Abstract
Anti-adenovirus serotype 5 antibodies are capable of neutralizing adenovirus serotype 5-based vaccines. In mice and guinea pigs, intranasal delivery of adenovirus serotype 5-based vaccine bypasses induced adenovirus serotype 5 preexisting immunity, resulting in protection against species-adapted Ebola virus challenge. In this study, nonhuman primates were vaccinated with adenovirus serotype 5-based vaccine either intramuscularly or via the airway route (intranasally/intratracheally) in the presence or absence of adenovirus serotype 5 preexisting immunity. Immune responses were evaluated to determine the effect of both the vaccine delivery route and preexisting immunity before and after a lethal Ebola virus (Zaïre strain Kikwit 95) challenge. Intramuscular vaccination fully protected nonhuman primates in the absence of preexisting immunity, whereas the presence of preexisting immunity abrogated vaccine efficacy and resulted in complete mortality. In contrast, the presence of preexisting immunity to adenovirus serotype 5 did not alter the survival rate of nonhuman primates receiving the adenovirus serotype 5-based Ebola virus vaccine in the airway. This study shows that airway vaccination with adenovirus serotype 5-based Ebola virus vaccine can efficiently bypass preexisting immunity to adenovirus serotype 5 and induce protective immune responses, albeit at lower efficacy than that using an intramuscular vaccine delivery route.
INTRODUCTION
Ebola virus (EBOV) is a pathogen that causes hemorrhagic fever, resulting in mortality rates as high as 90% among infected humans (1). While there have been relatively few incidences of human EBOV infection worldwide, this virus presents a significant concern to public health authorities due to its high mortality rate, lack of prophylactic or therapeutic interventions, and potential use as a biological weapon (2). In recent years, several vaccine platform candidates have proven efficacious against lethal EBOV infection in several animal models. These include virus-like particle preparations (3–6), vesicular stomatitis virus (7–11), human parainfluenza virus type 3 (12–14), and replication-deficient human adenovirus serotype 5 (AdHu5) vectors (15–18) expressing an EBOV gene(s).
Adenovirus causes mild respiratory disease, gastroenteritis, and conjunctivitis in humans (19). However, replication-deficient AdHu5-based vectors are an attractive vaccine platform, as they induce a strong innate and adaptive immune response (19). Recombinant adenoviruses expressing the EBOV glycoprotein (GP) mixed with AdHu5 expressing the EBOV nucleoprotein (NP) offered complete protection in nonhuman primates (NHP) against lethal EBOV challenge (18, 20). Such successes have encouraged the development of more replication-deficient adenovirus-based vaccine strategies and have led to a phase I clinical trial (http://clinicaltrials.gov/show/NCT00374309).
Despite the promising attributes associated with the adenoviral-based vaccine platforms, an inherent drawback exists if prior exposure and subsequent immune response to AdHu5 leads to preexisting immunity (PEI). Approximately 90% of sub-Saharan African and southeast Asian populations and an estimated 35% of the North America population have anti-AdHu5 antibodies capable of neutralizing AdHu5-based vaccines (21, 22). It is possible to circumvent PEI to AdHu5 by either increasing the adenoviral vaccine dose (23), by using rare human serotypes, such as AdHu12, AdHu35 (24), and AdHu6 (25), or by using adenoviruses from other species, such as simian (26), bovine (27), and porcine. Alternatively, it is possible to bypass AdHu5 PEI by altering the route of vaccine delivery from the conventional intramuscular (i.m.) injection which typically stimulates systemic responses (10, 20, 28) to an airway route of vaccination capable of inducing both mucosal and systemic immune responses in both the mouse and guinea pig animal models (29–33).
The first-generation AdHu5-GP vaccine was previously shown to protect mice, guinea pigs, and NHP from an otherwise lethal challenge of Zaire EBOV when administered i.m. (20, 34). AdHu5-GP was also efficacious in the mouse animal model when administered intranasally (i.n.) in the presence or absence of AdHu5-induced PEI (15, 16). A more potent second-generation Ad-based EBOV vaccine (Ad-CAGoptZGP) demonstrated improved T and B cell responses as well as protection in mice at doses of up to 100-fold lower than that needed with the first-generation AdHu5-GP vaccine (17). Upon further vaccine development, Ad-CAGoptZGP was combined with an AdHu5-expressing alpha interferon (Ad-IFN-α), providing both an antiviral activity to EBOV and adjuvant effect to Ad-CAGoptZGP. Ad-CAGoptZGP/Ad-IFN-α elicited complete protection in both mice and guinea pigs when administered 30 to 60 min after lethal challenge with adapted EBOV (postexposure treatment) (35).
The goal of the present study was to assess if airway administration of Ad-CAGoptZGP/Ad-IFN-α could induce protection from EBOV challenge in NHP in the presence or absence of PEI to AdHu5. Additionally, specific cellular and humoral immune responses were monitored following airway vaccination with Ad-CAGoptZGP/Ad-IFN-α, and the T and B cell responses were analyzed in relation to survival after lethal EBOV challenge.
MATERIALS AND METHODS
Construction of adenoviral vaccine.
Construction and production of Ad-CAGoptZGP vaccine was described previously (17). Particle number and infectivity were determined by optical density and immunodetection of the hexon protein of AdHu5 (Adeno-X rapid titer kit; Clontech, Mountain View, CA). Adenovirus preparations were quantified for both infectious (infection-forming units [IFU]), PFU, and total particle number. Ad-IFN-α was provided by Defyrus Inc. (Toronto, Canada). Ad-CAGoptZGP/Ad-IFN-α consists of 1 × 1010 IFU of Ad-CAGoptZGP mixed with 1 × 109 PFU of Ad-IFN-α. Ad-CAGoptZGP and an AdHu5 vector expressing LacZ (Ad-lacZ) were constructed using the Adeno-X expression system I (BD Biosciences, Palo Alto, CA), where E1/E3 deletions render this human adenovirus serotype 5 replication incompetent. Ad-IFN-α (Defyrus) is also a recombinant human adenovirus serotype 5 that is replication incompetent.
Animal model, sample collection, and challenge.
