Abstract
Adenovirus serotype 5 (Ad5) naturally infects the liver after intravenous injection, making it a candidate for hepatocyte-directed gene transfer. While Ad5 can be efficient, most of the dose is destroyed by liver Kupffer cells before it can reach hepatocytes. In contrast, Ad5 bearing the hexon from Ad6 (Ad5/6) evades Kupffer cells. While Ad5/6 dramatically increases hepatocyte transduction in BALB/c mice, it has surprisingly little effect on C57BL/6 mice. To determine the source of this strain-specific difference, the roles of Kupffer cells, liver sinusoidal endothelial cells (LSECs), hepatocytes, scavenger receptors, clotting factors, and immunoglobulins were analyzed. The numbers of Kupffer cells and LSECs, the level of clotting factor X, and hepatocyte infectibility did not differ between different strains of mice. In contrast, high levels of immunoglobulins correlated negatively with Ad5 liver transduction in different mouse strains. Removal of immunoglobulins by use of Rag-deficient mice restored Ad5 transduction to maximal levels. Removal of Kupffer cells by predosing or by testing in colony-stimulating factor knockout mice restored Ad5 transduction in the presence of immunoglobulins. Partial reconstitution of IgM in Rag mice resulted in significant reductions in liver transduction by Ad5 but not by Ad5/6. These data suggest a role for IgM-mediated clearance of Ad5 via Kupffer cells and may explain the mechanism by which Ad5/6 evades these cells. These mechanisms may play a vital role in Ad pharmacology in animals and in humans.
INTRODUCTION
Adenovirus serotype 5 (Ad5) is one of the most robust vectors for in vivo liver-directed gene transfer. Ad5 liver transduction is mediated in part by its surprisingly high affinity for vitamin K-dependent blood clotting factors. In particular, factor X (FX) has been shown to bind to the hexon protein of Ad5 with subnanomolar affinity and, in one model, may act as a bridge to the virus, retargeting it to heparan sulfate proteoglycans on hepatocytes (1–4).
As much as 98% of systemically delivered Ad5 is eliminated by liver Kupffer cells before reaching hepatocytes (5). Kupffer cells can phagocytose particles as large as 2 μm in diameter, an appropriate size for a virus bound to host proteins and cells (6). Uptake into these cells is likely mediated by broadly specific scavenger receptors (7) that can recognize hypervariable loops 1, 2, 5, and 7 of the hexon (8). While there is good evidence for interactions between Ad5 and scavenger receptors (9), Kupffer cells in wild-type and scavenger receptor SR-A knockout mice take up similar amounts of Ad5, suggesting that the host may have additional, redundant mechanisms for viral recognition and removal from the systemic circulation (10).
Kupffer cells are not the only components of the reticuloendothelial system that remove foreign particles from the bloodstream. Under normal conditions, liver sinusoidal endothelial cells (LSECs) can pinocytose particles with a diameter of <0.23 μm, also allowing the uptake of 90-nm adenoviral virions (6). LSECs also express scavenger receptors SREC-I and SREC-II (11) and also absorb Ad5 from the bloodstream. Beyond LSECs and Kupffer cells, there are likely a variety of other nonproductive pharmacological elimination routes for intravenously injected Ad5 (reviewed in reference 12).
While Ad5 appears to be effective at mediating liver transduction, there is surprisingly wide variation between it and other highly conserved members of species C adenoviruses (13). For instance, Ad6, a lower-seroprevalence species C Ad (14), mediates three-times-higher liver transduction than Ad5, but both Ad5 and Ad6 mediate higher transduction than Ad1 and Ad2 (13). These differences were observed in BALB/c mice, although surprisingly, when Ad5 and Ad6 were compared in C57BL/6 mice, Ad6 no longer mediated higher transduction than Ad5 (15).
The variability of adenovirus pharmacology in different strains of mice has been noted previously. Tao et al. demonstrated earlier that at moderate doses (1 × 1010 virus particles [vp]), Ad5 displays 400-fold differences in transgene expression in immunocompetent C57BL/6, BALB/c, and C3H mice or in immunodeficient nude or Rag-1 mice (16). Similarly, Snoeys et al. observed that intravenous (i.v.) injection of Ad5 expressing apolipoprotein A-I at high doses of 5 × 1010 vp produced 3-fold-higher expression in C57BL/6 mice than in BALB/c mice (17). When doses were reduced to 1.5 × 1010 vp, expression was 60-fold higher in C57BL/6 mice than in BALB/c mice. This lower level of transduction coincided with the observation that BALB/c mice sequestered 20 times more Ad5 DNA in their Kupffer cells and C57BL/6 mice absorb 3 times more Ad5 in their LSECs than BALB/c mice.
These data indicate that the large differences in the ways in which different strains of mice remove adenovirus vectors from their circulation may make extrapolation of vector pharmacology between inbred mouse strains and outbred humans difficult. To better understand adenovirus pharmacology, we have investigated the roles of cells of the reticuloendothelial system and the effects of natural antibodies in different strains of mice.
MATERIALS AND METHODS
Viruses.
Ad5 and Ad5/6 vectors were generated as described previously (15). Briefly, Ad5 was produced using the AdEasy system (Qbiogene/MP Biomedicals) in HEK293 cells, and Ad5/6 was created by recombining hypervariable regions 1 to 7 (HVRs 1 to 7) of Ad6 into the Ad5 backbone. Both vectors are replication defective and contain an enhanced green fluorescent protein (eGFP)-luciferase fusion transgene.
Animals.
Female BALB/c, C57BL/6, aC57BL/6 (albino C57BL/6), CB6F1, or FVB mice between the ages of 6 and 8 weeks were obtained from Harlan Sprague-Dawley. Rag−/− BALB/c and C57BL/6 mice between the ages of 5 and 6 weeks were obtained from Jackson Laboratories. One male and one female mouse (B6;C3Fe a/a-Csf1op/J) were purchased from Jackson Laboratories and were bred in-house with aC57BL/6 mice. Homozygote knockout offspring were selected by the absence of teeth and were fed a soft dough diet (BioServ). Only female mice were used in luciferase imaging experiments. All animals were housed and bred in the Mayo Clinic Animal Facility, and experiments were conducted according to the policies and procedures of the Mayo Clinic.
Monitoring luciferase transgene expression.
