Abstract
HIV-1 entry involves the viral envelope glycoproteins (Env gps) and receptors on the target cell. Receptor binding channels the intrinsic high potential energy of Env into the force required to fuse the membranes of virus and target cell. For some HIV-1 strains, prolonged incubation on ice decreases Env potential energy and results in functional inactivation. By characterizing chimeras between two primary clade C HIV-1 strains that differ in sensitivities to cold, soluble CD4, and neutralizing antibodies, we found that these properties were largely determined by discrete elements within the gp120 variable regions V1V2 and V3.
TEXT
The envelope glycoprotein (Env gp) complex of human immunodeficiency virus (HIV-1) is composed of the surface glycoprotein gp120 and transmembrane glycoprotein gp41, which are organized into trimeric spikes on the surface of the virus (1–3). HIV-1 entry is initiated by interaction of gp120 with the CD4 receptor. CD4 interaction induces conformational changes in gp120 that allow binding to CCR5 or CXCR4 coreceptors, promoting virus entry (4, 5). The unliganded Env gps exist in a high-potential-energy state. Binding to the receptors induces Env conformational changes that lead to lower-energy states and activate the entry pathway (6, 7). However, it is not yet fully understood how HIV Env channels its intrinsic high potential energy into the force required to fuse the membranes of the virus and target cell. Under some circumstances (e.g., incubation with soluble CD4 [sCD4] or some neutralizing antibodies [Abs]), HIV-1 Env undergoes conformational changes that lead to functional inactivation (8). We recently reported that for some HIV-1 Env isolates, prolonged incubation on ice results in functional inactivation (cold inactivation) (9). Importantly, cold inactivation depended on the ability of the HIV-1 gp120 to sample the CD4-bound conformation; for example, changes in layer 1 of the HIV-1 gp120 inner domain that decreased the spontaneous sampling of the CD4-bound conformation were resistant to cold inactivation (9). Interestingly, we recently reported that the major variable regions V1, V2, and V3 restrain the unliganded gp120 from “snapping” into the CD4-bound conformation (10). Alteration of a glycosylation site in the V1V2 stem can influence both cold sensitivity and the propensity of Env to transit into the CD4-bound state (18). These observations suggested the hypothesis that the major gp120 variable regions are able to modulate cold inactivation.
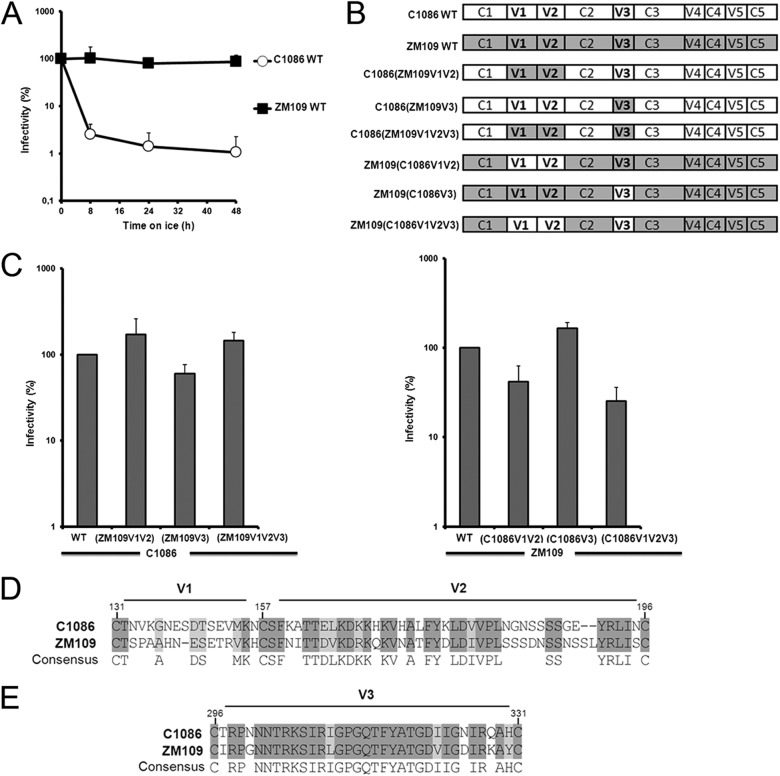
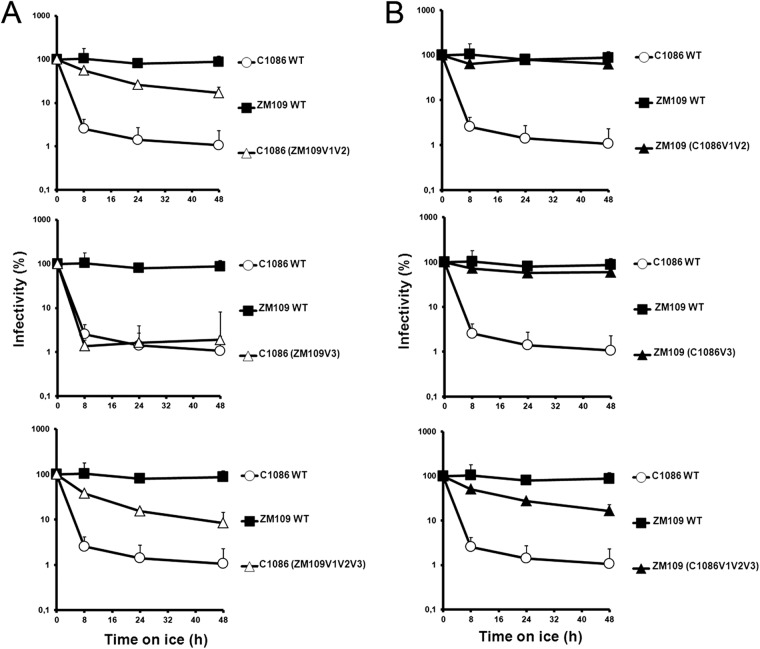
