Abstract
Immunotherapeutic herpes simplex virus 2 (HSV-2) vaccine efficacy depends upon the promotion of antigen-specific immune responses that inhibit reactivation or reactivated virus, thus controlling both recurrent lesions and viral shedding. In the present study, a candidate subunit vaccine, GEN-003/MM-2, was evaluated for its ability to induce a broad-spectrum immune response in mice and therapeutic efficacy in HSV-2-infected guinea pigs. GEN-003 is comprised of HSV-2 glycoprotein D2 (gD2ΔTMR340-363) and a truncated form of infected cell polypeptide 4 (ICP4383-766), formulated with Matrix M-2 (MM-2) adjuvant (GEN-003/MM-2). In addition to eliciting humoral immune responses, CD4+ and CD8+ T cells characterized by the secretion of multiple cytokines and cytolytic antigen-specific T cell responses that were able to be recalled at least 44 days after the last immunization were induced in immunized mice. Furthermore, vaccination with either GEN-003 or GEN-003/MM-2 led to significant reductions in both the prevalence and severity of lesions in HSV-2-infected guinea pigs compared to those of phosphate-buffered saline (PBS) control-vaccinated animals. While vaccination with MM-2 adjuvant alone decreased recurrent disease symptoms compared to the PBS control group, the difference was not statistically significant. Importantly, the frequency of recurrent viral shedding was considerably reduced in GEN-003/MM-2-vaccinated animals but not in GEN-003- or MM-2-vaccinated animals. These findings suggest a possible role for immunotherapeutic GEN-003/MM-2 vaccination as a viable alternative to chronic antiviral drugs in the treatment and control of genital herpes disease.
INTRODUCTION
Herpes simplex virus 2 (HSV-2) is one of the most prevalent sexually transmitted diseases, having infected more than 500 million people worldwide, with an estimated 23 million new infections occurring annually (1). HSV-2 infects epithelial cells of the genital mucosa during primary infection, followed by the establishment of latency in neuronal dorsal root ganglia via retrograde transport along nerve axons. Throughout latency, virus can reactivate, causing genital lesions and/or asymptomatic shedding of virus. Although suppressive antiviral therapy has shown promise in reducing both symptomatic recurrent lesions and overall viral shedding, subclinical HSV reactivation persists, likely contributing significantly to the observed continued transmission (2). The development of an efficacious immunotherapeutic vaccine targeting HSV-2 likely represents the best strategy for preventing both lesion outbreaks and the continued spread of virus.
Despite considerable effort, all vaccine candidates to date have failed to meet their defined endpoints in clinical trials. The majority of clinical trials to date have focused on prophylactic subunit vaccines, largely using the HSV-2 surface glycoproteins as immunogens. The viral envelope glycoproteins gD and gB are the dominant targets for neutralizing antibody production (3, 4), making them logical candidates for vaccine development. A prophylactic vaccine comprised of truncated gD2 and gB2 combined with MF59 adjuvant, however, failed to demonstrate efficacy in placebo-controlled trials (5). In a recent study, a gD2 subunit vaccine, formulated with an alum/monophospholipid A adjuvant, was found to be ineffectual in men and HSV-1-seropositive women; however, it initially demonstrated significantly reduced HSV-2 disease in a subgroup analysis of HSV-1- and HSV-2-seronegative women (6). Unfortunately, a subsequent clinical trial focused on this subgroup failed to reproduce this protection against either HSV-2 disease or infection (7).
Considerable and varied efforts have been made to develop vaccines aimed at preventing HSV-2 transmission and recurrent disease. Preclinical studies of vaccines utilizing DNA, recombinant HSV glycoproteins, live attenuated viruses, and combinations have demonstrated some success (8–13). A phase 2 clinical trial was conducted to assess the efficacy of a vaccine comprised of a gH-deleted disabled infectious single-cycle (DISC) mutant in symptomatic HSV-2-infected individuals, but neither viral shedding nor recurrence rates were reduced (14). When vaccines similar to those used in prophylactic trials were evaluated, treatment with a gD2/gB2 subunit vaccine led to a slight reduction in the duration and severity of recurrent lesions, suggesting that an immunotherapeutic vaccine might be achievable (15). The results of these clinical trials demonstrate the need to improve protection, which may require changes in dosing schemes, adjuvant optimization, and the addition of other antigenic targets capable of inducing antigen-specific CD4+ and CD8+ T cell populations.
T cells have been shown to play a major role in anti-HSV immunity in both animal models and humans. While CD8+ T cells have been shown to be important for the clearance of HSV infection, CD4+ T cells are necessary to provide helper functions that sustain anti-HSV CD8+ T cell immunity and promote antibody class switching. Reactivation of HSV-1 in infected mouse trigeminal ganglia is blocked by CD8+ T cells secreting gamma interferon (IFN-γ) (16–18), and CD4+ T cells secreting IFN-γ are critical for immune protection against lethal genital HSV-2 infection in mice (19, 20). In humans, HSV-specific CD8+ T cells with IFN-γ effector functions have been recovered from trigeminal ganglia (21). In addition, reduced numbers of CD4+ T cells among patients with AIDS have been associated with a significant increase in HSV-2 shedding (22). CD4+ and CD8+ T cells have been shown to recognize epitopes in HSV glycoproteins, tegument proteins, and immediate-early (IE) proteins (18, 23, 24).
Given the relative importance of T cell immune responses for controlling viral reactivation and HSV disease, our approach for development of an immunotherapeutic HSV-2 vaccine was to combine gD2, a primary target antigen for antibody and CD4+ T cells in HSV-infected humans, with ICP4, a CD8+ T cell antigen identified by our high-throughput HSV-2 proteomic screens using T cells from HSV-2-exposed or -infected individuals. This T cell antigen was first identified by comparing T cell responses of hundreds of human donors against our proteomic library of HSV-2 proteins. It was prioritized by comparing T cell responses of HSV-2-seropositive but asymptomatic or HSV-2-exposed seronegative individuals with those of individuals who were infected and experience recurrent lesion outbreaks (25) (M. Skoberne, D. Long, T. Gierahn, and J. B. Flechtner, unpublished data). This bivalent subunit vaccine candidate, called GEN-003, combined with Matrix M-2 (MM-2) adjuvant (26), activates both humoral and cellular immunities in mice. Importantly, therapeutic immunization of HSV-2-infected guinea pigs with GEN-003, adjuvanted with MM-2 (GEN-003/MM-2), significantly reduces both the frequency and severity of recurrent lesions, as well as recurrent viral shedding, compared to those of phosphate-buffered saline (PBS)-vaccinated control animals.
MATERIALS AND METHODS
Cells and viruses.
Vero (ATCC CLL-81) cells were purchased from ATCC and maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen Life Technologies, Grand Island, NY) supplemented with 10% (vol/vol) fetal bovine serum (FBS; HyClone, Logan, UT), 100 U penicillin, and 100 μg streptomycin per ml. HSV-2 strain MS (ATCC VR540) was propagated in Vero cells. HSV-2 (strain 333)/Gal, which has a lacZ expression cassette inserted between the UL3 and UL4 genes (27) (kindly provided by P. Spear, Northwestern University, Chicago, IL), was propagated and plaque assayed on Vero cells.
Recombinant baculovirus construction and production of antigens.
Nucleotide sequences encoding gD2ΔTMR were synthetically constructed and codon optimized for baculovirus expression by GeneArt (Invitrogen Life Technologies, Grand Island, NY). The final gD2ΔTMR protein sequence consisted of a six-histidine tag at the C terminus, deletion of the transmembrane region (GLIIGALAGSTLAALVIGGIAFWV), and replacement of the native signal sequence (MGRLTSGVGTAALLVVAVGLRVVCA), with a sequence encoding honey bee melittin (MKFLVNVALVFMVVYISYIYANRW) that directed protein secretion (28). The resulting synthetic DNA construct was then cloned into the baculovirus pFastBacA vector. This clone was subsequently used to prepare a recombinant baculovirus that expressed gD2ΔTMR by replacing the polyhedron gene of Autographa californica with the gD2ΔTMR sequence under the control of the polyhedron promoter.
Nucleotide sequences encoding a fragment of ICP4 (residues 383 to 766) and full-length ICP5 (UL19) were PCR amplified from HSV-2 DNA G strain (Advanced Biotechnologies, Inc., Columbia, MD) and cloned into an expression plasmid derived from pDEST17 (Invitrogen Life Technologies). This plasmid was used as a template to amplify the gene for cloning into the baculovirus transfer vector pFastBacHT (Invitrogen Life Technologies), which contains an N-terminal six-histidine tag. The bacmid was then used to generate an ICP5- or ICP4383-766-expressing recombinant baculovirus, as described above for gD2ΔTMR. Baculovirus-expressed gD2ΔTMR, ICP5, and ICP4383-766 were purified from either infected insect cell supernatants (gD2ΔTMR) or cell pellets (ICP5 and ICP4383-766) with nickel and ion-exchange chromatography.
Western blot analysis was performed as previously described (29) with the following modifications. Membranes were probed with either an antiserum prepared against ICP4383-766 peptides (Covance, Princeton, NJ) or an anti-gD monoclonal antibody (Virusys, Taneytown, MD). The secondary antibodies consisted of either rabbit anti-mouse or goat anti-guinea pig IgG conjugated to alkaline phosphatase (Sigma-Aldrich, St. Louis, MO), and protein bands were visualized by the addition of p-nitrophenyl phosphate (pNPP) substrate (Sigma-Aldrich).
OLPs.
Overlapping peptides (OLPs) for both gD2ΔTMR and ICP4383-766 were synthesized by JPT Peptide Technologies GmbH (Berlin, Germany). The peptides comprising each OLP pool were 15 amino acids in length, with an 11-amino-acid overlap between sequential peptides. OLPs for gD2 spanned the full length of the native protein, excluding the transmembrane domain. OLPs for HIV (Con B gag motif; PM-HIVgag) were purchased from JPT and employed as negative controls.
(i) Mouse immunogenicity.
Female C57BL/6 mice, 6 to 8 weeks of age, were purchased from Charles River Laboratories (Wilmington, MA). Vaccine formulations in all experiments involving mice were comprised of 1 to 2 μg of each recombinant protein, with 10 to 24 μg of the immune-stimulating complex adjuvant Matrix M-2 (MM-2; Isconova, Uppsala, Sweden) formulated in phosphate-buffered saline (PBS). All animals were vaccinated subcutaneously (s.c.) in the scruff of the neck. For acute immunogenicity studies, animals were immunized twice, three weeks apart, and immunogenicity analysis was performed seven days after the second immunization. For in vivo cytotoxicity studies, mice were immunized three times, three weeks apart, and in the recall studies, the mice were rested until day 86, when responses were recalled with a fourth immunization. Control animals for the memory studies were immunized only on day 86 (control for recall immunization), and immunogenicity and cytotoxicity were evaluated for all groups 5 days later (day 91). All animal care and procedures were conducted with the approval of the Institutional Animal Care and Use Committee guidelines of Vivisource Laboratories, Cambridge, MA.