Mice were injected intravascularly with 1 × 1010 vp of Ad vectors via the tail vein. For in vivo bioluminescence imaging, mice were anesthetized with ketamine/xylazine and were injected intraperitoneally (i.p.) with 100 μl of d-luciferin (20 mg/ml; Molecular Imaging Products). Albino mice were used, or else black/brown fur was shaved from the mouse midsection. Light output was measured with the Lumazone imaging system (Photometrics, Roper Scientific) for 3 min with 1 × 1 binning using no filters or photomultiplication. Lumazone imaging software was used to determine total light intensity per mouse (photons) for data analysis (15). For in vitro luciferase assays, cells were plated onto a 96-well plate and were infected with either 1,000 or 10,000 vp of Ad5/cell. After incubation at 37°C for 24 h, 25 μl of 5× cold passive lysis buffer and 50 μl of the luciferase assay reagent were added to each well. Relative luminescence units (RLU) were measured with the Beckman Coulter DTX 880 Multimode Detector system.
Immunohistochemistry.
The large liver lobe was harvested from mice and was incubated overnight (o/n) at room temperature (RT) in formalin. The tissue was embedded in paraffin and was cut into 4-μm sections. To stain for F4/80+ cells, slides were probed with a rat anti-F4/80 primary antibody (MCA497EL; AbD Serotec) at a 1:200 dilution and with a horseradish peroxidase (HRP)-conjugated anti-rat secondary antibody, with diaminobenzidine (DAB) as the substrate. To stain for CD31+ cells, slides were probed with rabbit anti-CD31 at a 1:200 dilution and with an HRP-conjugated anti-rabbit secondary antibody, with DAB as the substrate. To quantify positively stained cells, images of 5 fields of view for each mouse section were taken. ImageJ software was used to convert these to binary images, and the “Threshold” feature was used to separate out positively stained cells from background nuclei. “Analyze particles” was used to count the numbers of stained regions (from 25 to infinity). Normal human liver biopsy tissue was received from the Mayo Clinic and was sectioned and stained with an anti-CD68 primary antibody.
Uptake by MPMs.
Mice were euthanized, and mouse peritoneal macrophages (MPMs) were immediately harvested by peritoneal lavage with 5 ml of endotoxin-free phosphate-buffered saline (PBS) (Gibco). Cells from 3 mice were pooled, counted, plated to confluence onto 24-well plates, and incubated at 37°C for 1 h. The adherent cells were washed twice with PBS, infected with 10,000 or 100,000 vp of Ad5 or Ad5/6, and incubated in Dulbecco's modified Eagle medium (DMEM) containing 10% heat-inactivated fetal calf serum (Gibco) and penicillin-streptomycin at 100 U/ml (Gibco). After 24 h at 37°C, the cells were washed twice to remove unbound virus and were then harvested for DNA extraction with a DNeasy kit (Qiagen). Real-time PCR was performed with primers against cellular glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and cytomegalovirus (CMV) (15), and the uptake per cell is presented as a ratio between copies of CMV DNA and copies of host GAPDH gene.
Predosing.
For the Ad5 predose, 3 × 1010 vp of Ad5-DsRed in 100 μl of PBS was injected i.v. After 4 h, 1 × 1010 vp of Ad5 or Ad5/6 was injected i.v. For the poly(I) predose, 100 μg of poly(I) in 100 μl PBS was injected 5 min before Ad vector injection. Luciferase expression was measured as the light output 24 h later by in vivo imaging.
IgM complementation.
BALB/c mice were exsanguinated, and serum was used for IgM purification with the CaptureSelect IgM affinity matrix (BAC BV, the Netherlands), yielding approximately 0.3 mg IgM per mouse. IgM purity was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Endotoxin levels were less than 0.6 endotoxin unit (EU)/ml. Pathogen testing by the Impact III PCR profile (Idexx-Radil) detected no mouse pathogens. PBS or the maximal feasible injection amount of purified IgM (445 μg) was injected i.v. into each mouse, followed 1 h later by 1 × 1010 vp of Ad5 or Ad5/6 via the i.v. route. Luciferase expression was measured 24 h later by in vivo imaging.
ELISAs.
To determine antibody levels, serum was harvested from different mouse strains by a submandibular bleed into serum separator tubes (BD Microtainer) and was allowed to clot for 30 min. The serum was separated by spinning at 13,000 × g for 2 min. The mouse hybridoma subisotyping kit (EMD Millipore) was used to assess circulating antibody isotypes. For antibody binding to Ad vectors, 120 ng of Ad5 or Ad5/6 was applied to 96-well ultrahigh-binding enzyme-linked immunosorbent assay (ELISA) plates for 1 h at 37°C (Immunolon 4HBX). The wells were washed 3 times with PBS and were then blocked for 2 h at RT with 3% bovine serum albumin (BSA) in PBS. The wells were washed 3 times with PBS, and then serum (diluted 1:50 in PBS) was added. After 2 h at RT, the wells were washed 3 times with PBS, and the procedure for the hybridoma subisotyping kit was used to determine specific isotype binding.
To determine FX levels, mice were anesthetized, and whole blood was collected by a retroorbital bleed into 3.2% sodium citrate. The Mouse Factor X total antigen ELISA kit (Molecular Innovations) was used to determine FX levels.
Isolating and infecting primary hepatocytes.
Mice were anesthetized. The inferior vena cava was cannulated, and the portal vein was cut. The blood in the liver was replaced with 24 g/liter HEPES buffer with 9.5 g/liter EGTA. The liver and catheter were cut away and were placed in an ex vivo system that maintains a steady temperature of 37°C. The liver was perfused with 300 mg/liter of collagenase and 4 g/liter of an albumin solution for 10 min. After perfusion, the liver cells were gently teased apart, and hepatocytes were separated out with two gentle spins at 30 × g. Cells counted by using a hemacytometer were plated onto a 96-well plate and were infected with 1,000 or 10,000 vp of Ad5/cell. After 24 h, transduction was assessed by a luciferase assay.
Data analysis.
Graphs were made, and statistical analyses were performed, using GraphPad Prism software. Analyses comparing two and three groups were carried out by use of an unpaired t test and one-way analysis of variance (ANOVA) (Bonferroni posttest), respectively. To determine the correlation coefficient (R2) for correlations between relative amounts of antibody isotypes and expression, a semilog (log values along the x axis and linear values along the y axis) trend line was fitted.
RESULTS
Mouse strains show dramatic differences in liver expression by Ad5 and Ad5/6.
To test differences in adenoviral pharmacology, we compared replication-defective Ad5 and Ad5/6 (15), both expressing luciferase. Ad5/6 is an Ad5 vector whose hexon hypervariable regions (HVRs) were replaced with those from species C family member Ad6. Previous work with BALB/c mice demonstrated that Ad5/6 mediates a level of liver transduction as much as 10-fold higher than that with Ad5 in BALB/c mice (15). This effect correlated with reduced recognition of Ad5/6 scavenger receptors (8). Therefore, Ad5/6 was used in these studies as a vector with reduced tropism for Kupffer cells, at least in BALB/c mice.