Here we investigated whether the gp120 variable regions V1, V2, and V3 determine the dramatic difference in sensitivities to cold inactivation of two primary HIV-1 clade C isolates, HIV-1C1086 and HIV-1ZM109 (accession no. FJ444395 and AY424138, respectively) (Fig. 1A). We focused our study on these variable regions because they represent the elements that differs the most between these two Env isolates. Importantly, no major differences were observed in the composition of the inner-domain layers or the Phe 43 cavity, previously shown to modulate cold inactivation (9), or in the CD4-binding site (not shown). Whereas the lengths of the V3 regions are identical and differ by only 12%, the V1V2 region is one residue longer in ZM109 (59 residues) but differs significantly in its amino acid sequence, by 45%, compared to that of C1086 (Fig. 1D and E). We also tested whether these variable regions influenced virus sensitivity to CD4-binding site, CD4-induced, and quaternary-dependent neutralizing Abs. Therefore, we swapped the V1V2, V3 or V1V2V3 regions between these two isolates (Fig. 1B) and tested their sensitivity to prolonged incubation on ice, as reported previously (9). Briefly, HIV-1 virions encoding a luciferase reporter (pNL4.3 E-Luc) and bearing wild-type (wt) Env from HIV-1C1086 or HIV-1ZM109 or chimeric Envs were normalized by reverse transcriptase and incubated for different amounts of time on ice before being used to infect Cf2Th cells expressing CD4 and CCR5 (12). Luciferase activity was measured 48 h later, as described previously (11). Viruses bearing wt HIV-1C1086 Env were dramatically sensitive to cold inactivation, whereas the infectivity of virions bearing wt HIV-1ZM109 Env was not affected by cold incubation (Fig. 1A and Table 1). Of note, swapping V1V2 from C1086 into the ZM109 backbone slightly decreased infectivity; the reverse was true when the ZM109 V1V2 region was introduced into the C1086 backbone. Substitution of V3 from C1086 had a positive effect on the infectivity of ZM109; substitution of the V3 region from ZM109 into C1086 slightly decreased infectivity. Nonetheless, all chimeras supported robust HIV-1 entry (Fig. 1C) and were highly resistant to weakly neutralizing CD4-binding site Abs, such as b12 and VRC03, as well as the CD4-induced 48d Ab (Table 1). Thus, the swaps of the variable regions did not impose a global neutralization-sensitive phenotype. Interestingly, the cold sensitivity of HIV-1C1086 was reduced by substitution of the V1V2 region from the cold-resistant HIV-1ZM109; substitution of the HIV-1ZM109 V3 region did not have this effect (Fig. 2A). However, the reciprocal chimeras (i.e., ZM109 with either the V1V2 or V3 region from C1086) exhibited the same cold-resistant phenotype as the parental ZM109. Even substitution of all three variable regions (V1, V2, and V3) from C1086 only slightly enhanced ZM109 sensitivity to cold inactivation (Fig. 2B). These results indicate that variable regions V1, V2, and V3 play a major role in modulating cold inactivation; however, their effects appear to be context dependent.
Fig 1.
