Abstract
Bhanja virus (BHAV) and its antigenically close relatives Forecariah virus (FORV), Kismayo virus (KISV), and Palma virus (PALV) are thought to be members of the family Bunyaviridae, but they have not been assigned to a genus or species. Despite their broad geographical distribution and reports that BHAV causes sporadic cases of febrile illness and encephalitis in humans, the public health importance of the Bhanja serogroup viruses remains unclear, due in part to the lack of sequence and biochemical information for the virus proteins. In order to better define the molecular characteristics of this group, we determined the full-length sequences of the L, M, and S genome segments of multiple isolates of BHAV as well as FORV and PALV. The genome structures of these Bhanja viruses are similar to those of viruses belonging to the genus Phlebovirus. Functional domains and amino acid motifs in the viral proteins that are conserved among other known phleboviruses were also identified in proteins of the BHAV group. Phylogenetic and serological analyses revealed that the BHAVs are most closely related to the novel emerging tick-borne phleboviruses severe fever with thrombocytopenia syndrome virus and Heartland virus, which have recently been implicated as causing severe acute febrile illnesses associated with thrombocytopenia in humans in China and the United States. Our results indicate that the Bhanja serogroup viruses constitute a single novel species in the genus Phlebovirus. The results of this study should facilitate epidemiological surveillance for other, similar tick-borne phleboviruses that may represent unrecognized causes of febrile illness in humans.
INTRODUCTION
The family Bunyaviridae constitutes the largest group of RNA viruses, with more than 350 viruses identified (1). Bunyaviruses, particularly those belonging to the genera Orthobunyavirus, Phlebovirus, and Nairovirus, are found worldwide and are capable of replicating alternately in vertebrates and arthropods. Different viruses in these three genera are transmitted to vertebrates by the bites of infected mosquitoes, ticks, phlebotomine sand flies, or culicoid midges. Bunyaviruses cause a number of important diseases in humans and in livestock (2).
Bunyaviruses possess a genome consisting of three negative-stranded RNA segments: the L segment, encoding the RNA-dependent RNA polymerase (RdRp); the M segment, encoding the envelope glycoproteins (GPs) Gn and Gc; and the S segment, encoding a nucleocapsid protein (N). The M and/or S segments of some viruses also encode nonstructural proteins (NSm and/or NSs). Despite the discovery of numerous bunyaviruses that are pathogenic to humans and/or domestic animals, genetic information about these viruses is still rather limited. This lack of knowledge has hampered our understanding of many biological and ecological aspects of these viruses, and it has impeded the development of much-needed diagnostic tools. Several novel bunyaviruses causing disease in human and domestic animals have recently emerged in Western Europe, China, and the United States. These include severe fever with thrombocytopenia syndrome virus (SFTSV; also known as Huaiyangshan virus or Henan fever virus), Schmallenberg virus (SBV), and Heartland virus. SFTSV is a novel tick-borne phlebovirus (TBPV) that emerged recently in China and causes a severe and sometimes fatal febrile illness with thrombocytopenia and hemorrhagic manifestations in humans (3–5). SBV is midge borne and appeared recently in Western Europe, where it causes congenital malformations and reduced milk production in cattle, goats, and sheep (6). Heartland virus is a novel phlebovirus (presumably tick borne) that was recently reported in the central United States, where it is associated with a severe febrile illness in humans (7). The continual emergence of new pathogenic bunyaviruses and the propensity of these segmented viruses to undergo reassortment in nature (8–12) emphasize the need for a more comprehensive understanding of their genetics.
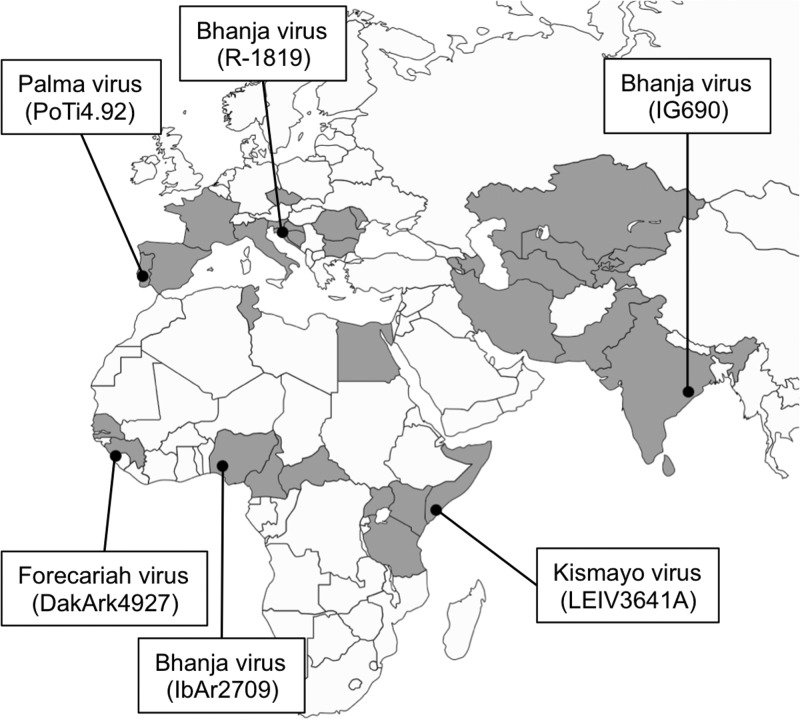
At present, 40 viruses listed as members of the family Bunyaviridae have not been assigned to genera or approved as species, due to the lack of genetic information and/or serologic cross-reactivity with other known bunyaviruses (1). Bhanja virus (BHAV), Forecariah virus (FORV), and Kismayo virus (KISV) are included in this group of unassigned bunyaviruses, although they are antigenically related to one another based on serologic tests and form a Bhanja serogroup together with Palma virus (PALV), which has not been assigned to the Bhanja group in the current list of the International Committee on Taxonomy of Viruses (ICTV). These four viruses have been isolated from ticks (fed and unfed), livestock, and wild animals in India, southern Europe, and Africa (Fig. 1) (13–23). Three laboratory-acquired human BHAV infections (two clinical and one subclinical) have been reported (24, 25), and a single case of encephalitis due to natural BHAV infection has been described (26). In addition, serological surveys in Eastern Europe suggest that BHAV is endemic in that region and causes undetected human infection (27, 28).
Fig 1.
Geographic distribution of Bhanja group viruses. The countries or regions where Bhanja group viruses have been isolated or where specific antibodies to Bhanja group viruses have been detected in animals or humans are shaded. The geographic locations where the viruses used in the present study were first isolated are indicated by black dots. The names and strain numbers of the isolates are given.
In order to gain a better understanding of the molecular biology and ecology of the Bhanja group viruses, we performed a full sequence determination and analysis of representative Bhanja group viruses from different geographic locations. This communication reports the results of our phylogenetic and serologic studies with the Bhanja group viruses, which indicate that viruses in this group constitute a new species within the genus Phlebovirus and that they are related to other TBPVs in the Uukuniemi group.
MATERIALS AND METHODS
Cells and viruses.
The Vero E6 (African green monkey kidney) and DH82 (canine histiocytosis-derived macrophage-like) cell lines, obtained from the American Type Culture Collection (ATCC), were grown in Dulbecco's modified Eagle medium (DMEM; Sigma-Aldrich) supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin (Gibco, Life Technologies). Two strains of BHAV (IG690 [20] and IbAr2709 [29]), KISV strain LEIV3641A (14), and PALV strain PoTi4.92 (30) were obtained from the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) at the University of Texas Medical Branch (UTMB), Galveston. BHAV strain R-1819 (25) and FORV strain DakArk4927 (15) were provided by the Division of Vector-Borne Infectious Diseases (DVBID), National Center for Infectious Diseases, Centers for Disease Control and Prevention (Table 1).
Table 1.
Bhanja group viruses used in the present study
| Virus | Strain | Yr | Country | Source | Reference |
|---|---|---|---|---|---|
| Bhanja virus (BHAV) | IG690 | 1954 | India | Ticks (Haemaphysalis intermedia) | 20 |
| IbAr2709 | 1968 | Nigeria | Ticks (Rhipicephalus decoloratus) | 29 | |
| R-1819 | 1974 | United States | Human (lab infection)a | 25 | |
| Forecariah virus (FORV) | DakArk4927 | 1983 | Guinea | Ticks (Rhipicephalus geiyi) | 15 |
| Palma virus (PALV) | PoTi4.92 | 1992 | Portugal | Ticks (Haemaphysalis punctata) | 30 |
| Kismayo virus (KISV) | LEIV3641A | 1992 | Somalia | Ticks (Rhipicephalus pulchellus) | 14 |
The infecting agent was a BHAV tick isolate from Yugoslavia.