(ii) Guinea pig therapy.
Female Hartley guinea pigs, weighing approximately 250 to 300 g, were purchased from Charles River Breeding Laboratories (Wilmington, MA). Vaccine formulations were comprised of 15 μg of each recombinant protein in the presence or absence of 50 μg MM-2 adjuvant in PBS or PBS alone. All animals were vaccinated subcutaneously in the scruff of the neck. The therapeutic model is described below. All animal care and procedures were conducted with the approval of the Institutional Animal Care and Use Committee guidelines of Cincinnati Children's Hospital Medical Center, Cincinnati, OH.
Cell-mediated immune response. (i) CBA and IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assays.
Antigen-specific IFN-γ-positive CD4+ and CD8+ T cells were detected and quantitated as previously described (30). Briefly, splenic CD4+ and CD8+ T cells, derived from immunized mice, were enriched by magnetic bead isolation, resuspended at a final concentration of 4 × 106/ml in RPMI 1640 medium (Mediatech, Manassas, VA) supplemented with 10% (vol/vol) heat-inactivated FBS (HyClone, Logan, UT), 100 U penicillin, and 100 μg streptomycin per ml, 2 mM l-glutamine, 1 mM sodium pyruvate (Mediatech), and 55 μM β-mercaptoethanol (Invitrogen Life Technologies), and plated at a density of 2 × 105 cells per well on polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA) precoated with anti-mouse IFN-γ capture antibody (Mabtech, Nacka Strand, Sweden). Naïve mouse splenocytes were used as antigen-presenting cells (APC) by being pulsed for 2 h at 37°C with 1 μg/ml of OLPs or 50 ng/ml of phorbol myristate acetate (PMA) plus 1 μm of ionomycin calcium salt (EMD Chemicals, San Diego, CA). A total of 1 × 105 pulsed APCs per well were added to the plated T cells and incubated for 18 h at 37°C. Supernatants were harvested and subjected to cytometric bead array (CBA) analysis to detect levels of tumor necrosis factor alpha (TNF-α), interleukin 2 (IL-2), IL-10, IL-4, and IL-6 production by following the manufacturer's instructions (BD Biosciences, San Jose, CA).
After supernatant harvest, remaining cells were removed and PVDF membranes were washed extensively. Captured IFN-γ was detected by incubation with a biotinylated anti-IFN-γ antibody (Mabtech), and spots were visualized using the Vector Biolabs Vectastain ABC kit (Philadelphia, PA) and amino-9-ethylcarbazole (AEC) substrate kit (BD Biosciences). Spot-forming units (SFU) were quantitated using an ELISPOT reader system (ZellNet Consulting, Fort Lee, NJ).
(ii) In vivo cytotoxicity.
An in vivo killing assay was performed as described elsewhere (31) with minor modifications. Splenocytes from naïve C57BL/6 mice were pulsed with the indicated OLPs or left unpulsed and then labeled with 5 (and 6)-carboxyfluorescein (FAM) diacetate succinimidyl ester (CFSE; Invitrogen Life Technologies). Unpulsed cells were labeled with 250 nM CFSE, and the two OLP-pulsed splenocyte populations were separately labeled with 1 μM and 4 μM CFSE. After CFSE staining was quenched with ice-cold Optimizer Cell Therapy Systems medium (Invitrogen Life Technologies), the three differentially CFSE-labeled splenocyte populations were combined in equal ratios, and an aliquot was analyzed by flow cytometry to ensure proper CFSE labeling. A total of 1 × 107 labeled cells were intravenously (i.v.) transferred into groups of mice (n ≥ 5 to 6) that had been previously immunized with GEN-003/MM-2 or negative controls. Sixteen hours later, recipient mice were euthanized and their splenocytes were examined for the presence of CFSE-labeled cells by flow cytometry. Labeled cells were distinguished from one another based on their fluorescence intensity in the FL1 channel. Peptide-pulsed splenocyte populations were compared separately against the control population. Percent cytotoxicity was calculated as (1 − Rimmunized/Rnaive) × 100%, where Rimmunized is the percentage of OLP-pulsed cells and Rnaive is the percentage of unpulsed cells.
Humoral response. (i) Antigen-specific antibody titers.
Antibody titers were determined by standard antigen-specific enzyme-linked immunosorbent assays (ELISA) as previously described (30). Briefly, ELISA plates were coated overnight at 4C° with either 0.5 μg/ml gD2ΔTMR or 1.5 μg/ml ICP4383-766 protein diluted in coating buffer. Serially diluted mouse serum or guinea pig plasma samples were added to washed and blocked antigen-coated plates, followed by incubation for 2 h at room temperature. Bound gD2ΔTMR- or ICP4383-766--specific antibodies were detected with either alkaline phosphatase (AP)-conjugated anti-mouse IgG (Sigma-Aldrich, St. Louis, MO), IgG1 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), or IgG2c (Southern Biotech, Birmingham, AL) or AP-conjugated anti-guinea pig IgG (Sigma-Aldrich) and SigmaFAST p-nitrophenyl phosphate (pNPP; Sigma-Aldrich) substrate. The resultant optical density at 405 nm (OD405) and the regression line representing the best fit for the data were calculated via GraphPad Prism and Excel software. The endpoint titer was defined as the reciprocal of the serum dilution resulting in an OD405 equal to twice the background.
(ii) Neutralization analysis.
Antibody-mediated neutralization of viral infectivity was determined via a β-galactosidase (β-Gal) colorimetric assay. Briefly, in 96-well microtiter plates, plasma or serum samples were serially diluted 3-fold in DMEM supplemented with 2% (vol/vol) heat-inactivated FBS in a 96-well microtiter plate. Approximately 500 PFU of HSV-2 (strain 333)/Gal were added to each well and incubated for 60 min at 37°C. Two-hundred-microliter aliquots were plated in duplicate on Vero cells seeded in 96-well tissue culture plates and incubated for 18 h at 37°C. Cells were then lysed with 0.2% Triton X-100, and the plates were incubated for 30 min at 37°C in chlorophenol red-β-d-galactopyranoside (CPRG; Roche Applied Sciences, Indianapolis, IN) substrate. The resultant absorbance was measured at 590 nm, and neutralizing antibody titer was defined as the reciprocal of the plasma or serum dilution that produced a 50% reduction in the OD590 of the virus control.
Guinea pig genital herpes therapeutic model.
Guinea pigs were infected intravaginally with HSV-2 and randomized into treatment groups based on acute lesion scores as previously described (32). On day 2 postinfection, vaginal swabs were assayed for infection by quantitative real-time PCR. Animals with no detectable virus were removed from the study. On days 14, 21, and 36 postinfection, animals were immunized s.c. with either (i) PBS, (ii) GEN-003 (gD2ΔTMR and ICP4383-766), or (ii) GEN-003/MM-2 (gD2ΔTMR and ICP4383-766 with MM-2 adjuvant). Animals were scored daily for 48 days (days 15 to 63 postinfection) for the severity of recurrent lesions using a previously described severity scale (33). In this scale, lesions range from 0, representing no disease, to 4, representing severe vesiculoulcerative skin disease of the perineum.
Viral shedding.
Vaginal swabs were collected from HSV-2-infected guinea pigs as previously described (32). DNA was extracted from 200 μl of vaginal swab samples using the QIAamp 96 DNA Swab BioRobot kit (Qiagen, Germantown, MD). Each plate included no template wells (negative controls) and four 10-fold dilutions of HSV-2 strain 333 (positive controls). The number of HSV-2 DNA copies was quantified by real-time PCR using the TaqMan assay and the StepOnePlus real-time PCR system (Invitrogen Life Technologies) with primers and probes specific for HSV-2 glycoprotein G2 (gG2) designed by the Primer Express Software (Invitrogen Life Technologies). The gG2 primer and probe sequences were as follows: forward, 5′-CGGAGACATTCGAGTACCAGATC-3′; reverse, 5′-GCCCACCTCTACCCACAACA-3′; and probe, 5′-(FAM)ACCCACGTGCAGCTCGCCG(6-carboxytetramethylrhodamine [TAMRA])-3′. Each 20-μl reaction was performed in duplicate and contained 5 μl of DNA, 1× TaqMan Universal Master Mix (Invitrogen Life Technologies), 0.45 μM (each) forward and reverse primers, and 0.10 μM probe. A standard curve, based on a known amount of genomic HSV-2 DNA (Advanced Biotechnologies Inc., Columbia, MD), was used to determine copy number. The limit of detection was determined to be one copy per 20-μl reaction, with excellent linearity (R ≥ 0.98) over 5 logs of HSV-2 genomic DNA content.
Statistical analysis.
Student's t tests were performed using two-tailed analysis with equal variance. Recurrent lesion scores and viral shedding were analyzed by two-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparisons using Prism 5.04 (GraphPad, San Diego, CA). A P value of <0.05 was considered significant.
RESULTS
Generation of purified recombinant protein antigens.
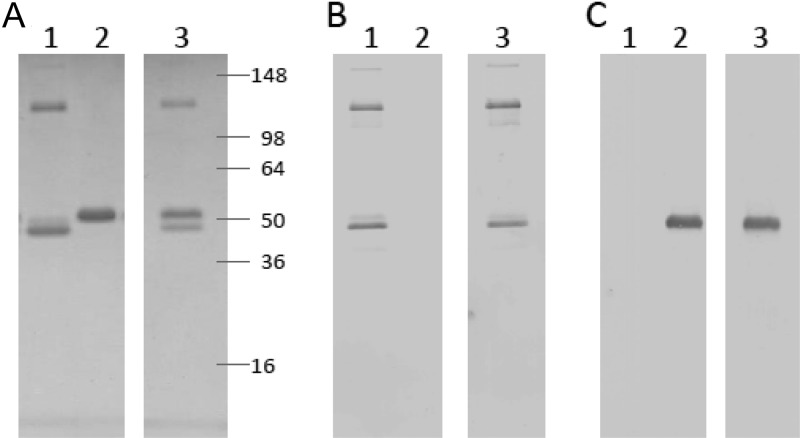
Recombinant proteins were expressed by baculovirus-infected insect cells. As expected, gD2ΔTMR was secreted into the culture medium and ICP4383-766 was present in infected cell lysate (data not shown). Nickel and ion-exchange chromatography were used to purify the proteins, and SDS-PAGE analysis demonstrated that recombinant ICP4383-766 and gD2ΔTMR proteins migrated with the expected apparent molecular masses of ∼52 kDa and ∼48 kDa, respectively (Fig. 1A). The purity of both recombinant proteins was calculated to be >95% based on densitometry analysis (data not shown). The respective identities of ICP4383-766 and gD2ΔTMR were confirmed by Western blotting (Fig. 1B and C). Based on the Western blot analysis, the protein band observed at approximately 130 kDa is predicted to be an ICP4383-766 dimer formed through hydrophobic interactions.
