGF and transfected RasV12 constructs are not due to poor transfection, as the transfection efficiency was ∼90%. In order to assess for dosage effects, we transfected increasing amounts of K-RasV12, showing that under the conditions used the activation of ERK and AKT is saturated (Fig. 4C). Thus, our data suggest that EGF interrupts MST2 signaling by stimulating the inhibitory phosphorylation of MST2 by AKT, whereas mt Ras is ineffective. The lower level of ERK1/2 and AKT1 activation caused by oncogenic Ras isoforms is likely due to the activation of negative feedback loops that downregulate these pathways in response to chronic stimulation (6). To test this hypothesis in our cell systems, we performed an EGF stimulation time course experiment in MCF7 and monitored the effect of chemical inhibition of the AKT/ERK pathways. Our data indicate that in MCF7 cells there is an ERK-dependent negative regulation of AKT activation (Fig. 4D; see Fig. S8 in the supplemental material). Furthermore, this feedback loop is also modulated by RASSF1A (Fig. 4E; see Fig. S9 in the supplemental material), indicating that the regulation of AKT and ERK signaling is also regulated by the MST2 pathway.
Fig 4.
(A) HeLa cells were transfected with HA-K-Ras where indicated. After serum starvation for 20 h the cells were treated with 10 nM EGF and 10 nM LY294002 as indicated. MST2 was immunoprecipitated and blotted for LAST1 interaction. Lysates were blotted with the indicated antibodies. The lower panel was spliced from the same gel. (B) HeLa cells were transfected with 1 μg of Flag-H-, -K-, or -N-RasV12 expression plasmids or treated with EGF for 5 min as indicated. MST 2 was immunoprecipitated and examined for phosphorylation on AKT consensus sites by an anti-AKT substrate antibody. Protein expression and phosphorylation in lysates were monitored with the indicated antibodies. (C) HeLa cells were transfected with 0.5 or 1 μg of Flag-K-RasV12 plasmid. Cells were serum starved for 20 h before lysates were blotted with the indicated antibodies. (D) MCF7 cells were serum deprived where indicated and treated with 10 μM UO126 for 1 h as indicated. Cells were then incubated with 10 nM EGF for the indicated times. Lysate protein levels and phosphorylation were monitored with the indicated antibodies. (E) MCF7 cells transfected with increasing amounts of HA-RASSF1A were serum starved for 20 h and treated with 10 nM EGF for the indicated times. Lysates were blotted with the indicated antibodies.
Mutant K-Ras-induced apoptosis is due to chronic K-Ras activation.
The above results suggested that the sustained activation of mt Ras may be responsible for the inability to constrain MST2 activity. Another explanation for this paradoxical effect of K-Ras signaling upon MST2 may be based on the qualitatively different biochemical properties of oncogenic mt K-Ras and wt K-Ras. To distinguish between these two scenarios, we used Hke3 cells (KRAS−/wt), which are an isogenic derivative of HCT116 (KRASmt/wt) cells where the mt KRAS allele has been knocked out (35). We previously used this cell line pair to show that mt K-Ras induces apoptosis via activation of the MST2 pathway (7). We overexpressed two Ras guanidine exchange factors (RasGEFs), SOS1 and GRF2, that cause chronic activation of endogenous K-Ras (25) or stimulated the cells with EGF for 5 min or 16 h. Overexpression of SOS1 and GRF2 resulted in a constitutive activation of endogenous K-Ras (Fig. 5A), while EGF stimulation resulted in a transient activation of K-Ras, which had returned to basal levels at the 16-h time point. Importantly, the overexpression of SOS1 or GRF2 induced apoptosis (Fig. 5B), suggesting that apoptosis is a consequence of the chronic hyperactivation of K-Ras, i.e., a quantitative trait rather than a qualitative difference between mt and wt K-Ras signaling.
Fig 5.