Ad5 and Ad5/6 were injected intravenously (i.v.) by the tail vein into BALB/c and C57BL/6 mice. Mice were injected with 1 × 1010 vp, a dose that is susceptible to Kupffer cell absorption (16, 18), and light output from luciferase transgene expression was measured 24 h later. In agreement with previous results (15), Ad5/6 mediated 50-fold-higher transduction than Ad5 in the livers of BALB/c mice (Fig. 1A). Ad5 transduction was similar in the two strains of mice; expression was only 1.5-fold higher in C57BL/6 mice. In contrast to the results with BALB/c mice, Ad5/6 mediated no increase in transduction in C57BL/6 mice. These results were consistent whether C57BL/6 mice were imaged with their normal black fur, were shaved, or were on an albino background (Fig. 1B). To determine if this effect was dominant or recessive, Ad5 and Ad5/6 were compared in F1 progeny of C57BL/6 and BALB/c mice (CB6F1) (Fig. 1A). In this case, levels of transduction by both Ad5 and Ad5/6 were intermediate between those for BALB/c and C57BL/6 mice, suggesting a more subtle genotype-phenotype relationship.
Fig 1.
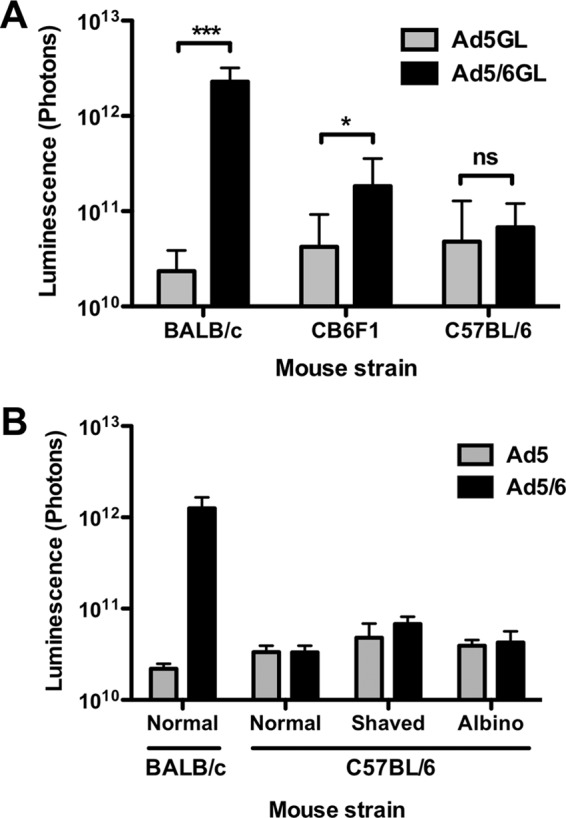
Ad vector expression is highly variable between mouse strains. (A) BALB/c mice, C57BL/6 mice, and their F1 progeny (CB6F1) were injected i.v. with 1 × 1010 vp of Ad5 or Ad5/6. After 24 h, light output was quantified as a measure of luciferase expression (n, 10 to 18). (B) BALB/c mice, unshaved or shaved C57BL/6 mice, and albino C57BL/6 mice were injected i.v. with 1 × 1010 vp of Ad5 or Ad5/6. *, P < 0.05; ***, P < 0.001; ns, not significant.
Mouse strains have similar levels of liver Kupffer cells and LSECs.
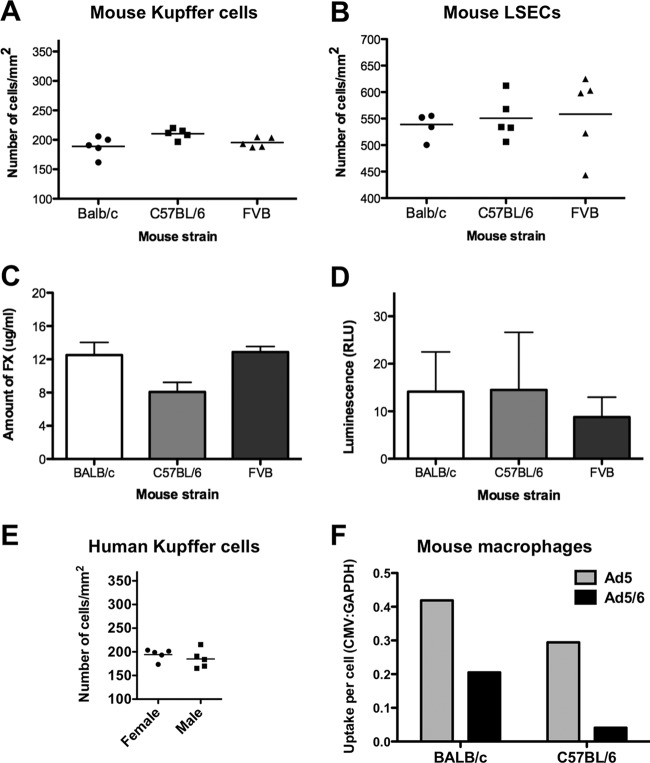
Adenoviruses can be absorbed by a variety of cells in the body (reviewed in reference 12). Liver Kupffer cells have become the focus of interest, since they may play the largest role in Ad5 absorption after intravenous injection. More recently, LSECs have been recognized as another cell type that can absorb Ad after i.v. injection (10). A simple explanation for differences in virus pharmacology among mouse strains could be that they have large differences in raw numbers of Kupffer cells and/or LSECs. To test this, livers from the mice were immunohistochemically stained for Kupffer cells using an anti-F4/80 antibody and for LSECs using an anti-CD31 antibody. Gross histological stains and counts failed to find any obvious numerical differences in either cell type in any of the mouse strains analyzed (Fig. 2A and 3A and B). Staining of human liver sections revealed a Kupffer cell density similar to that of mice (Fig. 2B and 3E).
Fig 2.