Fig 1.
SDS-PAGE analysis of purified recombinant proteins. After purification from baculovirus-infected cell cultures, antigens were electrophoresed through a 4 to 20% Tris-glycine polyacrylamide gel under reducing conditions and visualized by Coomassie blue staining (A) or Western blot analysis with either an ICP4-specific polyclonal antiserum (B) or a gD-specific monoclonal antibody (C). Prestained molecular mass markers were run on the same gel. Lane 1, ICP4383-766 (300 ng); lane 2, gD2ΔTMR (300 ng); lane 3, ICP4383-766 mixed with gD2ΔTMR (300 ng each).
GEN-003/MM-2 vaccine induces virus- and antigen-specific antibody responses in mice.
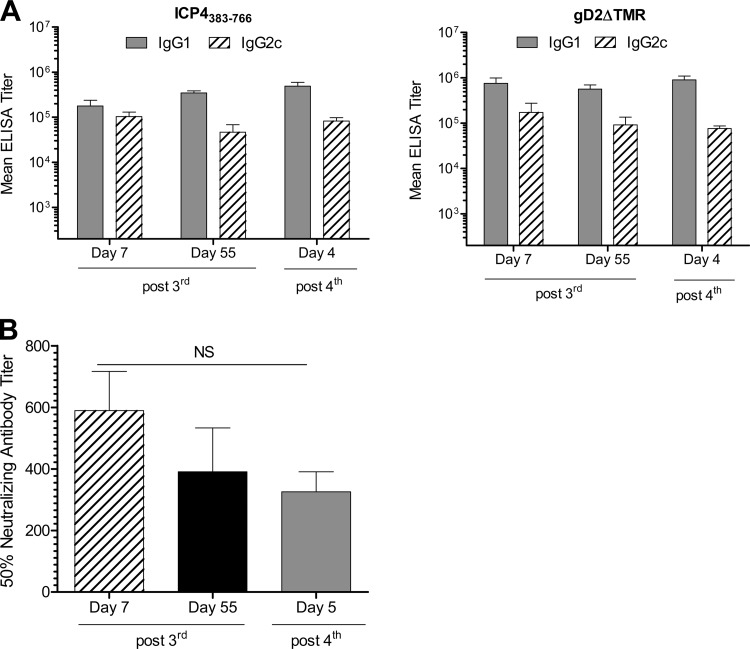
Sera collected from mice immunized with GEN-003/MM-2 vaccine 7 and 55 days after the third immunization and 5 days after a fourth immunization were analyzed for anti-gD2ΔTMR or -ICP4383-766 IgG1 and IgG2c antibodies by antigen-specific ELISA. As shown in Fig. 2A, immunization with GEN-003/MM-2 elicited robust anti-gD2ΔTMR and anti-ICP4383-766 IgG1 and IgG2c titers, indicating a balanced Th1/Th2 response. We observed a slightly higher, but not significantly so (P > 0.05), antibody response to gD2ΔTMR than to ICP4383-766 after three or four immunizations with GEN-003/MM-2. No significant boost in IgG1 or IgG2c antibody titers was observed following the fourth immunization compared to the titers measured after three immunizations for either ICP4383-766 or gD2ΔTMR antigens. The IgG1 and IgG2c antibody responses were sustained for at least 55 days after the third immunization, suggesting that a durable and balanced Th1/Th2 antibody response is elicited by vaccination with GEN-003/MM-2.
Fig 2.
Antibody response to GEN-003/MM-2 vaccine formulation. C57BL/6 mice (n = 15) were immunized s.c. three times at three-week intervals with the GEN-003/MM-2 vaccine formulation (2 μg of each antigen plus 20 μg MM-2). Five mice per group received a fourth immunization 44 days after the third immunization. Serum samples were taken from five mice by cardiac puncture seven and 55 days after the third immunization and four days after the fourth immunization. (A) Serum samples were serially diluted 5-fold, and antibody titers to ICP4383-766 and gD2ΔTMR protein were measured by endpoint ELISA. IgG1 and IgG2c endpoint titers are shown as the mean ± the standard error of the mean (SEM) per group. (B) Neutralizing antibody titers to virus were determined using a colorimetric assay as described in Materials and Methods. Titers are shown as the mean ± SEM titer that resulted in 50% neutralizing activity per GEN-003/MM-2 vaccine-immunized group. No ELISA or neutralizing antibody titers were detected in serum from naïve mice or MM-2 adjuvant-immunized mice. P values were calculated by Student's t test. Data are representative of two experiments. NS, not significant.
There is considerable evidence in animal models that an efficacious prophylactic HSV-2 vaccine will most likely include a robust neutralizing antibody response (19, 34, 35); therefore, we theorized that neutralizing antibodies would also be essential for limiting secondary or cell-to-cell spread of virus replication after reactivation from latency. To determine the effect of GEN-003/MM-2 vaccination on neutralizing antibody production and durability, neutralizing antibody titers were measured in sera from GEN-003/MM-2-immunized mice. We developed a microneutralization assay based on the colorimetric quantitation of β-galactosidase expression from HSV-2/Gal infection (27). Antibody titers inducing HSV-2 neutralization were determined by linear regression analysis of the optical density values. Previous studies showed a good correlation between the microneutralization assay and a direct assessment of 50% plaque reduction, with less than 25% variation in antibody titers between the two methods. As shown in Fig. 2B, a strong HSV-2 neutralizing antibody titer was elicited in mice receiving three or four immunizations of GEN-003/MM-2. The mean neutralizing antibody titer decreased from 589 ± 127 to 391 ± 142 between 7 and 55 days after the third immunization (not significant; P = 0.34), suggesting that a durable neutralizing antibody response is elicited by the GEN-003/MM-2 vaccine.
In summary, robust antibody responses are generated against the protein antigens contained in the GEN-003/MM-2 vaccine. The responses were high titer, neutralizing, and a balanced Th1 and Th2 phenotype. Both the antigen-specific IgG1 and IgG2c antibody titers and the HSV-2 neutralization activity persisted for at least 55 days after the third immunization.
The GEN-003/MM-2 vaccine elicits antigen-specific cellular immune responses in mice.
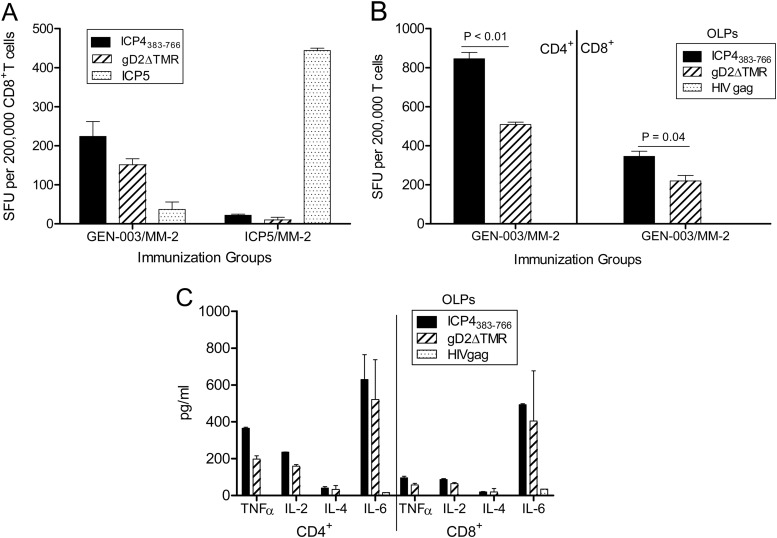
ELISPOT analysis was performed to measure IFN-γ production by CD4+ and CD8+ T cells in mice immunized with the GEN-003/MM-2 vaccine formulation. Mice were immunized s.c. twice, three weeks apart, with a 100-μl formulation containing 1 μg each of gD2ΔTMR and ICP4383-766 plus 24 μg of MM-2 diluted in PBS or appropriate controls. Splenic T cells were enriched from immunized mice 1 week after the second immunization. CD8+ T cells isolated from splenocytes of mice immunized with the GEN-003/MM-2 vaccine or another baculovirus-derived recombinant HSV-2 protein, ICP5/MM-2, produced only background levels of IFN-γ in response to irrelevant antigens (i.e., ICP5, 10 to 37 SFU per 200,000 T cells) compared with vaccine antigen-specific IFN-γ production (150 to 444 SFU per 200,000 T cells). This demonstrates that observed IFN-γ levels are not resulting from nonspecific activation by contaminating baculovirus or cellular proteins (Fig. 3A). Subsequent T cell simulations were performed with antigen-specific overlapping peptides (OLPs) to limit the possibility that artifacts, if present in different batches of purified protein preparations, would generate high background levels of IFN-γ in mice immunized with the GEN-003/MM-2 vaccine. Robust, antigen-specific IFN-γ responses to both gD2ΔTMR and ICP4383-766 antigens were measured in both CD4+ and CD8+ T cell subsets, and there were no detectable responses to the negative-control peptides (Fig. 3B). At this immunization dose, the response to the ICP4383-766 antigen was significantly higher than the response to gD2ΔTMR for CD4+ and CD8+ T cell subsets (P < 0.01 and 0.04, respectively). The magnitude of response to both antigens was quite high, however, with the frequency of responding cells ranging from 0.1 to 0.5% of the overall T cell population. There were no measurable T cell responses in mice immunized with the antigens alone in the absence of MM-2 (data not shown).
Fig 3.