Viruses were grown in Vero E6 cells or DH82 cells in DMEM supplemented with 2% FCS, 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin (Gibco, Life Technologies), and 10 μg/ml MycoKill AB (GE Healthcare). Virus growth was monitored based on the appearance and progression of cytopathic effect (CPE) in cells. When advanced CPE was observed, the culture supernatants were harvested for further experiments. Virus titers were determined by plaque assays in Vero E6 cells. Experiments with these viruses were performed in the biosafety level 2 (BSL-2) and BSL-3 laboratories at the Rocky Mountain Laboratories (RML), Division of Intramural Research (DIR), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Hamilton, MT, and the University of Texas Medical Branch, Galveston.
Serological tests.
Antigens for use in serological tests were prepared from frozen lysates of Vero cells or from newborn mouse brains infected with the indicated viruses (Table 1). Complement fixation (CF) tests were carried out using antigens prepared from infected mouse brains and extracted by the sucrose-acetone method (31). We were unable to obtain good hemagglutinating activity with mouse brain antigens prepared for many of the tick-associated bunyaviruses, so frozen harvests of infected Vero cell cultures (undiluted) were used as an alternative antigen in the hemagglutination inhibition (HI) tests. The CF and HI tests were performed by the microtiter technique, as described previously (31). CF titers were recorded as the reciprocals of the highest antibody/highest antigen dilutions giving 3+ or 4+ fixation of complement on a scale of 0 to 4+. HI antibody titers of 1:20 or greater were considered positive.
Immune reagents.
Virus-specific antibodies (mouse hyperimmune ascitic fluids and rabbit hyperimmune sera) used in serological tests were obtained from the WRCEVA collection at the UTMB. The methods used to prepare mouse immune ascitic fluids (MIAFs) have been described previously (31, 32). The immunizing antigens were 10% homogenates of infected newborn mouse brain in phosphate-buffered saline (PBS); MIAFs were made in adult ICR mice given four weekly intraperitoneal injections of the mouse brain antigen mixed with Freund's adjuvant (31, 32). Sarcoma 180 cells (ATCC) were used to induce ascites (31). A rabbit hyperimmune serum to SFTSV was also prepared, using the same immunization schedule, except that the immunogen was infected rabbit kidney cells grown in a medium with 5% rabbit serum instead of bovine serum. All animal work was done at the UTMB under an approved animal protocol.
In addition, convalescent-phase sera from three severe fever with thrombocytopenia syndrome (SFTS) patients were included in HI tests. These sera were obtained by one of us (X.-J.Y.) during field studies in China (5).
Electron microscopy.
DH82 cells infected with BHAV IbAr2709 were harvested 24 h postinfection. Cells were washed with PBS and were fixed with 2.5% glutaraldehyde in phosphate buffer (pH 7.4) overnight at room temperature, according to a previously published protocol (33). After embedding, cells were sectioned into 70-nm layers with a diamond knife, poststained with 1% uranyl acetate and 1% lead citrate, and examined with a model H7500 electron microscope (Hitachi High-Technologies) at 80 kV. Digital images were collected with an XR100 charge-coupled-device camera (Advanced Microscopy Techniques).
RNA isolation.
After centrifugation of cell culture supernatants twice at 2,500 × g to remove debris, samples were concentrated to a small final volume (<1,000 μl) using an Amicon Ultra-15 centrifugal filter unit with an Ultracel-50 membrane (Millipore). Concentrated supernatants were lysed in 3 volumes of TRIzol LS reagent (Life Technologies), and RNA was extracted using a modified guanidine thiocyanate method (34). To 550 μl of lysate, 147 μl of 1-bromo-3-chloropropane (Sigma) was added, and the phases were separated by centrifugation for 20 min at 4°C. An equal amount of guanidine isothiocyanate-containing RLT buffer (Qiagen) was added to the aqueous phase, and RNA was subsequently DNase treated and purified by using RNeasy columns according to the manufacturer's protocol (Qiagen).
cDNA synthesis.
cDNA was synthesized using a modification of the protocol described by Palacios et al. (35). Briefly, the first-strand cDNA was synthesized using the SuperScript III reverse transcriptase system (Invitrogen) with 100 to 1,000 ng of total RNA. Reverse transcription (RT) of the RNA strand was performed with 4.5 μg of a random octamer linked to a defined 17-mer primer (5′-GTT TCC CAG TAG GTC TCN NNN NNN N-3′), 1,500 U of SuperScript III reverse transcriptase, 10 mM dithiothreitol (DTT), and 0.5 mM deoxynucleoside triphosphates (dNTPs). Following first-strand synthesis, RNA was hydrolyzed in 0.2 N NaOH by incubating the cDNA product for 30 min at 65°C and was then neutralized with 0.2 N HCl. The single-stranded cDNA (ss-cDNA) products were purified using the QIAquick system (Qiagen). Then the ss-cDNAs were randomly amplified using a 1:9 mixture of the arbitrary 17-octamer primer and a primer targeting a specific 17-mer sequence (5′-CGC CGT TTC CCA GTA GGT CTC-3′) by modification of a procedure described by Palacios et al. (35). Five microliters of each ss-cDNA template was used as the template in 50-μl reaction mixtures consisting of 0.2 μM primer mix, 1× Platinum Taq polymerase buffer, 2.5 U Platinum Taq polymerase, 0.2 mM each dNTP, and 1.5 mM MgCl2 (Invitrogen). PCRs were performed in triplicate in two phases; the initial cycles were performed at a low annealing temperature of 25°C and were followed by cycles at a more stringent annealing temperature of 55°C. The cycling conditions were as follows: 95°C for 2 min; 5 cycles at 95°C for 15 s, 25°C for 1 min, and 72°C for 1 min; 25 cycles at 95°C for 15 s, 55°C for 1 min, and 72°C for 1 min; and a final extension at 72°C for 1 min. Replicate PCR products were pooled and purified using the QIAquick kit according to the manufacturer's protocol (Qiagen). The final PCR products were visualized by gel electrophoresis on a 2% SeaKem agarose gel (Lonza), quantified by UV spectrophotometry at 260 nm and 280 nm, and used as the template for sequencing on the 454 Genome Sequencer FLX system (454 Life Sciences).
High-throughput sequencing and data analysis.
cDNA samples were quantitated using PicoGreen reagent (Life Technologies) and were prepared according to the Rapid Library Preparation Method Manual: GS FLX Titanium Series, by 454 Life Sciences (36). A pool consisting of 12 samples was titrated in medium-volume emulsion (MVE) format to determine the optimal copy-per-bead (CPB) ratio, which produced the best sequencing quality. A 454 Titanium sequencing run was then performed using 1 CPB.
Genomic viral sequences run on the Genome Sequencer FLX system generated an average of 16,000 usable fragment reads, with coverage ranging from 100× to 400×. De novo genome assembly was performed using the GS De Novo Assembler, version 2.6 (454 Life Sciences), and the CLC Genomics Workbench, version 4.0 (CLC bio). Translated BLAST (blastx) was performed to remove nonviral contaminants, and the initial assembly was performed using Sequencher, version 5.0 (Gene Codes). Assembled contigs were then verified, refined, or corrected by mapping the 454 reads using GS Reference Mapper, version 2.6 (454 Life Sciences). Where needed, several rounds of manual assembly and trimming were performed in Sequencher, and verification was done using GS Reference Mapper to eliminate discrepancies or errors discovered during the prior reference mapping procedure.
RT-PCR and Sanger sequencing.
Based on the data obtained from the high-throughput sequencing described above, primers for RT-PCR and Sanger sequencing were designed (primer sequences are available upon request). RT-PCR was performed with SuperScript III reverse transcriptase (Life Technologies) and iProof DNA polymerase (Bio-Rad). The 3′ and 5′ termini of each genome RNA segment were amplified as a single fragment by RT-PCR following RNA ligation with T4 RNA ligase (New England BioLabs) (primer sequences are available upon request). The amplified fragments were cloned into the TOPO TA cloning vector (Life Technologies) for sequencing.
Sequence analyses.