Infectious work was performed in the biosafety level 4 (BSL-4) laboratory at the National Microbiology Laboratory, and it was approved by the Canadian Science Centre for Human and Health Animal Care Committee, following the guidelines of the Canadian Council on Animal Care. Fourteen healthy female cynomolgus macaques (Macaca fascicularis), ages 5 to 25 years old, were obtained from a Health Canada NHP colony. Tracheal intubations were performed on NHP scheduled to receive the vaccine via the airway route. The vaccine bolus was administered with a 5-ml syringe attached to a catheter tube inserted into the endotracheal tube and delivered to the lower lobes of one lung. One ml of the vaccine was also administered directly into the nasal cavity. For EBOV challenge, animals were infected i.m. at two sites with 1 ml in total of freshly prepared EBOV (strain Kikwit 95; passage 2 on VeroE6 cells) inoculum containing 1,000 50% tissue culture infectious doses (TCID50) in diluent (minimum essential medium containing 0.3% bovine serum albumin). Two injections were used to minimize variation between animals. EBOV titers were confirmed (936 TCID50) by back-titration of the challenge preparation following administration of the virus.
Vaccination schedule.
For group A, three NHP were vaccinated i.m. 28 days prior to challenge (day −28) in the deltoid muscle with a single dose of Ad-CAGoptZGP/Ad-IFN-α diluted to a total volume of 1 ml in phosphate-buffered saline (PBS). This group was the positive control to confirm that the vaccine is efficacious in naïve (no induced PEI to AdHu5) NHP using the conventional i.m. vaccination route. For group B, three NHP were vaccinated i.n./intratracheally (i.t.) 28 days prior to challenge (day −28) with a single dose of Ad-CAGoptZGP/Ad-IFN-α diluted with PBS to a total volume of 6 ml (0.5 ml per nostril and 5 ml directly into the lung). For group C, four NHP were each administered a single i.m. dose of 1 × 1010 IFU of Ad-lacZ (diluted to a total volume of 1 ml in PBS) in the deltoid muscle 35 days prior to vaccination (day −63). Ad-lacZ was used to induce PEI in NHP with the intent of generating a robust immune response against subsequent exposures to AdHu5. This group was then vaccinated i.n./i.t. 28 days prior to challenge (day −28) with a single dose of Ad-CAGoptZGP/Ad-IFN-α diluted with PBS to a total volume of 6 ml (0.5 ml per nostril and 5 ml directly into the lung). For group D, three NHP were administered a single dose of 1 × 1010 IFU of Ad-lacZ i.m. in the deltoid muscle 35 days prior to vaccination (day −63). Ad-lacZ was diluted to a total volume of 1 ml in PBS. This group was then vaccinated i.m. 28 days prior to challenge in the deltoid muscle with a single dose of Ad-CAGoptZGP/Ad-IFN-α diluted to a total volume of 1 ml with PBS. For group E, one control NHP was administered a single dose of 1 × 1010 IFU of Ad-lacZ i.m. in the deltoid muscle 28 days prior to EBOV challenge (day −28).
Anti-AdHu5 neutralizing antibodies.
PEI against AdHu5 was assessed by determining the amount of neutralizing antibodies (NAb) present in serum or bronchiolar lavage (BAL) samples according to established methods (15). Briefly, samples were inactivated at 56°C for 40 min and then 2-fold diluted with Dulbecco's modified Eagle medium (DMEM), starting from a 1:20 dilution. Each dilution was mixed with an equal volume of AdHu5 expressing Escherichia coli β-galactosidase (Ad-lacZ) at a concentration of 100 transducing units/well and incubated for 4 h at 37°C. The mixture was then added to subconfluent VeroE6 cells in 96-well flat-bottom plates and incubated for 48 h at 37°C. Sample dilutions which showed a 50% reduction in the number of cells that were stained blue by visual inspection (per the in situ β-galactosidase staining kit protocol [Agilent Technologies]) compared to controls were scored positive for NAb presence. NAb data were reported as reciprocal dilution values. Consent was obtained for all human serum samples (using the Consent for Research Phlebotomy Form, modified from the Consent for Serum Storage Form SES-F-064B-1 from the Public Health Agency of Canada).
Anti-EBOV neutralizing antibodies.
Serum and BAL samples were prepared and serially diluted as described above. Each dilution was mixed with equal volumes of recombinant EBOV expressing the enhanced green fluorescent protein (EGFP) reporter gene (EBOV-EGFP) at a concentration of 100 transducing units/well and incubated at 37°C for 4 h as previously described (36). The mixture was then added to subconfluent VeroE6 cells in 96-well flat-bottom plates and incubated for 48 h at 37°C. Sample dilutions which showed a 50% reduction in the number of fluorescing cells compared to controls were scored positive for NAb presence.
Enzyme-linked immunosorbent assay (ELISA).
Costar EIA/RIA 96-well flat-bottom high-binding polystyrene microtiter plates were coated overnight at 4°C with 30 μl/well of 1 μg/ml His-EBOV-GP capture antigen. The His-EBOV-GP capture antigen was generated using truncated EBOV glycoproteins (strain Zaire) as previously described (37). Plates were blocked for 1 h with blocking buffer (5% milk powder–PBS [100 μl/well] at 37°C). Serum was serially diluted to 1:50, 1:100, 1:200, 1:400, 1:800, 1:1,600, 1:3,200, and 1:6,400 in 2% skim milk powder–PBS–0.05% Tween 20, and 30 μl of the dilution was added to each well and incubated for 1 h at 37°C. The plates were washed eight times with Tris-buffered saline (TBS)–0.1%–Tween 20 (150 μl/well). A secondary antibody (goat anti-monkey IgAα-horseradish peroxidase [HRP] conjugate [1:30,000 dilution; Fitzgerald], goat anti-monkey IgMμ-HRP conjugate [1:2,500 dilution; KPL], or goat anti-human IgG [H+L] [1:2,000 dilution; KPL]) was added to the wells and then incubated for 1 h at 37°C. The plates were washed eight times with TBS–0.1%–Tween 20 (150 μl/well). Horseradish peroxidase substrate {3% hydrogen peroxide solution with ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)]} was then added and incubated at room temperature for 30 min. The plates were read using a VMax Kinetic ELISA microplate reader (Molecular Devices), and the data were analyzed using CellMaxPro software for the detection of ZGP-specific IgG, IgA, and IgM antibodies. The data are reported as the optical density at 405 nm (OD405).
Flow cytometry analysis.
