Abstract
We have identified a gene that encodes the polypeptide cytochrome b in the avian malarial parasite Plasmodium gallinaceum. The gene containing the open reading frame was found to be located on a 6.2-kilobase multimeric extrachromosomal element. The amino acid translation from this gene demonstrated significant similarities to cytochrome b sequences from yeast, mammal, and fungus genomes. We present evidence that the P. gallinaceum cytochrome b transcript is part of a larger primary transcript from the element that is subsequently processed. The message for P. gallinaceum cytochrome b was found to be 1.2 kilobases in size. This is the first report identifying a mitochondrial nucleic acid sequence in malaria-causing organisms and suggests that a functional cytochrome system may exist in these parasites.
Full text
PDF
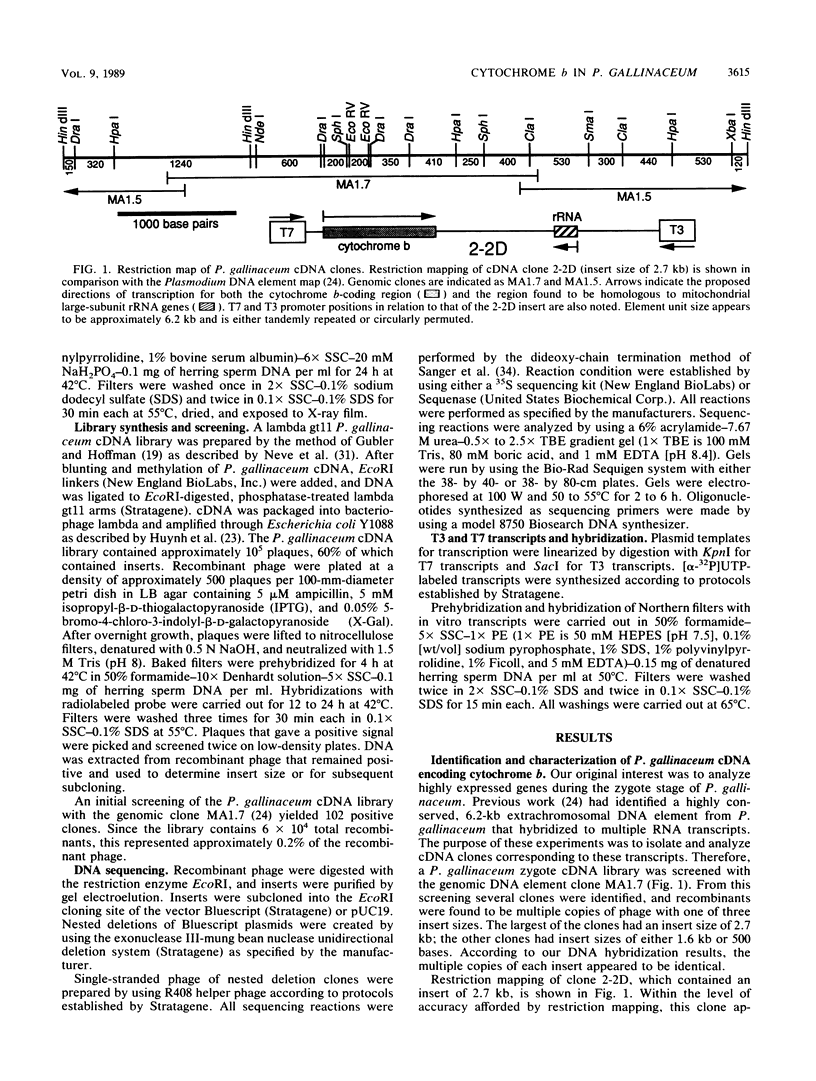
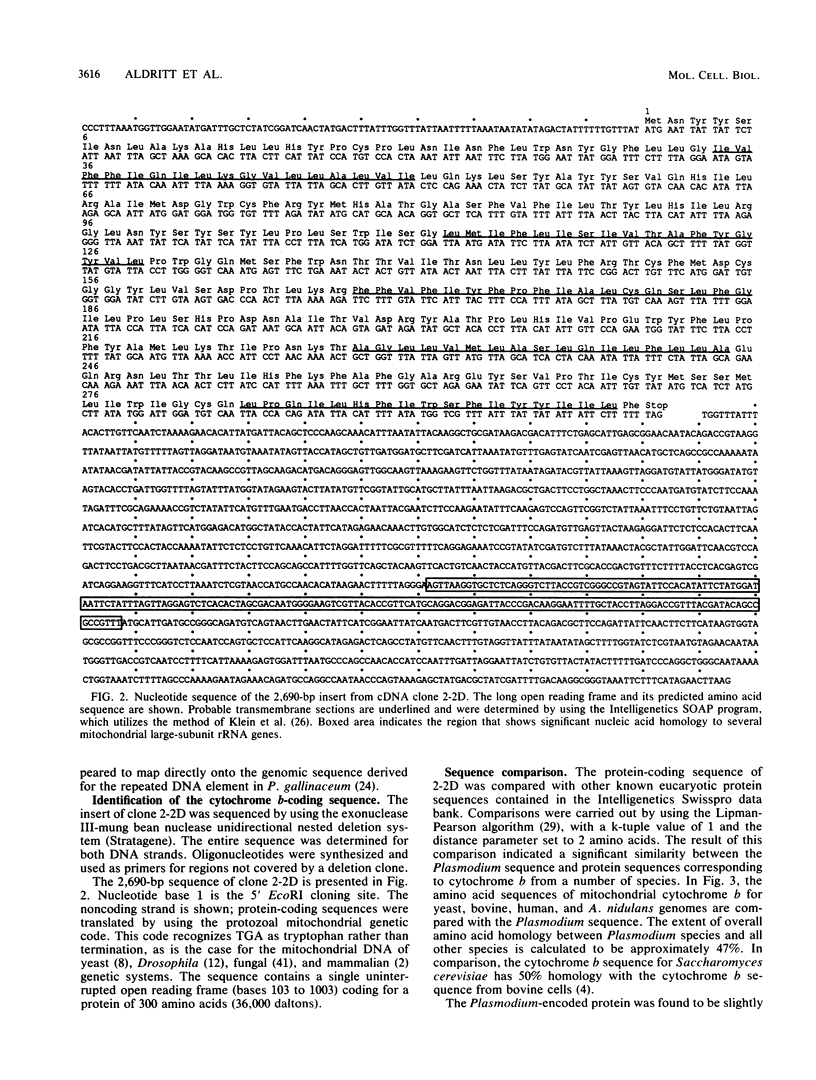
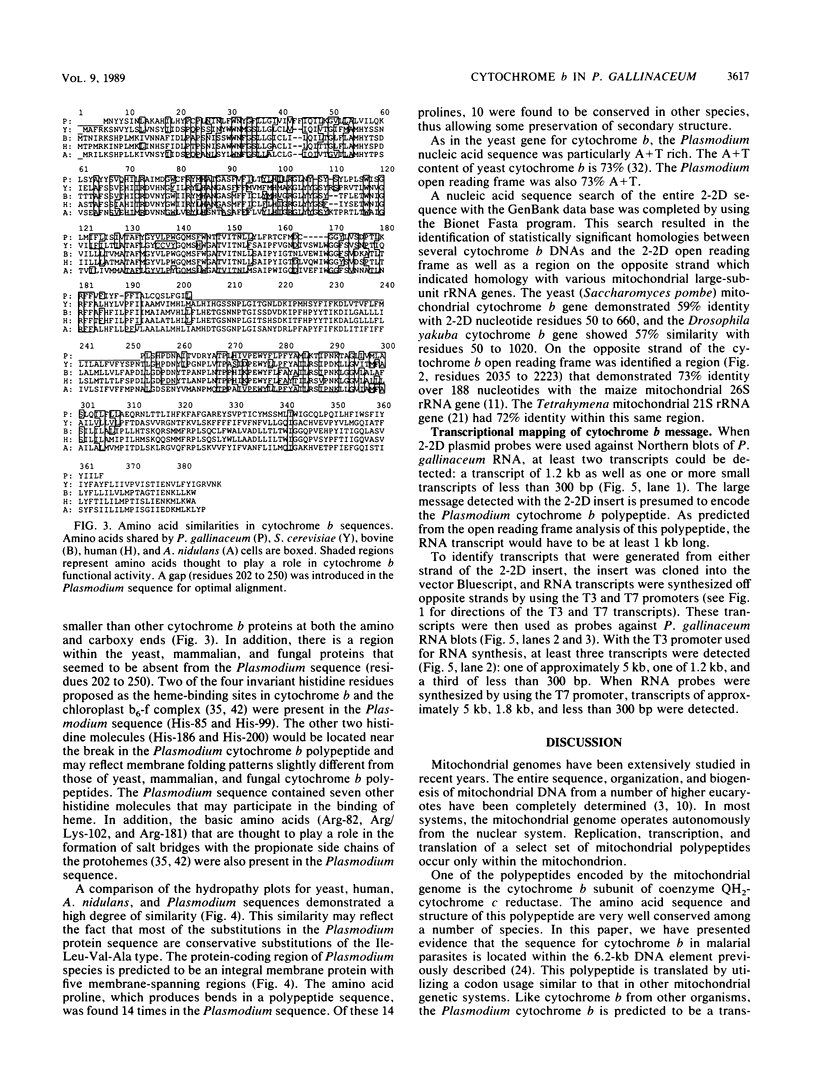


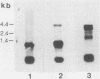
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M. Parasitological review. Plasmodium: the fine structure of malarial parasites. Exp Parasitol. 1971 Oct;30(2):284–320. doi: 10.1016/0014-4894(71)90094-4. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. J., Ginsburg H. Absence of alpha-ketoglutarate dehydrogenase activity and presence of CO2-fixing activity in Plasmodium falciparum grown in vitro in human erythrocytes. J Protozool. 1984 Feb;31(1):167–169. doi: 10.1111/j.1550-7408.1984.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Blum J. J., Yayon A., Friedman S., Ginsburg H. Effects of mitochondrial protein synthesis inhibitors on the incorporation of isoleucine into Plasmodium falciparum in vitro. J Protozool. 1984 Aug;31(3):475–479. doi: 10.1111/j.1550-7408.1984.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Bonitz S. G., Berlani R., Coruzzi G., Li M., Macino G., Nobrega F. G., Nobrega M. P., Thalenfeld B. E., Tzagoloff A. Codon recognition rules in yeast mitochondria. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3167–3170. doi: 10.1073/pnas.77.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Mendu N., Ginsburg H., Kridl J. C. Sequence analysis of the maize mitochondrial 26 S rRNA gene and flanking regions. Plasmid. 1984 Mar;11(2):141–150. doi: 10.1016/0147-619x(84)90019-2. [DOI] [PubMed] [Google Scholar]
- Divo A. A., Geary T. G., Jensen J. B., Ginsburg H. The mitochondrion of Plasmodium falciparum visualized by rhodamine 123 fluorescence. J Protozool. 1985 Aug;32(3):442–446. doi: 10.1111/j.1550-7408.1985.tb04041.x. [DOI] [PubMed] [Google Scholar]
- Dore E., Frontali C., Forte T., Fratarcangeli S. Further studies and electron microscopic characterization of Plasmodium berghei DNA. Mol Biochem Parasitol. 1983 Aug;8(4):339–352. doi: 10.1016/0166-6851(83)90080-4. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Bates P. A., Ling I. T., Moore D. J., McCready S., Gunasekera M. B., Wilson R. J., Williamson D. H. Mitochondrial DNA of the human malarial parasite Plasmodium falciparum. Mol Biochem Parasitol. 1988 Oct;31(1):11–17. doi: 10.1016/0166-6851(88)90140-5. [DOI] [PubMed] [Google Scholar]
- Geary T. G., Jensen J. B. Effects of antibiotics on Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1983 Mar;32(2):221–225. doi: 10.4269/ajtmh.1983.32.221. [DOI] [PubMed] [Google Scholar]
- Gero A. M., Brown G. V., O'Sullivan W. J. Pyrimidine de novo synthesis during the life cycle of the intraerythrocytic stage of Plasmodium falciparum. J Parasitol. 1984 Aug;70(4):536–541. [PubMed] [Google Scholar]