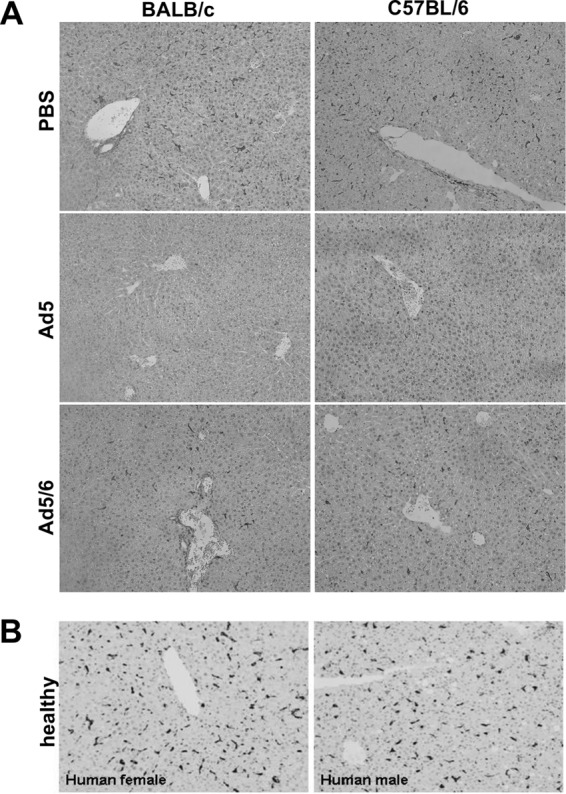
Kupffer cell numbers in mice and humans. (A) BALB/c and C57BL/6 mice were injected with 3 × 1010 vp of PBS, Ad5GL, or Ad5/6GL expressing GFP-luciferase. After 6 h, the large liver lobe was harvested, formalin fixed, and paraffin embedded. Immunohistochemistry for F4/80+ cells was performed on 4-μm sections. (B) Kupffer cell density in human liver. Normal human liver biopsy tissue was harvested, paraffin embedded, and sectioned (4 μm thick). Immunohistochemical staining for CD68 was used to visualize Kupffer cells.
Fig 3.
Cellular and functional measurements in mice and humans. Immunohistochemical detection of F4/80+ Kupffer cells (A) and CD31+ endothelial cells (B) in the livers of different strains of mice was performed on 4-μm tissue sections. The numbers of positively stained cells from 5 fields of view were quantified with ImageJ. (C) FX levels in different strains of mice. Whole blood was collected into sodium citrate, and an ELISA was used to determine the amount of mouse FX in the plasma. Results from 4 wells are shown. (D) Infectibility of purified mouse hepatocytes from different strains. Mice (pools of 3) were perfused ex vivo with collagenase, and hepatocytes were separated by gentle centrifugation. The cells were infected in suspension with 1,000 vp of Ad5/cell and were measured for luciferase expression after 24 h. Results from 8 wells are shown. (E) Kupffer cell numbers in human liver. Normal human liver biopsy tissue was harvested, paraffin embedded, and sectioned (4 μm thick). Immunohistochemical staining for CD68 was used to visualize Kupffer cells (Fig. 2B), and positively stained cells from 5 fields of view were counted with ImageJ. (F) Uptake of Ad5 or Ad5/6 by MPMs. MPMs were collected from 3 BALB/c or 3 C57BL/6 mice and were pooled. These samples were infected in vitro with 1,000 vp of the indicated vector/cell. After 24 h, DNA was harvested from the cells, and real-time PCR was used to determine the number of viral genomes taken up in relation to cellular genomes (CMV/GAPDH ratio). Results from 3 wells are shown.
Host factor X (FX) levels and hepatocyte susceptibility to infection do not correlate with Ad5 expression in the murine liver.
FX facilitates liver transduction by both Ad5 and Ad5/6 (15). To determine if FX levels could explain differences in liver transduction between strains, plasma was assayed for FX by ELISA (Fig. 3C). FX levels were insignificantly different between the strains. To test whether Ad vectors might transduce hepatocytes from different strains to different degrees at the cellular level, hepatocytes from various strains of mice were purified and infected with 1,000 vp of Ad5/cell (Fig. 3D). Transduction was assessed by luciferase assay after 24 h and showed no significant difference in Ad5 expression in hepatocytes between the different mice.
Ad5/6 evades macrophages and Kupffer cells regardless of the mouse strain.
Phagocytosis of Ad5 by Kupffer cells not only destroys the virus but also kills the cell (19). Previous work with BALB/c mice showed that Ad5/6 causes less Kupffer cell depletion than Ad5 at the same dose (15). To compare this in the two mouse strains, both were injected with 3 × 1010 vp of PBS, Ad5, or Ad5/6. After 6 h, their livers were harvested for immunohistochemistry with an anti-F4/80 antibody (Fig. 2A). In both strains of mice, Ad5 injection markedly reduced F4/80-positive staining, whereas Ad5/6 showed considerably lower clearance of these cells.
Differences in Ad tropism between the strains could be due to differences in the phagocytic activities of their cells. To compare the phagocytic functions of macrophages in the two mouse strains, mouse peritoneal macrophages (MPMs) were harvested from BALB/c and C57BL/6 mice. MPMs were collected, and Ad5 or Ad5/6 was incubated with the cells at 1,000 vp/cell for 24 h. DNA was harvested from the cells, and real-time PCR was used to determine the uptake of Ad genomes by the cells. C57BL/6 macrophages took up approximately 25% fewer Ad5 virions and 75% fewer Ad5/6 virions than BALB/c cells, in agreement with observations that C57BL/6 Kupffer cells take up less Ad5 after i.v. injection (Fig. 3F). In agreement with previous results (15), Ad5/6 was taken up less efficiently than Ad5 by macrophages from both BALB/c and C57BL/6 mice.
Predosing of BALB/c and C57BL/6 mice.
Negatively charged poly(I) can be used to predose mice and block scavenger receptors on both Kupffer cells and LSECs. As a result, a subsequent dose of Ad evades uptake and more effectively transduces the liver (7). In BALB/c mice, poly(I) boosted both Ad5 and Ad5/6 transduction in the liver to peak levels (Fig. 4A). Poly(I) also boosted Ad5 and Ad5/6 transduction in C57BL/6 mice, although overall levels of transduction were nearly 10-fold lower (Fig. 4B).
Fig 4.
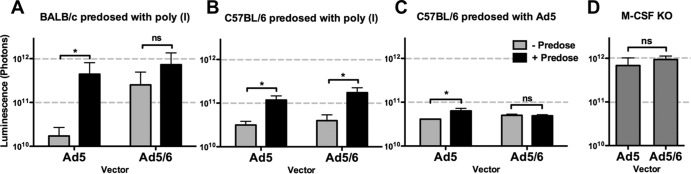
Predosing effects in different mouse strains. (A and B) BALB/c (A) or C57BL/6 (B) mice were predosed i.v. with PBS or 100 μg of poly(I) and were injected 5 min later with 1 × 1010 vp of Ad5 or Ad5/6. (C) C57BL/6 mice were predosed i.v. with PBS or 3 × 1010 vp of an irrelevant Ad5-DsRed vector and were then injected i.v. 4 h later with 1 × 1010 vp of Ad5 or Ad5/6. (D) M-CSF knockout (KO) mice were injected i.v. with 1 × 1010 vp of Ad5 or Ad5/6. All injected mice were imaged for luciferase expression after 24 h (n, 2 to 5). *, P < 0.05; ns, not significant.
The lethal effects of Ad5 on Kupffer cells can also be used to predose mice (20). Previous work with BALB/c mice confirmed that predosing with Ad5 enhances Ad5, but not Ad5/6, transduction (15). To test whether this effect was consistent in the different mouse strains, C57BL/6 mice were predosed with Ad5 and were then dosed 4 h later with either Ad5 or Ad5/6 (Fig. 4C). As before, Ad5 predosing enhanced Ad5, but not Ad5/6, transduction in C57BL/6 mice. Although statistically significant, the effect of Ad5 predosing was considerably weaker in C57BL/6 mice, remaining 10 times below the peak photon level achieved in BALB/c mice.
Vector expression in M-CSF knockout mice.
Predosing with Ad5 can eliminate Kupffer cells; however, this first dose of virus could also block many other receptors and cell types. Similarly, anionic poly(I) likely blocks the interactions of many charged surfaces in the mice. To probe Ad5 and Ad5/6 interactions with Kupffer cells by alternate means, macrophage colony-stimulating factor (M-CSF) mutant C57BL/6 mice were used (21). M-CSF knockout mice have a 77% reduction in the numbers of Kupffer cells and an 84% reduction in the level of phagocytosis (22). When M-CSF knockout mice were injected with Ad5 or Ad5/6, the two vectors mediated similar levels of liver transduction (Fig. 4D). Unlike pharmacologic predosing with either Ad5 or poly(I), genetic knockdown of liver Kupffer cells was able to raise the level of liver transduction in C57BL/6 mice to the peak photon level observed under optimal conditions in BALB/c mice.
Antibody levels correlate inversely with Ad5 expression in the liver.
These data suggest that Ad5 and Ad5/6 differ primarily in their interactions with Kupffer cells and/or macrophages in different mouse strains. Antibody opsonization can play a key role in macrophage-dependent clearance of foreign particles. Pathogen recognition can be mediated by antibodies generated by adaptive immune responses or by natural antibodies that are encoded in the germ line. Prior data suggest that Ad5 may be targeted to Kupffer cells by natural antibodies (7).
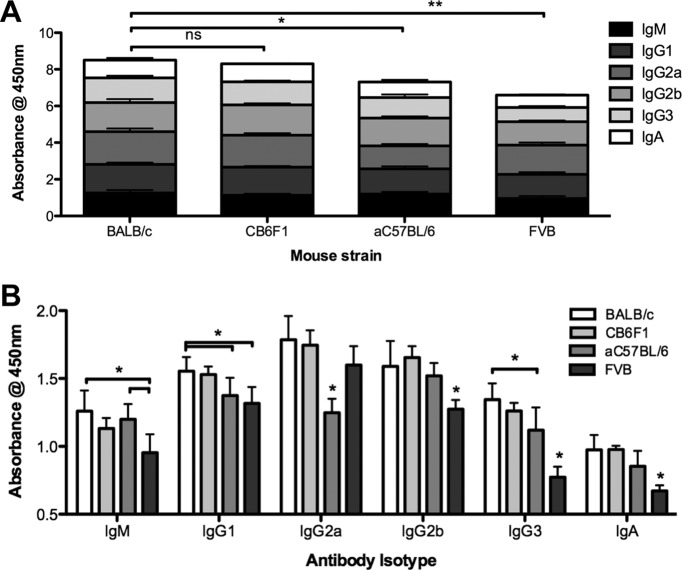
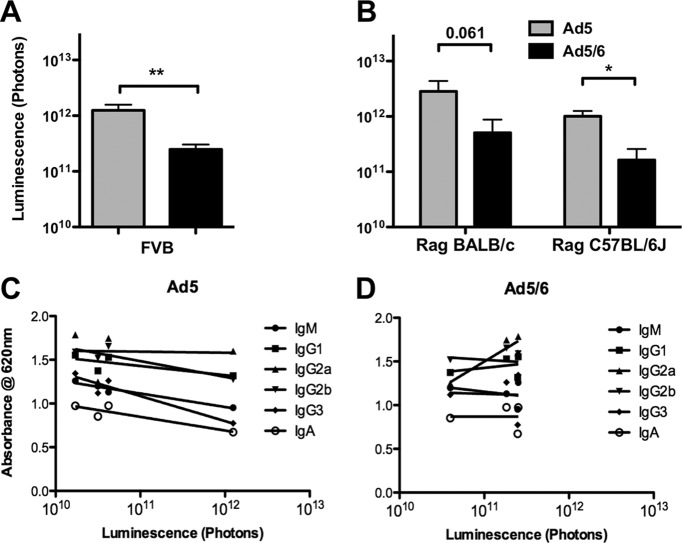
To test whether antibody levels differ in different mouse strains, fresh serum was harvested from naïve mice and was analyzed for isotype distribution by ELISA. BALB/c mice had higher levels of antibodies than C57BL/6 mice by ELISA (Fig. 5A and B). F1 progeny of BALB/c and C57BL/6 mice (CB6F1) had total antibody levels (expressed by the optical density at 450 nm [OD450]) nearly as high as those of BALB/c mice; levels of most isotypes were slightly lower than those in BALB/c mice. On the other hand, FVB mice had total antibody levels that were actually lower than those in C57BL/6 mice. Interestingly, FVB mice were unique in mediating very robust Ad5 liver transduction up to the peak photon level with no manipulation (Fig. 6A). When the observed isotype profiles and transduction data were taken together, liver transduction by Ad5 was inversely correlated with levels of IgM, IgG2b, IgG3, and IgA antibodies (Fig. 6C and Table 1). Ad5/6 expression did not correlate negatively with IgM, IgG2b, IgG3, and IgA antibody OD levels but instead correlated positively with higher IgG2a isotype levels observed in BALB/c and CB6F1 mice (Fig. 5 and Table 1).
Fig 5.
Antibody levels differ distinctly between murine strains. Serum was harvested from mice (pool of two mice per strain) and was analyzed for circulating antibody isotypes by ELISA. Shown are total antibody levels (A) or antibody levels divided by isotype (B). Results from 6 wells are shown. *, P < 0.05; **, P < 0.005; ns, not significant.
Fig 6.
Ad expression is related to circulating antibody levels. (A and B) CB6F1 (F1 progeny of BALB/c and C57BL/6 mice) and FVB mice (A) and Rag−/− BALB/c and Rag−/− C57BL/6 mice (B) were injected i.v. with 1 × 1010 vp of Ad5 or Ad5/6. Luciferase expression, measured by light output, was quantified (n, 2 to 10). *, P < 0.05; **, P < 0.01; ns, not significant. (C and D) Ad5 (C) and Ad5/6 (D) expression levels in the BALB/c, CB6F1, C57BL/6, and FVB mouse strains were plotted against relative levels of antibody isotypes.