The frequency of CD4- or CD8-positive cells producing IFN-γ was assessed by flow cytometry. Whole blood collected in EDTA-containing Vacutainer tubes was diluted with an equal volume of PBS and layered on a Ficoll density gradient (GE Healthcare). Peripheral blood mononuclear cells (PBMCs) were collected from the interphase after centrifugation at 750 × g for 45 min. Cells were washed with L-15 media (Invitrogen) and seeded at a density of 5 × 105 cells per well in RPMI 1640 supplemented with 0.1 mM minimal essential medium (MEM) nonessential amino acids (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 10 mM HEPES, 2 mM l-glutamine (Invitrogen), 55 μM β-mercaptoethanol, 10% fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 μg/ml streptomycin (enzyme-linked immunosorbent spot [ELISpot] medium). PBMCs were incubated at 37°C in a 5% CO2 atmosphere for 18 h with AdHu5 hexon or EBOV GP peptide pools to reach a final concentration of 5 μg/ml in order to stimulate the IFN-γ production. PBMC were seeded at 1 × 106 cells per well in ELISpot medium. Cells were stimulated overnight with 5 μg/ml of AdHu5 hexon or EBOV GP peptide pools in the presence of 1 μl/ml of the protein transport inhibitor GolgiPlug (BD Biosciences). Peripheral blood lymphocytes were then stained with peridinin chlorophyll protein (PerCP)-Cy5.5-conjugated mouse anti-human CD4 and allophycocyanin (APC)-conjugated mouse anti-human CD8 antibodies (clones L200 and RPA-T8, respectively; BD Biosciences), followed by a 20-min incubation in Cytofix/Cytoperm (BD Biosciences). Intracellular IFN-γ was detected after staining with a fluorescein isothiocyanate (FITC)- or Alexa 700-conjugated mouse anti-human IFN-γ antibody (BD Biosciences) diluted in Perm/Wash buffer (BD Biosciences). At least 300,000 events were analyzed using a 4-color (under BSL-4 conditions) or a 16-color flow cytometer (ACCURI or LSRII; BD Biosciences).
Statistical analysis.
Data were analyzed for statistical differences by performing two-tailed unpaired t tests. The differences in the mean or raw values among treatment groups were considered significant when P < 0.05.
RESULTS
Generation of AdHu5 immunity in NHP.
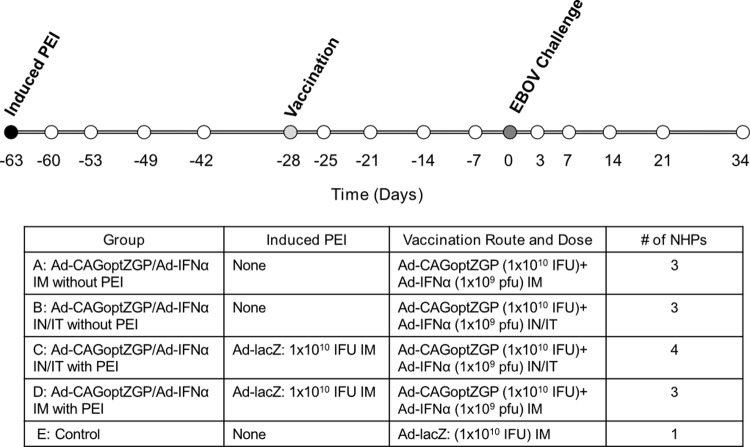
Systemic anti-AdHu5 antibodies that are often present in the human population are thought to be capable of neutralizing and negatively affecting the efficacy of AdHu5-based vaccines (38). Ideally, induction of an immune response to AdHu5 in NHP would be established using replication-competent AdHu5 in a manner that mimics community-acquired AdHu5 and subsequent immune response in humans. However, this is not possible, as wild-type AdHu5 does not replicate in macaques (39). In order to determine if altering the route of vaccination from i.m. to an airway route (i.n./i.t.) can circumvent PEI in NHP, cynomolgus macaques were administered 1 × 1010 IFU of Ad-lacZ intramuscularly (i.m.) to induce systemic PEI to AdHu5. The schedule of inducing PEI is outlined in Fig. 1. Briefly, NHP were either given 1 × 1010 IFU Ad-lacZ i.m. (group C and D) or remained naïve to AdHu5 (groups A, B, and E).
Fig 1.
Schematic representation of NHP treatment, sample, and challenge schedule. The experimental schedules shown represent the timeline for the induction of PEI in NHP by i.m. administration of 1 × 1010 IFU of Ad-lacZ on day −63 (black circle), vaccination with Ad-CAGoptZGP/Ad-IFN-α (light gray circle), and i.m. challenge day with 1,000 TCID50 of EBOV (dark gray circle) on serum sample collection day −63, −60, −53, −49, −42, −28, −25, −21, −14, −7, 0, 3, 7, 14, 27, and 34 (all circles). The route of administration and the vaccine used are shown for each group in the lower panel.
Antibody and cellular immune responses to AdHu5 were monitored following exposure of NHP to Ad-lacZ. Serum samples collected from NHP at day −63 (prior to Ad-lacZ administration), day −28 (prior to vaccination with Ad-CAGoptZGP/Ad-IFN-α), day 0 (prior to EBOV challenge), and day 34 (34 days after EBOV challenge) were analyzed for anti-AdHu5 NAb levels. At day −63, there were no detectable anti-AdHu5 NAb for any of the 14 naïve NHP (Fig. 2a). Day −28 serum collected from NHP preexposed to AdHu5 had anti-AdHu5 systemic NAb titers of 533 ± 185, 853 ± 370, 320 ± 0, and 853 ± 370 for NHP in group C and 3,413 ± 1,478, 1,280 ± 1,109, and 1,280 ± 0 reciprocal dilutions for NHP in group D (Fig. 2a). The average level of anti-AdHu5 NAb for NHP with induced PEI (group C and D) was 1,219 ± 1,030 reciprocal dilutions at day −28, which was comparable to the average level of NAb to AdHu5 detected in 30 seropositive human patients and reported in the literature (Fig. 2b) (32). NHP serum collected at day 0 (prior to EBOV challenge) was assayed, and in all cases the levels of anti-AdHu5 NAb ranges were equal or elevated for groups A, B, C, and D due to exposure to Ad-lacZ or the vaccine or both (Fig. 2a). The control NHP had no detectable anti-AdHu5 NAb level. In all NHP sampled at day 34, the levels of anti-AdHu5 NAb remained elevated, suggesting stable circulation of systemic anti-AdHu5 NAb (Fig. 2a).