Differential sensitivity to cold of two primary clade C Env isolates. (A) Recombinant HIV-1 strains bearing wt C1086 or ZM109 Env gps were incubated on ice for different amounts of time. At the indicated time points, aliquots were removed and frozen at −80°C. After completion of the longest incubation, all samples were thawed and infectivity on Cf2Th-CD4/CCR5 cells was measured. Data are representative of results from at least three independent experiments performed in quadruplicate. (B) Schematic representation of the chimeric Env gps variants used in this study. Sequences derived from C1086 and ZM109 Env gps are represented in white or gray, respectively. (C) The infection of Cf2Th-CD4/CCR5 cells by 10,000 reverse transcriptase (RT) units of virus containing each Env gp variant was measured. Data are representative of results from at least three independent experiments, performed in quadruplicate. (D) Primary sequence alignment of variable regions 1 (V1) and 2 (V2) from two primary HIV-1 clade C isolates, HIV-1C1086 and HIV-1ZM109 (accession no. FJ444395 and AY424138, respectively). (E) Alignment of the variable region 3 (V3). The shading highlights residues that are conserved.
Table 1.
Phenotypes of variable region (V1V2 and V3) chimeras between Envs from two primary clade C HIV-1 isolatesa
| Strain | Relative CD4-Ig binding | Cold inactivation t1/2 (h) | Neutralization Ab IC50 (μg/ml) |
||||
|---|---|---|---|---|---|---|---|
| sCD4 | VRC01 | VRC03 | b12 | 48d | |||
| Wild-type strains | |||||||
| ZM109 | 1.0 | >48 | 2.0 | 1.0 | >10 | >10 | >10 |
| C1086 | 0.4 | 6.0 | >10 | 3.0 | >10 | >10 | >10 |
| Chimeras | |||||||
| ZM109(C1086 V1V2) | 1.0 | >48 | 3.5 | 2.0 | >10 | >10 | >10 |
| ZM109(C1086 V3) | 0.4 | >48 | 10 | 1.0 | >10 | >10 | >10 |
| ZM109(C1086 V1V2V3) | 0.6 | >18 | 7.0 | 1.5 | >10 | >10 | >10 |
| ZM109 N160K | 1.0 | >48 | 4.0 | 1.0 | >10 | >10 | >10 |
| C1086(ZM109 V1V2) | 0.6 | 12.0 | >10 | 2.5 | >10 | >10 | >10 |
| C1086(ZM109 V3) | 0.9 | 6.0 | 1.5 | 3.0 | >10 | >10 | >10 |
| C1086(ZM109 V1V2V3) | 0.7 | 9.0 | 4.0 | 2.0 | >10 | >10 | >10 |
| C1086 K160N | 0.9 | 6.0 | >10 | 5.0 | 9.5 | >10 | >10 |
The phenotypes of the wt and chimeric Env gps were determined as described in the text. Results represent the mean values derived from at least two independent experiments performed in quadruplicate. Less than 20% deviation from the reported mean value was typically observed.
Fig 2.
Effect of swapping variable regions V1V2 and V3 on cold inactivation. Cold inactivation sensitivity of recombinant HIV-1 bearing wt C1086, ZM109, or chimeric Env gps was measured in the C1086 (A) or ZM109 (B) backbone, as described in the legend to Fig. 1A. Data are representative of results from at least three independent experiments, performed in quadruplicate.