Table 1.
Correlation between Ad vector expression and relative amounts of natural antibody isotypes
| Antibody isotype | Correlation (R2) with: |
|
|---|---|---|
| Ad5 | Ad5/6 | |
| IgM | 0.9532 | 0.1061 |
| IgG1 | 0.571 | 0.1139 |
| IgG2a | 0.001692 | 0.8391 |
| IgG2b | 0.82 | 0.01987 |
| IgG3 | 0.9047 | 0.001871 |
| IgA | 0.8323 | 0.0000183 |
Liver transduction in mice with no antibodies.
Previous work showed that C57BL/6 Rag−/− mice, which lack all immunoglobulins, have markedly reduced uptake of Ad5 by Kupffer cells, implicating natural antibodies in this sequestration (23). To further confirm the link between circulating antibodies and Ad expression in different strains of mice, Ad5 and Ad5/6 were tested in Rag−/− mice on either a BALB/c or a C57BL/6 genetic background (Fig. 6B). In these mice lacking immunoglobulins, transduction by each vector was similar on the two backgrounds. Ad5 transduction reached the 1012 photon level in the absence of immunoglobulins, in a manner similar to that observed in M-CSF knockout mice. Interestingly, as in FVB mice, which have low levels of circulating natural antibodies, Ad5/6 transduction was lower than Ad5 transduction in Rag−/− mice.
Natural antibodies can bind directly to the Ad capsid.
Direct binding of immunoglobulins to virions would be the simplest means to affect Ad pharmacology. To test this, fresh serum from naïve mice was tested for isotype-specific binding to Ad5 and Ad5/6 by ELISA (Fig. 7). All isotypes bound directly to both Ad vectors, although IgM from all murine strains and IgG2b from C57BL/6 mice mediated the highest binding.
Fig 7.
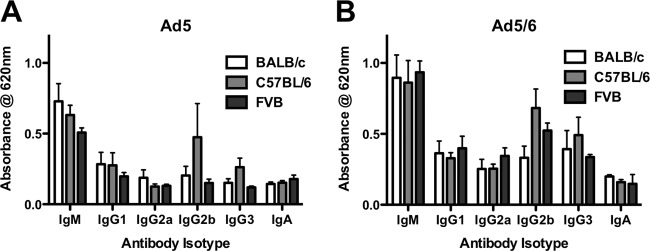
Antibodies can bind directly to the Ad capsid. Ad5 (A) or Ad5/6 (B) was applied to ELISA plates, bound with serum from BALB/c, C57BL/6, or FVB mice, and specifically probed with isotype-specific antibodies. Relative levels of natural antibody binding were determined with an HRP-conjugated secondary antibody. Results for 6 wells are shown.
Administration of IgM antibodies to Ig-deficient BALB/c Rag−/− mice reduces liver transduction by Ad5.
BALB/c mice have circulating IgM levels of approximately 1,000 μg/ml. To test if IgM can mediate effects on Ad liver transduction, IgM antibodies were injected into Ig-deficient Rag knockout mouse on the BALB/c background. Bulk IgM from untreated BALB/c mice was purified. Eight Rag mice each received 445 μg of this IgM and 8 mice received PBS as a control. One hour later, the animals were injected i.v. with 1 × 1010 vp of Ad5 or Ad5/6, and luciferase expression was measured 24 h later (Fig. 8). Under these conditions, IgM complementation reduced liver transduction by Ad5 more than 5-fold from that for mice receiving PBS (P, <0.05 by one-way ANOVA). In contrast, IgM reduced Ad5/6 transduction slightly, but this reduction was not statistically significant. These data suggest that bulk IgM antibodies can mediate reduced liver transduction observed for different strains of mice.
Fig 8.
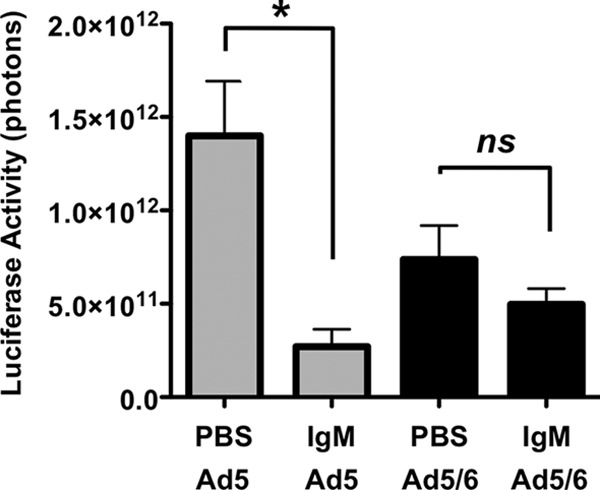
Partial restoration of IgM in Ig-deficient Rag−/− mice reduces liver transduction by Ad5. Groups of 4 BALB/c Rag−/− mice were injected i.v. with either PBS or 445 μg of purified IgM from BALB/c mice. One hour later, the mice were injected i.v. with 1 × 1010 vp of Ad5 or Ad5/6. All injected mice (n = 4) were imaged for luciferase expression after 24 h. The asterisk indicates a significant difference (P < 0.05) by one-way ANOVA.
DISCUSSION
Ad5 is a hepatotropic virus that has been studied extensively as a potential vector for liver-directed gene therapy. Mice have been used as the preferred small-animal model in the vast majority of preclinical investigations, although dramatic differences in Ad5 liver expression have been documented in different murine strains (7, 16).
Transduction of the liver by Ad5 after i.v. injection depends on interactions with blood components such as circulating cells, blood factors, antibodies, and complement (reviewed in reference 12). These interactions increase the delivery of Ad5 to the liver, where resident liver macrophages called Kupffer cells can deplete more than 98% of an injected dose. There is also evidence to suggest that other cells in the reticuloendothelial system of the liver, such as liver sinusoidal endothelial cells (LSECs), also sequester some proportion of virus particles. To enter the space of Disse and transduce hepatocytes, virions must evade phagocytosis by Kupffer cells or LSECs. Those that escape the liver can be distributed to other organs, such as the spleen and lungs.
While it is certainly known that mice differ from humans in many respects, recent data have suggested strong differences in Ad5 pharmacology between inbred mouse strains. After we observed dramatically better liver transduction by Ad5/6 than by Ad5 in BALB/c mice, we were surprised to find essentially no improvement in C57BL/6 mice. This study aimed to resolve these differences with the hope of ultimately extrapolating the underlying biological differences in these inbred models to humans.