Cellular responses elicited in mice following immunization with HSV-2 antigens plus MM-2 adjuvant. (A) ELISPOT analysis of IFN-γ secretion by CD8+ T cells elicited in C57BL/6 mice (n = 3) vaccinated twice, nine days apart, with 10 μg of ICP4383-766 and 5 μg of gD2ΔTMR with 12 μg of MM-2 (GEN-003/MM-2) or 10 μg of ICP5 plus 12 μg of MM-2. One week after the second immunization, sorted CD8+ T cells were restimulated with naïve splenocytes pulsed with the indicated protein plus MM-2 and washed extensively. (B) ELISPOT analysis of IFN-γ secretion by CD4+ and CD8+ T cells from mice vaccinated twice, three weeks apart, with 2 μg of GEN-003 plus 24 μg of MM-2 adjuvant. Seven days after the second immunization, spleens were harvested, and sorted T cells were restimulated via naïve splenocytes pulsed with the indicated overlapping peptide (OLP) pools. IFN-γ production is represented by the mean number of spot-forming units (SFU) per 2 × 105 T cells from three assay replicates ± SEM. (C) Supernatants obtained in the ELISPOT assay in panel B were evaluated for the presence of TNF-α, IL-2, IL-4, and IL-6 by cytometric bead array (CBA). CD4+ and CD8+ T cell responses to antigen-specific and irrelevant OLPs, respectively, are shown as the mean cytokine concentration (pg/ml) of triplicate wells ± the standard deviation (SD). Data are representative of three replicate experiments.
Previous research has indicated that effector T cell responses, characterized by the secretion of the Th1-type cytokines IFN-γ, IL-2, and TNF-α, are required for mounting an effective immune response to HSV infection (19). To determine the antigen-specific cytokine profile induced after GEN-003/MM-2 immunization, cytometric bead array (CBA) analysis was performed using supernatants harvested from the IFN-γ ELISPOT assay to determine the relative levels of Th1- and Th2-type cytokines present in the supernatants. The responses to the negative-control peptides ranged from undetectable for TNF-α and IL-2 to below 40 pg/ml for IL-6. Significant antigen-specific TNF-α, IL-2, and IL-6 cytokine responses were measured for CD4+ and CD8+ T cells derived from the GEN-003/MM-2-immunized mice (Fig. 3C), whereas IL-4 responses were low (20 to 40 pg/ml) and IL-10 was undetectable (<5 pg/ml; data not shown). The pattern of these cytokines mirrored that seen in the IFN-γ ELISPOT assay, in which the responses to the ICP4383-766 antigen were greater than those to the gD2ΔTMR antigen. These data suggest that multiple cytokines associated with polyfunctionality are produced by antigen-specific CD4+ and CD8+ T cells after GEN-003/MM-2 immunization.
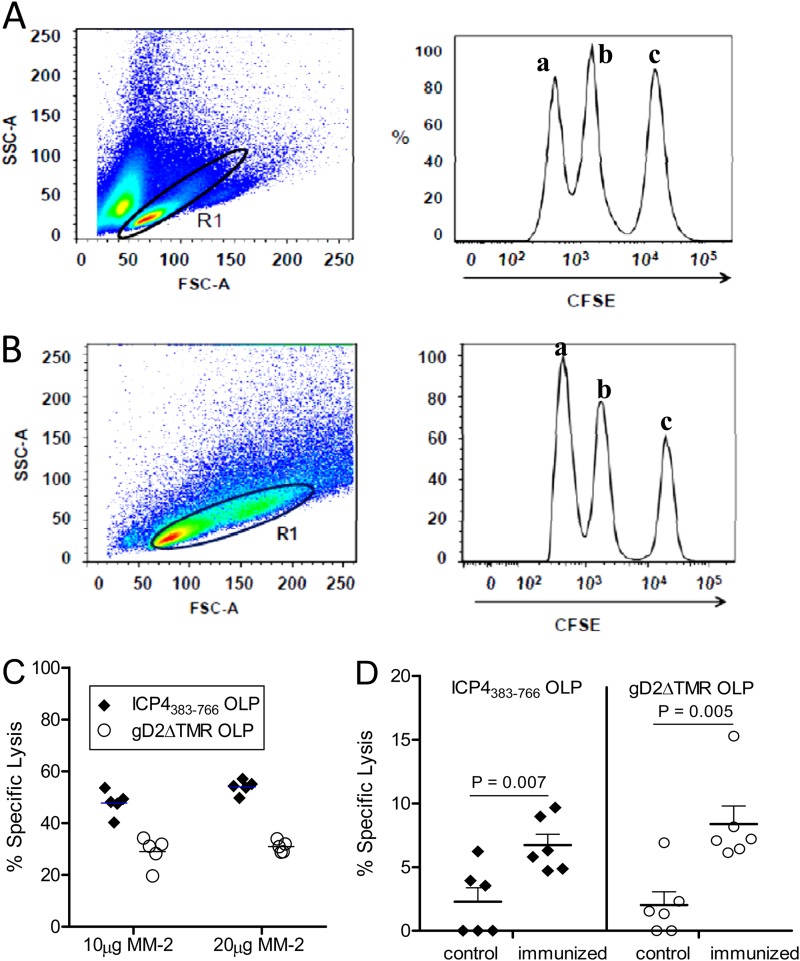
Resolution of herpetic lesions has been correlated with the presence of HSV-specific cytotoxic T lymphocytes (CTLs), indicating their critical role in limiting recurrent disease (24, 36). Thus, the effector function of antigen-specific T cells was evaluated in GEN-003/MM-2-immunized mice by an in vivo cytotoxicity assay. C57BL/6 mice were immunized s.c. three times, 21 days apart, with 2 μg of each antigen plus 10 μg or 20 μg MM-2. Six days after the third immunization, a mixed population of CFSE-labeled and OLP-pulsed splenocytes derived from naïve mice was adoptively transferred by i.v. injection. Sixteen hours later, spleens were harvested and the proportion of CFSE-labeled cells was determined by flow cytometry. As shown in Fig. 4C, there was more than 50% specific lysis of ICP4383-766 OLP-pulsed target cells and approximately 30% specific lysis of gD2ΔTMR OLP-pulsed target cells relative to the unpulsed CFSE-labeled population (Fig. 4B, peak a) adoptively transferred into the same immunized mice. These data indicate that the T cells elicited by immunization with the GEN-003/MM-2 vaccine have functional cytolytic activity in addition to secreting effector cytokines.
Fig 4.
Antigen-specific in vivo cytotoxicity in GEN-003/MM-2 vaccine-immunized mice. (A) Splenocytes derived from naïve mice were pulsed with gD2 or ICP4383-766 OLPs and then separately labeled with CFSE at 1 μM and 4 μM CFSE, respectively. As negative controls, unpulsed naïve splenocytes were labeled with 250 nM CFSE. CFSE-labeled splenocytes were analyzed by flow cytometry to ensure appropriate separation of peaks. (Left) Dot plot shows the live gate (R1) in which 1 × 105 events were acquired. (Right) Histogram shows gated events evaluated for fluorescence intensity of the respective CFSE-labeled populations. (B) Five C57BL/6 mice per group were immunized three times on days 0, 21, and 42 with 2 μg of each antigen and 10 μg or 20 μg of MM-2. On day 48, equal mixtures of each of the pulsed and CFSE-labeled target populations were adoptively transferred into the GEN-003/MM-2 vaccine-immunized mice. After 16 h, mice were euthanized and splenocytes were analyzed by flow cytometry. (Left) Dot plot shows the live gate (R1) in which 1 × 105 events were acquired. (Right) Histogram shows gated events evaluated for fluorescence intensity of the respective CFSE-labeled populations. Peaks represent unpulsed cells (a), gD2 OLP-pulsed cells (b), and ICP4383-766 OLP-pulsed cells (c). Representative data from one mouse are shown. (C) In vivo cytolytic activity for individual mice against each of the OLP-pulsed targets relative to the unpulsed controls is shown as the mean percentage (%) of specific lysis ± SEM. Data are representative of 2 similar experiments. (D) One group of mice (n = 6) was immunized subcutaneously (s.c.) three times, three weeks apart, with 2 μg of each antigen and 20 μg of MM-2; the other group (control; n = 5) did not receive vaccinations. The mice were rested until day 86, when they all received a recall immunization. Five days later, CFSE-labeled and OLP-pulsed target cells were generated as described above and adoptively transferred into all mice. After 16 h, mice were euthanized and splenocytes were analyzed for the presence of CFSE-positive (CFSE+) cells by flow cytometry as previously described. Values represent specific lysis ± SEM of target cells in control and immunized mice. P values were calculated by comparing immunized and control groups using two-way Student's t test.
To determine if this antigen-specific cytolytic activity persists over time, CFSE-labeled and OLP-pulsed target splenocytes were adoptively transferred into immunized mice 91 days after their primary immunization. Briefly, as described above, one group of mice received three immunizations, 21 days apart, with 2 μg of each antigen plus 20 μg of MM-2 while the control group of mice did not receive the vaccine. The mice were then rested to allow development of memory responses. On day 86 (44 days after the third dose), any existing antigen-specific cells were recalled by in vivo stimulation of vaccinated and control mice with GEN-003/MM-2 followed by in vivo cytolytic evaluation of antigen-specific T cells 5 days later, as described in Materials and Methods. The recall was performed to activate and expand the persisting T cells so that antigen-specific cytolytic activity would be above the assay's sensitivity level and therefore measurable. GEN-003/MM-2-immunized mice demonstrated antigen-specific lysis of OLP-pulsed target cells. The time between recall in vivo stimulation and in vivo cytolytic activity measurement was short (5 days); however, theoretically, it may be sufficient to allow priming of antigen-specific T cells in the control group of mice. Therefore, the cytolytic activities of vaccinated and control mice were compared, and a statistically significant increase in cytolysis by T cells of vaccinated animals compared to that by T cells of the control animals was confirmed (Fig. 4).
Taken together, these data indicate that the GEN-003/MM-2 vaccine formulation elicits both ICP4383-766- and gD2ΔTMR-specific CD4+ and CD8+ T cell responses that secrete multiple cytokines in response to antigen stimulation. Moreover, the T cell responses are functionally cytolytic in vivo and can be recalled at least 44 days after the third immunization.
Effect of therapeutic immunization on recurrent HSV-2 disease in guinea pigs.