- Ginsburg H., Divo A. A., Geary T. G., Boland M. T., Jensen J. B. Effects of mitochondrial inhibitors on intraerythrocytic Plasmodium falciparum in in vitro cultures. J Protozool. 1986 Feb;33(1):121–125. doi: 10.1111/j.1550-7408.1986.tb05570.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., Dave D., Richards W. H. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979 Feb 1;582(3):390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Heinonen T. Y., Schnare M. N., Young P. G., Gray M. W. Rearranged coding segments, separated by a transfer RNA gene, specify the two parts of a discontinuous large subunit ribosomal RNA in Tetrahymena pyriformis mitochondria. J Biol Chem. 1987 Feb 25;262(6):2879–2887. [PubMed] [Google Scholar]
- Joseph J. T., Aldritt S. M., Unnasch T., Puijalon O., Wirth D. F. Characterization of a conserved extrachromosomal element isolated from the avian malarial parasite Plasmodium gallinaceum. Mol Cell Biol. 1989 Sep;9(9):3621–3629. doi: 10.1128/mcb.9.9.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A. Circular mitochondrial DNA from the avian malarial parasite Plasmodium lophurae. Biochim Biophys Acta. 1975 May 16;390(3):276–284. doi: 10.1016/0005-2787(75)90348-2. [DOI] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Jensen J. B., Reese R. T., Trager W. Fine structure of human malaria in vitro. J Protozool. 1978 Nov;25(4):443–452. doi: 10.1111/j.1550-7408.1978.tb04167.x. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Matsuda S., Yoshida Y. Ultrastructure of erythrocytic stages of Plasmodium ovale in humans. Am J Trop Med Hyg. 1986 Jul;35(4):689–696. doi: 10.4269/ajtmh.1986.35.689. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Harris P., Kosik K. S., Kurnit D. M., Donlon T. A. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986 Dec;387(3):271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Rush M. G., Misra R. Extrachromosomal DNA in eucaryotes. Plasmid. 1985 Nov;14(3):177–191. doi: 10.1016/0147-619x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M. Location of haem-binding sites in the mitochondrial cytochrome b. FEBS Lett. 1984 Jan 30;166(2):367–372. doi: 10.1016/0014-5793(84)80114-3. [DOI] [PubMed] [Google Scholar]