Fig 2.
Immune response following induction of AdHu5 PEI in NHP. (a) Serum anti-AdHu5 NAb. Serum samples were collected on day −63 (gray bars), day −28 (white bars), day 0 (black bars), and day 34 (wave) and assayed for the presence of anti-AdHu5 NAb. The values represent the reciprocal dilutions for each NHP assayed in triplicate; error bars represent the standard deviations. (b) Comparison of serum anti-AdHu5 NAb prior to vaccination (day −28) in induced NHP to those from seropositive human samples. The values represent the reciprocal dilutions for each sample done in triplicate; error bars represent the standard errors. P = 0.7042 by two-tailed unpaired t test (NHP, n = 7; human, n = 30). (c) Anti-AdHu5 NAb in BAL samples. BAL samples were collected on day 0 (black bars) and day 34 (white bars) and assayed for the presence of anti-AdHu5 NAb. The values represent the reciprocal dilutions for each NHP done in triplicate, and error bars represent the standard deviations. (d) Cellular immunity against AdHu5 measured by flow cytometry. Cells were stimulated by ex vivo exposure to AdHu5 hexon peptide pools 21 days after administration of AdHu5 vector expressing LacZ. Data represent the sum of the IFN-γ-positive cells per million lymphocytes counted for all of the peptides pools; error bars represent the standard errors. P = 0.354 by two-tailed unpaired t test (group C, n = 4; group D, n = 3). The background, unstimulated control is shown (CTR). In all instances, the star symbols represent NHP that succumbed to the EBOV infection.
Bronchoalveolar lavage (BAL) samples collected from NHP at days 0 and 34 were also analyzed for anti-AdHu5 NAb levels in order to compare the systemic levels versus the levels of anti-AdHu5 NAb in the airway. i.m.-vaccinated NHP did not have detectable anti-AdHu5 NAb in their airway at either time point (Fig. 2c). NHP vaccinated i.n./i.t. had anti-AdHu5 NAb levels of 0, 13 ± 12, and 13 ± 12 at day 0 and 20 ± 20 or 13 ± 12 at day 34 (Fig. 2c). NHP vaccinated i.n./i.t. with PEI had anti-AdHu5 NAb levels of 7 ± 12, 133 ± 46, 0, and 133 ± 46 at day 0 and 0, 7 ± 12, and 0 at day 34 (Fig. 2c). The control NHP had no detectable airway anti-AdHu5 NAb level at day 0 (Fig. 2c).
While it has been postulated and mainly accepted that the humoral immune response is responsible for, and leads to, AdHu5-based vaccine neutralization (38), we also assessed the levels of cellular immunity against AdHu5 as measured by flow cytometry. PBMCs were isolated from NHP in group C and group D on day −42 (21 days after administration of Ad-lacZ). The PBMCs were stimulated by ex vivo exposure to AdHu5 hexon peptide pools to determine the number of IFN-γ-secreting cells. Groups C and D had 138.8 ± 30.2 and 331.7 ± 221.7 IFN-γ producing lymphocytes per million, respectively (Fig. 2d). This difference was not statistically significant (P = 0.354) (Fig. 2d).
Immune response to vaccination.
To determine systemic NAb levels against ZGP following vaccination, serum samples were collected from NHP at day −28 (prior to vaccination), day −21, day −14, day −7, and day 0 (prior to EBOV challenge) and assayed for NAb levels against ZGP. Background levels of anti-ZGP NAb were observed up to day 0, and at this time point the anti-ZGP NAb levels increased slightly to 16 ± 4, 10 ± 0, 13 ± 1, and 16 ± 4 reciprocal dilutions for groups A, B, C, and D, respectively (Fig. 3a). Levels of anti-ZGP NAb detected in the serum of the control animal remained below 10 reciprocal dilutions.
Fig 3.
Immune response in NHP following vaccination and EBOV challenge. (a) Serum anti-EBOV NAb. Serum samples collected on days −28, −21, −14, −7, 0, 7, 14, 21, and 34 were assayed for the presence of anti-EBOV NAb. The values represent the reciprocal dilutions for each NHP done in triplicate and then averaged for the groups, and error bars represent the standard deviations between the groups. (b) ZGP-specific IgG ELISA. Microtiter plates were coated with His-EBOV-GP capture antigen. All serum samples were assayed in triplicate, and each sample was diluted 2-fold from 1:50 to 1:6,400. Goat anti-human IgG was used as the secondary antibody. ZGP-specific IgG levels for the 1:1,600 serum dilution were shown as a representative set of data, and similar trends were observed for the other dilutions. Error bars were excluded for clarity. For groups A and D versus group B and C (day −7), P = 0.0123 by two-tailed unpaired t test. For group C versus group B (day −7), P = 0.8232 by two-tailed unpaired t test. For group C versus group B (day 0), P = 0.9374 by two-tailed unpaired t test. For group D versus group A (day −7), P = 0.7134 by two-tailed unpaired t test. For group D versus group A (day 0), P = 0.0021 by two-tailed unpaired t test. (c) ZGP-specific IgM ELISA. The assay was preformed identically to the IgG ELISA, except goat anti-monkey IgM was used as the secondary antibody. Serum was serially diluted, and data represent the 1:100 dilutions. Error bars were excluded for clarity. (d) Cellular immune response against EBOV GP as measured by flow cytometry. The data represent the number of IFN-γ-positive cells per million lymphocytes. Error bars represent the standard errors. For group D versus group A (day −14), P = 0.0054 by two-tailed unpaired t test (groups A, B, C, and D, n = 3, 3, 4, and 3, respectively). For group C versus group B (day −14), P = 0.3978 by two-tailed unpaired t test. The response of the unvaccinated NHP (CTR) is also shown.