To predict the secondary structure of the intergenic regions of the S segment RNAs from each virus, CLC Main Workbench (CLC bio) was used with default settings. N-linked glycosylation sites in the GPs were predicted with NetNGlyc, version 1.0 (Center for Biological Sequence Analysis at the Technical University of Denmark). Transmembrane domains were predicted with the MEMSAT algorithm on the PSIPRED server (37, 38), and signal sequences were predicted using the SignalP 4.0 server (39).
Sequence alignment and phylogenetic analysis.
The nucleotide sequences obtained for each genome segment, or the deduced amino acid sequences of each of the open reading frames (ORFs), were aligned with the representative sequences of other known phleboviruses from GenBank (see Table S1 in the supplemental material) using MUSCLE as implemented in MEGA, version 5 (40). Phylogenetic trees were constructed using the neighbor-joining (NJ) and maximum likelihood (ML) methods. For NJ analysis, the Tamura 3-parameter model and the Poisson model built into MEGA 5 were used for nucleotide sequences and amino acid sequences, respectively. The robustness of the nodes was tested by 2,000 bootstrap replications. For ML analysis, RAxML was used with 1,000 bootstrap replications. The general time-reversible (GTR) + Γ + I model was selected for tree searches of nucleotide and amino acid sequences.
Nucleotide sequence accession numbers.
The genome sequences of all Bhanja group viruses were deposited in GenBank under the following accession numbers (S segment, M segment, and L segment): JX961619, JX961620, and JX961621 (BHAV IG690); JX961622, JX961623, and JX961624 (BHAV R-1819); JX961616, JX961617, and JX961618 (BHAV IbAr2709); JX961625, JX961626, and JX961627 (FORV DakArk4927); and JX961628, JX961629, and JX961630 (PALV PoTi4.92).
RESULTS AND DISCUSSION
Serological studies.
Table 2 shows the results of CF tests comparing four Bhanja group viruses: BHAV (strain IG690), PALV, FORV, and KISV. The CF test indicates the degree of serological divergence of the N protein. In this test, BHAV, PALV, and FORV were essentially indistinguishable. KISV was distinct, although there was some cross-reactivity between BHAV, PALV, and KISV.
Table 2.
Results of complement fixation tests with Bhanja group viruses
| Antigen | CF titera with antibody against: |
|||
|---|---|---|---|---|
| BHAV | PALV | FORV | KISV | |
| Bhanja virus | 1,024/512 | 256/512 | 256/512 | 16/128 |
| Palma virus | 1,024/128 | 512/128 | 256/128 | 8/32 |
| Forecariah virus | 1,024/32 | 512/32 | 512/32 | 0 |
| Kismayo virus | 16/128 | 16/128 | 0 | 1,024/128 |
Expressed as the reciprocal of the highest antibody dilution/reciprocal of the highest antigen dilution. 0, <8/<8.
Table 3 gives the results of the HI test comparing Uukuniemi virus (UUKV), BHAV, and Heartland virus antigens against UUKV, BHAV, and SFTSV MIAF, as well as hyperimmune SFTSV rabbit serum and convalescent-phase sera from three human cases of SFTS. To date, we have not produced an SFTSV preparation with potent hemagglutination activity or a Heartland virus MIAF. Despite these limitations, a close antigenic relationship between Heartland virus and SFTSV was demonstrated by the HI test. The BHAV antigen also reacted with the mouse and rabbit antibodies against SFTSV, as well as with the convalescent-phase sera from human SFTS patients. The anti-UUKV antibody also reacted slightly with the Heartland virus antigen. These data support other recent work (41) indicating that on the basis of serologic relationships, BHAV, SFTSV, and Heartland virus are all members of an expanded Uukuniemi group within the genus Phlebovirus.
Table 3.
Results of hemagglutination inhibition tests, comparing Uukuniemi virus, Bhanja virus, SFTSV, and Heartland virus
| Antibody | HI titera with the following antigen: |
||
|---|---|---|---|
| Uukuniemi virus | Bhanja virus | Heartland virus | |
| Anti-Uukuniemi virus | 1:320 | 0 | 1:20 |
| Anti-Bhanja virus | 0 | 1:320 | 1:20 |
| Anti-SFTSV (mouse) | 0 | 1:40 | 1:1,280 |
| Anti-SFTSV (rabbit) | NT | 1:40 | 1:320 |
| SFTS serum 1 (human)b | 0 | 1:80 | 1:160 |
| SFTS serum 2 (human)b | 0 | 1:20 | 1:40 |
| SFTS serum 3 (human)b | 0 | 1:40 | 1:80 |
NT, not tested; 0, <1:20.
SFTS convalescent-phase human serum.
Growth of Bhanja virus in vertebrate cells.
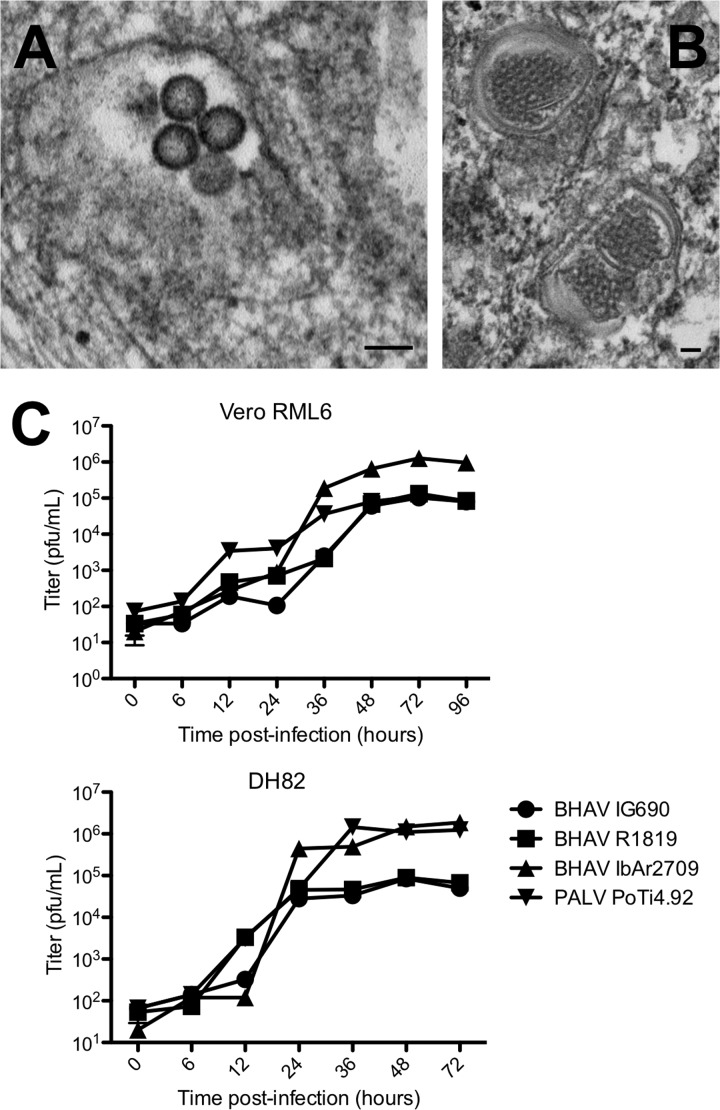
The growth characteristics of several Bhanja group viruses were determined in both DH82 and Vero cells. BHAV, PALV, and FORV all produced cytopathic effect (CPE) and plaques in Vero and DH82 cells. In contrast, no CPE or plaques were observed in either of these two vertebrate cell lines with KISV. RT-PCR primers, based on the sequences of conserved regions of Bhanja group virus genomes, also failed to amplify KISV RNA or cDNA fragments, suggesting that KISV did not grow in the two vertebrate cell lines. By electron microscopy, DH82 cells infected with BHAV strain IbAr2709 showed bunyavirus-like particles, not exceeding 100 nm in diameter, located in cytoplasmic vacuoles (Fig. 2A). Inclusion bodies were also observed within the infected cells (Fig. 2B).
Fig 2.
Growth of Bhanja viruses in cell lines. (A and B) The growth of Bhanja virus (BHAV) IbAr2709 in DH82 cells was confirmed by electron microscopy. (A) Virions in an intracellular vacuole; (B) inclusion bodies. Bars, 100 nm. (C) Three isolates of BHAV and an isolate of Palma virus (PALV) were tested for growth in Vero E6 and DH82 cells. The cells were infected at an MOI of 0.05, and the supernatants of the cells at each time point were titrated by a plaque assay using Vero E6 cells. The experiments were performed in triplicate. Error bars indicate the standard errors of the means.