- Scheibel L. W., Ashton S. H., Trager W. Plasmodium falciparum: microaerophilic requirements in human red blood cells. Exp Parasitol. 1979 Jun;47(3):410–418. doi: 10.1016/0014-4894(79)90094-8. [DOI] [PubMed] [Google Scholar]
- Scheibel L. W., Pflaum W. K. Cytochrome oxidase activity in platelet-free preparations of Plasmodium falciparum. J Parasitol. 1970 Dec;56(6):1054–1054. [PubMed] [Google Scholar]
- Sherman I. W. Biochemistry of Plasmodium (malarial parasites). Microbiol Rev. 1979 Dec;43(4):453–495. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Myers A. M. Genetics of mitochondrial biogenesis. Annu Rev Biochem. 1986;55:249–285. doi: 10.1146/annurev.bi.55.070186.001341. [DOI] [PubMed] [Google Scholar]
- Warhurst D. C., Thomas S. C. The chemotherapy of rodent malaria, XXXI. The effect of some metabolic inhibitors upon chloroquine-induced pigment clumping (CIPC) in Plasmodium berghei. Ann Trop Med Parasitol. 1978 Jun;72(3):203–211. doi: 10.1080/00034983.1978.11719307. [DOI] [PubMed] [Google Scholar]
- Waring R. B., Davies R. W., Lee S., Grisi E., Berks M. M., Scazzocchio C. The mosaic organization of the apocytochrome b gene of Aspergillus nidulans revealed by DNA sequencing. Cell. 1981 Nov;27(1 Pt 2):4–11. doi: 10.1016/0092-8674(81)90354-8. [DOI] [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Herrmann R. G., Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Wilson R. J., Bates P. A., McCready S., Perler F., Qiang B. U. Nuclear and mitochondrial DNA of the primate malarial parasite Plasmodium knowlesi. Mol Biochem Parasitol. 1985 Feb;14(2):199–209. doi: 10.1016/0166-6851(85)90038-6. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H. Drosophila melanogaster mitochondrial DNA, a novel organization and genetic code. Nature. 1983 Jul 21;304(5923):234–241. doi: 10.1038/304234a0. [DOI] [PubMed] [Google Scholar]