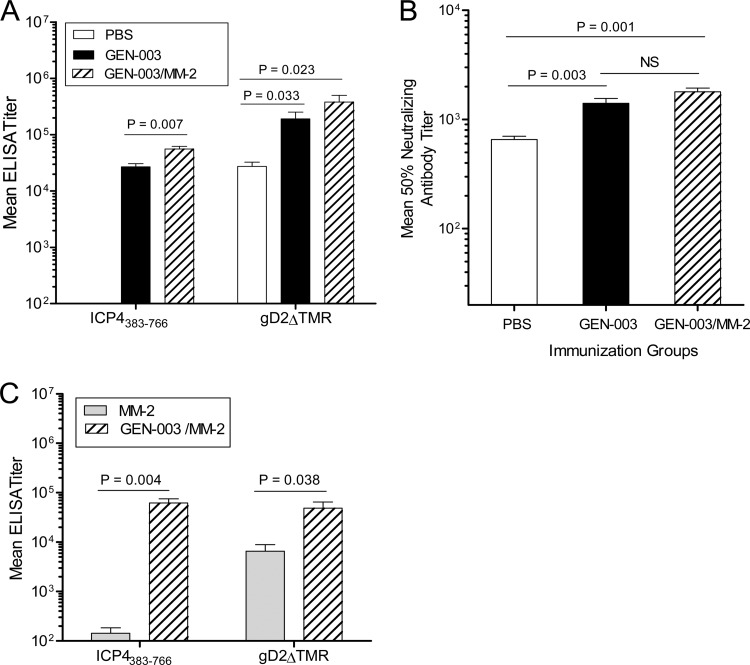
HSV-2 infection in guinea pigs induces recurrent lesions and viral shedding, similar to the human course of infection and disease symptoms (37). To examine the potential therapeutic effect of vaccination with either of the HSV-2 proteins alone (plus MM-2), GEN-003, or the GEN-003/MM-2 vaccine, guinea pigs were intravaginally infected with 5 × 105 PFU of HSV-2 strain MS and subsequently immunized s.c. three times at 14, 21, and 36 days postinfection. Anti-ICP4383-766 and -gD2ΔTMR IgG titers were measured by ELISA on day 42 postinfection, six days after the third immunization. Infected, PBS- or MM-2-immunized animals developed detectable antibody to gD2ΔTMR but not ICP4383-766 (Fig. 5A and C). A statistically significant increase in antibody titers to ICP4383-766 protein was observed in the GEN-003/MM-2 vaccine-immunized group compared to that in the groups receiving GEN-003 or PBS. Relative to the infected, PBS-immunized group, HSV-2-infected animals therapeutically immunized with GEN-003 or the GEN-003/MM-2 vaccine had a 7- or 14-fold increase in gD2ΔTMR antibody titer, respectively (Fig. 5A). Immunization with GEN-003/MM-2 boosted neutralizing antibody titers as well, with approximately 3-fold higher levels than in PBS-immunized, HSV-2-infected animals (Fig. 5B) (P = 0.001), presumably due to the increase in gD2 antibody.
Fig 5.
Antibody responses of HSV-2-infected guinea pigs vaccinated with GEN-003 or GEN-003/MM-2 vaccine. Guinea pigs were infected intravaginally with 5 × 105 PFU of HSV-2 strain MS, allowed to recover from primary disease, and then vaccinated s.c. three times on days 14, 21, and 36 postinfection with PBS, 15 μg of each antigen only (GEN-003), or 15 μg of each antigen plus 50 μg MM-2 adjuvant (GEN-003/MM-2). (A) Antibody titers in plasma samples (n = 4) to ICP4383-766 or gD2ΔTMR were measured by endpoint ELISA at day 42 postinfection (six days after the third immunization). Data are shown as the mean ELISA endpoint titer of each group ± SEM. (B) Neutralizing antibody titers were determined by a colorimetric assay as described in Materials and Methods. The titer was defined as the reciprocal of the serum dilution required to decrease the OD590 obtained using the virus control by 50%. Data are shown as the mean neutralizing antibody titer of each group ± SEM. P values compare the GEN-003-immunized group to the GEN-003/MM-2-immunized group or to the PBS control group using Student's t test. (C) Antibody titers in plasma samples (n = 4) to ICP4383-766 or gD2ΔTMR were measured by endpoint ELISA at day 42 postinfection (six days after the third immunization). P values compare the GEN-003/MM-2-vaccinated group to the MM-2 control group using Student's t test.
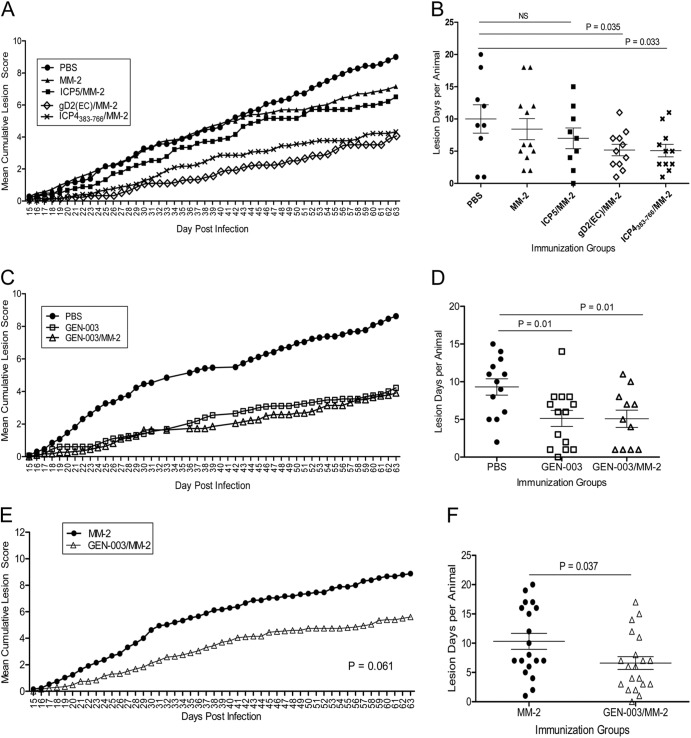
The effect of vaccination on recurrent disease is shown in Fig. 6. HSV-2-infected guinea pigs were examined daily for the severity of lesions as well as the appearance of new recurrent lesions from day 15 through day 63 postinfection. As shown in Fig. 6A and Table 1, therapeutic immunization with ICP4383-766 or gD2 plus MM-2 significantly reduced the mean cumulative recurrent lesion scores by 49% or 53%, respectively, compared to the PBS control group (P < 0.05). In comparison, immunization of HSV-2-infected guinea pigs with ICP5 plus MM-2 was less effective, with only a 26% reduction in the severity of the clinical lesion score (P > 0.05) relative to the PBS control group. A slight decrease in severity scores was also noted with MM-2 alone (17%), but only after the third immunization, and it was not statistically significant compared to the PBS control group. Vaccination with either gD2 or ICP4383-766 significantly decreased the number of recurrent lesion days compared to that in the PBS-vaccinated group (Fig. 6B) (P = 0.035 and P = 0.033, respectively). MM-2 alone or ICP5/MM-2 vaccination had a small effect that was not significant relative to the PBS-vaccinated control group. In a second experiment, shown in Fig. 6C and Table 1, the mean recurrent lesion scores for the GEN-003- and GEN-003/MM-2-vaccinated groups were reduced compared to that of the PBS control group (4.2 ± 2.9 and 3.9 ± 3.0 versus 8.6 ± 3.8, respectively), representing a 51% reduction for GEN-003 and a 55% reduction for GEN-003/MM-2 (P values of <0.05 for both versus the PBS control). The frequency of recurrent lesion days was also significantly reduced by approximately 45% in the GEN-003- and GEN-003/MM-2-immunized groups compared to that in the PBS control group (Fig. 6D) (P = 0.01). There was no significant difference between the GEN-003 and GEN-003/MM-2 groups. In another experiment, we compared the effectiveness of GEN-003/MM-2 vaccination to that of MM-2 adjuvant alone on recurrent lesion symptoms. Although cumulative lesion scores were not significantly reduced in animals receiving GEN-003/MM-2 vaccine relative to those in animals receiving MM-2 alone (Fig. 6E) (P = 0.061), the number of days with recurrences was significantly less in the GEN-003/MM-2 group (Fig. 6F) (P = 0.037),
Fig 6.
Therapeutic treatment of infected guinea pigs with HSV-2 antigens or GEN-003 vaccine. Guinea pigs were infected intravaginally with 5 × 105 PFU of HSV-2 strain MS, allowed to recover from primary disease, and then vaccinated s.c. three times on days 14, 21, and 36 postinfection. (A and B) PBS (n = 9), 50 μg of MM-2 alone (n = 12), and 15 μg of each antigen alone plus 50 μg of MM-2 adjuvant (n = 9 to 11) (Table 1, experiment 1). (A) Cumulative mean lesion severity scores (as described in Materials and Methods) are shown for each group. (B) The number of days with a recurrent lesion is shown. Each symbol indicates an individual animal from the same group. The P value compares the PBS control group to each antigen-immunized group using unpaired, two-tailed Student's t test. (C and D) Fifteen micrograms of each antigen (GEN-003) or 15 μg of each antigen plus 50 μg of MM-2 (GEN-003/MM-2) (Table 1, experiment 2). (C) Cumulative mean lesion severity scores (as described in Materials and Methods) are shown for each group: PBS (n = 13), GEN-003 (n = 14), and GEN-003/MM-2 (n = 11). (D) The number of days with a recurrent lesion is shown. Each symbol indicates individual animals from the same group. The P value compares the PBS control group to the GEN-003 or GEN-003/MM-2 vaccine-immunized group using unpaired, two-tailed Student's t test. (E and F) Fifty micrograms of MM-2 or 15 μg of gD2ΔTMR plus 15 μg of ICP4383-766 with 50 μg of MM-2 (GEN-003/MM-2). (E) Cumulative mean lesion severity scores are shown for each group: MM-2 (n = 19) and GEN-003/MM-2 (n = 20). (F) The number of days with a recurrent lesion is shown. Each symbol indicates an individual animal from the same group. P values compare the GEN-003/MM-2-vaccinated group to the MM-2 control group using unpaired, two-tailed Student's t test.
Table 1.