To determine the growth rates of representative Bhanja group viruses, flasks of Vero E6 and DH82 cells were inoculated at a multiplicity of infection (MOI) of 0.05 with BHAV strains IG690, R-1819, and IbAr2709 and with PALV. Supernatants of the cultures were taken at various time points after infection (6, 12, 24, 36, 48, and 72 h) and were subsequently titrated by plaque assays in Vero E6 cells. The growth rates of the four viruses were higher in DH82 cells, although the final titers were similar and stabilized between 105 and 106 PFU/ml (Fig. 2C). In general, the highest titers were obtained with BHAV strain IbAr2709.
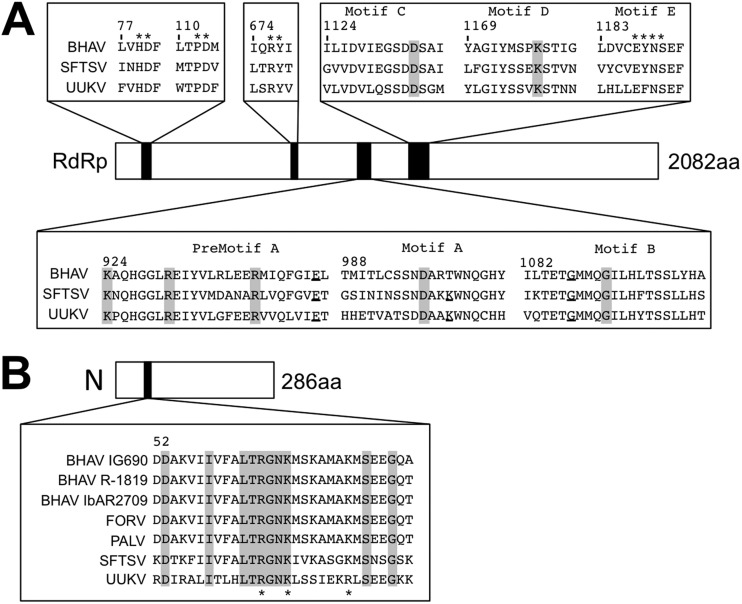
Gene structure/organization and identification of conserved motifs in the predicted viral protein sequences.
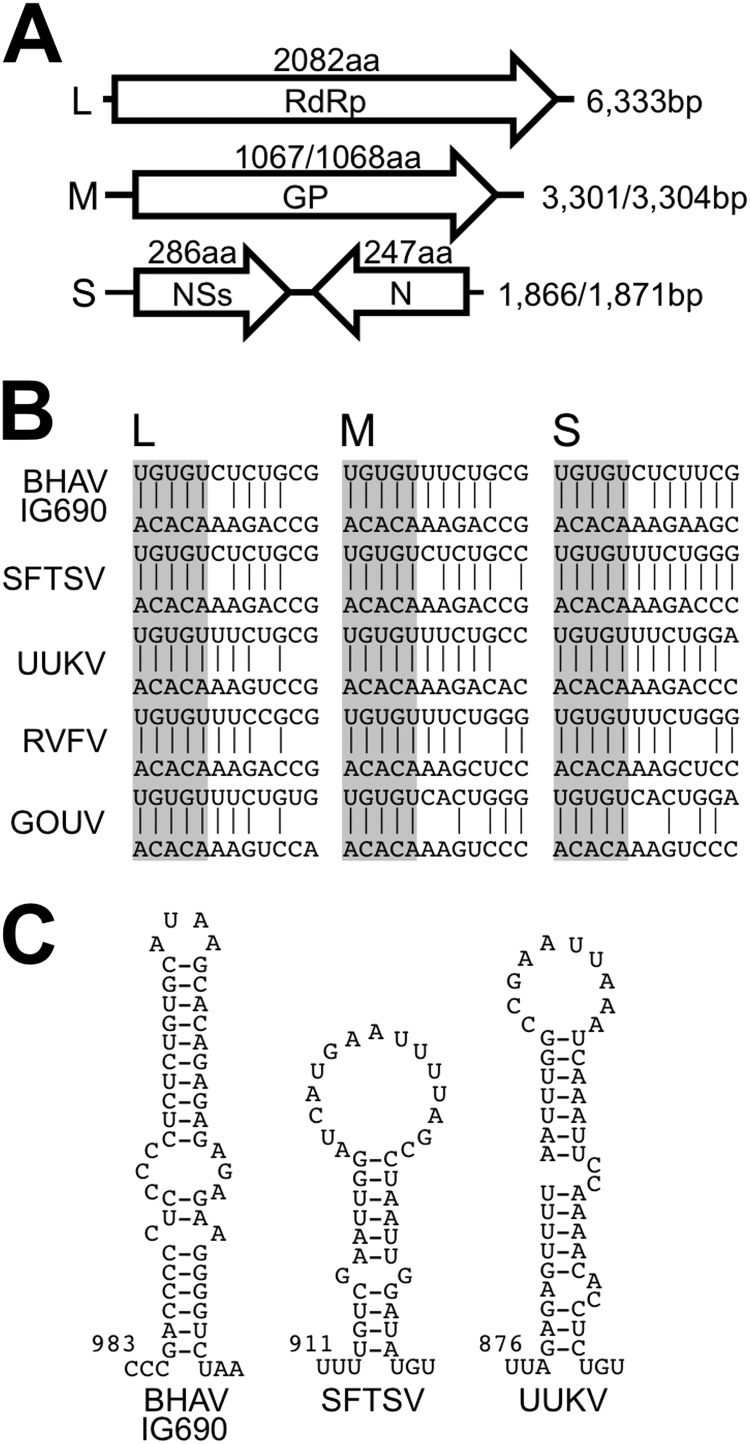
The full-length genome RNA sequences of three strains of BHAV (IG690, IbAr2709, and R-1819) and one strain of PALV (PoTi4.92) were determined by high-throughput sequencing and were subsequently confirmed by Sanger sequencing. The full-length genome sequence of FORV (DakArk4927) was determined by conventional RT-PCR and Sanger sequencing with primers binding to the conserved sequences of the BHAVs. The genome structure/organization of BHAVs revealed that they belong to the genus Phlebovirus of the family Bunyaviridae. The genome structures of BHAV isolates and of PALV and FORV were almost identical, consisting of three RNA segments with different lengths (Fig. 3A). The large (6,333 nucleotides [nt]) and medium (3,301 nt or 3,304 nt) segments (the L and M segments) each contained one long deduced open reading frame (ORF) in the negative-sense orientation. The nucleotide sequences of the L and M genome segments and the deduced amino acid sequences of their ORFs showed homology with the L segments (RdRp) and M segments (GPs Gn and Gc) of other phleboviruses (see Tables S2 and S3 in the supplemental material). The small segment (S segment; 1,866 nt for BHAV R-1819 and PALV PoTi4.92 and 1,871 nt for the others) contained two open reading frames in opposing orientations (ambisense coding strategy). As with the L and M segments, the nucleotide and deduced amino acid sequences of these S segments showed homology with S segment nucleotide sequences and N and NSs amino acid sequences of other known phleboviruses (see Tables S4 and S5 in the supplemental material).
Fig 3.
Genome structure of Bhanja virus. (A) Schematic diagram of the coding strategy of Bhanja virus. BHAV has three genome segment RNAs with different lengths (nucleotide sequence lengths are shown on the right). The largest segment (L) encodes an RNA-dependent RNA polymerase (RdRp), and the second largest segment (M) encodes a glycoprotein (GP). The smallest RNA segment (S) encodes a nonstructural protein (NSs) and a nucleocapsid protein (N) in opposing directions. The amino acid (aa) sequence length of each protein is indicated above the corresponding open reading frame (shown as an open arrow). (B) Terminal conserved complementary sequences that constitute a “panhandle structure” in each genome segment. The upper strand in each alignment starts from the 3′ end of the genome, while the lower strand starts from the 5′ end. Vertical lines between the two strands indicate complementary nucleotides in the 3′ and 5′ ends. The conserved 3′ and 5′ terminal nucleotides of each genome segment are shaded. SFTSV, severe fever with thrombocytopenia syndrome virus; UUKV, Uukuniemi virus; RVFV, Rift Valley fever virus; GOUV, Gouleako virus (55). (C) The predicted secondary structure of the intergenic region between the two ORFs of the S segment RNA was calculated using CLC Main Workbench (CLC bio). Each number indicates the nucleotide position from the 3′ end of the virus genome.