(A) Hke3 cells were transfected with AU5-SOS1 or Flag-GRF2 expression plasmids or treated with EGF (100 ng/ml) for the indicated times. The levels of activated K-Ras were measured by pulldown. (B) Hke3 cells were transfected with AU5-SOS1 or GRF2 or treated with EGF for 20 h, and the levels of apoptosis were determined by measuring DNA fragmentation. Error bars show standard deviations (n = 6). (C) HeLa cells were transfected as for panel A, and lysates were blotted with the indicated antibodies. (D) HeLa cells were transfected with AU5-SOS1 and Flag GRF2. After 16 h in 0.1% serum the cells were lysed and immunoprecipitated with anti-MST2. Lysates were blotted with the indicated antibodies. (E) HeLa cells were transfected with the indicated plasmids or siRNAs, and the levels of apoptosis were measured as described above. SCRB, scrambled, nontargeting siRNA. Error bars show standard deviations (n = 4).
We also confirmed this hypothesis by experiments in HeLa cells. EGF activated both ERK and AKT. Overexpression of SOS1 and GRF2 induced a slight stimulation of ERK activity, but no AKT activation was observable as detected by the phosphorylation of AKT substrates or AKT itself (Fig. 5C). Furthermore, overexpression of SOS and GRF2 strongly increased the MST2-LATS1 interaction (Fig. 5D), Their effect on MST2 autophosphorylation was less dramatic and rather subtle with GRF2 compared to that with SOS. The reason for this differential behavior is unclear at present. As seen in Hke3 cells, SOS1 and GRF2 overexpression increased apoptosis, which could be abrogated by siRNA-mediated downregulation of K-Ras or MST2 (Fig. 5E). These results strongly indicated that the chronic activation of K-Ras either by mutation or overexpression of RasGEFs is sufficient for the activation of apoptosis and that the MST2 pathway is mediating this effect.
DISCUSSION
K-Ras mutation is one of the most frequent events in cancer (2), but paradoxically K-Ras is the only member of the Ras family that can activate apoptosis (36). Our recent work has helped to identify the MST2 pathway as one of the signaling pathways that mediates K-Ras proapoptotic effects and has shown that this apoptosis signal is repressed to allow CRC progression (23). Oncogenic K-Ras activates the RASSF1A-MST2-LATS1 pathway, resulting in the stabilization and activation of the tumor suppressor p53 due to LATS1-mediated sequestration of the p53 ubiquitin ligase MDM2. We also have shown a statistically significant inverse correlation between MST2 expression and KRAS gene mutation in human CRC patients with metastatic disease (7), suggesting that shutting down the proapoptotic signal mediated by MST2 is necessary for mt K-Ras-driven cancer progression. Interestingly, we also observed that wt K-Ras can prevent mt K-Ras-induced apoptosis by inhibiting MST2 signaling. This antiapoptotic effect of wt K-Ras requires EGF receptor (EGFR) signaling and the activation of AKT. The evidence described here provides a mechanistic explanation of the differences in MST2 pathway regulation by wt K-Ras and mt K-Ras.
RASSF1A is a specific effector of K-Ras. Both mt K-Ras and wt K-Ras bind to RASSF1A, promoting the formation of the RASSF1A-MST2 interaction. However, they have very different effects on the downstream regulation of the pathway. Wt K-Ras activated by EGF interrupts the pathway by physically decoupling MST2 from LATS1. On the other hand, mt K-Ras increases the formation of the MST2-LAST1 complex, leading to enhanced apoptosis. These differences are encoded by the activation kinetics rather than qualitative differences in signaling between mt and wt K-Ras, as the chronic activation of wt K-Ras by overexpression of RasGEFs also induces apoptosis.
According to our data, a main difference between mt K-Ras and EGF-activated wt K-Ras is their ability to activate AKT. While mt K-Ras only weakly activates AKT, EGF causes robust AKT activation and induces AKT-mediated phosphorylation of MST2. AKT phosphorylation of MST2 blocks MST2 activity and binding to RASSF1A but promotes MST2 interaction with Raf-1 (33). This mechanism can change the ratio between the inactive and active populations of MST2 that are bound to Raf-1 and RASSF1A, respectively. Therefore, K-Ras promotes two competing pathways: one by recruiting RASSF1A and MST2 and another via AKT activation that stimulates binding of MST2 to Raf-1. Indeed, EGF enhances the formation of both MST2-Raf-1 and MST2-RASSF1A complexes. These complexes compete with each other in terms of both formation and effects. The existence of negative feedback from ERK1/2 and AKT is likely to explain the differential regulation of the MST2 pathway mediated by mt K-Ras and wt K-Ras. Chronic activation of K-Ras downstream pathways results in the activation of several positive and negative feedback loops (6) that may be responsible for the different biological effects mediated by this protein. The nature of the feedback loops that are regulating the cross talk among the ERK and AKT pathways seems to be cell type specific and may explain the resistance to drugs specifically developed to target these pathways (4, 6).