Effect of ICP5, ICP4383-766, gD2, or GEN-003 vaccination on recurrent lesion scores and viral shedding in HSV-2-infected guinea pigsa
| Expt and treatment group | n | Effects of each vaccination: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| After 1st immunization |
After 2nd immunization |
After 3rd immunization |
||||||||
| Recurrent mean lesions |
Shedding frequencyc [% (no. of samples from which DNA was detected/total no. of samples analyzed)] | Recurrent mean lesions |
Shedding frequencyc [% (no. of samples from which DNA was detected/total no. of samples analyzed)] | Recurrent mean lesions |
Shedding frequencyc [% (no. of samples from which DNA was detected/total no. of samples analyzed)] | |||||
| Score | % reductionb | Score | % reductionb | Score | % reductionb | |||||
| Expt 1 | ||||||||||
| PBS | 9 | 9.0 ± 5.7 | ND | 7.8 ± 5.0 | 26.9 (29/108) | 5.3 ± 2.9 | 22.2 (12/54) | |||
| MM-2 | 12 | 7.5 ± 4.7 | 17 | ND | 6.0 ± 4.0 | 23 | 16.0 (23/144) | 3.7 ± 2.6 | 30 | 15.3 (11/72) |
| ICP5/MM-2 | 9 | 6.7 ± 3.9 | 26 | ND | 5.8 ± 3.2 | 26 | 22.2 (24/108) | 3.6 ± 2.0 | 32 | 20.4 (11/54) |
| ICP4383-766/MM-2 | 11 | 4.6 ± 2.5 | 49d | ND | 4.3 ± 2.5 | 45d | 7.7 (10/130)e | 2.4 ± 1.7 | 59d | 4.6 (3/65)e |
| gD2 (EC)/MM-2 | 11 | 4.2 ± 2.4 | 53d | ND | 3.9 ± 2.3 | 50d | 13.0 (17/131) | 3.0 ± 1.9 | 48d | 13.6 (9/66) |
| Expt 2 | ||||||||||
| PBS | 13 | 8.6 ± 3.8 | 15.0 (43/286) | 6.8 ± 3.0 | 10.0 (26/260) | 3.8 ± 2.8 | 4.4 (8/182) | |||
| GEN-003 | 14 | 4.2 ± 2.9 | 51d | 14.0 (43/308) | 3.6 ± 2.5 | 47d | 8.6 (24/280) | 2.5 ± 2.2 | 34 | 2.6 (5/196) |
| GEN-003/MM-2 | 11 | 3.9 ± 3.0 | 55d | 12.8 (31/242) | 3.6 ± 2.6 | 47d | 8.6 (19/220) | 2.3 ± 2.1 | 40d | 0.0 (0/154)e |
Guinea pigs were infected by intravaginal inoculation of 5 × 105 PFU of HSV-2 strain MS and immunized on days 14, 21, and 36 postinfection (p.i.). Experiment 1 included PBS, 50 μg MM-2 adjuvant, and 15 μg of ICP5, ICP4383-766, or Escherichia coli-derived gD2 (EC) with 50 μg MM-2. Experiment 2 included 15 μg of gD2ΔTMR and 15 μg ICP4383-766 (GEN-003) or 15 μg of each antigen with 50 μg MM-2 adjuvant (GEN-003/MM-2). Vaginal swab samples were collected from each animal between days 22 and 63 (experiment 1, n = 12) and days 16 and 63 (experiment 2, n = 22) and processed as described in Materials and Methods.
% reduction, percent reduction compared to the PBS control.
Shedding frequency = (number of vaginal swab samples from which viral DNA was detected/total number of vaginal swab samples analyzed) × 100. ND, not determined.
P value of <0.05 compared to the PBS control group using a two-way ANOVA with Bonferroni's multiple comparisons.
P value of <0.05 compared to the PBS control group using a chi-square test.
To determine the effect of therapeutic vaccination on viral shedding by HSV-2-infected guinea pigs, vaginal swabs were collected and evaluated for viral DNA by real-time PCR (Table 1). In the first experiment, 12 vaginal swab samples per guinea pig were collected after the second immunization, from days 22 to 63 postinfection, and assayed for the presence of HSV-2 Us4 DNA by real-time PCR. Only the ICP4383-766-vaccinated group had a significantly reduced frequency for recurrent virus shedding compared to that of the PBS control group (7.7% versus 26.9%; P < 0.05) (Table 1). Immunization with ICP5 or gD2 plus MM-2 did not significantly affect the frequency of recurrent viral shedding relative to that of the control (22.2% and 13.6%, respectively). In the second experiment, 22 vaginal swab samples per animal were collected, from days 16 to 63 postinfection, and assayed for the presence of HSV-2 Us4 DNA. In the GEN-003/MM-2 vaccine-immunized group, 12.8% of the 242 total swabs assayed were positive for HSV-2 DNA, compared to 14.0% and 15.1% positive for the GEN-003- and PBS-immunized groups, respectively (not significant) (Table 1). No significant difference in the magnitude of viral shedding was measured for either the GEN-003- or GEN-003/MM-2-immunized group (data not shown). When viral shedding was measured after the second immunization (days 21 to 63), 8.6% of samples obtained from the GEN-003 or GEN-003/MM-2 vaccine-immunized group were positive for HSV-2 DNA, compared to 10.0% of samples in the PBS-immunized group. Importantly, after the third vaccination (days 36 to 63), viral shedding was completely undetectable in GEN-003/MM-2-immunized animals while 2.6% and 4.4% of samples in the GEN-003- and PBS-immunized groups, respectively, contained detectable HSV-2 DNA (P value of <0.05 compared to the PBS control). Taken together with the analysis of the severity of recurrent lesions, these data suggest that ICP4383-766 and gD2ΔTMR each contribute to the efficacy of the GEN-003/MM-2 vaccine and that the addition of MM-2 adjuvant to the GEN-003 vaccine antigens will create a more effective immunotherapeutic vaccine than just the antigens alone.
DISCUSSION
This study represents the first in vivo demonstration of the immunogenicity and therapeutic efficacy of a candidate subunit herpes vaccine, GEN-003/MM-2, comprised of ICP4 (residues 383 to 766) and a soluble form of gD2 (transmembrane region deleted; gD2ΔTMR) with a potent adjuvant, Matrix M-2 (MM-2) (38). In the experiments presented here, therapeutic vaccination of HSV-2-infected guinea pigs with the GEN-003/MM-2 vaccine significantly reduced recurrent lesion scores and the frequency of vaginal virus shedding, critical goals for an effective HSV-2 immunotherapeutic. Furthermore, the finding that GEN-003/MM-2 stimulates antigen-specific and cytolytic CD4+ and CD8+ T cells that secrete multiple Th1-type cytokines in mice suggests that this vaccine formulation meets the theorized requirements for controlling HSV infection (16, 18, 19, 39–45).
Our goals for a therapeutic HSV-2 vaccine are not only to reduce or prevent symptomatic recurrences but also to control asymptomatic viral shedding, providing a public health benefit by decreasing virus transmission to uninfected persons (46, 47). Past studies in humans have suggested that a vaccine may be able to change the course of recurrent disease. When given therapeutically, a subunit vaccine formulation containing 100 μg of gD2 with alum reduced the frequency of recurrences by 24% over a 12-month period compared to that in the alum-only group (48). Virus-specific neutralizing antibody titers were boosted over the baseline in vaccine recipients, but no correlation was found between a rise in antibody responses and vaccine efficacy among the treated group, suggesting that induction of T cell responses, in addition to functional antibodies, may be required for therapeutic protection. When the dose of gD2 was lowered and administered with gB2 and MF59 adjuvant, the duration and severity of the first recurrence were significantly reduced although the frequency was not (15). The reasons for this difference may include that (i) the HSV-specific antibody responses were lower with the gD2gB2/MF59 vaccine than with the gD2-alum vaccine and/or (ii) MF59 did not elicit the cell-mediated immune responses that are important in controlling recurrent HSV infections (5, 40, 49). The result of these trials also emphasized the importance of antigen and adjuvant selection for a therapeutic HSV subunit vaccine in facilitating the induction of protective immune responses.
ICP4 and gD2 with Matrix M-2 adjuvant was chosen as a therapeutic vaccine candidate for the following reasons. (i) ICP4, an HSV immediate-early (IE) gene product, was identified by our antigen screening platform (ATLAS) as a T cell target in HSV-2-seropositive asymptomatic and/or HSV-exposed seronegative subjects (25). This antigen was also identified independently by others studying HSV-seronegative individuals (50). This selection is further supported by the fact that IE gene products have been shown to provide target antigens for major histocompatibility complex (MHC) class I-restricted CTLs (18, 50). (ii) gD is a confirmed target of neutralizing antibody, antibody-dependent cellular cytotoxicity (ADCC) responses, and virus-specific immune responses mediated by CD4+ and CD8+ T cells (3, 19, 51–54). This protein was also identified in our T cell screens as one of the frequently recognized glycoproteins in HSV-2-infected individuals (M. Skoberne, unpublished data). (iii) Immune-stimulating complex (ISCOM) technology-based Matrix M adjuvant potentiates both cell- and antibody-mediated responses to protein antigens and has a good safety profile in humans (55, 56).
Here, we have demonstrated that GEN-003/MM-2 was highly effective at generating strong Th1/Th2 humoral and Th1 cellular immune responses in vaccine-immunized mice. Activation of Th1 cytokine expression, especially IFN-γ, and induction of cytotoxic T lymphocytes (CTLs) are thought to play an important role in controlling HSV infections (39, 41). Following immunization, antigen-specific peptides were able to activate splenic T cells to respond with the production of Th1-type cytokines (IFN-γ, TNF-α, and IL-2) and the development of cytolytic activity. By using an in vivo cytotoxicity assay, we demonstrated that GEN-003/MM-2 immunization induced CTL activity that was able to be recalled for at least 44 days in response to both gD2ΔTMR and ICP4383-766 protein antigens. In addition, we measured potent levels of antigen-specific IFN-γ expression in splenic CD4+ and CD8+ T cells. This builds on the findings by other researchers, who have shown cellular responses, including IFN-γ expression and induction of cytotoxic CD4+ and CD8+ T cells by recombinant gD vaccination, in mouse HSV immunization models (19, 57). Limited studies to characterize the cellular responses elicited by ICP4 in mice have been performed (58, 59). Martin et al. (59) showed that HSV-specific T cells lysed ICP4-expressing target cells in vitro, but none of the immunized mice generated antigen-specific cytotoxic T cell responses. These results are inconsistent with our findings showing significant ICP4-specific cytotoxic activity elicited in vivo but can be explained by poor expression of the ICP4 gene by the vaccinia vector used for immunization in the study by Martin et al.
Adjuvant selection is critical for facilitating the desired immune response to protein antigens. Matrix M is a novel, saponin-based adjuvant that has demonstrated a balanced B and T cell immunostimulatory profile (55). In our mouse studies, immunization with 2 μg of each recombinant HSV-2 protein and 20 μg of MM-2 elicited strong antigen-specific IgG1 and IgG2c titers that persisted for at least 55 days after the third immunization, indicating a durable and balanced Th1/Th2 cytokine profile. In our studies, both gD2 and ICP4 antigens activated CD4+ and CD8+ T cells to produce Th1-type cytokines, IFN-γ and IL-2, that promote CD8+ T cell proliferation and facilitate isotype switching to IgG2a production (equivalent to the IgG2c subtype in C57BL/6 mice [60]) by antibody-secreting cells (61). Induction of IgG2a with enhanced complement-fixing capability is one of the hallmarks of a Th1 response profile in mice and provided superior protection against HSV challenge (62, 63). We also observed the induction of strong HSV-2 neutralizing antibody titers in GEN-003/MM-2-immunized mice, which was induced by the gD2 antigen (data not shown). Previous studies demonstrated a more potent neutralizing capacity associated with the IgG2a serum fraction than with the IgG1 isotype, suggesting that a greater proportion of IgG1 antibody may be directed to nonneutralizing epitopes (64). Moreover, investigators have reported that HSV infection in humans preferentially induces the IgG1 isotype, distinguishing HSV from many other viral infections (65, 66). These studies support the idea that induction of virus-specific neutralizing antibodies that skew to a Th1-type response, with the promotion of functional IgG2a antibody production, may be facilitated by an immunotherapeutic vaccine and effectively control HSV infection.
Guinea pigs vaginally infected with HSV-2 demonstrate a course of acute and recurrent disease similar to that observed in humans. The guinea pig model has not been predictive of HSV-2 vaccine efficacy in human clinical trials; however, work by Bourne et al. showed that a gD2 vaccine with monophosphoryl lipid A (MPL)/alum significantly decreased acute and recurrent clinical disease symptoms but did not prevent mucosal infection in guinea pigs (67). Similarly, in clinical trials, immunization with the gD2/AS04 vaccine, which has a similar composition, provided HSV-seronegative women significant protection against HSV-2 genital disease symptoms but limited protection against infection (6). Depending on the vaccine composition and adjuvant used, studies from several groups reported reductions in outbreak frequency of 25% to 70% in immunized, infected guinea pigs (10, 12, 68, 69). The results presented here are very encouraging, since vaccination of infected guinea pigs with the GEN-003/MM-2 vaccine significantly reduced recurrent lesion outbreaks by approximately 45%, decreased the total lesion days per animal (Fig. 6D), and decreased lesion severity scores by 55% (Fig. 6C) relative to the PBS-vaccinated control group. Furthermore, and of particular interest, after the third immunization, no HSV-2 DNA was detected in any vaginal swab sample from GEN-003/MM-2 vaccine-immunized animals, suggesting that viral shedding was limited by vaccine administration. We also showed that vaccination of infected guinea pigs with ICP4383-766 or gD2ΔTMR with MM-2 significantly decreased recurrent lesion scores by 49% or 53%, respectively. While the frequency with which animals shed virus was reduced after gD2/MM-2 vaccination, the decrease was not significantly different relative to the PBS control group, whereas in animals receiving ICP4383-766/MM-2, the reduction in viral shedding was significantly different compared to the PBS-vaccinated control group, suggesting that immune responses to ICP4383-766 and gD2ΔTMR play different roles in the control of viral reactivation. Thus, the combination of ICP4383-766 and gD2ΔTMR with MM-2 may have an advantage over gD2ΔTMR/MM-2 alone as a therapeutic vaccine. The decrease in the number of days with recurrent lesions, lesional severity, and shedding may be due to enhanced immune responses that control reactivation and subsequent viral replication. Although reagents are not commercially available for measuring T cell responses in guinea pigs, the unique impact on viral shedding may be attributed to T cell responses, as results from studies using the same antigens and adjuvant as immunogens in mice demonstrate potent T cell activation. Antibody titers were also measured in the guinea pig therapeutic model, from which a couple observations can be drawn: (i) immunization of previously infected animals with this subunit vaccine results in a significant boost in antigen-specific antibody titer beyond that generated by infection alone, and (ii) the virus neutralizing antibody titer is 3-fold higher in the vaccine group than in the PBS-immunized control animals.
An effective therapeutic vaccine may help to reduce HSV-2 shedding in addition to limiting symptomatic recurrences. The data presented in these studies show that the GEN-003/MM-2 vaccine meets the predicted requirements of an effective therapeutic vaccine. Specific T cell responses to antigens comprising the vaccine are cytolytic and durable, and high-titer Th1/Th2 balanced antibody responses that are virus neutralizing are induced. Finally, therapeutic administration of the GEN-003/MM-2 subunit vaccine influences the course of genital herpes disease in infected guinea pigs by reducing the frequency and severity of lesions and ameliorating viral shedding.
ACKNOWLEDGMENTS
We thank J. Earwood and J. Clark for excellent technical assistance with the guinea pig model. We are grateful to Audrey Eisen-Vandervelde for manuscript support and Louis Crowley and Bharat Dixit for recombinant protein production and SDS-PAGE analysis.
Footnotes
Published ahead of print 30 January 2013
REFERENCES
- 1. Looker KJ, Garnett GP, Schmid GP. 2008. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. World Health Organ. 86:805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnston C, Saracino M, Kuntz S, Magaret A, Selke S, Huang M-L, Schiffer JT, Koelle DM, Corey L, Wald A. 2012. Standard-dose and high-dose antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet 379:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen GH, Dietzschold B, Leon MP, Long D, Golub E, Varrichio A, Pereira L, Eisenberg RJ. 1984. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J. Virol. 49:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muggeridge MI, Robert SR, Isola VJ, Cohen GH, Eisenberg RJ. (ed). 1990. Herpes simplex virus glycoproteins. Elsevier, New York, NY [Google Scholar]
- 5. Corey L. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331–340 [DOI] [PubMed] [Google Scholar]
- 6. Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G, GSK Herpes Vaccine Efficiency Study Group 2002. Glycoprotein-d-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652–1661 [DOI] [PubMed] [Google Scholar]
- 7. Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Ashley Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for Women 2012. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 366:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burke RL, Goldbeck C, Ng P, Stanberry L, Ott G, Nest GV. 1994. The influence of adjuvant on the therapeutic efficacy of a recombinant genital herpes vaccine. J. Infect. Dis. 170:1110–1119 [DOI] [PubMed] [Google Scholar]
- 9. Harrison CJ, Miller RL, Bernstein DI. 2001. Reduction of recurrent HSV disease using imiquimod alone or combined with a glycoprotein. Vaccine 19:1820–1826 [DOI] [PubMed] [Google Scholar]
- 10. Hoshino Y, Dalai SK, Wang K, Pesnicak L, Lau TY, Knipe DM, Cohen JI, Straus SE. 2005. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J. Virol. 79:410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McLean CS, Ertuck M, Jennings R, Challanain DN, Minson AC, Duncan I, Boursnell ME, Inglis SC. 1994. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J. Infect. Dis. 170:1100–1109 [DOI] [PubMed] [Google Scholar]
- 12. Manservigi R, Boero A, Argnani R, Caselli E, Zucchini S, Miriagou V, Mavromara P, Cilli M, Grossi MP, Balboni PG, Cassai E. 2005. Immunotherapeutic activity of a recombinant combined gB-gD-gE vaccine against recurrent HSV-2 infections in a guinea pig model. Vaccine 23:865–872 [DOI] [PubMed] [Google Scholar]
- 13. Simms JR, Heath AW, Jennings R. 2000. Use of herpes simplex virus (HSV) type 1 ISCOMS 703 vaccine for prophylactic and therapeutic treatment of primary and recurrent HSV-2 infection in guinea pigs. J. Infect. Dis. 181:1240–1248 [DOI] [PubMed] [Google Scholar]
- 14. de Bruyn G, Vargas-Cortez M, Warren T, Tyring SK, Fife KH, Lalezari J, Brady RC, Shahmanesh M, Kinghorn G, Beutnen KR, Patel R, Drehobl MA, Horner P, Kurtz TO, McDermott S, Wald A, Corey L. 2006. A randomized controlled trial of a replication-defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent herpes among immunocompetent subjects. Vaccine 24:914–920 [DOI] [PubMed] [Google Scholar]
- 15. Straus SE, Wald A, Kost RG, McKenzie R, Langenberg AGM, Hohman P, Lekstrom J, Cox E, Nakamura M, Sekulovich R, Izu A, Dekker C, Corey L. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoprotein D and B: results of a placebo controlled vaccine trial. J. Infect. Dis. 176:1129–1134 [DOI] [PubMed] [Google Scholar]
- 16. Khanna KM, Lepisto AJ, Decman V, Hendricks RL. 2004. Immune control of herpes simplex virus during latency. Curr. Opin. Immunol. 16:463–469 [DOI] [PubMed] [Google Scholar]
- 17. Liu T, Khanna KM, Carriere BN, Hendricks RL. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75:11178–11184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mikloska Z, Ruckholdt M, Ghadiminejad I, Dunckley H, Denis M, Cunningham AL. 2000. Monophosphoryl lipid A and QS21 increase CD8 T lymphocyte cytotoxicity to herpes simplex virus-2 infected cell proteins 4 and 27 through IFN-γ and IL-12 production. J. Immunol. 164:5167–5176 [DOI] [PubMed] [Google Scholar]
- 19. Cooper D, Pride MW, Cutler M, Mester JC, Nasar F, She J, Souza V, York L, Mishkin EM, Eldridge J, Natuk RJ. 2004. Interleukin-12 redirects murine immune responses to soluble or aluminum phosphate adsorbed HSV-2 glycoprotein D toward TH1 and CD4+ CTL responses. Vaccine 23:236–246 [DOI] [PubMed] [Google Scholar]
- 20. Harandi AM, Svennerholm B, Holmgren J, Eriksson K. 2001. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 82:845–853 [DOI] [PubMed] [Google Scholar]