Differences in transduction by Ad5 and Ad5/6 among the BALB/c, C57BL/6, CB6F1, FVB, Rag−/−, and M-CSF−/− mouse strains support the hypothesis that different strains have host-specific factors that influence Ad5 and Ad5/6 transduction. Several experiments in this work confirm that Ad5/6 evades macrophages regardless of the mouse strain. For instance, Ad5/6 was taken up less efficiently than Ad5 in both BALB/c and C57BL/6 peritoneal macrophages in vitro. Furthermore, mice that were predosed with Ad5 to destroy Kupffer cells showed an increase in Ad5 expression and no increase in Ad5/6 expression. Finally, genetic reduction of the number of Kupffer cells in M-CSF mutant mice ablated the difference between the two vectors on the C57BL/6 mouse background.
Predosing with poly(I) boosted transduction by both Ad5 and Ad5/6. This difference in predosing between Ad5 and poly(I) suggests that non-Kupffer cells may also be involved in virus sequestration. Kupffer cells and LSECs both express several classes of scavenger receptors, and poly(I) can disrupt the uptake of Ad into either of these cell types. Therefore, one hypothesis is that Ad5 is absorbed by both Kupffer cells and LSECs, while Ad5/6 is absorbed primarily by LSECs, particularly in C57BL/6 mice. This hypothesis is consistent with previously published data showing that C57BL/6 mice take up 3 times more Ad DNA in their LSECs than in their Kupffer cells, whereas the pattern of uptake in BALB/c mouse cells is reversed (17). While this hypothesis may be accurate at some level, the observed equalization of Ad5 and Ad5/6 transduction in C57BL/6 mice with M-CSF knocked out suggests that Kupffer cells, and perhaps other macrophages in the body, are the predominant actors on Ad5 and Ad5/6, even in C57BL/6 mice. To our knowledge, M-CSF is not directly involved in LSEC biology, so its knockout most likely does not affect the function of these cells. However, indirect effects may occur. Whether poly(I) inhibits the uptake of virus into another location remains to be determined.
Pathogens can be neutralized by a number of pathways (Fig. 9). Antibodies can trigger uptake directly, through Fc receptors, or indirectly, by activating complement and binding complement receptors (reviewed in references 24 and 25). Earlier work with Ad5 demonstrated binding of IgM antibodies to the Ad5 capsid and weak binding of IgG to the virus (7). This work also showed strong complement factor C3 activation during the elimination of Ad5 by Kupffer cells.
Fig 9.
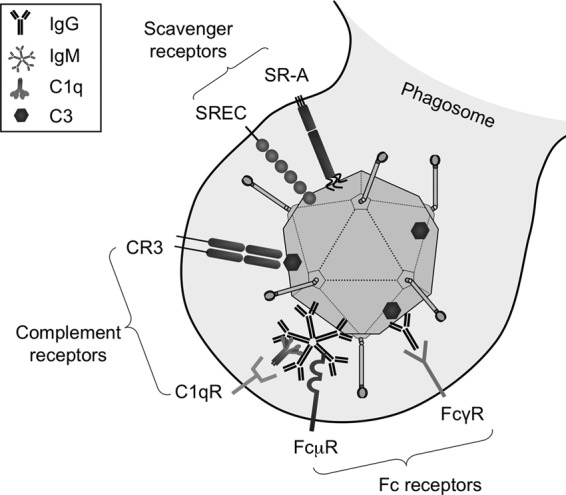
Representation of multiple receptor systems on Kupffer cells for Ad recognition and uptake. Adenovirus type 5 can bind scavenger receptors (such as SR-AI, SR-AII, and SREC) based on negative-charge interactions. Natural or preexisting neutralizing antibodies can bind directly to capsid proteins or can bind indirectly, via C3 binding. Monomeric IgG or pentameric IgM antibodies are recognized by different Fc receptors. Complement receptors can attach to complement proteins bound either directly or indirectly to the adenoviral surface.
We show here that the addition of IgM to Ig-deficient mice reduces liver transduction by Ad5. This finding supports the observed correlation between IgM levels in different mice and liver transduction by Ad5. It should be noted that we were able to introduce only 445 μg of IgM into each of the Rag mice due to constraints on i.v. injection volumes. Considering that BALB/c mice have approximately 1,000 μg/ml of IgM and approximately 2 mg of total IgM, this antibody reconstitution would result in only 25% of normal IgM levels. Therefore, the 5-fold effect on Ad5 transduction observed may underrepresent the possible effects of natural antibodies on the virus. Natural antibody effects are also likely affected by differences in other important factors, such as complement factor C3 in the Rag mice (7).
Higher levels of IgM, IgG2b, IgG3, and IgA antibodies in mice correlated negatively with liver transduction by Ad5. What was surprising was the observation that none of these antibody levels correlated with Ad5/6 transduction. This was consistent with the relatively weak effect of IgM reconstitution in Rag mice on Ad5/6 transduction. In contrast, IgG2a antibodies correlated positively with Ad5/6 activity. Given that antibodies can mediate opsonization and phagocytosis through a number of mechanisms (Fig. 9), exactly how, or if, IgG2a antibody binding exerts direct effects or more complicated effects remains to determined.
IgG2a and IgG2b, and their human homologs IgG1 and IgG3, are described as strongly opsonizing (26). Comparison of the abilities of different isotypes of murine antibodies to opsonize Escherichia coli showed that IgM had the strongest antibacterial effect and IgG2a was the next most potent (27). In this case, antibacterial effects were directly correlated with the ability to deposit complement factor, a finding consistent with previous work on Ad5 clearance (7). In contrast, comparison of anticancer effector functions between antibodies bearing the same idiotype but with different Fc regions from IgG1, IgG2a, IgG2b, and IgG3 demonstrated that IgG2a was by far the most potent at facilitating Fc receptor-mediated anticancer functions (25). Therefore, it is possible that Ad5 is indeed cleared by IgM and complement, as well as by IgG antibodies via Fc receptors (Fig. 9). Most of the biology of Ad5/6 appears to be inverted from that of Ad5, perhaps suggesting disruption at some step between Ig binding, complement binding, and phagocytic uptake. Ad5/6 or Ad6 may bind IgM or other immunoglobulins in such a way that the virus can avoid phagocytosis, as has been shown in both RAW 264.7 cells and, here, in BALB/c and C57BL/6 macrophages. Ad5/6 is able to avoid scavenger receptors in vitro, but it remains to be determined whether it also avoids complement receptors and/or Fc receptors.