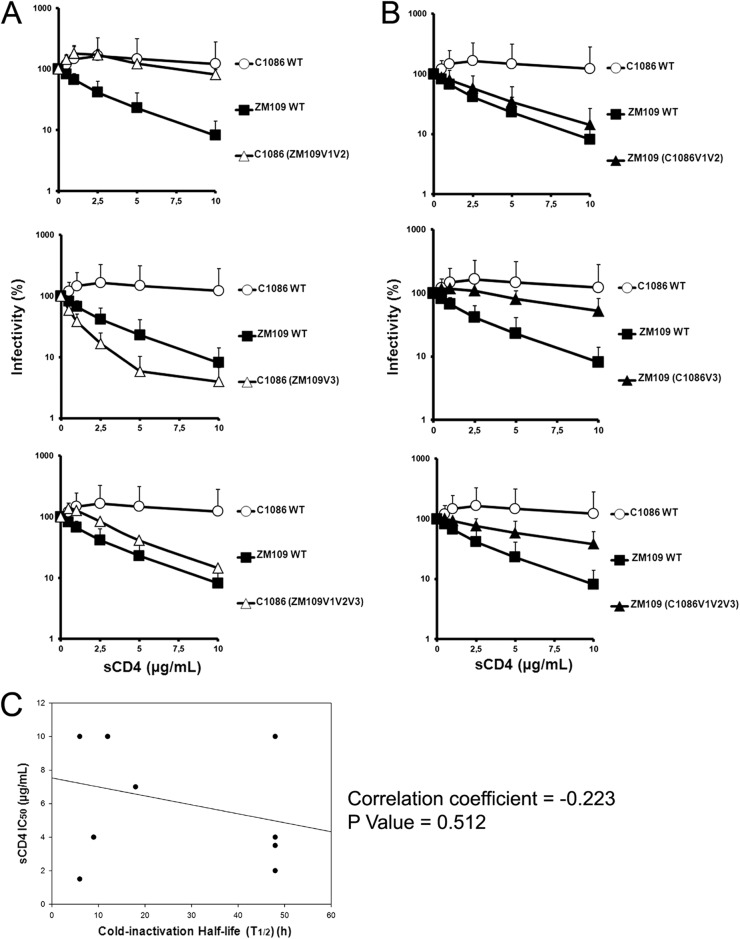
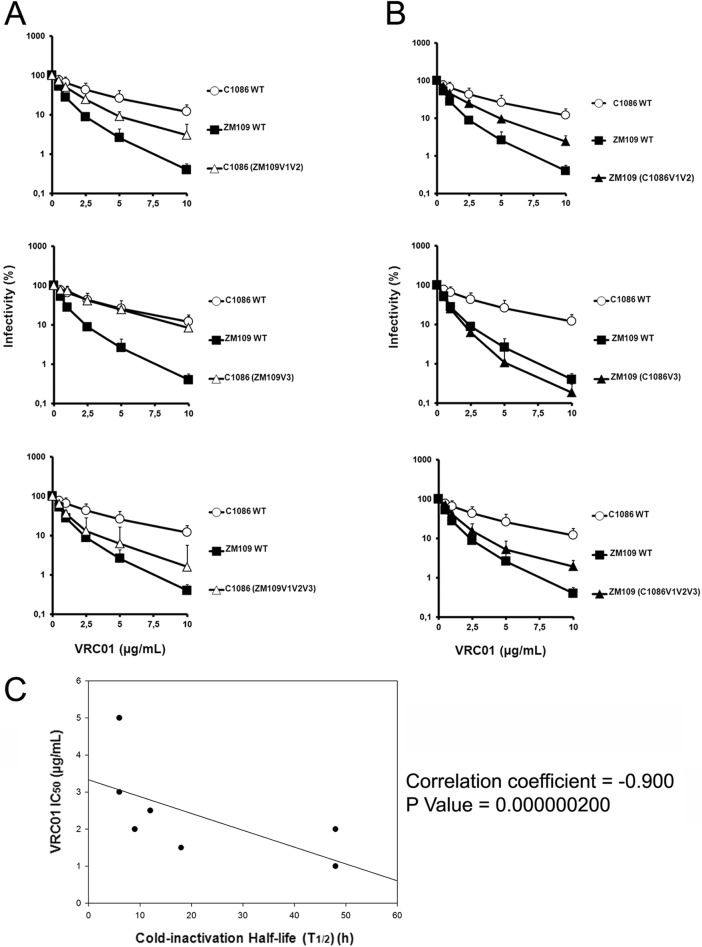
Changes in the conformation sampled by the HIV-1 Env gps have been shown to modulate sensitivity to both sCD4 neutralization (11, 13) and cold inactivation (8, 18). Therefore, we asked whether the cold inactivation phenotypes observed for our panel of chimeras correlated with their sensitivity to soluble CD4 (sCD4) or the CD4-binding site antibody VRC01. Recombinant viruses bearing wt or chimeric Env gps were produced, incubated with increasing concentrations of sCD4 or VRC01 for 1 h at 37°C, and used to infect Cf2Th CD4/CCR5 cells, as indicated above. The cold-sensitive HIV-1C1086 was completely resistant both to sCD4 and VRC01 neutralization (Fig. 3A and 4A and Table 1), whereas the cold-resistant HIV-1ZM109 was sCD4 and VRC01 sensitive (Fig. 3B and 4B and Table 1). Whereas, the V3 region was the major determinant of virus sensitivity for neutralization by sCD4, the V1V2 region determined VRC01 sensitivity. Thus, whereas for these two HIV-1 isolates, cold inactivation and sCD4 sensitivity did not directly correlate and were determined by different variable regions (Fig. 3C), we observed an inverse correlation between cold inactivation and VRC01 sensitivity that appears to be mainly determined by the V1V2 variable region (Fig. 4C).
Fig 3.
Effect of swapping variable regions V1V2 and V3 on sCD4 neutralization. The sCD4 sensitivity of recombinant HIV-1 bearing wt C1086, ZM109, or chimeric Env gps was measured in the C1086 (A) or ZM109 (B) backbone. Recombinant HIV-1 strains bearing wt C1086, ZM109 Env gps, or chimeric Env gps were incubated with serial dilutions of sCD4 at 37°C for 1 h before infection of Cf2Th-CD4/CCR5 cells. Forty-eight hours later, cells were lysed and infectivity was measured by luciferase. Infectivity at each dilution of sCD4 tested is shown as the percentage of infection seen in the absence of sCD4. Data are representative of results from at least three independent experiments performed in quadruplicate, with means and standard deviations (SD) indicated. (C) sCD4 neutralization does not correlate with cold inactivation. Sensitivity to cold inactivation of recombinant HIV-1 bearing wt C1086, ZM109, or chimeric Env gps (presented in Fig. 1B) as well as PNGS 160 variants (Table 1) was measured as described in the legend to Fig. 1A. The half-life (t1/2) of each virus incubated on ice was calculated from data presented in Fig. 2 and correlated by the Spearman rank correlation method (using SigmaPlot software) with the sCD4 neutralization 50% inhibitory concentration (IC50) (presented in panels A and B and Table 1).