In summary, the current work explains the differential regulation by wt K-Ras and mt K-Ras of the MST2 pathway. It also helps to explain how wt K-Ras collaborates with mt K-Ras to facilitate CRC progression. An interesting possibility is that MST2 activity is also regulated by the other Ras isoforms. Although H-Ras and N-Ras do not interact with RASSF1A, expression of their oncogenic mutants decreases MST2 kinase activity (Fig. 3B). Interestingly, we also have observed that the overexpression of oncogenic H-Ras and N-Ras decreases MST2 kinase activity in HCT116 CRC cells (7), pointing to the possibility that these isoforms also prevent the activation of the MST2 pathway in this cell system. Although we could not detect H-RasV12 and N-RasV12 induction of MST2 phosphorylation by AKT in HeLa cells, it is possible that these proteins induce such effects in other cell systems. There is clear evidence from the literature that the different Ras isoforms differentially activate PI3K-AKT in a cell- and tumor-specific fashion (37). For instance, collaboration of wt N-Ras with mt H-Ras has been proposed to be necessary for the maintenance of the transformed phenotypes in different cells (38). It has also been reported that cross talk between K-Ras and N-Ras signals may be necessary for the regulation of migration and proliferation in transformed cells (39, 40). Two recent reports have increased the evidence for collaboration between mt Ras and wt Ras isoforms in cancer, showing that SOS allosteric activation by mt K-Ras is responsible for the activation of wt Ras isoforms and necessary for tumorigenesis and that the wt Ras isoforms are regulating EGFR downstream signals to facilitate mt Ras transformation (41, 42). Interestingly, mutations in all three Ras isoforms have been related to poor prognosis in thyroid tumors, and concomitant mutations of K- and N-Ras are observed in myeloma (43, 44). All these data strongly support the notion that cross talk between the different Ras isoforms and their wt and mt versions can play a major role in tumor maintenance (39).
The observation that the activation of MST2 proapoptotic signals is closely regulated by the ERK and the AKT pathways and EGFR signaling at different levels may be of use in the design of new therapeutic treatments. Conceivably, preventing the phosphorylation of MST2 by AKT or the inhibitory interaction of MST2 with Raf-1 would allow the activation of apoptosis by MST2. Drugs designed to activate MST2 kinase activity may help to prevent the resistance observed to the therapeutic agents specifically targeting the ERK and PI3K-AKT pathways shown in many clinical trials (4).
Supplementary Material
ACKNOWLEDGMENTS
We thank Alfonso Blanco from the Conway Core Technologies for help with FACS analysis and Amaya García and Ruth Pilkington from SBI for technical support.
This work was supported by the Science Foundation Ireland under grant no. 06/CE/B1129 and Cancer Research UK.
Footnotes
Published ahead of print 4 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01414-12.