In order to form “panhandle structures,” the 3′ and 5′ ends of the bunyavirus genome have complementary sequences (42), which are conserved within the Phlebovirus genus. Bhanja group viruses also have conserved sequences at their genome termini, and the sequences of the five terminal nucleotides (3′-UGUGU and ACACA-5′) are identical to those of other members of the genus Phlebovirus (Fig. 3B). The intergenic regions of Punta Toro virus and UUKV, located between the N and NSs ORFs, have been reported to potentially constitute a hairpin-like secondary structure (43, 44). Bhanja group viruses have conserved complementary nucleotide sequences in the region, which, in theory, are also able to constitute a hairpin-like structure (Fig. 3C). While the TBPVs have similar secondary structures in this region, the nucleotide sequences are not conserved. It is noteworthy that the S segment genome RNAs of other phleboviruses, such as Rift Valley fever virus (RVFV), do not form such a secondary structure, suggesting that the mechanism of transcription termination differs among phleboviruses (44). Overall, the characteristics of the genome RNA segments clearly indicated that Bhanja group viruses are members of the genus Phlebovirus.
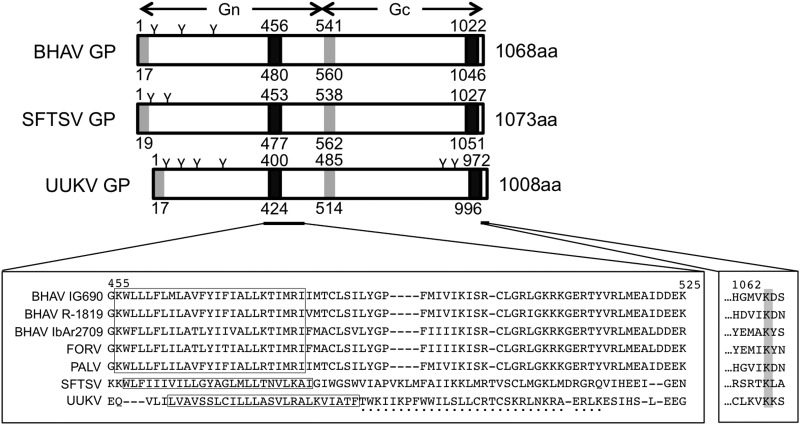
Next, we compared the amino acid sequences of the Bhanja group viruses with those of other phleboviruses, mainly the TBPVs. These amino acid sequence-based predictions revealed that the BHAV GP contains two potential transmembrane domains and two signal sequences. Like the viruses belonging to the Uukuniemi group, the BHAV GP ORF does not contain a nonstructural protein NSm in a pre-glycoprotein coding region (45) (Fig. 4). The transmembrane topology of the BHAV GP was predicted to be similar to those of the SFTSV and UUKV GPs, suggesting that it uses similar maturation machinery and that the GP will be cleaved into two membrane glycoproteins, Gn and Gc, which then constitute the heterodimer, as shown for UUKV GP maturation (45). Interestingly, the amino acids downstream of the first transmembrane domain, which form the cytoplasmic tail of Gn and are essential for the retention of the GP by the Golgi complex, are not conserved among phleboviruses (46, 47). Similarly, although the cytoplasmic tail of Gn was conserved among the Bhanja group viruses, these sequences differed from those in other TBPVs (Fig. 4). The cytoplasmic tail of Gc is involved in the budding of virions and the intracellular trafficking of the GPs (47). The lysine in the third position from the C terminus in the cytoplasmic tail of Gc, which is critical for the trafficking of the UUKV Gc, is also conserved among Bhanja group viruses and the other TBPVs (Fig. 4). These results suggest that the GP of BHAV retains many of the functions associated with other phlebovirus GPs.
Fig 4.
Comparison of the glycoproteins among tick-borne phleboviruses. (Top) Schematic diagrams of the predicted structure of GP for Bhanja virus IG690 (BHAV), severe fever with thrombocytopenia syndrome virus (SFTSV), and Uukuniemi virus (UUKV). The length of the total amino acid (aa) sequence for each protein is given on the right. Signal sequences (shaded rectangles) were predicted with SignalP 4.0, and transmembrane domains (filled rectangles) were predicted with MEMSAT3. Each region or domain starts from the amino acid position indicated above the rectangle and ends at the position indicated below the rectangle. N-linked glycosylation sites were predicted with NetNGlyc, version 1.0, and are indicated by the letter “Y” above each diagram. (Bottom) Alignment of the amino acid sequences of the areas around the transmembrane region of Gn (left) and the cytoplasmic tail of Gc (right) in tick-borne phleboviruses. Numbers above the alignment indicate the amino acid position in the BHAV IG690 GP. FORV, Forecariah virus; PALV, Palma virus. (Left) The deduced transmembrane domains are boxed, and the Golgi retention motif of UUKV GP (47) is indicated by black dots under the alignment. (Right) The conserved lysine at the third amino acid position from the C terminus of GP is shaded.
In the RdRp sequences of BHAVs, conserved functional motifs of negative-stranded RNA virus RNA polymerases (Fig. 5A) were identified: all three dipeptides (HD at positions 81 and 82, PD at positions 114 and 115, and RY at positions 678 and 679 of BHAV IG690 RdRp) found in arenavirus and bunyavirus polymerases are also conserved in the RdRp's of BHAV and the other TBPVs (i.e., SFTSV and UUKV). Six motifs that have been identified in a broad range of negative-stranded RNA virus RdRp's have also been identified in TBPV RdRp's. In the N protein sequences, critical amino acid residues for the RNA-binding motif, as identified in the crystal structure of the N protein of RVFV (48, 49), were also found in TBPV N proteins (Fig. 5B). The amino acid residues R64, K67, and K74 of the RVFV N protein are conserved among phleboviruses, including UUKV. Sequence alignment of the RNA-binding region shows that the three amino acid residues and the region around R64 are all highly conserved among the TBPVs. The conservation of these functional or potentially functional amino acids in the Bhanja virus N and RdRp proteins indicates a similarity in the RNA replication and RNA-packaging machineries between BHAVs and other phleboviruses. The amino acid sequences of phlebovirus NSs proteins, which inhibit host innate immune responses (50, 51), are more divergent than the other protein sequences (see Tables S2 to S5 in the supplemental material). Further analysis may reveal both common and different strategies of NSs to suppress host responses among phleboviruses.
Fig 5.
Comparison of amino acid sequences of the RNA-dependent RNA polymerase (RdRp) and nucleocapsid (N) among tick-borne phleboviruses. (A) The amino acid residues of the so-called ‘polymerase module’ of RdRp were compared among tick-borne phleboviruses: Bhanja virus (BHAV) IG690, Severe Fever and Thrombocytopenia virus (SFTSV), and Uukuniemi virus (UUKV). Amino acids conserved among negative-stranded RNA viruses have been highlighted in gray and ones conserved only among bunyaviruses have been underlined (53). Numbers above the aligned sequences indicate the amino acid positions in the BHAV IG690 RdRp. In the RNA-dependent RNA polymerases (RdRp) of negative-stranded RNA viruses, the polymerase modules are conserved among different virus families, including phlebovirus, suggesting that the modules represent the amino acids crucial for polymerase function. (B) Alignment of the RNA-binding region of the N protein. The asterisks on the bottom of the sequences indicate the amino acids crucial for the ability of N to bind viral RNAs, which are conserved among phleboviruses (54). The conserved amino acids among tick-borne phleboviruses are indicated by gray boxes. Numbers above the aligned sequences indicate the amino acid positions in the BHAV IG690 N protein.
Phylogenetic and serological evidence for the classification of Bhanja group viruses into a novel species in the genus Phlebovirus that is closely related to SFTSV.
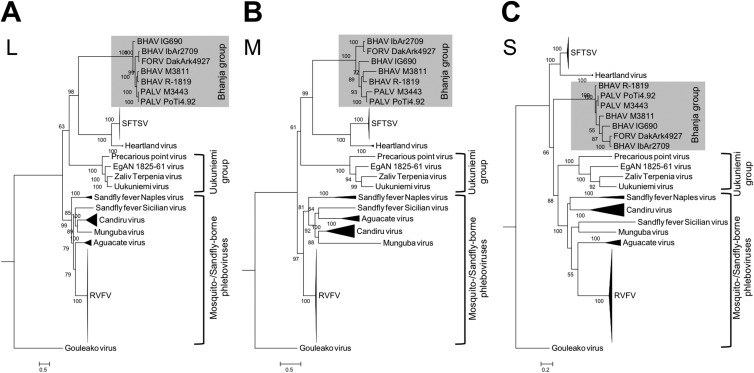
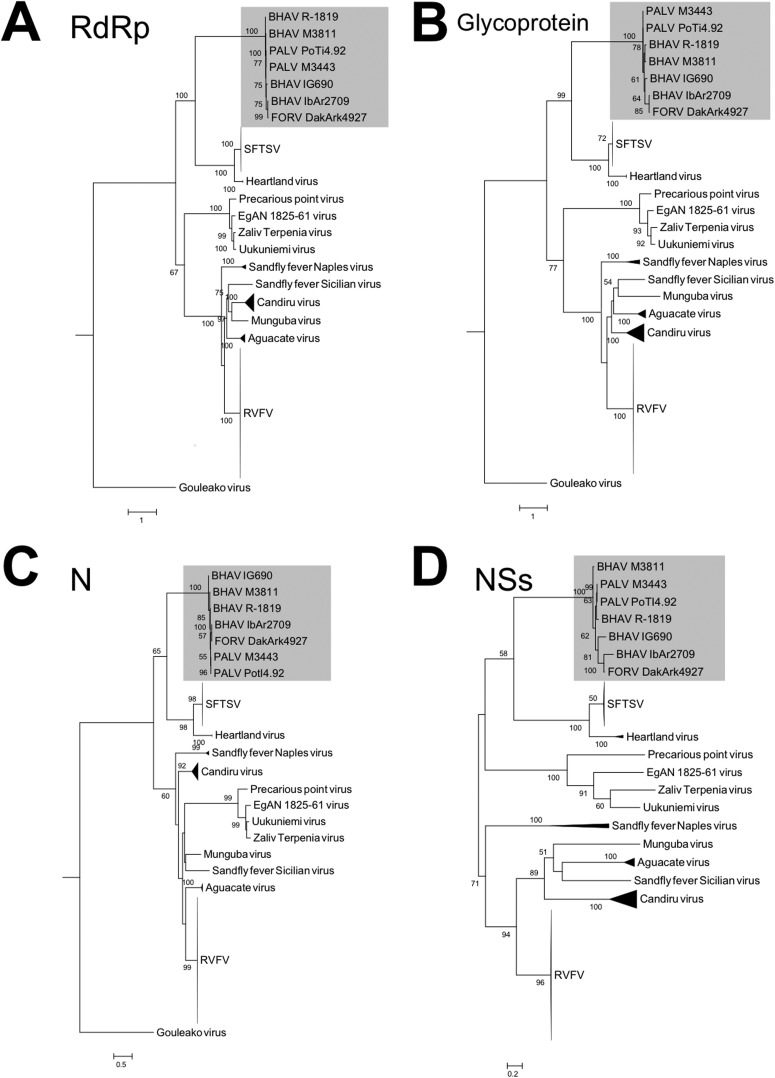
Phylogenetic trees based on the nucleotide sequences of the L, M, and S genome segments were constructed to define the molecular relationships between Bhanja group viruses and other phleboviruses (Fig. 6). Two additional BHAV and PALV sequences (M3811 and M3443, respectively), recently determined by Dilcher et al. (52), were also included in the construction of these phylogenetic trees. In all three phylogenetic trees (L, M, and S segments), the Bhanja group viruses constitute a single clade closely related to the clades formed by SFTSV and Heartland virus. Despite the similar conclusions regarding the genetic relationship between BHAV and SFTSV drawn by Dilcher et al. (52) and in our study, the phylogenetic dendrograms produced by these two studies were slightly different, which might reflect the selection of the evolutionary model, outgroup, and/or number of sequences used to construct the tree. Recently, Palacios et al. determined and reported additional full-length genome sequences of viruses belong to the UUKV group (41) (the sequence data are available in GenBank). Exploiting the additional data reported by Palacios et al., Dilcher et al., and the present study may provide a more confident classification of TBPVs based on molecular biological approaches. The other phleboviruses (such as RVFV, sandfly fever Naples virus [SFNV], and sandfly fever Sicilian virus [SFSV]), which are transmitted by mosquitoes or sand flies, appeared in a different branch distinct from the clades formed by BHAVs and SFTSVs. While the clades formed by other TBPVs, including Uukuniemi group viruses, were clearly divergent from the clades of sand fly- or mosquito-borne phleboviruses in trees based on the L and M segment sequences, clades of the TBPVs and sandfly- or mosquito-borne phleboviruses were not clearly separated in the trees based on the S segment sequences. This partial difference in tree topology between the L and M segments, on the one hand, and the S segments, on the other (i.e., instability of the UUKV clade), may be due to different evolutionary processes affecting the N protein versus the other proteins, as shown in the phylogenetic trees based on amino acid sequences (Fig. 7), suggesting that the N gene of Uukuniemi group viruses may evolve by selection under different environmental conditions (e.g., vector and mammalian host). The phylogenetic trees for BHAVs, SFTSV, and Heartland virus based on the amino acid sequences of the RdRp, GP, and NSs showed topologies similar to those of the trees based on nucleotide sequences. The Bhanja group viruses, SFTS and Heartland viruses, shared a most recent common ancestor among viruses in this genus and constitute a cluster distinct from the other viruses (Fig. 7).
Fig 6.
Phylogenetic relationships among phleboviruses. Maximum likelihood trees were constructed with the GTR + Γ + I model based on the nucleotide sequences of the L segment RNA (A), the M segment RNA (B), and the S segment RNA (C). Numbers on the trees represent bootstrap values of 1,000 replicates. Abbreviations: BHAV, Bhanja virus, FORV, Forecariah virus; PALV, Palma virus; SFTSV, severe fever with thrombocytopenia syndrome virus; RVFV, Rift Valley fever virus.
Fig 7.
Phylogenetic relationships among phlebovirus proteins. Maximum likelihood trees based on the amino acid sequences of each viral protein were constructed using the PROTGAMMA model. (A) RNA-dependent RNA polymerase (RdRp), encoded by the L segment; (B) glycoprotein, encoded by the M segment; (C) nucleocapsid protein (N), encoded by the S segment; (D) nonstructural protein (NSs), also encoded by the S segment. Numbers on the trees represent bootstrap values of 1,000 replicates. Abbreviations of taxa: BHAV, Bhanja virus; FORV, Forecariah virus; PALV, Palma virus; SFTSV, severe fever with thrombocytopenia syndrome virus; RVFV, Rift Valley fever virus.
The results of HI tests (Table 3), which reflect mainly antigenic similarities and differences among the GPs of phleboviruses, also support a close relationship between BHAV, SFTSV, and Heartland virus. Antiserum against BHAV reacted with homologous antigens (BHAV IG690) but not with the UUKV antigen, and vice versa. In contrast, antisera to SFTSV and convalescent-phase sera from SFTS patients reacted with the BHAV and Heartland virus antigens. These serological and phylogenetic results support the classification of BHAVs and PALV into a novel species of the genus Phlebovirus. However, although the serological relationship of KISV to BHAV is closer than that to the other TBPVs (Table 2), taxonomic assignment of KISV to the genus Phlebovirus still remains elusive due to the lack of sequence data. Thus, at present, KISV can be identified only as a “probable phlebovirus.”
Among the Bhanja group viruses, three geographic lineages (African, Asian, and European) might be implied by the phylogenetic analysis (Fig. 6) and the amino acid sequence identities (see Tables S2 to S5 in the supplemental material). As discussed above, the three phylogenetic trees (L, M, and S segments) displayed different topologies within the Bhanja group virus branches, suggesting that different evolutionary processes have occurred among the three RNA genome segments of Bhanja group viruses (e.g., genetic reassortment). Nevertheless, Bhanja group viruses seem to be divided into African and Eurasian lineages. This may reflect or be defined by the geographic distribution of their tick vectors and/or mammalian reservoirs. In general, Bhanja group viruses have been isolated from multiple species of hard ticks. To date, the Eurasian Bhanja group viruses have been isolated only from the Haemaphysalis species of ticks, whereas BHAV group viruses of the African lineage have been isolated from several different genera of ticks, including Amblyomma, Dermacentor, Rhipicephalus, and Hyalomma. Further studies will be required to define the vector competence of different tick species for these viruses and the relationship between the geographic distributions of ticks and viruses.
