Abstract
The conserved alphaherpesviral serine/threonine kinase US3 causes dramatic actin rearrangements, associated with increased viral spread. Here, we show that US3 of pseudorabies virus (PRV) leads to activation (dephosphorylation) of the central actin regulator cofilin. A mutation that impairs US3 kinase activity and the group I p21-activated kinase inhibitor IPA-3 inhibited US3-mediated cofilin activation. Additionally, expression of phosphomimetic S3D cofilin significantly suppressed the ability of US3 to cause cell projections and cell rounding. In conclusion, the US3 kinase of PRV leads to activation (dephosphorylation) of cofilin, and cofilin contributes to US3-mediated actin rearrangements.
TEXT
The US3 kinase is conserved among Alphaherpesvirinae. We and others have shown that this kinase induces dramatic rearrangements of the actin cytoskeleton, including disassembly of actin stress fibers and formation of cellular projections, which are associated with increased viral spread in cell culture (1–7). For the alphaherpesvirus pseudorabies virus (PRV), we previously reported that the US3-induced changes in the actin cytoskeleton are mediated through p21-activated kinases (PAKs), central regulators in RhoGTPase signaling (8). Apart from the involvement of PAKs, relatively little is known about the factors contributing to US3-mediated actin rearrangements.
Cofilin, a member of the actin depolymerizing factor (ADF)/cofilin family, is a central player in actin dynamics known to be activated through dephosphorylation on serine residue 3 (S3) (9). Phosphorylation and dephosphorylation of cofilin at S3 is complexly regulated by multiple kinases and phosphatases (10). Increasing evidence indicates that cofilin constitutes an important cellular target affected by both bacterial and viral infections (11–15). With regard to alphaherpesviruses, herpes simplex virus 1 (HSV-1) has been reported to induce a cell-type-dependent upregulation of cofilin levels and modulation of cofilin activity (16, 17). This may affect viral replication, although the underlying mechanism is unclear (15, 16). The best-characterized viral modulation of cofilin activity has been documented for HIV, which triggers cofilin S3 phosphorylation and thus inactivation through gp120-mediated activation of the Rac-PAK-LIMK pathway, which is involved in initiation of infection of CD4 T cells (17). HIV Nef also leads to cofilin inactivation through the activity of PAK2, thereby restricting migration of infected T lymphocytes (14). On the other hand, HIV-mediated activation of cofilin has also been described to affect initiation of infection (17–19).
In the current report, we investigated whether the US3 protein of the alphaherpesvirus PRV affects cofilin phosphorylation, and, if so, whether this contributes to the US3-mediated effects on the actin cytoskeleton.
US3 is required for PRV-mediated suppression of cofilin phosphorylation.
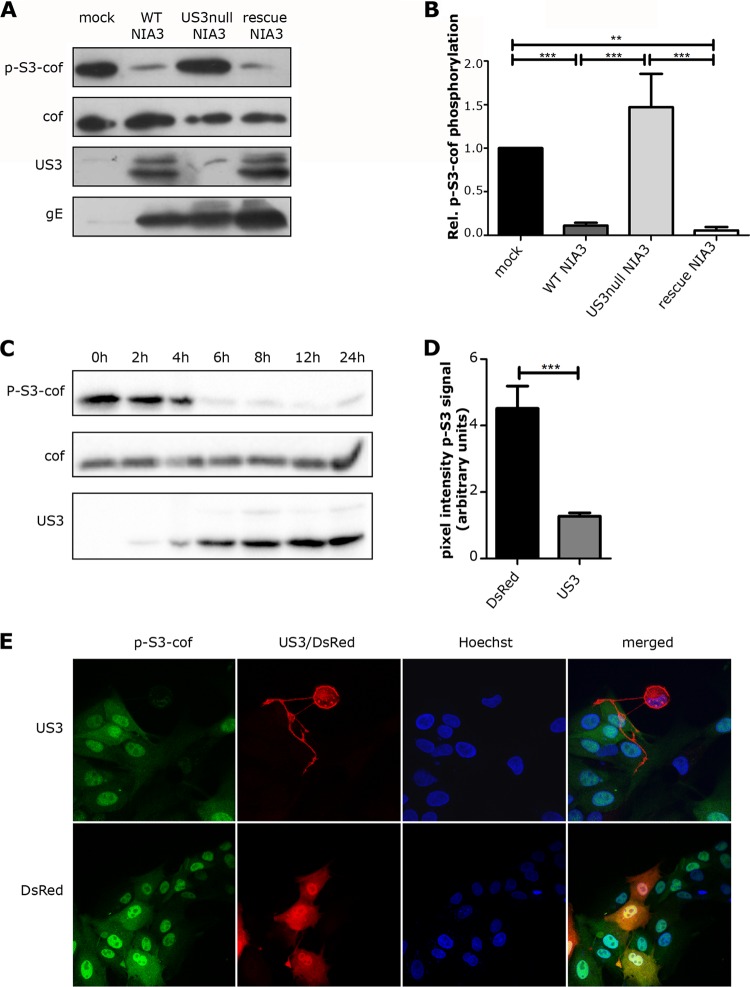
We determined whether US3 modulates the activity of cofilin through altered phosphorylation at the critical S3 residue in cofilin. ST cells (seeded at 150,000 cells/ml, cultured as described in reference 20) were inoculated with the previously described isogenic NIA3 strains wild-type (WT) PRV or US3null PRV (containing a translational stop codon in US3) or a revertant virus of the latter (21). At 6 h postinoculation (hpi), cells were subjected to Western blotting (WB). Antibodies used were directed against S3 phospho-cofilin (Santa Cruz; sc-12912), total cofilin (Santa Cruz; sc-42824), US3 (kindly provided by LeighAnne Olsen and Lynn Enquist, Princeton University), and the viral membrane protein gE (18E8) (22). Band intensity was measured with the “Analyze gels” option in ImageJ, and phospho-S3 cofilin levels were normalized to mock levels. Figure 1 shows that, compared to mock-infected ST cells, WT and US3rescue PRV infection led to a strong decrease in S3 cofilin phosphorylation, in contrast to US3null PRV (Fig. 1A and B). Phospho-S3 cofilin levels in US3null PRV-infected cells were even increased, albeit not significantly, compared to those of mock-infected cells. In line with the early kinetics of US3 expression, the decrease in phospho-S3 cofilin could be observed already early in infection (from 4 hpi onward) (Fig. 1C). The ability of US3 to modulate cofilin activity levels is underscored by the fact that transfection of a WT US3-encoding construct in ST cells was sufficient to suppress phospho-S3 cofilin levels, as shown in Fig. 1D and E. Transfection with a control plasmid encoding red fluorescent protein DsRed (kindly provided by R. Y. Tsien, UCSD, La Jolla, CA) was used as a control (23). Hence, US3 leads to suppressed phospho-S3 cofilin levels in infected and transfected ST cells.
Fig 1.
PRV infection leads to a US3-dependent suppression in S3 cofilin phosphorylation. (A) ST cells were mock inoculated or inoculated (MOI of 10) with WT PRV, US3null PRV, or US3rescue PRV. At 6 hpi, total cell lysates were subjected to Western blotting to detect phospho-S3 cofilin, total cofilin, US3, and gE. (B) Relative cofilin phosphorylation levels based on the phospho-S3 cofilin/cofilin ratio (with mock infection set to 1) are represented as means + standard errors of the means of data from three independent experiments, with ** indicating P values of <0.01 and *** indicating P values of <0.001. (C) ST cells were inoculated with WT PRV (MOI of 10) and lysed at 0 h, 2 h, 4 h, 6 h, 8 h, 12 h, or 24 h postinfection. Total cell lysates were subjected to Western blotting to detect phospho-S3 cofilin, total cofilin, and US3. (D and E) ST cells were transfected with US3 or with a control plasmid encoding DsRed (23) and stained for US3 and phospho-S3 cofilin. Panel D shows quantification of fluorescein isothiocyanate (FITC) (p-S3-cof) pixel intensities of 8 randomly chosen US3- or control plasmid-transfected cells, which were determined using ImageJ. Data shown represent means + standard errors of the means, with * indicating P values of <0.05.
The kinase activity of US3 is required to suppress phosphorylation of cofilin.
To assess the involvement of the kinase activity of US3 in suppressing cofilin phosphorylation in ST cells, cells were inoculated with a previously described PRV strain expressing a kinase-inactive US3 protein, containing a point mutation (D223A) in the catalytic base required for phosphotransfer (PRV-GS976) (24, 25). At 6 hpi, phospho-S3 cofilin, total cofilin, US3, and gE levels were evaluated by WB (Fig. 2A and B). The PRV strain Becker expressing a kinase-inactive US3, unlike isogenic wild-type PRV (multiplicity of infection [MOI] of 10; PRV-GS847), did not suppress phospho-S3 levels of cofilin. A rescue strain in which the D223A mutation in US3 was restored (PRV-GS3000) (24, 25) acted like the WT virus and induced a strong suppression in cofilin phosphorylation. As observed for US3null PRV (Fig. 1), infection with PRV encoding kinase-inactive US3 resulted in increased phosphorylation of cofilin compared to that of mock-infected cells. Hence, the ability of US3 to suppress S3 phosphorylation of cofilin in ST cells relies on its kinase activity.
Fig 2.
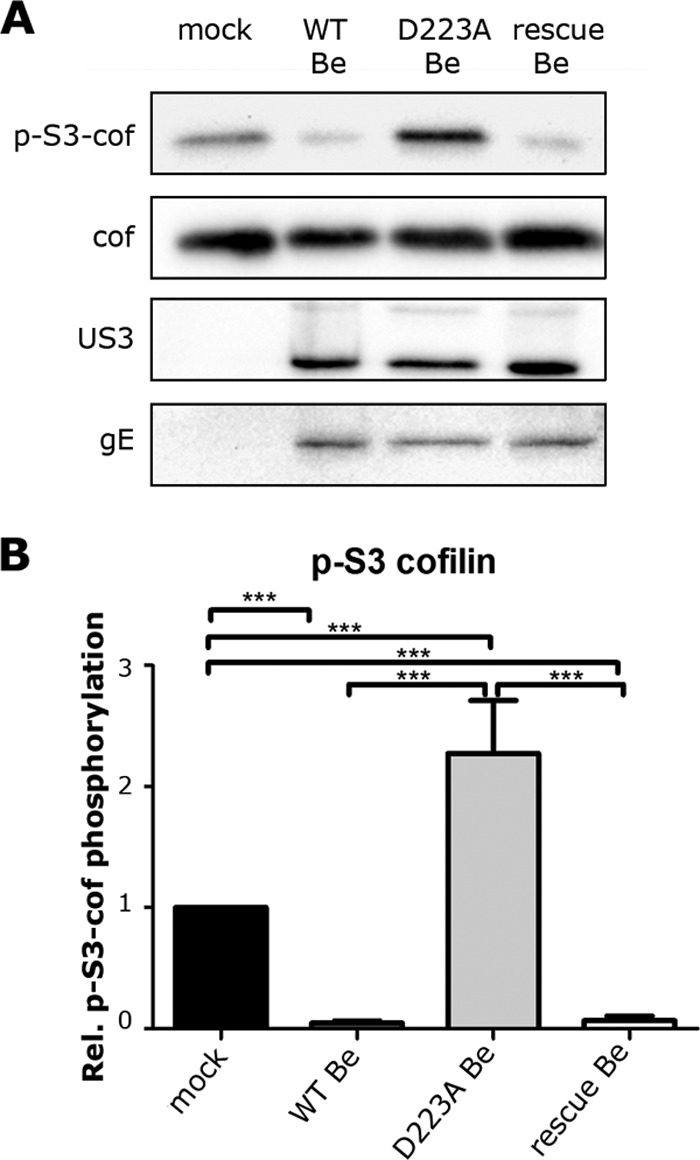
The kinase activity of US3 is required to suppress phosphorylation of cofilin. (A) ST cells were mock inoculated or inoculated (MOI of 10) with WT PRV, kinase-inactive D223A US3 PRV, or D223Arescue PRV. At 6 hpi, total cell lysates were subjected to Western blotting to detect phospho-S3 cofilin, total cofilin, US3, and gE. (B) Means + standard errors of relative cofilin phosphorylation levels from three independent experiments, with *** indicating P values of <0.001.
Interestingly, infection with US3null PRV or D223A US3 PRV resulted in increased phospho-S3 cofilin levels compared to those of mock-infected cells (Fig. 1 and 2). One hypothetical way to explain this may be that infection leads to cofilin inactivation (S3 phosphorylation) and that US3 activity counteracts this and even reduces phospho-S3 cofilin levels below normal levels. Why would infection lead to increased phospho-S3 cofilin levels? Viral infection is known to lead to a stress response in cells (26–28), which may perhaps be involved in increased phosphorylation of cofilin. Indeed, other cellular stress stimuli have been reported to lead to increased S3 cofilin phosphorylation, including heat shock (29), fluid shear stress (30, 31), and scavenging of reactive oxygen species (32). It will be interesting to investigate the potential biological consequences of increased levels of phospho-S3 cofilin during US3null PRV infection for both virus and cell.
A constitutively inactive, S3D phosphomimetic cofilin variant interferes with US3-mediated cell rounding and cell projections.
The experiments described above indicate that US3 leads to substantial S3 cofilin dephosphorylation, a hallmark of cofilin activation (9). If this cofilin activation is important for PRV US3-induced actin rearrangements, one would expect that overexpression of a constitutively inactive (phosphomimetic) S3D cofilin mutant will interfere with US3-mediated actin rearrangements, whereas overexpression of wild-type cofilin or a constitutively active S3A cofilin mutant should not. Likewise, overexpression of S3D (but not S3A) cofilin has been reported to suppress the formation of long actin-containing dendritic cell protrusions in hippocampal neurons (33).
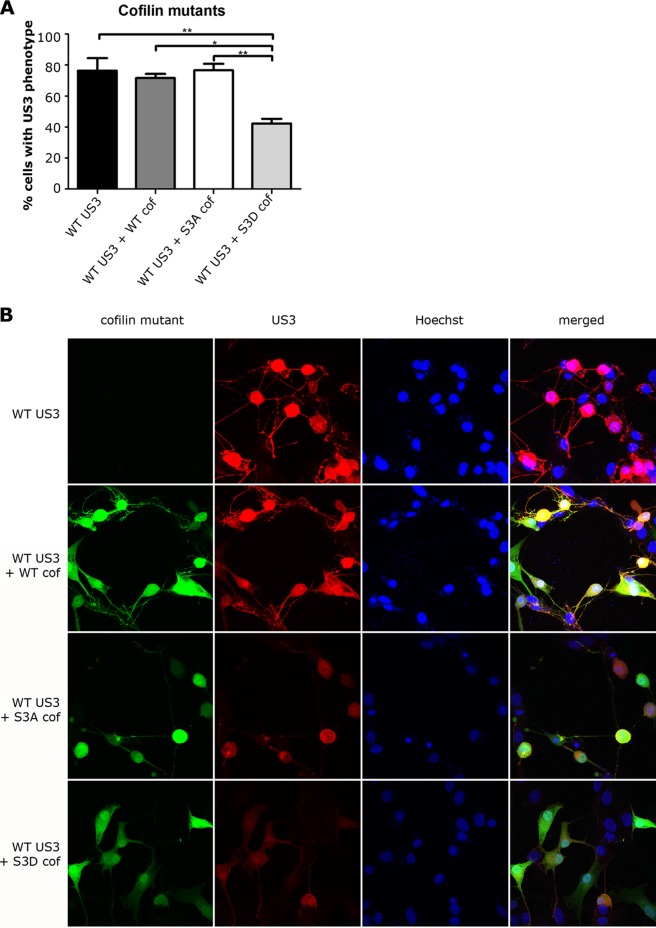
To assess this, ST cells were cotransfected with US3 and constructs expressing previously described green fluorescent protein (GFP) fusions of wild-type cofilin, S3D cofilin, or S3A cofilin (34). At 24 h posttransfection, cells were stained with anti-US3 antibody and scored for US3-mediated effects on the actin cytoskeleton. In brief, 200 randomly chosen transfected cells per condition were scored for cell rounding (actin stress fiber disassembly) and cell projection formation. Phosphomimetic S3D cofilin, but not wild-type or S3A cofilin, significantly suppressed the ability of US3 to induce actin rearrangements in ST cells (Fig. 3). Overexpression of either WT or S3D cofilin on itself did not cause apparent changes in cell morphology. Overexpression of S3A cofilin on itself did not lead to obvious cell rounding but did induce cell projections that were shorter and less branched than observed upon transfection of US3 (data not shown). Notwithstanding the apparent colocalization of cofilin with US3 in some of the immunofluorescence images, immunoprecipitation experiments were not indicative for a direct interaction between US3 and cofilin (data not shown). In conclusion, expression of phosphomimetic S3D cofilin in ST cells interferes with the ability of US3 to induce actin rearrangements.
Fig 3.
Overexpression of S3D phosphomimetic cofilin interferes with the ability of US3 to cause cell rounding and cell projections. (A and B) ST cells were transfected with US3 encoding plasmid or cotransfected with plasmids encoding US3 and GFP-tagged WT cofilin, S3A cofilin, or S3D cofilin. At 24 h posttransfection, cells were fixed and stained for US3 and nuclei and analyzed for expression of cofilin (GFP; green) and US3 (red). Panel A shows the percentage of transfected cells displaying actin rearrangements, as assessed by cell rounding and the formation of cell projections (means + standard errors of the means; data from three independent experiments), with * indicating P values of <0.05 and ** indicating P values of <0.01. Small blue dots in panel B represent leftover plasmid DNA-containing transfection reagent in cells and on the cover glass.
Group I PAKs are involved in the US3-mediated dephosphorylation of cofilin.
The ability of PRV US3 to induce actin rearrangements has been shown to depend on the ability of US3 to phosphorylate and thereby activate group I PAKs (8). As a consequence, the group I PAK inhibitor IPA-3 is able to inhibit US3-mediated actin rearrangements in ST cells (25, 35). We investigated whether IPA-3 is also capable of reverting the observed US3-mediated suppression of S3 cofilin phosphorylation. To this end, ST cells were either mock inoculated or inoculated with WT PRV in the absence or presence of 33 μM IPA-3, used as described before (21). At 6 hpi, cells were lysed and phospho-S3 cofilin, total cofilin, and US3 levels were evaluated. The addition of IPA-3 restored the phospho-S3 cofilin signal in PRV-infected cells (Fig. 4B), while it did not influence phospho-S3 cofilin levels in mock-infected cells (Fig. 4A). Hence, the use of an inhibitor of group I PAK activity in ST cells interferes with the US3-mediated suppression of S3 cofilin phosphorylation.
Fig 4.
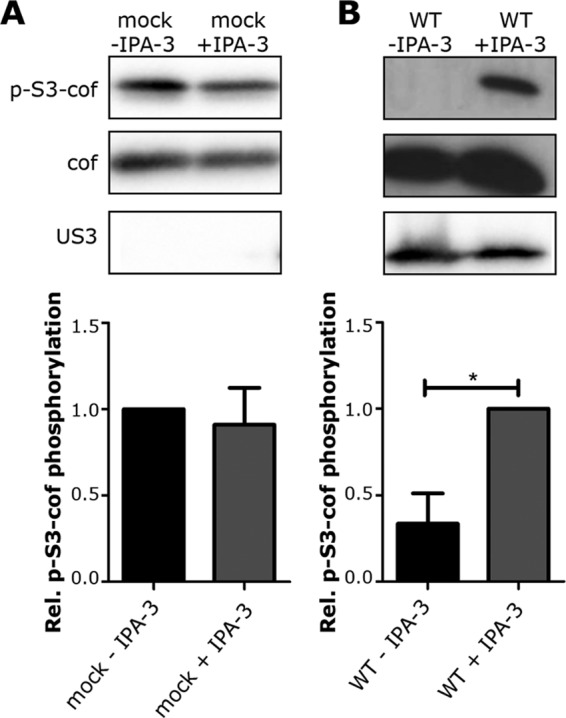
Group I PAKs are involved in US3-mediated suppression of S3 cofilin phosphorylation. (A and B) ST cells treated with or without 33 μM group I PAK inhibitor IPA-3 were either mock inoculated (A) or inoculated with WT PRV (B). At 6 hpi, total cell lysates were subjected to Western blotting to detect phospho-S3 cofilin, total cofilin, and US3. Values were normalized to mock (A) or to PRV and IPA-3 (B). The graphs represent the means + standard errors of the means from three independent experiments, with * indicating P values of <0.05.
This is in apparent contradiction with studies in HIV, where virus-induced activation of PAK2 (a group I PAK member) leads to S3 cofilin phosphorylation and thus inactivation in Jurkat cells (14, 17, 36). Nevertheless, ambiguity exists in the literature as to whether PAK activation leads to cofilin phosphorylation or dephosphorylation at S3. On the one hand, PAK activity has been associated with cofilin phosphorylation, mainly because LIM kinase isoforms are important downstream substrates of PAK that can lead to phosphorylated cofilin (37–40). On the other hand, more recently, increasing evidence indicates that group I PAK activity may also signal to several of the phosphatases, like PP2A, chronophin (CIN), and/or the slingshot (SSH) family, that are known to dephosphorylate and activate cofilin (41–44). Most likely, cell-type-specific or environmental factors may influence the outcome of PAK activation on cofilin activity (35, 45–48). Future research aimed at further dissecting the mechanistic details of US3-PAK-mediated cofilin dephosphorylation will further clarify the other molecular players in this pathway and may therefore generate important cell biological insights on PAK-mediated cofilin regulation.
ACKNOWLEDGMENTS
Thary Jacob is supported by a Ph.D. grant from the Agency for Innovation by Science and Technology in Flanders (IWT-Vlaanderen).
We thank C. Van Waesberghe for technical assistance, L. A. Olsen and L. Enquist (Princeton University) for the mouse monoclonal anti-US3 antibodies, G. A. Smith (Northwestern University) for PRV strains GS847 and GS976, the ID-DLO (The Netherlands) for PRV NIA-3 strains, R. Tsien (UCSD, La Jolla, CA) for the DsRed plasmid, and J. Chernoff (Fox Chase Cancer Center) for IPA-3.
Footnotes
Published ahead of print 30 January 2013
REFERENCES
- 1. Calton CM, Randall JA, Adkins MW, Banfield BW. 2004. The pseudorabies virus serine/threonine kinase Us3 contains mitochondrial, nuclear and membrane localization signals. Virus Genes 29:131–145 [DOI] [PubMed] [Google Scholar]
- 2. