Abstract
Ocular herpes simplex virus 1 (HSV-1) infection can lead to multiple complications, including iritis, an inflammation of the iris. Here, we use human iris stroma cells as a novel in vitro model to demonstrate HSV-1 entry and the inflammatory mediators that can damage the iris. The upregulated cytokines observed in this study provide a new understanding of the intrinsic immune mechanisms that can contribute to the onset of iritis.
TEXT
Herpes simplex virus 1 (HSV-1) is a significant ocular pathogen affecting multiple regions, including the iris (1). The iris, a specialized eye tissue, is affected in various inflammatory ophthalmic conditions (2, 3). For instance, inflammation of the iris following HSV-1 infection may be associated with elevated intraocular pressure, ultimately resulting in glaucoma, although the possibility remains that this may be steroid induced (4–6). The iris has been shown to have histopathologic involvement in HSV-1 infection of the corneal stroma, herpetic stromal keratitis (HSK) (3). Inflammation of the iris is also seen in herpetic anterior uveitis, a condition that often presents as an inflammation of the iris and ciliary body (iridocyclitis) and is the leading cause of infectious anterior uveitis worldwide (2). In addition to iris involvement in primary inflammatory conditions of various ocular tissues, there had previously been a longstanding question as to whether HSV-induced iritis could occur without concurrent or precedent keratitis. Studies performed since have indicated that HSV may cause iritis that predisposes to glaucoma without necessarily causing keratitis (6). Although these results indicate the possible pathogenesis mediated by primary HSV-1 infection of the iris, primary infection of human iris stromal (HIS) cells has not been previously investigated. Likewise, chemokine signaling, which plays a significant role in many inflammatory eye conditions (7), has been poorly studied in the context of HSV-1 infection of HIS cells. The current study demonstrates the potential of an in vitro HIS cell model to uncover the molecular basis of viral entry and the identity of the inflammatory mediators involved.
HSV-1 starts its infectious journey into cells via interactions of its envelope glycoproteins with their respective host cell receptors (8). The initial attachment involves glycoprotein B (gB) and glycoprotein C (gC) interactions with host cell surface heparan sulfate proteoglycans (9, 10). HSV-1 glycoprotein D (gD), with its subsequent conformational changes, can bind to any of its host cell receptors, such as nectin-1, herpesvirus entry mediator (HVEM) (11), or 3-O-sulfated heparan sulfate (3-OS HS) (12). This conformational change is important since it allows for the gD-host cell receptor complex to associate with the heterodimer complex of glycoprotein H-glycoprotein L (gH-gL). This allows gH-gL to bind gB, a process that allows the viral and host cell membranes to fuse (12, 13).
Following infection by HSV-1, the intrinsic inflammatory responses contribute strongly to subsequent disease manifestations. In cases of HSK, an outburst of cytokine and chemokine activities by local and infiltrating immune cells can serve as the main contributor to the observed scarring of the corneal stromal tissue and vision loss (14, 15). Inhibiting the overreactive inflammatory response elicited by HSV-1 replication may effectively taper the damaging effects on the ocular tissues (16). This possibility has been evidenced with the frequent use of steroids to treat HSK. Here, we demonstrate primary cultures of HIS cells as an excellent model to study and highlight cytokine induction that occurs in the iris in the absence of the other mediators of inflammation, such as neutrophils that are usually recruited to the site upon infection (17).
Cultured HIS cells are susceptible to HSV-1 entry and plaque formation.
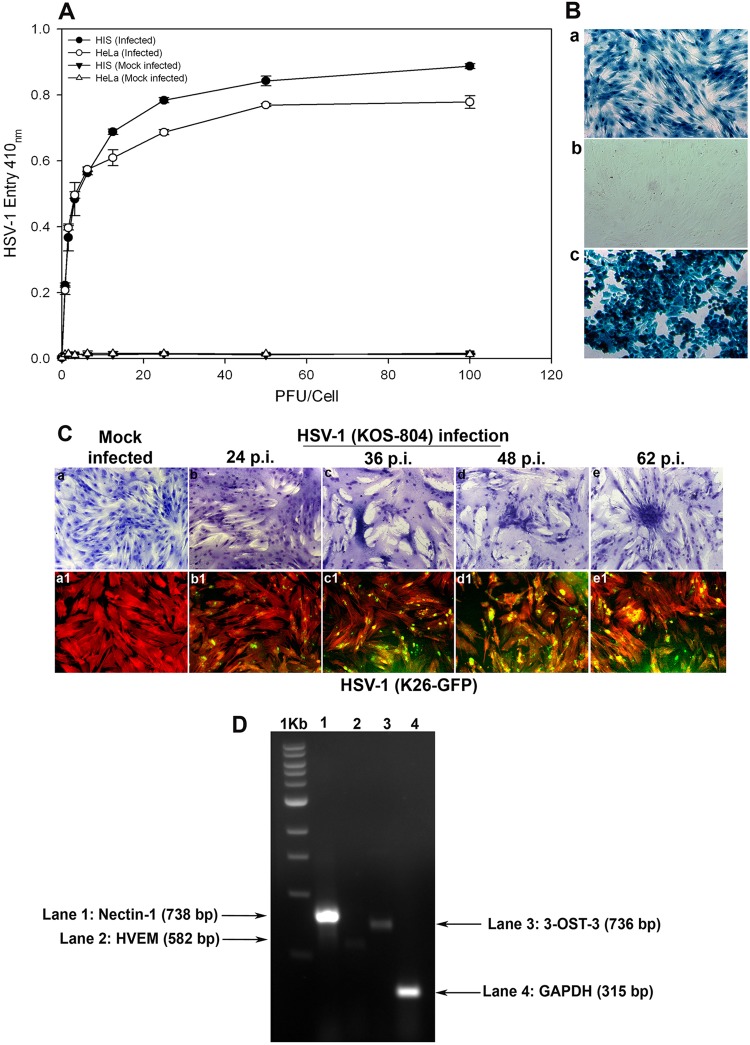
In order to demonstrate susceptibility to HSV-1 infection, HIS cell cultures were prepared in accordance with institutional review board-approved protocols and were isolated from anonymously donated human eyes (provided by the Illinois Eye Bank, Chicago, IL) via sterile dissection of the iris and pigmented epithelial layer and subsequent removal with a sterile cotton swab. The tissue was then digested with 0.2% type II collagenase (Sigma-Aldrich, St. Louis, MO) in cell culture medium, MCDB-131 (Sigma-Aldrich, St. Louis, MO) at 37°C with gentle stirring for 20 to 30 min. Digested tissues were next centrifuged to remove tissue debris, and HIS cells were cultured in MCDB-131 containing 10% fetal bovine serum (FBS) and antibiotics. To establish an HSV-1 entry model for the cells of the iris, both HIS and HeLa cells were infected with serial dilutions of a recombinant form of HSV-1 (KOS) gL86 that expresses β-galactosidase under the HSV-1 immediate-early promoter following cell entry (18). Mock infections with PBS and without virus of both cell lines were used as controls. As shown in Fig. 1A, compared to the controls, treatment of HIS and HeLa cells demonstrated viral entry in a dose-dependent manner. We further confirmed the above-described result via X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining after infecting HIS cells with reporter HSV-1. As shown in Fig. 1B, cultured HIS cells that were exposed to HSV-1 (KOS) gL86 were 100% stained blue (Fig. 1B, panel a) compared to uninfected HIS cells (Fig. 1B, panel b). HeLa cells, with HSV-1 KOS gL86-infected cells shown in blue, were used as a positive control (Fig. 1B, panel c). Next, in order to verify viral replication and spread to the neighboring cell, we used two recombinant forms of HSV-1 (KOS) 804, a mutant known to form high numbers of syncytia (19) and green fluorescent protein (GFP)-tagged HSV-1, HSV-1 (K26) GFP driven by the UL35 promoter sequences (20). As shown in Fig. 1C (panels a to e), more plaques were visualized at greater lengths of time postinfection (p.i.), while no plaques were observed in the mock-infected control. As shown in Fig. 1C (panels a1 to e1), fluorescence imaging was used to observe HSV-1 (K26) GFP spread into HIS cells stained with tetramethyl rhodamine isocyanate (TRITC)-conjugated phalloidin binding to F-actin. HSV-1-infected cells demonstrated both green fluorescence due to the HSV-1 capsid and red fluorescence from F-actin expression. Mock-infected cells showed only red fluorescence. Next, using reverse transcriptase PCR (RT-PCR), we focused on demonstrating the expression of common HSV-1 entry receptors in HIS cells. RNA samples were isolated from HIS cells and converted to first-strand cDNAs using the high-capacity cDNA reverse transcription kits (Applied Biosystems, Foster City, CA) by following the manufacturer's protocol. The RNA isolation and the primers were based on our previously published study (21). As shown in Fig. 1D, expression of the gD receptor nectin-1 was evident in HIS cells, while levels of HVEM and 3-OST-3 were found to be insignificant, suggesting that it is nectin-1 that is the gD receptor being used. Compared to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was used as the control, the signal for nectin-1 expression was the highest, followed by those of 3-OST-3 and HVEM. A relatively low expression of HVEM has also been reported with other cell types (21–23). Combined together, our results demonstrated that cultured HIS cells are highly susceptible to HSV-1 entry and spread. In addition, HIS cells express the required gD receptors that are expressed by other, more studied target cell types, including corneal epithelial cells (21).
Fig 1.
Analysis of herpes simplex virus 1 (HSV-1) entry in primary cultures of HIS cells. (A) Entry of HSV-1 into cultured HIS cells. Cultured HIS cells, along with HeLa cells, were plated in 96-well plates and inoculated with serial dilutions of a recombinant form of HSV-1 (KOS) gL86 that expresses β-galactosidase following cell entry at the indicated PFU/cell. After 6 h, the cells were washed, permeabilized, and incubated with o-nitrophenyl-β-d-galactopyranoside (ImmunoPure ONPG; Pierce) substrate for quantitation of β-galactosidase activity expressed from the input viral genome. The enzymatic activity was measured by spectrophotometer (Molecular Devices) at an optical density (OD) at 410 nm. Values in the figure were plotted as the means from three determinations (±standard deviations [SD]). (B) X-Gal staining of HSV-1 entry. Cultured HIS cells inoculated with HSV-1 (KOS) gL86 at an MOI of 0.01 (a) were 100% stained blue, indicating 100% viral infection, whereas uninfected cells (b) did not demonstrate any staining. (c) HeLa cells, with HSV-1 KOS gL86-infected cells stained blue, were used as a positive control. (C) Viral cell-to-cell spread. HIS cells were inoculated with serial dilutions of two recombinant forms of HSV-1, (KOS) 804, a mutant known to form high numbers of syncytia, and green fluorescent protein-tagged HSV-1 (K26) GFP. Confluent monolayer cultures inoculated with HSV-1 (KOS) 804 for 2 h were then washed and incubated with methyl cellulose containing media and stained at the various time points p.i. as indicated (a to e). Cells inoculated with HSV-1 (K26) GFP were subjected to fluorescence imaging (a1 to e1). Confluent monolayer cultures were stained with TRITC-conjugated phalloidin (red color), which binds to F-actin. HSV-1-infected cells showed both green fluorescence from HSV-1 capsid and red fluorescence from F-actin expression (green and red overlapping areas appear yellow). The images were taken with a fluorescence microscope at 20× magnification. (D) Standard nonquantitative RT-PCR analysis for entry receptor expression. Total RNA samples were isolated from HIS cells and converted to first-strand cDNAs and analyzed by PCR for the receptors as indicated. A housekeeping gene, GAPDH, was used as a control.
Inflammation in HIS cells upon HSV-1 infection.
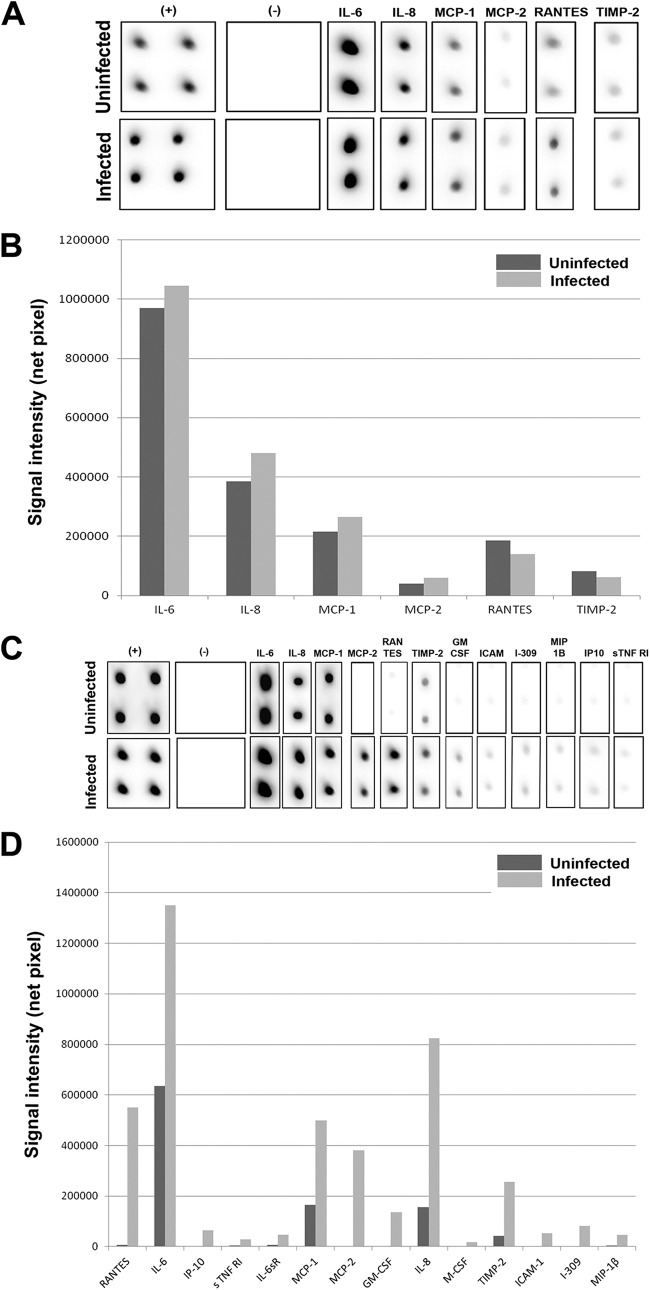
Intrinsic mediators of iritis are poorly understood. An in vitro cell culture model is the best way to identify the mediators of inflammation that are solely generated by the resident cells of the iris. Using a RayBio cytokine antibody array (RayBio, Norcross, GA) per the manufacturer's instructions, we assessed inflammatory markers induced as a result of HSV-1 infection of HIS cells in vitro. We used HSV-1 (KOS) 804 virus strain at a low (0.01) multiplicity of infection (MOI) to infect HIS cells and also mock-infected HIS cells with PBS to monitor inflammation. As shown in Fig. 2A and B, at 1 day p.i., there were no significant qualitative differences in the signal intensities of the inflammatory markers interleukin 6 (IL-6), IL-8, monocyte chemotactic protein 1 (MCP-1), MCP-2, regulated on activation normal T cell expressed and secreted (RANTES), or TIMP metallopeptidase inhibitor 2 (TIMP-2) between the HSV-1-infected and uninfected cells. However, at 2 days p.i., as noted in Fig. 2C and D, there was a greater signal intensity of MCP-2, TIMP-2, IL-6, and IL-8 in the infected cells. In addition, expression of new inflammatory markers, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), I-309, intercellular adhesion molecule 1 (ICAM-1), IL-6 soluble receptor (IL-6sR), interferon-inducible protein 10 (IP-10), macrophage inflammatory protein 1β (MIP-1β), and soluble tumor necrosis factor receptor 1 (sTNFR1), was documented in order of decreasing qualitative signaling at 2 days p.i. in infected cells, although none are present at 1 day p.i. Although levels of ICAM-1 have been suggested to have only moderate effects on immune response in cases of HSK in a murine model (24), upregulation of other markers at 2 days p.i. may have greater implications in terms of HSV-1-mediated pathology. For instance, GM-CSF has previously been shown to have a role in HSK via a supportive function of the neutrophils infiltrating corneal tissue, an important finding, as neutrophils are believed to contribute greatly to the permanent corneal damage seen in HSK. GM-CSF is also known to induce a maturation stimulus important for antigen-presenting cell (APC) function (25). The fact that this cytokine is first detected 2 days p.i. gives detailed insight into the temporal changes in the inflammatory response in infected HIS cells. The upregulation of IL-6 seems critical, too, as previous studies have suggested that IL-6 stimulates the secretion of vascular endothelial growth factor (VEGF) in the cornea (26, 27), implicating possible roles for initiating neovascularization by HSV-1 infection of HIS cells as well. The detection of IL-6sR with upregulation of IL-6 is not surprising then, as IL-6sR has been shown to increase the activity levels of IL-6 (28). An antiviral effect against HSV-1 is also documented in IP-10 detection at 2 days p.i., since this is known to directly inhibit HSV-1 replication (29). Upregulation of other inflammatory markers, such as I-309 and sTNFR1, may support viral infection since these chemokines are believed to have antiapoptotic effects (30, 31). The fact that these are seen in an in vitro model suggests that infected cells, independent of the host's immune response, are an important source of the mediators and cytokines seen in HSV-1 infection.
Fig 2.
Inflammation in HIS cells upon HSV-1 infection. (A) A RayBio cytokine antibody array (RayBio, Norcross, GA) per the manufacturer's instructions was used to assess inflammatory markers produced in HSV-1 (KOS) 804 (MOI of 0.01) infection of an in vitro HIS cell culture at 1 day p.i.; (B) signal intensities of various inflammatory markers are plotted in uninfected versus infected cells at 1 day p.i.; (C) inflammatory markers measured at 2 days p.i. utilizing same material and methods as described above; (D) signal intensities of various inflammatory markers are plotted in uninfected versus infected cells at 2 days p.i.
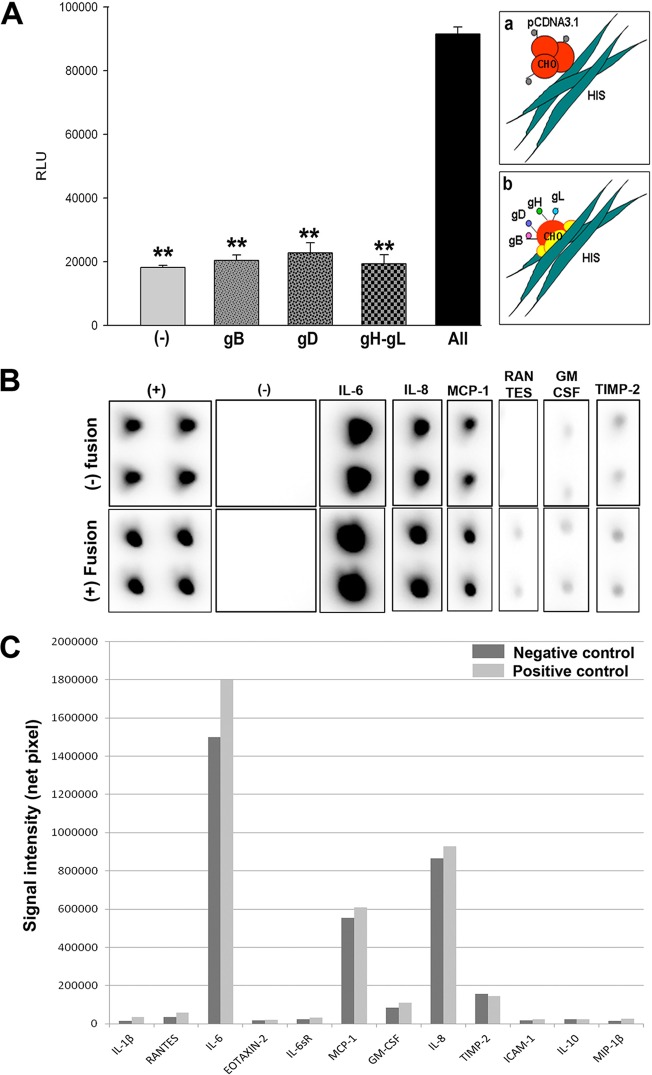
We next used the cytokine array to evaluate if expression of cytokines is altered during HSV-1-glycoprotein-induced cell fusion. As briefly mentioned, virus-mediated cell fusion requires the binding of gD to one of its host cell receptors, HVEM, 3-OS HS, or nectin-1. The subsequent conformational change in gD allows for this gD-host cell receptor complex to interact with gB and gH-gL to initiate the fusion of the viral and host cell membranes. This mechanism initially helps with virus entry and subsequently for the spread of the virus from cell to cell (13). A surrogate form of this assay can be performed by transiently expressing four HSV-1 glycoproteins, gB, gD, gH-gL, in cells and then allowing the cells to fuse with cells expressing a gD receptor such as nectin-1. We hypothesized that this biologically relevant assay can improve our understanding of the proinflammatory role of some key glycoproteins involved in HSV-1 entry. In order to proceed with this assay, we first verified whether HIS cells show the ability to fuse with Chinese hamster ovary (CHO-K1) cells expressing HSV-1 glycoproteins. CHO-K1 cells were either transfected with an empty vector (pCDNA3.1) or plasmids expressing one or more of the glycoproteins. Data and the schematic in Fig. 3A verify that CHO-K1 cells expressing HSV-1 glycoproteins gB, gD, and gH-gL were able to successfully fuse with HIS cells. Next, the cytokine array was used to determine the changes in the cytokine expression upon cell fusion. The assay was performed 2 days after mixing the target HIS cells with glycoprotein-expressing CHO-K1 cells. As shown in Fig. 3B, there was overall greater signal intensities detected for IL-6, MCP-1, IL-8, GM-CSF, and RANTES in the test than in the negative control. The variations in cytokines between the test and negative controls are plotted in Fig. 3C. These results suggest that it is possible that the upregulation of inflammatory cytokines seen in HSV-1 infection of HIS cells as noted earlier may be partly a result of the membrane fusion process that is mediated by the viral glycoproteins. Therefore, targeting some of these common cytokines may have therapeutic effects against HSV-1 entry as well as viral spread between the cells and an overall reduction in the complications seen with iritis. Further studies would be needed to confirm this possibility.
Fig 3.
Cytokine response following membrane fusion. (A) HIS cell fusion with HSV-1 glycoprotein-expressing Chinese hamster ovary (CHO-K1) cells. CHO-K1 cells were either transfected with an empty vector (pCDNA3.1) or plasmids expressing one or more of the glycoproteins gB, gD, or gH-gL. A luciferase-based reporter system was used to measure fusion. Relative luciferase activity was measured in relative luciferase units (RLU) (y axis). Cell fusion was measured in RLU. CHO-K1 cells expressing the empty vector were used as a negative control. The schematic shows both unsuccessful (a) and successful (b) cell fusion between CHO-K1 and HIS cells. (B) A RayBio cytokine antibody array (RayBio, Norcross, GA) per the manufacturer's instructions was used to assess inflammatory markers produced in HIS cell interactions with CHO-K1 cells in test versus negative-control cases. (C) Signal intensities of various inflammatory markers are plotted in negative-control (no fusion) versus test (fusion) cells.
Our results provide evidence for the first time that HIS cells are susceptible to HSV-1 entry/spread and verify the essential viral and host receptors involved, thus establishing an in vitro model to study iris stromal infection and the intrinsic mediators of inflammation. The fact that many of the inflammatory mediators are seen in the absence of the host immune response suggests a great importance for a usage of an in vitro HIS cell model. Future studies using this model may further help distinguish between the contributions from infected cells and those from the infiltrating immune cells during HSV-1 infection. This model may also serve as a platform to test for potential therapeutics that may help against HSV-1-induced iritis. For instance, G2, a peptide isolated against the receptor 3-OS HS, has previously been shown to decrease HSV-1-induced mouse corneal infection (32). With this in vitro model, we may be able to assess how inhibiting various stages of viral attachment and entry may affect the chemokine induction. Along the same line, this model will also be a new and an easy step to screen for effective drugs that reduce inflammatory conditions in context with HSV-1 infection of the iris stroma.
ACKNOWLEDGMENTS
This work was supported by NIH grants to D.S. (AI057860, AI081869) and V.T. (AI 088429-01A1) and a Midwest Eye Bank research grant to M.V.V. J.B. was supported by a Midwestern University (Downers Grove, IL)-sponsored Kenneth A. Suarez Summer Research Fellowship (10-2014-8172).
Footnotes
Published ahead of print 23 January 2013
REFERENCES
- 1. Dawson CR, Togni B. 1976. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv. Ophthalmol. 21:121–135 [DOI] [PubMed] [Google Scholar]
- 2. Rathinam SR, Namperumalsamy P. 2007. Global variation and pattern changes in epidemiology of uveitis. Indian J. Ophthalmol. 55:173–183 [DOI] [PubMed] [Google Scholar]
- 3. Teitelbaum CS, Streeten BW, Dawson CR. 1987. Histopathology of herpes simplex virus keratouveitis. Curr. Eye Res. 6:189–194 [DOI] [PubMed] [Google Scholar]
- 4. Sungur GK, Hazirolan D, Yalvac IS, Ozer PA, Aslan BS, Duman S. 2010. Incidence and prognosis of ocular hypertension secondary to viral uveitis. Int. Ophthalmol. 30:191–194 [DOI] [PubMed] [Google Scholar]
- 5. Tugal-Tutkun I, Ötük-Yasar B, Altinkurt E. 2010. Clinical features and prognosis of herpetic anterior uveitis: a retrospective study of 111 cases. Int. Ophthalmol. 30:559–565 [DOI] [PubMed] [Google Scholar]
- 6. Van der Lelij A, Ooijman FM, Kijlstra A, Rothova A. 2000. Anterior uveitis with sectoral iris atrophy in the absence of keratitis: a distinct clinical entity among herpetic eye diseases. Ophthalmology 107:1164–1170 [DOI] [PubMed] [Google Scholar]
- 7. Lundberg P, Cantin E. 2003. A potential role for CXCR3 chemokines in the response to ocular HSV infection. Curr. Eye Res. 26:137–150 [DOI] [PubMed] [Google Scholar]
- 8. Spear PG, Longnecker R. 2003. Herpesvirus entry: an update. J. Virol. 77:10179–10185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herold BC, WuDunn D, Soltys N, Spear PG. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 65:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scanlan PM, Tiwari V, Bommireddy S, Shukla D. 2005. Spinoculation of heparan sulfate deficient cells enhances HSV-1 entry, but does not abolish the need for essential glycoproteins in viral fusion. J. Virol. Methods 128:104–112 [DOI] [PubMed] [Google Scholar]
- 11. Krummenacher C, Baribaud F, Ponce de Leon M, Baribaud I, Whitbeck JC, Xu R, Cohen GH, Eisenberg RJ. 2004. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology 322:286–299 [DOI] [PubMed] [Google Scholar]
- 12. Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13–22 [DOI] [PubMed] [Google Scholar]
- 13. Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biswas PS, Banerjee K, Kim B, Rouse BT. 2004. Mice transgenic for IL-1 receptor antagonist protein are resistant to herpetic stromal keratitis: possible role for IL-1 in herpetic stromal keratitis pathogenesis. J. Immunol. 172:3736–3744 [DOI] [PubMed] [Google Scholar]
- 15. Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. 2001. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J. Virol. 75:9828–9835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carr DJ, Chodosh J, Ash J, Lane TE. 2003. Effect of anti-CXCL10 monoclonal antibody on herpes simplex virus type 1 keratitis and retinal infection. J. Virol. 77:10037–10046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tumpey TM, Cheng H, Cook DN, Smithies O, Oakes JE, Lausch RN. 1998. Absence of macrophage inflammatory protein-1α prevents the development of blinding herpes stromal keratitis. J. Virol. 72:3705–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montgomery RI, Warner MS, Lum BJ, Spear PG. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427–436 [DOI] [PubMed] [Google Scholar]
- 19. Little SP, Schaffer PAA. 1981. Expression of the syncytial (syn) phenotype in HSV-1, strain KOS: genetic and phenotypic studies of mutants in two syn loci. Virology 112:686–702 [DOI] [PubMed] [Google Scholar]
- 20. Desai P, Person S. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72:7563–7568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shah A, Farooq AV, Tiwari V, Kim MJ, Shukla D. 2010. HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection. Mol. Vis. 16:2476–2486 [PMC free article] [PubMed] [Google Scholar]
- 22. Akhtar J, Tiwari V, Oh MJ, Kovacs M, Jani A, Kovacs SK, Valyi-Nagy T, Shukla D. 2008. HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium. Invest. Ophthalmol. Vis. Sci. 49:4026–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shukla SY, Singh YK, Shukla D. 2009. Role of nectin-1, HVEM, and PILR-α in HSV-2 entry into human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 50:2878–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung HW, Jung CR, Choi BK, Vinay DS, Hill JM, Gebhardt BM, Kwon BS. 2004. Herpesvirus infection of ICAM-1-deficient mice. Curr. Eye Res. 29:201–208 [DOI] [PubMed] [Google Scholar]
- 25. Miyazaki D, Haruki T, Takeda S, Sasaki S, Yakura K, Terasaka Y, Komatsu N, Yamagami S, Touge H, Touge C, Inoue Y. 2011. Herpes simplex virus type 1-induced transcriptional networks of corneal endothelial cells indicate antigen presentation function. Invest. Ophthalmol. Vis. Sci. 52:4282–4293 [DOI] [PubMed] [Google Scholar]
- 26. Biswas PS, Banerjee K, Kinchington PR, Rouse BT. 2006. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp. Eye Res. 82:46–54 [DOI] [PubMed] [Google Scholar]
- 27. Terasaka Y, Miyazaki D, Yakura K, Haruki T, Inoue Y. 2010. Induction of IL-6 in transcriptional networks in corneal epithelial cells after herpes simplex virus type 1 infection. Invest. Ophthalmol. Vis. Sci. 51:2441–2449 [DOI] [PubMed] [Google Scholar]
- 28. Gaillard JP, Liautard J, Klein B, Brochier J. 1997. Major role of the soluble interleukin-6/interleukin-6 receptor complex for the proliferation of interleukin-6-dependent human myeloma cell lines. Eur. J. Immunol. 27:3332–3340 [DOI] [PubMed] [Google Scholar]
- 29. Lokensgard JR, Hu S, Sheng W, vanOijen M, Cox D, Cheeran MC, Peterson PK. 2001. Robust expression of TNF-alpha, IL-1beta, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J. Neurovirol. 7:208–219 [DOI] [PubMed] [Google Scholar]
- 30. Louahed J, Struyf S, Demoulin JB, Parmentier M, Van Snick J, Van Damme J, Renauld JC. 2003. CCR8-dependent activation of the RAS/MAPK pathway mediates anti-apoptotic activity of I-309/ CCL1 and vMIP-I. Eur. J. Immunol. 33:494–501 [DOI] [PubMed] [Google Scholar]
- 31. Waetzig GH, Rosenstiel P, Arlt A, Till A, Bräutigam K, Schäfer H, Rose-John S, Seegert D, Schreiber S. 2005. Soluble tumor necrosis factor (TNF) receptor-1 induces apoptosis via reverse TNF signaling and autocrine transforming growth factor-β1. FASEB J. 19:91–93 [DOI] [PubMed] [Google Scholar]
- 32. Tiwari V, Liu J, Valyi-Nagy T, Shukla D. 2011. Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J. Biol. Chem. 286:25406–25415 [DOI] [PMC free article] [PubMed] [Google Scholar]