Here we have shown a closer genetic and serological relationship of Bhanja group viruses to the more pathogenic SFTSV and Heartland virus than to UUKVs and the other phleboviruses. Although reported human cases of BHAV infection are rare, the close relationship between Bhanja group viruses, SFTSV, and Heartland virus may imply that Bhanja group viruses have pathogenic potential for humans. In addition, the broad geographical distribution of those TBPVs suggests the existence of other, still unrecognized but related TBPVs that could be pathogenic for humans. Further clarification of the evolutionary processes leading to the emergence of these known human pathogens, to prepare for the potential emergence of other novel, highly pathogenic TBPVs, would seem prudent. The genetic and serological data presented here should facilitate the development of diagnostic tools for the surveillance and recognition of tick-associated illnesses caused by unrecognized pathogens. Also, further investigations into the molecular biology of Bhanja group virus pathogenesis and transmission should contribute to a better understanding of the pathogenesis, ecology, and epidemiology of these viruses.
Supplementary Material
ACKNOWLEDGMENTS
We thank the following individuals at the RML, DIR, NIAID, NIH: Daniel Bruno, Kimmo Virtaneva, and Stacy Ricklefs for performing the 454 sequencing; Elizabeth Fischer for help with the electron microscopy experiments; Allison Groseth for assistance in editing the article; and Martha Thayer for conducting the literature searches. We also thank Brandy Russell, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, for providing BHAV (strain R-1819) and FORV.
This work was supported by the Intramural Research Program of the NIH. A.P.A.T.D.R., X.-J.Y., and R.B.T. were supported by NIH contract HHSN272201000040I/HHSN27200004/D4.
The opinions, interpretations, conclusions, and recommendations presented here are those of the authors and are not necessarily endorsed by the NIH.
Footnotes
Published ahead of print 16 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02845-12.
REFERENCES
- 1. Plyusnin A, Beaty BJ, Elliott RM, Goldbach R, Kormelink R, Lundkvist A, Schmaljohn CS, Tesh RB. 2011. Family Bunyaviridae, p 725–741 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses, 1st ed Elsevier, London, United Kingdom [Google Scholar]
- 2. Schmaljohn CS, Nichol ST. 2007. Bunyaviridae, p 1741–1789 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 3. Xu B, Liu L, Huang X, Ma H, Zhang Y, Du Y, Wang P, Tang X, Wang H, Kang K, Zhang S, Zhao G, Wu W, Yang Y, Chen H, Mu F, Chen W. 2011. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province, China: discovery of a new bunyavirus. PLoS Pathog. 7:e1002369 doi:10.1371/journal.ppat.1002369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magiorkinis G. 2011. A novel bunyavirus in China. N. Engl. J. Med. 365:864–865 [DOI] [PubMed] [Google Scholar]
- 5. Yu X-J, Liang M-F, Zhang S-Y, Liu Y, Li J-D, Sun Y-L, Zhang L, Zhang Q-F, Popov VL, Li C, Qu J, Li Q, Zhang Y-P, Hai R, Wu W, Wang Q, Zhan F, Wang X, Kan B, Wang S-W, Wan K-L, Jing H-Q, Lu J-X, Yin W-W, Zhou H, Guan X-H, Liu J-F, Bi Z-Q, Liu G-H, Ren J, Wang H, Zhao Z, Song J-D, He J-R, Wan T, Zhang J-S, Fu X-P, Sun L-N, Dong X-P, Feng Z-J, Yang W-Z, Hong T, Zhang Y, Walker DH, Wang Y, Li D-X. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364:1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffmann B, Scheuch M, Höper D, Jungblut R, Holsteg M, Schirrmeier H, Eschbaumer M, Goller KV, Wernike K, Fischer M, Breithaupt A, Mettenleiter TC, Beer M. 2012. Novel orthobunyavirus in cattle, Europe, 2011. Emerg. Infect. Dis. 18:469–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albariño CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. 2012. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 367:834–841 [DOI] [PubMed] [Google Scholar]
- 8. Goller KV, Höper D, Schirrmeier H, Mettenleiter TC, Beer M. 2012. Schmallenberg virus as possible ancestor of Shamonda virus. Emerg. Infect. Dis. 18:1644–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Briese T, Bird B, Kapoor V, Nichol ST, Lipkin WI. 2006. Batai and Ngari viruses: M segment reassortment and association with severe febrile disease outbreaks in East Africa. J. Virol. 80:5627–5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Briese T, Kapoor V, Lipkin WI. 2007. Natural M-segment reassortment in Potosi and Main Drain viruses: implications for the evolution of orthobunyaviruses. Arch. Virol. 152:2237–2247 [DOI] [PubMed] [Google Scholar]
- 11. Palacios G, Tesh R, Travassos Da Rosa AP, Savji N, Sze W, Jain K, Serge R, Guzman H, Guevara C, Nunes MRT, Nunes-Neto JP, Kochel T, Hutchison S, Vasconcelos PFC, Lipkin WI. 2011. Characterization of the Candiru antigenic complex (Bunyaviridae: Phlebovirus), a highly diverse and reassorting group of viruses affecting humans in tropical America. J. Virol. 85:3811–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yanase T, Aizawa M, Kato T, Yamakawa M, Shirafuji H, Tsuda T. 2010. Genetic characterization of Aino and Peaton virus field isolates reveals a genetic reassortment between these viruses in nature. Virus Res. 153:1–7 [DOI] [PubMed] [Google Scholar]
- 13. Pavlov P, Rosický B, Hubálek Z, Daniel M, Bárdos V, Minár J, Juricová Z. 1978. Isolation of Bhanja virus from ticks of the genus Haemaphysalis in southeast Bulgaria and presence of antibodies in pastured sheep. Folia Parasitol. 25:67–73 [PubMed] [Google Scholar]
- 14. Butenko AM, Gromashevsky VL, Lvov DK, Popov VL. 1979. Kismayo virus—a representative of the Bhanja antigenic group. Vop. Virus. 24:661–665 [PubMed] [Google Scholar]
- 15. Boiro I, Lomonossov NN, Malenko GV, Balde C, Bah A. 1986. Forécariah virus, a new representative of the Bhanja antigenic group, isolated in the Republic of Guinea. Bull. Soc. Pathol. Exot. Filiales 79:183–186 (In French.) [PubMed] [Google Scholar]
- 16. Ungureanu A, Popovici V, Cătănaş F, Ioniţă I, Tutoveanu A, Safta M. 1990. Isolation of Bhanja virus in Romania. Arch. Roum. Pathol. Exp. Microbiol. 49:139–145 [PubMed] [Google Scholar]
- 17. Hubálek Z, Mittermayer T, Halouzka J, Cerný V. 1988. Isolation of “exotic” Bhanja virus (Bunyaviridae) from ticks in the temperate zone. Arch. Virol. 101:191–197 [DOI] [PubMed] [Google Scholar]
- 18. Hubálek Z, Mittermayer T, Halouzka J. 1987. Bhanja virus (Bunyaviridae) isolated from Dermacentor marginatus ticks in Czechoslovakia. Acta Virol. 32:526 [Google Scholar]
- 19. Hubálek Z. 1987. Geographic distribution of Bhanja virus. Folia Parasitol. 34:77–86 [PubMed] [Google Scholar]
- 20. Shah KV, Work TH. 1969. Bhanja virus: a new arbovirus from ticks Haemaphysalis intermedia Warburton and Nuttall, 1909, in Orissa, India. Indian J. Med. Res. 57:793–798 [PubMed] [Google Scholar]
- 21. Verani P, Balducci M, Lopes MC, Sacca G. 1970. Isolation of Bhanja virus from Haemaphysalis ticks in Italy. Am. J. Trop. Med. Hyg. 19:103–105 [DOI] [PubMed] [Google Scholar]
- 22. Zakaryan VA, Gromashevsky VL, Chubkova AI, Akopyan GS, Skvortseva TM. 1974. Isolation of Bhanja virus from Dermacentor marginatus Suiz (1776) ticks in Armenian SSR. Sborn. Trud. Ekol. Virus. 2:82–84 [Google Scholar]
- 23. Vesenjak-Hirjan J, Calisher CH, Brudnjak Z, Tovornik D, Skrtic N, Lazuick JS. 1977. Isolation of Bhanja virus from ticks in Yugoslavia. Am. J. Trop. Med. Hyg. 26:1003–1008 [DOI] [PubMed] [Google Scholar]
- 24. Punda V, Beus I, Calisher CH, Vesenjak-Hirjan J. 1980. Laboratory infections with Bhanja virus. Zentralbl. Bakteriol. Suppl 9:273–275 [Google Scholar]