Fig 4.
Effect of swapping variable regions V1V2 and V3 on VRC01 neutralization. The VRC01 sensitivity of recombinant HIV-1 bearing wt C1086, ZM109, or chimeric Env gps was measured in the C1086 (A) or ZM109 (B) backbone. Recombinant HIV-1 bearing wt C1086, ZM109 Env gps, or chimeric Env gps was incubated with serial dilutions of VRC01 at 37°C for 1 h before infection of Cf2Th-CD4/CCR5 cells. Forty-eight hours later, cells were lysed and infectivity was measured by luciferase. Infectivity at each dilution of VRC01 tested is shown as the percentage of infection seen in the absence of VRC01. Data are representative of results from at least four independent experiments performed in quadruplicate, with means and standard deviations indicated. (C) VRC01 sensitivity inversely correlates with cold inactivation. Sensitivity to cold inactivation of recombinant HIV-1 bearing wt C1086, ZM109, or chimeric Env gps (presented in Fig. 1B) as well as PNGS 160 variants (Table 1) was measured as described in the legend to Fig. 1A. The half-life (t1/2) of each virus incubated on ice was calculated from data presented in Fig. 2 and correlated by the Spearman rank correlation method (using SigmaPlot software) with the VRC01 neutralization IC50 (presented in panels A and B and Table 1).
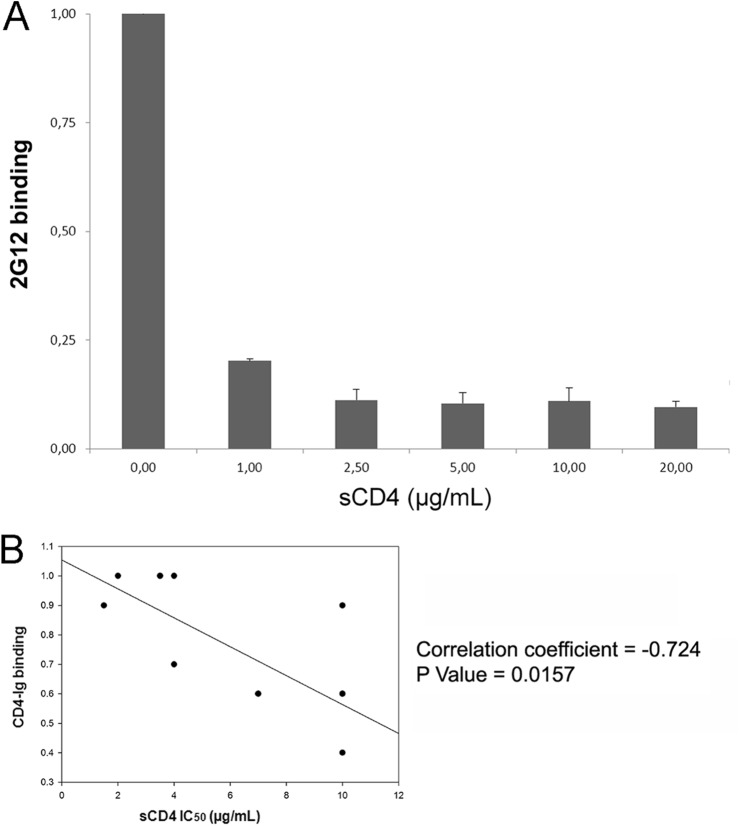
To further investigate whether sCD4 sensitivity correlated with the ability of the trimeric Env gps to interact with CD4, as previously suggested (14), we introduced a stop codon at position 711 to enhance cell surface expression of our panel of Env gp chimeras and examined their interaction with CD4-Ig by a cell-based enzyme-linked immunosorbent assay (ELISA) (8, 15) that specifically measures binding of selected ligands to cell-associated trimers (Fig. 5A). Of note, truncation of the gp41 cytoplasmic tail did not affect the cold inactivation phenotypes of the chimeras (data not shown). Importantly, the sCD4 sensitivity of our panel of chimeras correlated with the binding of CD4-Ig to the trimeric Env gps on the cell surface (Spearman rank order correlation coefficient, −0.724; P = 0.0157) (Fig. 5B). Thus, for this panel of chimeras, the ability of trimeric Env to interact with CD4 predicts sensitivity to sCD4 neutralization.
Fig 5.