REFERENCES
- 1.Barbacid M. 1987. ras genes. Annu. Rev. Biochem. 56:779–827 [DOI] [PubMed] [Google Scholar]
- 2.Downward J. 2003. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 3:11–22 [DOI] [PubMed] [Google Scholar]
- 3.Buday L, Downward J. 2008. Many faces of Ras activation. Biochim. Biophys. Acta 1786:178–187 [DOI] [PubMed] [Google Scholar]
- 4.Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Basecke J, Stivala F, Donia M, Fagone P, Malaponte G, Mazzarino MC, Nicoletti F, Libra M, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Laidler P, Milella M, Tafuri A, Bonati A, Evangelisti C, Cocco L, Martelli AM, McCubrey JA. 2011. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget 2:135–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubbert S, Shannon K, Bollag G. 2007. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 7:295–308 [DOI] [PubMed] [Google Scholar]
- 6.Aksamitiene E, Kiyatkin A, Kholodenko BN. 2012. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem. Soc. Trans. 40:139–146 [DOI] [PubMed] [Google Scholar]
- 7.Matallanas D, Romano D, Al-Mulla F, O'Neill E, Al-Ali W, Crespo P, Doyle B, Nixon C, Sansom O, Drosten M, Barbacid M, Kolch W. 2011. Mutant K-Ras activation of the proapoptotic MST2 pathway is antagonized by wild-type K-Ras. Mol. Cell 44:893–906 [DOI] [PubMed] [Google Scholar]
- 8.Ortiz-Vega S, Khokhlatchev A, Nedwidek M, Zhang XF, Dammann R, Pfeifer GP, Avruch J. 2002. The putative tumor suppressor RASSF1A homodimerizes and heterodimerizes with the Ras-GTP binding protein Nore1. Oncogene 21:1381–1390 [DOI] [PubMed] [Google Scholar]
- 9.Agathanggelou A, Cooper WN, Latif F. 2005. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res. 65:3497–3508 [DOI] [PubMed] [Google Scholar]
- 10.Donninger H, Vos MD, Clark GJ. 2007. The RASSF1A tumor suppressor. J. Cell Sci. 120:3163–3172 [DOI] [PubMed] [Google Scholar]
- 11.Yee KS, Grochola L, Hamilton G, Grawenda A, Bond EE, Taubert H, Wurl P, Bond GL, O'Neill E. 2012. A RASSF1A polymorphism restricts p53/p73 activation and associates with poor survival and accelerated age of onset of soft tissue sarcoma. Cancer Res. 72:2206–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baksh S, Tommasi S, Fenton S, Yu VC, Martins LM, Pfeifer GP, Latif F, Downward J, Neel BG. 2005. The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol. Cell 18:637–650 [DOI] [PubMed] [Google Scholar]
- 13.Vos MD, Dallol A, Eckfeld K, Allen NP, Donninger H, Hesson LB, Calvisi D, Latif F, Clark GJ. 2006. The RASSF1A tumor suppressor activates Bax via MOAP-1. J. Biol. Chem. 281:4557–4563 [DOI] [PubMed] [Google Scholar]
- 14.Avruch J, Praskova M, Ortiz-Vega S, Liu M, Zhang XF. 2006. Nore1 and RASSF1 regulation of cell proliferation and of the MST1/2 kinases. Methods Enzymol. 407:290–310 [DOI] [PubMed] [Google Scholar]
- 15.Guo C, Tommasi S, Liu L, Yee JK, Dammann R, Pfeifer GP. 2007. RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr. Biol. 17:700–705 [DOI] [PubMed] [Google Scholar]
- 16.Matallanas D, Romano D, Hamilton G, Kolch W, O'Neill E. 2008. A Hippo in the ointment: MST signalling beyond the fly. Cell Cycle 7:879–884 [DOI] [PubMed] [Google Scholar]
- 17.Mauviel A, Nallet-Staub F, Varelas X. 2012. Integrating developmental signals: a Hippo in the (path)way. Oncogene 31:1743–1756 [DOI] [PubMed] [Google Scholar]
- 18.Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, Baccarini M, Vass JK, Kolch W, O'Neill E. 2007. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol. Cell 27:962–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Neill E, Rushworth L, Baccarini M, Kolch W. 2004. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science 306:2267–2270 [DOI] [PubMed] [Google Scholar]
- 20.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. 2010. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 24:72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan D. 2010. The hippo signaling pathway in development and cancer. Dev. Cell 19:491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, Mantovani F, Damalas A, Citro G, Sacchi A, Del Sal G, Levrero M, Blandino G. 2005. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol. Cell 18:447–459 [DOI] [PubMed] [Google Scholar]
- 23.Arozarena I, Aaronson DS, Matallanas D, Sanz V, Ajenjo N, Tenbaum SP, Teramoto H, Ighishi T, Zabala JC, Gutkind JS, Crespo P. 2000. The Rho family GTPase Cdc42 regulates the activation of Ras/MAP kinase by the exchange factor Ras-GRF. J. Biol. Chem. 275:26441–26448 [DOI] [PubMed] [Google Scholar]