It has been suggested that IgG plays a protective role against EBOV infection (40–43). We hoped to determine if i.m. or i.n./i.t. vaccination leads to differential IgG levels in NHP in the presence or absence of PEI to AdHu5. IgG levels were assayed using a ZGP-specific ELISA on sera collected at days −28 (prior to vaccination), −21, −14, −7, and 0 (prior to EBOV challenge). NHP vaccinated i.m. (group A) had rising anti-ZGP IgG levels starting 21 days after vaccination (day −7) until day 0 (Fig. 3b). NHP vaccinated i.m. with PEI (group D) had low but detectable IgG levels 21 to 28 days after vaccination (day −7 to day 0) prior to EBOV challenge. Interestingly, levels of IgG for group B (vaccinated i.n./i.t.) and group C (vaccinated i.n./i.t. but having PEI) increased earlier, after day −14, than NHP from groups A (vaccinated i.m.) and D (vaccinated i.m. but having PEI) (Fig. 3b). No substantial levels of ZGP-specific IgG were detected from the control animal. At day −7 or day 0 there were no significant differences of ZGP-specific IgG levels in NHP vaccinated i.n./i.t. in the presence or absence of PEI (P = 0.8232 and 0.9374, respectively). Similarly, there was no significant difference in ZGP-specific IgG levels in NHP vaccinated i.m. in the presence or absence of PEI at day −7 (P = 0.7134). However, there were significantly higher levels of ZGP-specific IgG at day 0 for NHP vaccinated i.m. without PEI (group A) compared to levels observed for NHP with PEI (group D; P = 0.0021). Systemic ZGP-specific IgM levels were also evaluated following vaccination at the same time points. A ZGP-specific IgM response characterized by a pattern comparable to that of the IgG response was detected at earlier time points (elevating following day −21), representing faster kinetics (Fig. 3c).
To determine the effect of AdHu5 PEI on cellular immunity following vaccination, the cellular immune response against ZGP was measured by flow cytometry. PBMCs were isolated from all NHP at day −14 (corresponding to day 14 postvaccination) and then stimulated by ex vivo exposure to ZGP peptide pools to determine the number of IFN-γ-secreting lymphocytes. NHP from groups A, B, C, and D had 713 ± 86, 648 ± 338, 343 ± 145, and 166 ± 50 IFN-γ-positive cells per million lymphocytes, respectively (Fig. 3d). Among the NHP vaccinated i.m., the number of IFN-γ-secreting lymphocytes was significantly lower (P = 0.0054) in animals with PEI (group D) than in animals without PEI (group A). The NHP vaccinated i.n./i.t. with PEI (group C) generated a lower average number of positive cells than group B NHP receiving the same treatment without PEI; however, the difference was not statistically significant (P = 0.3978).
Survival following lethal EBOV challenge.
All NHP were challenged with 1,000 TCID50 of Kikwit 95 EBOV intramuscularly at day 0. This virus preparation, administered with identical dosing and conditions, had been fully lethal in 13 control macaques challenged in our laboratory in the previous 18 months (44). NHP vaccinated i.m. in the absence of PEI (group A) all survived the EBOV challenge. However, NHP vaccinated i.m. with AdHu5 PEI (group D) all succumbed to the challenge (Fig. 4a). NHP administered the vaccine i.n./i.t. (group B) were partially protected, with 2 of 3 animals surviving the lethal challenge, while 3 out of 4 survived in the presence of AdHu5 PEI (group C) (Fig. 4a). The control animal succumbed to infection at day 7 after EBOV infection. Prominent clinical signs typical of EBOV infection in NHP result in some or all of the following features: fever, reduced activity, lower food and water intake leading to weight loss, and occasional early signs of subcutaneous hemorrhage, which are recorded daily and reflected in an overall clinical score. The range of clinical scores at day 1 post-EBOV challenge ranged from 1 to 4 (Fig. 4b). The NHP from group A (vaccinated i.m.) were asymptomatic for the duration of the experiment. All surviving NHP reached an asymptomatic clinical state at day 18 postchallenge, where the highest clinical score recorded by a survivor was 7 on day 9 after challenge (Fig. 4b). All NHP that reached a clinical score of 10 or higher ultimately reached the human endpoint and were euthanized.
Fig 4.
(a) Survival curve of NHP following EBOV challenge. Data represent the percent survival of group A (filled circles; n = 3), group B (open circles; n = 3), group C (open squares; n = 4), group D (filled squares; n = 3), and group E (gray diamonds; n = 1). Time represents days post-EBOV exposure. (b) NHP clinical scores. The NHP were scored daily for clinical signs that are typical of EBOV infections in NHP, including fever, reduced activity, lower food and water intake leading to weight loss, and occasional early signs of subcutaneous hemorrhage. Based on the severity of the clinical sign of disease, the NHP were assigned an arbitrary score. Colored symbols represent NHP that succumbed to the disease. Time represents days post-EBOV exposure.
Immune response following EBOV challenge.
All NHP had low levels of anti-ZGP NAb following vaccination until 7 days after EBOV challenge, when the levels increased and stabilized between 43 and 164 reciprocal dilutions (Fig. 3a). The IgG levels for all surviving NHP by challenge day (day 0) were elevated, and by day 21 they reached the maximal detectable level and remained approximately the same through day 34 (Fig. 3b). There were no considerable differences in the levels of IgG for survivors post-EBOV challenge. However, NHP that succumbed to the disease had low IgG levels to day 7 after challenge, where most levels were at or below the limit of detection of the assay (Fig. 3b). IgM levels reached a maximum at day 14 postchallenge and remained elevated for the duration of the experiment for survivors (Fig. 3c). Nonsurvivors had levels of IgM around or below the detection limit of the assay 7 days after challenge (Fig. 3c).
The cellular immune response following EBOV challenge was measured by flow cytometry against ZGP. Lymphocytes were isolated from NHP 14 days following EBOV challenge, stimulated by ex vivo exposure to ZGP peptide pools, and then assayed by flow cytometry for the number of IFN-γ-secreting cells. Surviving NHP did not have significantly different values, where groups A, B, and C had 4,709 ± 4,475, 381 ± 88, and 749 ± 519 IFN-γ-positive cells per million lymphocytes, respectively (Fig. 5).
Fig 5.
Cellular immune response following EBOV challenge. Cellular immune response against EBOV GP was measured by flow cytometry, where the data represent the number of IFN-γ-positive cells per million lymphocytes. Error bars represent the standard errors. The background measured with unstimulated cells never exceeded 50 IFN-γ-positive cells per million lymphocytes.
DISCUSSION
Replication-deficient AdHu5-based vaccines have been shown to induce a strong innate and adaptive immune response to both the vector backbone and the transgene (19). However, PEI to the AdHu5 vector backbone poses a major roadblock for widespread development of this platform in vaccine research. By altering the route of vaccination, a significant effect on the type and strength of the immune response can be induced (30, 31). Vaccines administered i.m. typically stimulate systemic responses, whereas mucosal vaccination induces both systemic and mucosal immune responses, albeit at overall reduced levels (10, 20, 28, 45). Mucosal vaccination using AdHu5-based vaccine platforms have been shown to confer protection from of a variety of pathogens in the presence of AdHu5 PEI in several animal models (15, 32, 46–49).