CD4 binding predicts sCD4 neutralization. We first measured the ability of our cell-based ELISA to discriminate between cell-associated Env gps versus shed gp120 by inducing shedding. Cells expressing HIV-1YU2 Env gps lacking their cytoplasmic tail were used to enhance cell surface expression. Env-expressing cells were incubated with concentrations of sCD4 previously shown to induce significant gp120 shedding (11, 15). Cell-associated Env was then detected with the outer-domain-recognizing 2G12 antibody (A). Data are representative of at least three independent experiments done in quadruplicate ± standard deviation. Please note that upon sCD4 addition, the signal of 2G12 decreases in a manner dependent on the sCD4 dose, indicating that shed gp120 is removed during the washing steps and does not reassociate with the cell membrane. (B) The IC50 of sCD4 neutralization (presented in Fig. 3 and Table 1) was inversely correlated with the ability of trimeric Env gp variants to interact with CD4-Ig by cell-based ELISA. The P value and the Spearman rank correlation coefficient are presented.
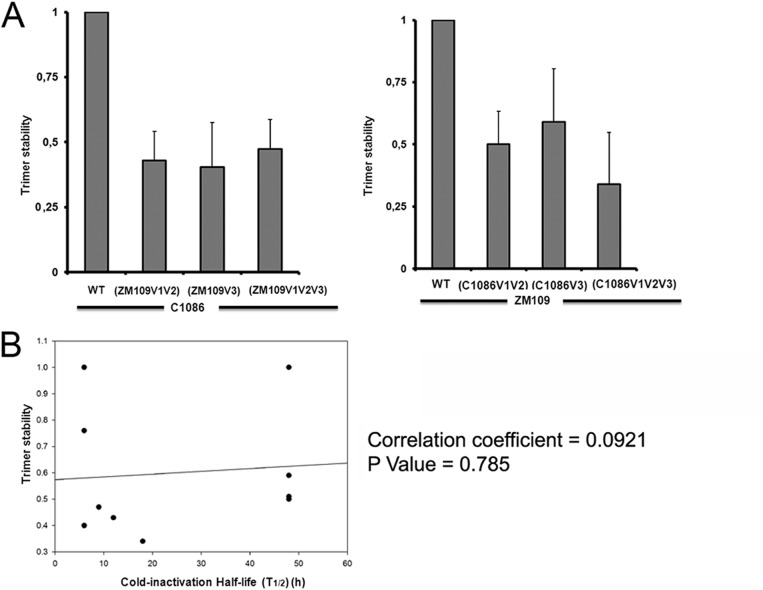
We then asked whether the differential sensitivity of HIV-1C1086 and HIV-1ZM109 to cold might be linked to the integrity of the Env trimer. Indeed, it has been reported that the variable regions V1V2 and V3 are part of the trimer-association domain (TAD), a region recently described as being important for trimer stability (16). We therefore evaluated the association of the gp120 and gp41 subunits of each Env trimer by precipitation of radiolabeled envelope glycoproteins from cell lysates and medium with a mixture of sera from HIV-1-infected individuals, as previously described (11, 15). Supporting a role of the variable regions V1V2 and V3 and the TAD in the stability of the Env trimer, swapping the V1V2 or V3 alone or in combination decreased the stability of the trimer, irrespective of the background (Fig. 6A). However, this property did not correlate with cold inactivation (Fig. 6B).
Fig 6.
Swapping the variable regions V1V2 and V3 affects gp120-trimer association, which does not correlate with cold inactivation. (A) Cell lysates and supernatants (SN) of 35S-labeled cells transiently expressing the HIV-1C1086 or HIV-1ZM109 wild-type or chimeric Env gps were precipitated with a serum mixture from HIV-1-infected individuals. The precipitated proteins were loaded onto SDS-PAGE gels and analyzed by autoradiography and densitometry. Trimer stability is a measure of the ability of the mutant gp120 molecule to remain associated with the envelope glycoprotein complex on the expressing cell relative to that of the wild-type envelope glycoproteins. The association index was calculated as previously described (11, 15). The data shown represent the average ± SD of at least two independent experiments. (B) The half-life (t1/2) of each virus incubated on ice was calculated from data presented in Fig. 2 and correlated with the trimer stability index (presented in panel A). The P value and the Spearman rank correlation coefficient are presented.