- 25. Calisher CH, Goodpasture HC. 1975. Human infection with Bhanja virus. Am. J. Trop. Med. Hyg. 24:1040–1042 [DOI] [PubMed] [Google Scholar]
- 26. Vesenjak-Hirjan J, Calisher CH, Beus I, Marton E. 1980. First natural clinical human Bhanja virus infection, p 297–301 In Vesenjak-Hirjan J, Porterfield JS, Arslanagić E. (ed), Arboviruses in the Mediterranean countries: 6th FEMS symposium Fischer, Stuttgart, Germany [Google Scholar]
- 27. Punda V, Ropac D, Vesenjak-Hirjan J. 1987. Incidence of hemagglutination-inhibiting antibodies for Bhanja virus in humans along the north-west border of Yugoslavia. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 265:227–234 [DOI] [PubMed] [Google Scholar]
- 28. Mittermayer T, Hubálek Z. 1986. Antibodies against the Bhanja virus in agricultural workers in Eastern Slovakia. Cesk. Epidemiol. Mikrobiol. Imunol. 35:355–359 (In Slovak.) [PubMed] [Google Scholar]
- 29. Theiler M, Downs WG. 1973. The arthropod-borne viruses of vertebrates, 1st ed, p 316–338 Yale University Press, New Haven, CT [Google Scholar]
- 30. Filipe AR, Alves MJ, Karabatsos N, de Matos AP, Núncio MS, Bacellar F. 1994. Palma virus, a new bunyaviridae isolated from ticks in Portugal. Intervirology 37:348–351 [DOI] [PubMed] [Google Scholar]
- 31. Beaty BJ, Calisher CH, Shope RE. 1989. Arboviruses, p 797–855 In Schmidt NJ, Emmons RW. (ed), Diagnostic procedures for viral, rickettsial and chlamydial infections, 6th ed American Public Health Association, Washington, DC [Google Scholar]
- 32. Travassos da Rosa AP, Tesh RB, Pinheiro FP, Travassos da Rosa JF, Peterson NE. 1983. Characterization of eight new phlebotomus fever serogroup arboviruses (Bunyaviridae: Phlebovirus) from the Amazon region of Brazil. Am. J. Trop. Med. Hyg. 32:1164–1171 [DOI] [PubMed] [Google Scholar]
- 33. Howe D, Shannon JG, Winfree S, Dorward DW, Heinzen RA. 2010. Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect. Immun. 78:3465–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siebert PD, Chenchik A. 1993. Modified acid guanidinium thiocyanate-phenol-chloroform RNA extraction method which greatly reduces DNA contamination. Nucleic Acids Res. 21:2019–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palacios G, Quan P-L, Jabado OJ, Conlan S, Hirschberg DL, Liu Y, Zhai J, Renwick N, Hui J, Hegyi H, Grolla A, Strong JE, Towner JS, Geisbert TW, Jahrling PB, Büchen-Osmond C, Ellerbrok H, Sanchez-Seco MP, Lussier Y, Formenty P, Nichol MST, Feldmann H, Briese T, Lipkin WI. 2007. Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg. Infect. Dis. 13:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. 454 Life Sciences October 2009. Rapid library preparation method manual: GS FLX titanium series. Roche Diagnostics, Mannheim, Germany [Google Scholar]
- 37. Buchan DWA, Ward SM, Lobley AE, Nugent TCO, Bryson K, Jones DT. 2010. Protein annotation and modelling servers at University College London. Nucleic Acids Res. 38:W563–W568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones DT. 2007. Improving the accuracy of transmembrane protein topology prediction using evolutionary information. Bioinformatics 23:538–544 [DOI] [PubMed] [Google Scholar]
- 39. Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8:785–786 [DOI] [PubMed] [Google Scholar]
- 40. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palacios G, Savji N, Travassos da Rosa AP, Guzman H, Yu X-J, Desai A, Rosen GE, Hutchison S, Lipkin WI, Tesh RB. 2 January 2013. Characterization of the Uukuniemi virus group (Phlebovirus: Bunyaviridae): evidence for seven distinct species. J. Virol. [Epub ahead of print.] doi:10.1128/JVI.02719-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kohl A, Dunn EF, Lowen AC, Elliott RM. 2004. Complementarity, sequence and structural elements within the 3′ and 5′ non-coding regions of the Bunyamwera orthobunyavirus S segment determine promoter strength. J. Gen. Virol. 85:3269–3278 [DOI] [PubMed] [Google Scholar]
- 43. Emery VC, Bishop DH. 1987. Characterization of Punta Toro S mRNA species and identification of an inverted complementary sequence in the intergenic region of Punta Toro phlebovirus ambisense S RNA that is involved in mRNA transcription termination. Virology 156:1–11 [DOI] [PubMed] [Google Scholar]
- 44. Giorgi C, Accardi L, Nicoletti L, Gro MC, Takehara K, Hilditch C, Morikawa S, Bishop DH. 1991. Sequences and coding strategies of the S RNAs of Toscana and Rift Valley fever viruses compared to those of Punta Toro, Sicilian sandfly fever, and Uukuniemi viruses. Virology 180:738–753 [DOI] [PubMed] [Google Scholar]
- 45. Andersson AM, Melin L, Persson R, Raschperger E, Wikström L, Pettersson RF. 1997. Processing and membrane topology of the spike proteins G1 and G2 of Uukuniemi virus. J. Virol. 71:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gerrard SR, Nichol ST. 2002. Characterization of the Golgi retention motif of Rift Valley fever virus GN glycoprotein. J. Virol. 76:12200–12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Overby AK, Popov VL, Pettersson RF, Neve EPA. 2007. The cytoplasmic tails of Uukuniemi virus (Bunyaviridae) GN and GC glycoproteins are important for intracellular targeting and the budding of virus-like particles. J. Virol. 81:11381–11391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Raymond DD, Piper ME, Gerrard SR, Smith JL. 2010. Structure of the Rift Valley fever virus nucleocapsid protein reveals another architecture for RNA encapsidation. Proc. Natl. Acad. Sci. U. S. A. 107:11769–11774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferron F, Li Z, Danek EI, Luo D, Wong Y, Coutard B, Lantez V, Charrel R, Canard B, Walz T, Lescar J. 2011. The hexamer structure of Rift Valley fever virus nucleoprotein suggests a mechanism for its assembly into ribonucleoprotein complexes. PLoS Pathog. 7:e1002030 doi:10.1371/journal.ppat.1002030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qu B, Qi X, Wu X, Liang M, Li C, Cardona CJ, Xu W, Tang F, Li Z, Wu B, Powell K, Wegner M, Li D, Xing Z. 2012. Suppression of the interferon and NF-κB responses by severe fever with thrombocytopenia syndrome virus. J. Virol. 86:8388–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Billecocq A, Spiegel M, Vialat P, Kohl A, Weber F, Bouloy M, Haller O. 2004. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J. Virol. 78:9798–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dilcher M, Alves MJ, Finkeisen D, Hufert F, Weidmann M. 2012. Genetic characterization of Bhanja virus and Palma virus, two tick-borne phleboviruses. Virus Genes 45:311–315 [DOI] [PubMed] [Google Scholar]
- 53. Müller R, Poch O, Delarue M, Bishop DH, Bouloy M. 1994. Rift Valley fever virus L segment: correction of the sequence and possible functional role of newly identified regions conserved in RNA-dependent polymerases. J. Gen. Virol. 75(Part 6):1345–1352 [DOI] [PubMed] [Google Scholar]
- 54. Katz A, Freiberg AN, Backström V, Holm L, Vaheri A, Flick R, Plyusnin A. 2012. Mutational analysis of positively charged amino acid residues of Uukuniemi phlebovirus nucleocapsid protein. Virus Res. 167:118–123 [DOI] [PubMed] [Google Scholar]
- 55. Marklewitz M, Handrick S, Grasse W, Kurth A, Lukashev A, Drosten C, Ellerbrok H, Leendertz FH, Pauli G, Junglen S. 2012. Gouleako virus isolated from West African mosquitoes constitutes a proposed novel genus in the family Bunyaviridae. 85:9227–9234 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.