Transmission of EBOV can occur through direct contact with body fluids of infected individuals, and as such it likely involves the mucosa as a route of natural exposure. For this reason there is a growing effort to target the mucosa for EBOV vaccination (15, 50). In previous studies, we demonstrated that PEI induced to AdHu5 could be circumvented in both mice and guinea pigs with i.n. vaccine administration (15, 32). We also observed that despite elevated systemic levels of anti-AdHu5 NAb in guinea pigs, the levels of corresponding mucosal NAb were relatively low.
Ideally, NHP immunity to adenovirus would have been induced following i.n. administration with a wild-type replication-competent AdHu5 vector to better mimic induced natural immunity in humans. However, adenoviruses are species specific, and while wild-type AdHu5 replicates in humans and chimpanzees, it does not replicate in macaques (39). Recently, an AdHu5 mutant that replicates partly in macaques has been used in a few studies related to simian immunodeficiency virus (SIV) for human immunodeficiency virus (HIV) vaccine development (39, 51). However, the AdHu5 host range required multiple administrations to stimulate consistent and uniform immunity in these studies. This is currently an incomplete but promising approach that will need more development and characterization to better understand its value in reproducing natural immunity to AdHu5 in humans (52). A previous study also demonstrated that humans with elevated levels of anti-AdHu5 neutralizing antibodies in serum samples (1:320 to <1:2,560 reciprocal dilutions) had anti-AdHu5 neutralizing antibody levels that were undetectable or just above the limit of detection for samples taken from the nasal cavity (nasal washes) or deeper in the lungs (bronchoalveolar lavages) (32). Overall, this indicates that natural mucosal immunity to AdHu5 is not as robust and long lasting as systemic immune responses. Therefore, the airway provides a promising route of immunization with AdHu5-based vaccines even after prior natural exposure to adenovirus serotype 5. The present study used a well-characterized approach with the use of a replication-deficient AdHu5 expressing an unrelated transgene, leading to controlled and uniform levels of immunity to AdHu5, as reported by several groups (15–18, 53). Consequently, this work sought to assess airway vaccination with an AdHu5-based Ebola virus vaccine in the presence or absence of immunity to homologous adenovirus in NHP. i.n./i.t. administration of the vaccine was selected to mimic voluntary nasal inhalation in humans.
In our study, 1 × 1010 IFU of Ad-lacZ was delivered i.m. to induce systemic PEI to AdHu5. The level of detectable anti-AdHu5 NAb in the NHP at the time of vaccination mimicked systemic levels previously observed in 30 samples from seropositive humans. None of the NHP receiving the vaccine i.m. with systemically induced AdHu5 PEI survived the lethal challenge, despite the relatively high vaccination dose (1 × 1010 IFU Ad-CAGoptZGP/1 × 109 PFU Ad-IFN-α). This highlights the impact of existing immunity to AdHu5 on vaccine efficacy of a relatively high dose of the AdHu5-based EBOV vaccine. In a previous NHP pilot study, we established a suboptimal i.n./i.t. vaccine dose in order to address the impact of PEI on i.n./i.t. vaccination with the highest precision. The suboptimal dose was used to increase sensitivity to detect a low impact from PEI. The dose employed in the current study resulted in incomplete survival (2 out of 3 NHP) without PEI, therefore providing conditions where just a slight reduction in vaccine efficacy, presumably from the presence of PEI, would result in a noticeable decrease in survival rate. No significant difference was observed in survival between groups B and C receiving the vaccine i.n./i.t. with or without PEI, with 3 out of 4 (75%) and 2 out of 3 (67%) showing protection, respectively. It has been reported that increasing the dose of Ad-based vaccine can circumvent PEI, but this also can reach levels linked to toxicity (15, 23, 53). If a higher dose was used in this study, full survival in the absence and in the presence of PEI could have been attributed to the system being oversaturated, resulting in circumvention of AdHu5 immunity. While this dose was necessary to observe subtle differences in NHP immune reactions to PEI, complete protection could be achieved with higher doses of the vaccine administered in the airway. Previously it was reported that toxic levels of adenovirus in NHP were reached when administered systemically at concentrations of 1.1 × 1013 Ad particles/kg of body weight, indicating that systemic delivery of adenovirus at this concentration leads to activation of the innate inflammatory response in a dose-dependent manner (54). In the current study, all NHP were given less than 1 × 1011 total Ad particles (versus per kg) by a nonsystemic delivery route (i.n.). One of the long-term goals is to achieve protection with the lowest dose of Ad-based vaccine to minimize toxicity. Of note, we have never observed toxicity following i.n./i.t. administration, and to our knowledge this has never been reported in the literature at the dose used in the current study.
To address how vaccination route and PEI influences the humoral immune response, we compared the levels of EBOV-specific immunoglobulin levels. Systemic levels of IgG in i.n./i.t.-vaccinated NHP with or without PEI were comparable to those of i.m.-vaccinated NHP without AdHu5 PEI. Of note, all NHP that succumbed to infection had lower detectable levels of ZGP-specific IgG. i.n./i.t. vaccination also stimulated a significant IgG antibody response at earlier time points compared to the i.m. route, making the airway route of immunization advantageous when rapid immune protection is desired (for example, for first responders to an outbreak). The cellular immune response to ZGP following vaccination was elevated in NHP vaccinated i.m. or i.n./i.t., and the difference between these vaccination routes was not significantly different 14 days following vaccination. Overall, the data suggest that i.n./i.t. vaccination elicited a cellular immune response comparable to that of i.m. vaccination. However, the presence of PEI significantly impacts the cellular response after the i.m. vaccination, while i.n./i.t.-vaccinated NHP had a cellular response mainly unaffected by the presence of PEI, which was also reflected in the similar survival rates.