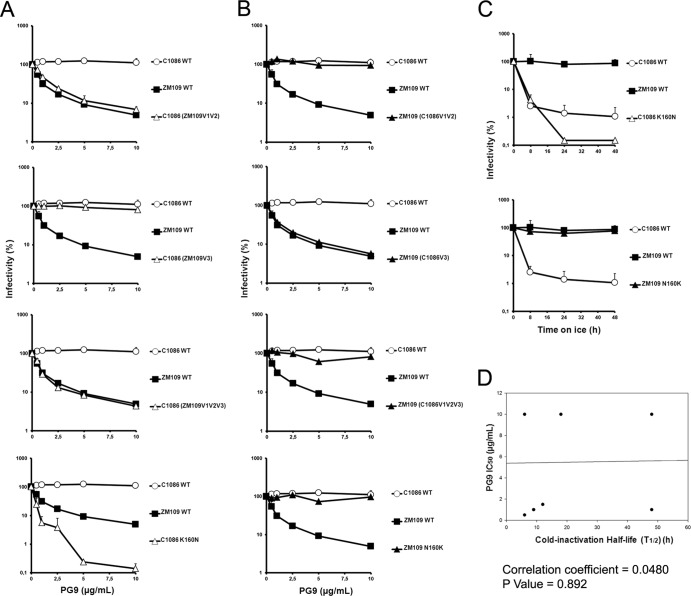
Finally, we asked whether cold inactivation could be linked to the ability of these Env gps to interact with the broadly neutralizing, quaternary-dependent PG9/PG16 antibodies that recognize epitopes present in conserved portions of the gp120 variable regions (17). These epitopes are located in the TAD, a region that could potentially influence sensitivity to cold. We produced viral particles expressing the different Env gps from our panel of chimeras and tested their sensitivity to neutralization by the PG9 antibody (Fig. 7 and Table 1). HIV-1C1086 was resistant to PG9 neutralization, whereas HIV-1ZM109 was sensitive. Interestingly, swapping of their respective V1V2 regions was sufficient to reverse their phenotypes. A glycan at residue 160 (in V1V2) has been shown to be essential for PG9/PG16 neutralization (17). As HIV-1ZM109 possesses this potential N-linked glycosylation site (PNGS), whereas HIV-1C1086 does not, we swapped the residues at position 160 and tested the sensitivity of these mutants to PG9 and PG16 neutralization. Of note, identical results were obtained for PG9 and PG16 (not shown) neutralization. As shown in Fig. 7, creation of a PNGS at position 160 (K160N) in HIV-1C1086 was sufficient to render it sensitive to PG9; however, this residue change did not result in increased sensitivity to cold (Fig. 7C). Alternatively, removal of the PNGS (N160K) in HIV-1ZM109 rendered this virus resistant to PG9 but did not alter its cold inactivation phenotype (Fig. 7C). Together these data indicate that cold inactivation and PG9 neutralization did not correlate (Fig. 7D) and that these phenotypes are regulated by different determinants within the gp120 variable regions.
Fig 7.
Effect of variable loops V1V2 and V3 or a PNGS at position 160 on PG9 neutralization. The PG9 sensitivity of recombinant HIV-1 bearing wt C1086, ZM109, or chimeric Env gps was measured in the C1086 (A) or ZM109 backbone (B). Recombinant HIV-1 strains bearing wt C1086, ZM109, or Env gp variants were incubated with serial dilutions of PG9 at 37°C for 1 h before infection of Cf2Th-CD4/CCR5 cells. Forty-eight hours later, cells were lysed and infectivity measured by luciferase. Infectivity at each dilution of PG9 tested is shown as the percentage of infection seen in the absence of PG9. (C) Cold inactivation profiles of PNGS mutants (at position 160) in C1086 and ZM109 Env gps. Data are representative of results from at least three independent experiments performed in quadruplicate, with means and standard deviations indicated. (D) Lack of correlation between PG9 neutralization and cold inactivation. Sensitivity to cold inactivation of recombinant HIV-1 bearing wt C1086, ZM109, or chimeric Env gps (presented in Fig. 1B), as well as PNGS 160 variants, was measured as described in the legend to Fig. 1A. (A) The half-life (t1/2) of each virus incubated on ice was calculated from data presented in Fig. 2 and correlated with the IC50 for PG9 neutralization (presented in panels A and B). The P value and the Spearman rank correlation coefficient are presented.
By characterizing chimeras between two primary clade C HIV-1 viruses that differ in sensitivity to cold, sCD4 neutralization and quaternary-dependent neutralizing Abs, we found that these properties were largely determined by the gp120 V1V2 and V3 regions. Whereas cold inactivation and VRC01 sensitivity were largely determined by the gp120 V1V2 region, the other properties were determined by distinct elements within the variable regions. These results underscore the importance of the gp120 major variable regions in dictating key Env phenotypes and reveal the independent relationships among sensitivities of HIV-1 to cold, sCD4, and PG9/PG16 neutralization.
ACKNOWLEDGMENTS
We thank Yvette McLaughlin and Elizabeth Carpelan for manuscript preparation and Barton Haynes for kindly sharing the HIV-1C1086 Env expressor. The ZM109F.PB4 Env expressor was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH from C. A. Derdeyn and E. Hunter: ZM109F.PB4, SVPC13. We thank Dennis Burton and Pascal Poignard and IAVI for their generous gift of PG9 and PG16 MAbs and James Robinson (48d) and Peter Kwong (VRC01) for kindly sharing MAbs.