- 24.Arozarena I, Matallanas D, Berciano MT, Sanz-Moreno V, Calvo F, Munoz MT, Egea G, Lafarga M, Crespo P. 2004. Activation of H-Ras in the endoplasmic reticulum by the RasGRF family guanine nucleotide exchange factors. Mol. Cell. Biol. 24:1516–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matallanas D, Arozarena I, Berciano MT, Aaronson DS, Pellicer A, Lafarga M, Crespo P. 2003. Differences on the inhibitory specificities of H-Ras, K-Ras, and N-Ras (N17) dominant negative mutants are related to their membrane microlocalization. J. Biol. Chem. 278:4572–4581 [DOI] [PubMed] [Google Scholar]
- 26.Feig LA, Buchsbaum RJ. 2002. Cell signaling: life or death decisions of ras proteins. Curr. Biol. 12:R259–261 [DOI] [PubMed] [Google Scholar]
- 27.Khokhlatchev A, Rabizadeh S, Xavier R, Nedwidek M, Chen T, Zhang XF, Seed B, Avruch J. 2002. Identification of a novel Ras-regulated proapoptotic pathway. Curr. Biol. 12:253–265 [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Viciana P, Sabatier C, McCormick F. 2004. Signaling specificity by Ras family GTPases is determined by the full spectrum of effectors they regulate. Mol. Cell. Biol. 24:4943–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stieglitz B, Bee C, Schwarz D, Yildiz O, Moshnikova A, Khokhlatchev A, Herrmann C. 2008. Novel type of Ras effector interaction established between tumour suppressor NORE1A and Ras switch II. EMBO J. 27:1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vos MD, Ellis CA, Bell A, Birrer MJ, Clark GJ. 2000. Ras uses the novel tumor suppressor RASSF1 as an effector to mediate apoptosis. J. Biol. Chem. 275:35669–35672 [DOI] [PubMed] [Google Scholar]
- 31.Creasy CL, Chernoff J. 1995. Cloning and characterization of a member of the MST subfamily of Ste20-like kinases. Gene 167:303–306 [DOI] [PubMed] [Google Scholar]
- 32.Vavvas D, Li X, Avruch J, Zhang XF. 1998. Identification of Nore1 as a potential Ras effector. J. Biol. Chem. 273:5439–5442 [DOI] [PubMed] [Google Scholar]
- 33.Romano D, Matallanas D, Weitsman G, Preisinger C, Ng T, Kolch W. 2010. Proapoptotic kinase MST2 coordinates signaling crosstalk between RASSF1A, Raf-1, and Akt. Cancer Res. 70:1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. 2004. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem. J. 381:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. 1993. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science 260:85–88 [DOI] [PubMed] [Google Scholar]
- 36.Cox AD, Der CJ. 2003. The dark side of Ras: regulation of apoptosis. Oncogene 22:8999–9006 [DOI] [PubMed] [Google Scholar]
- 37.Castellano E, Santos E. 2011. Functional specificity of ras isoforms: so similar but so different. Genes Cancer 2:216–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton M, Wolfman A. 1998. Oncogenic Ha-Ras-dependent mitogen-activated protein kinase activity requires signaling through the epidermal growth factor receptor. J. Biol. Chem. 273:28155–28162 [DOI] [PubMed] [Google Scholar]
- 39.Fotiadou PP, Takahashi C, Rajabi HN, Ewen ME. 2007. Wild-type NRas and KRas perform distinct functions during transformation. Mol. Cell. Biol. 27:6742–6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao J, Planchon SM, Wolfman JC, Wolfman A. 2006. Growth factor-dependent AKT activation and cell migration requires the function of c-K(B)-Ras versus other cellular ras isoforms. J. Biol. Chem. 281:29730–29738 [DOI] [PubMed] [Google Scholar]
- 41.Jeng HH, Taylor LJ, Bar-Sagi D. 2012. Sos-mediated cross-activation of wild-type Ras by oncogenic Ras is essential for tumorigenesis. Nat. Commun. 3:1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young A, Lou D, McCormick F. 2013. Oncogenic and wild-type Ras play divergent roles in the regulation of mitogen-activated protein kinase signaling. Cancer Discov. 3:112–123 [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Rostan G, Zhao H, Camp RL, Pollan M, Herrero A, Pardo J, Wu R, Carcangiu ML, Costa J, Tallini G. 2003. ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J. Clin. Oncol. 21:3226–3235 [DOI] [PubMed] [Google Scholar]
- 44.Portier M, Moles JP, Mazars GR, Jeanteur P, Bataille R, Klein B, Theillet C. 1992. p53 and RAS gene mutations in multiple myeloma. Oncogene 7:2539–2543 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.