Previously in mice, guinea pigs, and human patients, it was shown that nasal washes and BAL samples (15, 32) of individuals previously exposed to AdHu5 had relatively low mucosal anti-AdHu5 NAb despite elevated systemic levels. Similar to these findings, the levels of anti-AdHu5 NAb detected in the BAL samples of i.n./i.t.-vaccinated NHP with PEI were 10- to 100-fold lower than the systemic levels, possibly explaining why vaccine efficacy was not significantly reduced when administered by the i.n./i.t. route. The lack of vaccine-neutralizing antibodies detectable in the respiratory system even in NHP showing very high systemic levels suggests that the airway delivery system for AdHu5-based vaccine platforms is a promising option for adenovirus-based vaccine delivery, possibly including readministration. Mucosal vaccination may also offer increased protection against aerosol exposure to EBOV, a hypothesis we are actively exploring.
ACKNOWLEDGMENTS
This work was supported by grant CRTI-06-0218RD, awarded to G.P.K.
We thank Geoff Soule, Gary Wong, Shane Jones, Jim Strong, Judie Alimonti, Kaylie Tran, Xiangguo Qiu, Samantha Schindle, Max Abou, Gregg Schumer, Andrea Marzi, and Ayato Takada for either their technical assistance or donation of reagents. We also thank Nicole Beausoleil, Kim Azaransky, Christine De Graff, Michelle French, Cory Nakamura, Jaime Bernstein, Kevin Tierney, Maggie Forbes, Marlee Phair, and Andrea Casavant from Veterinary Technical Services for all animal husbandry support and Melanie VanderLoop for her veterinary assistance.
Footnotes
Published ahead of print 9 January 2013
REFERENCES
- 1. Wilson JA, Bosio CM, Hart MK. 2001. Ebola virus: the search for vaccines and treatments. Cell. Mol. Life Sci. 58:1826–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O'Toole T, Ascher MS, Bartlett J, Breman JG, Eitzen EM, Jr, Hamburg M, Hauer J, Henderson DA, Johnson RT, Kwik G, Layton M, Lillibridge S, Nabel GJ, Osterholm MT, Perl TM, Russell P, Tonat K. 2002. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA 287:2391–2405 [DOI] [PubMed] [Google Scholar]
- 3. Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. 2005. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine 23:3033–3042 [DOI] [PubMed] [Google Scholar]
- 4. Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, Aman MJ, Bavari S. 2003. Ebola virus-like particles protect from lethal Ebola virus infection. Proc. Natl. Acad. Sci. U. S. A. 100:15889–15894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Warfield KL, Olinger G, Deal EM, Swenson DL, Bailey M, Negley DL, Hart MK, Bavari S. 2005. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J. Immunol. 175:1184–1191 [DOI] [PubMed] [Google Scholar]
- 6. Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. 2007. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J. Infect. Dis. 196(Suppl. 2):S430–S437 [DOI] [PubMed] [Google Scholar]
- 7. Feldmann H, Jones S, Klenk HD, Schnittler HJ. 2003. Ebola virus: from discovery to vaccine. Nat. Rev. Immunol. 3:677–685 [DOI] [PubMed] [Google Scholar]
- 8. Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, Wagner R, Volchkov V, Klenk HD, Feldmann H, Stroher U. 2004. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J. Virol. 78:5458–5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, Feldmann H. 2009. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of Ebola virus. J. Virol. 83:7296–7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 11:786–790 [DOI] [PubMed] [Google Scholar]
- 11. Qiu X, Fernando L, Alimonti JB, Melito PL, Feldmann F, Dick D, Stroher U, Feldmann H, Jones SM. 2009. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong Ebola GP-specific immune responses. PLoS One 4:e5547 doi:10.1371/journal.pone.0005547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bukreyev A, Rollin PE, Tate MK, Yang L, Zaki SR, Shieh WJ, Murphy BR, Collins PL, Sanchez A. 2007. Successful topical respiratory tract immunization of primates against Ebola virus. J. Virol. 81:6379–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bukreyev A, Yang L, Zaki SR, Shieh WJ, Rollin PE, Murphy BR, Collins PL, Sanchez A. 2006. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J. Virol. 80:2267–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang L, Sanchez A, Ward JM, Murphy BR, Collins PL, Bukreyev A. 2008. A paramyxovirus-vectored intranasal vaccine against Ebola virus is immunogenic in vector-immune animals. Virology 377:255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, Feldmann H, Kobinger GP. 2008. Nasal delivery of an adenovirus-based vaccine bypasses preexisting immunity to the vaccine carrier and improves the immune response in mice. PLoS One 3:e3548 doi:10.1371/journal.pone.0003548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel A, Zhang Y, Croyle M, Tran K, Gray M, Strong J, Feldmann H, Wilson JM, Kobinger GP. 2007. Mucosal delivery of adenovirus-based vaccine protects against Ebola virus infection in mice. J. Infect. Dis. 196(Suppl. 2):S413–S420 [DOI] [PubMed] [Google Scholar]
- 17. Richardson JS, Yao MK, Tran KN, Croyle MA, Strong JE, Feldmann H, Kobinger GP. 2009. Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine. PLoS One 4:e5308 doi:10.1371/journal.pone.0005308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sullivan NJ, Geisbert TW, Geisbert JB, Shedlock DJ, Xu L, Lamoreaux L, Custers JH, Popernack PM, Yang ZY, Pau MG, Roederer M, Koup RA, Goudsmit J, Jahrling PB, Nabel GJ. 2006. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 3:e177 doi:10.1371/journal.pmed.0030177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nazir SA, Metcalf JP. 2005. Innate immune response to adenovirus. J. Investig. Med. 53:292–304 [DOI] [PubMed] [Google Scholar]
- 20. Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605–609 [DOI] [PubMed] [Google Scholar]
- 21. Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, Robbins PD, Gambotto A. 2004. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 11:351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gallo P, Dharmapuri S, Cipriani B, Monaci P. 2005. Adenovirus as vehicle for anticancer genetic immunotherapy. Gene Ther. 12(Suppl. 1):S84–S91 [DOI] [PubMed] [Google Scholar]