- 21. Verjans GM, Hintzen RQ, van Dun JM, Poot A, Millikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. U. S. A. 104:3496–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schacker T, Zeh J, Hu HL, Hill E, Corey L. 1998. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J. Infect. Dis. 178:1616–1622 [DOI] [PubMed] [Google Scholar]
- 23. Koelle DM, Corey L, Burke RL, Eisenberg RJ, Cohen GH, Pichyangkura R, Triezenburg SJ. 1994. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 68:2803–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koelle DM, Frank JM, Johnson ML, Kwok WW. 1998. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J. Virol. 72:7476–7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skoberne M, Larson S, Price J, Zielinski V, Lundberg A, Gierahn T, Cohane K, Warren T, Leone P, Scott EP, Grossman H, Davis A, Long D, Flechtner JB. 2012. Abstr. 37th Int. Herpesvirus Workshop, Calgary, Canada, abstr 6.05 [Google Scholar]
- 26. Pedersen G, Major D, Roseby S, Wood J, Madhun AS, Cox RJ. 2011. Matrix M adjuvanted virosomal H5N1 vaccine confers protection against lethal viral challenge in a murine model. Influenza Other Respi. Viruses 5:426–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, Ware CF, Pfeffer K, Miyoshi J, Takai Y, Spear PG. 2007. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe 2:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tessier DC, Thomas DY, Khouri HE, Laliberie F, Vernet T. 1991. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene 98:177–183 [DOI] [PubMed] [Google Scholar]
- 29. Kowalski J, Adkins K, Gangolli S, Ren J, Arendt H, DeStefano J, Obregon J, Tummolo D, Natuk RJ, Brown TP, Parks CL, Udem SA, Long D. 2007. Evaluation of neurovirulence and biodistribution of Venezuelan equine encephalitis replicon particles expressing herpes simplex virus type 2 glycoprotein D. Vaccine 25:2296–2305 [DOI] [PubMed] [Google Scholar]
- 30. Picard MD, Cohane KP, Gierahn TM, Higgins DE, Flechtner JB. 2012. High-throughput proteomic screening identifies Chlamydia trachomatis antigens that are capable of eliciting T cell and antibody responses that provide protection against vaginal challenge. Vaccine 30:4387–4393 [DOI] [PubMed] [Google Scholar]
- 31. Yewdall AW, Drutman SB, Jinwala F, Bahjat KS, Bhardwaj N. 2010. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS One 5:e11144 doi:10.1371/journal.pone.0011144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernstein DI, Farlay N, Bravo FJ, Earwood J, McNeal M, Fairman J, Cardin R. 2010. The adjuvant CLDC increases protection of a herpes simplex type 2 glycoprotein D vaccine in guinea pigs. Vaccine 28:3748–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stanberry LR, Bernstein DI, Burke RL, Pachl C, Myers MG. 1987. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J. Infect. Dis. 155:914–920 [DOI] [PubMed] [Google Scholar]
- 34. Bright H, Perez DL, Christy C, Cockle P, Eyles JE, Hammond D, Khodai T, Lang S, West K, Loudon RT. 2012. The efficacy of HSV-2 vaccines based on gD and gB is enhanced by the addition of ICP27. Vaccine 30:7529–7535 [DOI] [PubMed] [Google Scholar]
- 35. Hoshino Y, Pesnicak L, Dowdell KC, Burbelo PD, Knipe DM, Straus SE, Cohen JI. 2009. Protection from herpes simplex virus (HSV)-2 infection with replication-defective HSV-2 or glycoprotein D2 vaccines in HSV-1-seropositive and HSV-1-seronegative guinea pigs. J. Infect. Dis. 200:1088–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Posavad CM, Koelle DM, Corey L. 1996. High frequency of CD8+ cytotoxic T-lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J. Virol. 70:8165–8168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stanberry LR, Kern ER, Richards JT, Abbott TM, Overall JC., Jr 1982. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J. Infect. Dis. 146:397–404 [DOI] [PubMed] [Google Scholar]
- 38. Skoberne M, Cardin R, Kazimirova A, Larson S, Zielinski V, Garvie D, Lundberg A, Bernstein DI, Flechtner JB, Long D. 2012. Abstr. 37th Int. Herpesvirus Workshop, Calgary, Canada, abstr 6.09 [Google Scholar]
- 39. Dobbs ME, Srasser JE, Chu C-F, Chalk C, Milligan GM. 2005. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin-or fas-mediated cytolytic mechanisms. J. Virol. 79:14546–14554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koelle DM, Corey L. 2003. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin. Microbiol. Rev. 16:96–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cunningham AL, Diefenbach RJ, Miranda-Saksena M, Bosnjak L, Kim M, Jones C, Douglas MW. 2006. The cycle of human herpes simplex virus infection: virus transport and immune control. J. Infect. Dis. 194:S11–S18 [DOI] [PubMed] [Google Scholar]
- 42. Laing KJ, Dong L, Sidney J, Sette A, Koelle DM. 2012. Immunology in the Clinic Review Series; focus on host responses: T cell responses to herpes simplex viruses. Clin. Exp. Immunol. 167:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. 2000. CD8(+) T cells can block herpes virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franchini M, Abril C, Schwerdel C, Ruedel C, Ackerman M, Suter M. 2001. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J. Virol. 75:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Posavad CM, Huang ML, Barcy S, Koelle DM, Corey L. 2000. Long term persistence of herpes simplex virus-specific CD8+ CTL in persons with frequently recurring genital herpes. J. Immunol. 165:1146. [DOI] [PubMed] [Google Scholar]
- 46. Mertz GJ. 2008. Asymptomatic shedding of herpes simplex virus 1 and 2: implications for prevention of transmission. J. Infect. Dis. 198:1098–1100 [DOI] [PubMed] [Google Scholar]
- 47. Sacks SL, Griffiths PD. 2004. HSV shedding. Antiviral Res. 63(Suppl 1):S19–S26 [DOI] [PubMed] [Google Scholar]
- 48. Straus SE, Corey L, Burke RL, Savarese B, Barnum G, Krause PR, Kost RG, Meier JL, Sekulovich R, Adair SF, Dekker CL. 1994. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet 343:1460–1463 [DOI] [PubMed] [Google Scholar]
- 49. Stanberry LR. 2004. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes 11:161A–169A [PubMed] [Google Scholar]
- 50. Posavad CM, Remington M, Mueller DE, Zhao L, Margaret AS, Wald A, Corey L. 2010. Detailed characterization of T cell responses to herpes simplex virus-2 in immune seronegative persons. J. Immunol. 184:3250–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kohl S, Charlebois ED, Sigouroudinla M, Goldbeck C, Hartog K, Sekulovich RE, Langenberg AGM, Burke RL. 2000. Limited antibody-dependent cellular cytotoxicity antibody response induced by a herpes simplex virus type 2 subunit vaccine. J. Infect. Dis. 181:335–339 [DOI] [PubMed] [Google Scholar]
- 52. Mikloska Z, Kesson AM, Penfold ME, Cunningham AL. 1996. Herpes simplex virus protein targets CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal karinocytes treated with interferon-gamma. J. Infect. Dis. 173:7. [DOI] [PubMed] [Google Scholar]
- 53. Mikloska Z, Sanna P, Cunningham AL. 1999. Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro. J. Virol. 73:5934–5944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Torseth JW, Cohen GH, Eisenberg RJ, Berman PW, Laskey LA, Cerini CP, Heilman CJ, Kewar S, Merigan TC. 1987. Native and recombinant herpes simplex virus type 1 envelope proteins induce human immune T-lymphocyte response. J. Virol. 61:1532–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Madhun AS, Haaheim LR, Nilsen MV, Cox RJ. 2009. Intramuscular Matrix M-adjuvanted virosomal H5H1 vaccine induced high frequencies of multifunctional Th1 CD4+ cells and strong antibody responses in mice. Vaccine 27:7367–7376 [DOI] [PubMed] [Google Scholar]
- 56. Lövgren Bengtsson K, Morein M, Osterhaus A. 2011. ISCOM technology-based Matrix M adjuvant; success in future vaccine relies on formulation. Expert Rev. Vaccines 10:401–403 [DOI] [PubMed] [Google Scholar]
- 57. Meseda CA, Elkins KL, Merchlinsky MJ, Weir JP. 2002. Prime-boost immunization with DNA and modified vaccinia virus ankara vectors expressing herpes simplex virus-2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA alone. J. Infect. Dis. 186:1065–1073 [DOI] [PubMed] [Google Scholar]
- 58. Braun RP, Dong L, Jerome S, Herber R, Roberts LK, Payne LG. 2008. Multi-antigenic DNA immunization using herpes simplex virus type 2 genomic fragments. Hum. Vaccin. 4:36–43 [DOI] [PubMed] [Google Scholar]
- 59. Martin S, Zhu X, Silverstein SJ, Courtney RJ, Yao F, Jenkins FJ, Rouse BT. 1990. Murine cytotoxic T lymphocytes specific for herpes simplex virus type 1 recognize the immediate early protein ICP4 but not ICP0. J. Gen. Virol. 71:2391–2399 [DOI] [PubMed] [Google Scholar]
- 60. Martin RM, Brady JL, Lew AM. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 212:187–192 [DOI] [PubMed] [Google Scholar]
- 61. Mosmann TR. 1992. T lymphocyte subsets, cytokines and effector functions. Ann. N. Y. Acad. Sci. 664:89–92 [DOI] [PubMed] [Google Scholar]
- 62. Ishizaka ST, Piacente P, Silva J, Mishkin EM. 1995. IgG subtype is correlated with efficiency of passive protection and effector function of anti-herpes simplex virus glycoprotein D monoclonal antibodies. J. Infect. Dis. 172:1108–1111 [DOI] [PubMed] [Google Scholar]
- 63. Simms JR, Jennings R, Richardson VJ, Heath AW. 2002. Large-scale comparison of experimental adjuvants with herpes simplex virus vaccine reveals a correlation of protection with IgG2a and IgG2b responses. J. Med. Virol. 68:82–91 [DOI] [PubMed] [Google Scholar]
- 64. McKendall RR, Woo W. 1988. Murine IgG subclass responses to herpes simplex virus type 1 and polypeptides. J. Gen. Virol. 69:847–857 [DOI] [PubMed] [Google Scholar]
- 65. Coleman RM, Nahmias AJ, Williams SC, Phillips DJ, Black CM, Reimer CB. 1985. IgG subclass antibodies to herpes simplex virus. J. Infect. Dis. 151:929–936 [DOI] [PubMed] [Google Scholar]
- 66. Thomsen AR, Volkert M, Marker O. 1985. Different isotype profiles of virus-specific antibodies in acute and persistent lymphocyte choriomeningitis virus infection in mice. Immunology 55:213–223 [PMC free article] [PubMed] [Google Scholar]
- 67. Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB. 2005. Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. J. Infect. Dis. 192:2117–2123 [DOI] [PubMed] [Google Scholar]
- 68. Stanberry LR, Burke RL, Myers MG. 1988. Herpes simplex virus glycoprotein treatment of recurrent genital herpes. J. Infect. Dis. 157:156–163 [DOI] [PubMed] [Google Scholar]
- 69. Awasthi S, Zumbrun EE, Si H, Wang F, Shaw CE, Cai M, Lubinski JM, Barret SM, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. 2012. Live attenuated herpes simplex virus 2 glycoprotein E deletion mutant as a vaccine candidate defective in neuronal spread. J. Virol. 86:4586–4598 [DOI] [PMC free article] [PubMed] [Google Scholar]