Previous work implicated the binding of natural antibodies to the Ad5 capsid, combined with complement activation, as a primary opsonization mechanism for targeting the virus to Kupffer cells (7). This prior work is seminal in describing a clearance pathway for Ad5. We would stipulate that this clearance mechanism may be particular to Ad5, rather than common to all Ad serotypes, as evidenced by differences in tropism for Ad1, Ad2, Ad5, and Ad6 of species C adenoviruses (13), marked differences between Ad5 and Ad5/6 (15), and likely wider-ranging systemic and liver pharmacology for other Ad species (28). Though highly conserved, Ad5 and Ad6 (and Ad5/6) differ greatly in in vivo pharmacology and even in direct recognition by scavenger receptors SRA-II and SREC. Species B and species D viruses, with smaller HVRs in their hexons, appear less likely to bind FX, and at least a subset (Ad11, Ad35, Ad26, and Ad48) do not appear to infect mouse hepatocytes (as evidenced by minimal release of liver enzymes [28; unpublished observations]). Predosing mice with Ad11, Ad35, Ad26, or Ad48 does not enhance subsequent transduction by Ad5 (28; unpublished observations), suggesting that these other serotypes may not be absorbed by the same cells or at the same locations as Ad5.
ACKNOWLEDGMENTS
We thank Mary Barry and Shruthi Naik for technical assistance. We thank the Clinical Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567) and the Mayo Clinic Department of Histology and Pathology for tissue-embedding services.
We thank the Mayo Clinic Cancer Center TACMA shared resource, which provided immunohistological services that are supported in part by NCI grant 5P30 CA15083-37. This work was supported by a grant to M.A.B. from the NIH (R01-CA136945) and by funding from the Mayo Clinic Liver Regenerative Medicine Program. M.L.H. was supported by grant T32-DK00703, Kidney Disease Research Training Program at Mayo Clinic.
Footnotes
Published ahead of print 16 January 2013
REFERENCES
- 1. Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. 2008. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. U. S. A. 105:5483–5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, Denby L, Kemball-Cook G, Ni S, Lieber A, McVey JH, Nicklin SA, Baker AH. 2006. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 108:2554–2561 [DOI] [PubMed] [Google Scholar]
- 3. Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 79:7478–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409 [DOI] [PubMed] [Google Scholar]
- 5. Alemany R, Suzuki K, Curiel DT. 2000. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 81:2605–2609 [DOI] [PubMed] [Google Scholar]
- 6. Elvevold K, Smedsrod B, Martinez I. 2008. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am. J. Physiol. Gastrointest. Liver Physiol. 294:G391–G400 [DOI] [PubMed] [Google Scholar]
- 7. Xu Z, Tian J, Smith JS, Byrnes AP. 2008. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J. Virol. 82:11705–11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khare R, Reddy VS, Nemerow GR, Barry MA. 2012. Identification of adenovirus serotype 5 hexon regions that interact with scavenger receptors. J. Virol. 86:2293–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haisma HJ, Boesjes M, Beerens AM, van der Strate BW, Curiel DT, Pluddemann A, Gordon S, Bellu AR. 2009. Scavenger receptor A: a new route for adenovirus 5. Mol. Pharm. 6:366–374 [DOI] [PubMed] [Google Scholar]
- 10. Di Paolo NC, van Rooijen N, Shayakhmetov DM. 2009. Redundant and synergistic mechanisms control the sequestration of blood-borne adenovirus in the liver. Mol. Ther. 17:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plüddemann A, Neyen C, Gordon S. 2007. Macrophage scavenger receptors and host-derived ligands. Methods 43:207–217 [DOI] [PubMed] [Google Scholar]
- 12. Khare R, Chen CY, Weaver EA, Barry MA. 2011. Advances and future challenges in adenoviral vector pharmacology and targeting. Curr. Gene Ther. 11:241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weaver EA, Hillestad ML, Khare R, Palmer D, Ng P, Barry MA. 2011. Characterization of species C human adenovirus serotype 6 (Ad6). Virology 412:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Piedra PA, Poveda GA, Ramsey B, McCoy K, Hiatt PW. 1998. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics 101:1013–1019 [DOI] [PubMed] [Google Scholar]
- 15. Khare R, May SM, Vetrini F, Weaver EA, Palmer D, Rosewell A, Grove N, Ng P, Barry MA. 2011. Generation of a Kupffer cell-evading adenovirus for systemic and liver-directed gene transfer. Mol. Ther. 19:1254–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. 2001. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 3:28–35 [DOI] [PubMed] [Google Scholar]
- 17. Snoeys J, Mertens G, Lievens J, van Berkel T, Collen D, Biessen EA, De Geest B. 2006. Lipid emulsions potently increase transgene expression in hepatocytes after adenoviral transfer. Mol. Ther. 13:98–107 [DOI] [PubMed] [Google Scholar]
- 18. Bristol JA, Shirley P, Idamakanti N, Kaleko M, Connelly S. 2000. In vivo dose threshold effect of adenovirus-mediated factor VIII gene therapy in hemophiliac mice. Mol. Ther. 2:223–232 [DOI] [PubMed] [Google Scholar]
- 19. Manickan E, Smith JS, Tian J, Eggerman TL, Lozier JN, Muller J, Byrnes AP. 2006. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 13:108–117 [DOI] [PubMed] [Google Scholar]
- 20. Shashkova EV, Doronin K, Senac JS, Barry MA. 2008. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 68:5896–5904 [DOI] [PubMed] [Google Scholar]
- 21. Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442–444 [DOI] [PubMed] [Google Scholar]
- 22. Baer K, Roosevelt M, Clarkson AB, Jr, van Rooijen N, Schnieder T, Frevert U. 2007. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell. Microbiol. 9:397–412 [DOI] [PubMed] [Google Scholar]
- 23. Tian J, Xu Z, Smith JS, Hofherr SE, Barry MA, Byrnes AP. 2009. Adenovirus activates complement by distinctly different mechanisms in vitro and in vivo: indirect complement activation by virions in vivo. J. Virol. 83:5648–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ehrenstein MR, Notley CA. 2010. The importance of natural IgM: scavenger, protector and regulator. Nat. Rev. Immunol. 10:778–786 [DOI] [PubMed] [Google Scholar]
- 25. Nimmerjahn F, Ravetch JV. 2008. Analyzing antibody-Fc-receptor interactions. Methods Mol. Biol. 415:151–162 [DOI] [PubMed] [Google Scholar]
- 26. Janeway C. 2001. Immunobiology: the immune system in health and disease, 5th ed Garland Science, New York, NY [Google Scholar]
- 27. Oishi K, Koles NL, Guelde G, Pollack M. 1992. Antibacterial and protective properties of monoclonal antibodies reactive with Escherichia coli O111:B4 lipopolysaccharide: relation to antibody isotype and complement-fixing activity. J. Infect. Dis. 165:34–45 [DOI] [PubMed] [Google Scholar]
- 28. Shashkova EV, May SM, Barry MA. 18 September 2009,posting date Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology. doi:10.1016/j.virol.2009.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]