This work was supported by Canada Foundation for Innovation Program Leader grant 29866, by CIHR operating grant 257792, and by FRQS Establishment of Young Scientist grant 24639 to A.F. A.F. is the recipient of an FRSQ Chercheur Boursier Junior 1 fellowship (no. 24639). This work was also supported by grants from the National Institutes of Health (AI24755 and AI67854), the International AIDS Vaccine Initiative, and a gift from the late William F. McCarty-Cooper.
The authors have no conflicts of interest to report.
Footnotes
Published ahead of print 23 January 2013
REFERENCES
- 1. Chan DC, Fass D, Berger JM, Kim PS. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263–273 [DOI] [PubMed] [Google Scholar]
- 2. Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. 2008. Molecular architecture of native HIV-1 gp120 trimers. Nature 455:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426–430 [DOI] [PubMed] [Google Scholar]
- 4. Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958 [DOI] [PubMed] [Google Scholar]
- 5. Feng Y, Broder CC, Kennedy PE, Berger EA. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872–877 [DOI] [PubMed] [Google Scholar]
- 6. Allan JS, Coligan JE, Barin F, McLane MF, Sodroski JG, Rosen CA, Haseltine WA, Lee TH, Essex M. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091–1094 [DOI] [PubMed] [Google Scholar]
- 7. Myszka DG, Sweet RW, Hensley P, Brigham-Burke M, Kwong PD, Hendrickson WA, Wyatt R, Sodroski J, Doyle ML. 2000. Energetics of the HIV gp120-CD4 binding reaction. Proc. Natl. Acad. Sci. U. S. A. 97:9026–9031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haim H, Si Z, Madani N, Wang L, Courter JR, Princiotto A, Kassa A, DeGrace M, McGee-Estrada K, Mefford M, Gabuzda D, Smith AB, III, Sodroski J. 2009. Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state. PLoS Pathog. 5:e1000360 doi:10.1371/journal.ppat.1000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kassa A, Finzi A, Pancera M, Courter JR, Smith AB, III, Sodroski J. 2009. Identification of a human immunodeficiency virus type 1 envelope glycoprotein variant resistant to cold inactivation. J. Virol. 83:4476–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kwon YD, Finzi A, Wu X, Dogo-Isonagie C, Lee LK, Moore LR, Schmidt SD, Stuckey J, Yang Y, Zhou T, Zhu J, Vicic DA, Debnath AK, Shapiro L, Bewley CA, Mascola JR, Sodroski JG, Kwong PD. 2012. Unliganded HIV-1 gp120 core structures assume the CD4-bound conformation with regulation by quaternary interactions and variable loops. Proc. Natl. Acad. Sci. U. S. A. 109:5663–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finzi A, Xiang SH, Pacheco B, Wang L, Haight J, Kassa A, Danek B, Pancera M, Kwong PD, Sodroski J. 2010. Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol. Cell 37:656–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. LaBonte JA, Patel T, Hofmann W, Sodroski J. 2000. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J. Virol. 74:10690–10698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiang SH, Kwong PD, Gupta R, Rizzuto CD, Casper DJ, Wyatt R, Wang L, Hendrickson WA, Doyle ML, Sodroski J. 2002. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 76:9888–9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thali M, Olshevsky U, Furman C, Gabuzda D, Li J, Sodroski J. 1991. Effects of changes in gp120-CD4 binding affinity on human immunodeficiency virus type 1 envelope glycoprotein function and soluble CD4 sensitivity. J. Virol. 65:5007–5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desormeaux A, Coutu M, Medjahed H, Pacheco B, Herschhorn A, Gu C, Xiang SH, Mao Y, Sodroski J, Finzi A. 19 December 2012. The highly conserved layer 3 component of the HIV-1 gp120 inner domain is critical for CD4-required conformational transitions. J. Virol. [Epub ahead of print.] doi:10.1128/JVI.03104-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mao Y, Wang L, Gu C, Herschhorn A, Xiang SH, Haim H, Yang X, Sodroski J. 2012. Subunit organization of the membrane-bound HIV-1 envelope glycoprotein trimer. Nat. Struct. Mol. Biol. 19:893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haim H, Strack B, Kassa A, Madani N, Wang L, Courter JR, Princiotto A, McGee K, Pacheco B, Seaman MS, Smith AB, III, Sodroski J. 2011. Contribution of intrinsic reactivity of the HIV-1 envelope glycoproteins to CD4-independent infection and global inhibitor sensitivity. PLoS Pathog. 7:e1002101. [DOI] [PMC free article] [PubMed] [Google Scholar]