- 24. Nanda A, Lynch DM, Goudsmit J, Lemckert AA, Ewald BA, Sumida SM, Truitt DM, Abbink P, Kishko MG, Gorgone DA, Lifton MA, Shen L, Carville A, Mansfield KG, Havenga MJ, Barouch DH. 2005. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J. Virol. 79:14161–14168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Capone S, Meola A, Ercole BB, Vitelli A, Pezzanera M, Ruggeri L, Davies ME, Tafi R, Santini C, Luzzago A, Fu TM, Bett A, Colloca S, Cortese R, Nicosia A, Folgori A. 2006. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J. Virol. 80:1688–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobinger GP, Feldmann H, Zhi Y, Schumer G, Gao G, Feldmann F, Jones S, Wilson JM. 2006. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology 346:394–401 [DOI] [PubMed] [Google Scholar]
- 27. Singh N, Pandey A, Jayashankar L, Mittal SK. 2008. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of preexisting immunity against human adenovirus. Mol. Ther. 16:965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arvin AM, Greenberg HB. 2006. New viral vaccines. Virology 344:240–249 [DOI] [PubMed] [Google Scholar]
- 29. Basset C, Holton J, O'Mahony R, Roitt I. 2003. Innate immunity and pathogen-host interaction. Vaccine 21(Suppl. 2):S12–S23 [DOI] [PubMed] [Google Scholar]
- 30. Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. 2003. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J. Immunol. 170:5636–5643 [DOI] [PubMed] [Google Scholar]
- 31. Lajeunesse M, Zhang Q, Finn A. 2004. Mucosal immunity to infections and its importance in future vaccinology. Adv. Exp. Med. Biol. 549:13–22 [DOI] [PubMed] [Google Scholar]
- 32. Richardson JS, Abou MC, Tran KN, Kumar A, Sahai BM, Kobinger GP. 2011. Impact of systemic or mucosal immunity to adenovirus on Ad-based Ebola virus vaccine efficacy in guinea pigs. J. Infect. Dis. 204(Suppl. 3):S1032–S1042 [DOI] [PubMed] [Google Scholar]
- 33. Zhou D, Ertl HC. 2006. Therapeutic potential of adenovirus as a vaccine vector for chronic virus infections. Expert Opin. Biol. Ther. 6:63–72 [DOI] [PubMed] [Google Scholar]
- 34. Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, Koup RA, Jahrling PB, Nabel GJ. 2003. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424:681–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richardson JS, Wong G, Pillet S, Schindle S, Ennis J, Turner J, Strong JE, Kobinger GP. 2011. Evaluation of different strategies for post-exposure treatment of ebola virus infection in rodents. J. Bioterrorism Biodefense S1:007. doi:10.4172/2157-2526.S1-007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoenen T, Groseth A, Kolesnikova L, Theriault S, Ebihara H, Hartlieb B, Bamberg S, Feldmann H, Stroher U, Becker S. 2006. Infection of naive target cells with virus-like particles: implications for the function of Ebola virus VP24. J. Virol. 80:7260–7264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakayama E, Yokoyama A, Miyamoto H, Igarashi M, Kishida N, Matsuno K, Marzi A, Feldmann H, Ito K, Saijo M, Takada A. 2010. Enzyme-linked immunosorbent assay for detection of filovirus species-specific antibodies. Clin. Vaccine Immunol. 17:1723–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, Lockman S, Peter T, Peyerl FW, Kishko MG, Jackson SS, Gorgone DA, Lifton MA, Essex M, Walker BD, Goudsmit J, Havenga MJ, Barouch DH. 2005. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 174:7179–7185 [DOI] [PubMed] [Google Scholar]
- 39. Hidajat R, Kuate S, Venzon D, Kalyanaraman V, Kalisz I, Treece J, Lian Y, Barnett SW, Robert-Guroff M. 2010. Construction and immunogenicity of replication-competent adenovirus 5 host range mutant recombinants expressing HIV-1 gp160 of SF162 and TV1 strains. Vaccine 28:3963–3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pushko P, Bray M, Ludwig GV, Parker M, Schmaljohn A, Sanchez A, Jahrling PB, Smith JF. 2000. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 19:142–153 [DOI] [PubMed] [Google Scholar]
- 41. Sullivan NJ, Martin JE, Graham BS, Nabel GJ. 2009. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat. Rev. Microbiol. 7:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilson JA, Hart MK. 2001. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J. Virol. 75:2660–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu L, Sanchez A, Yang Z, Zaki SR, Nabel EG, Nichol ST, Nabel GJ. 1998. Immunization for Ebola virus infection. Nat. Med. 4:37–42 [DOI] [PubMed] [Google Scholar]
- 44. Qiu X, Audet J, Wong G, Pillet S, Bello A, Cabral T, Strong JE, Plummer F, Corbett CR, Alimonti JB, Kobinger GP. 2012. Successful treatment of Ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci. Transl. Med. 4:138ra81. doi:10.1126/scitranslmed.3003876 [DOI] [PubMed] [Google Scholar]
- 45. Kiyono H, Fukuyama S. 2004. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat. Rev. Immunol. 4:699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hashimoto M, Boyer JL, Hackett NR, Wilson JM, Crystal RG. 2005. Induction of protective immunity to anthrax lethal toxin with a nonhuman primate adenovirus-based vaccine in the presence of preexisting anti-human adenovirus immunity. Infect. Immun. 73:6885–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Santosuosso M, McCormick S, Xing Z. 2005. Adenoviral vectors for mucosal vaccination against infectious diseases. Viral Immunol. 18:283–291 [DOI] [PubMed] [Google Scholar]
- 48. Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. 2003. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 77:10780–10789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou D, Cun A, Li Y, Xiang Z, Ertl HC. 2006. A chimpanzee-origin adenovirus vector expressing the rabies virus glycoprotein as an oral vaccine against inhalation infection with rabies virus. Mol. Ther. 14:662–672 [DOI] [PubMed] [Google Scholar]
- 50. Mohamadzadeh M, Chen L, Schmaljohn AL. 2007. How Ebola and Marburg viruses battle the immune system. Nat. Rev. Immunol. 7:556–567 [DOI] [PubMed] [Google Scholar]
- 51. Klessig DF, Grodzicker T. 1979. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell 17:957–966 [DOI] [PubMed] [Google Scholar]
- 52. Qureshi H, Ma ZM, Huang Y, Hodge G, Thomas MA, DiPasquale J, DeSilva V, Fritts L, Bett AJ, Casimiro DR, Shiver JW, Robert-Guroff M, Robertson MN, McChesney MB, Gilbert PB, Miller CJ. 2012. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J. Virol. 86:2239–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lemiale F, Kong WP, Akyurek LM, Ling X, Huang Y, Chakrabarti BK, Eckhaus M, Nabel GJ. 2003. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J. Virol. 77:10078–10087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. 2004. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 15:35–46 [DOI] [PubMed] [Google Scholar]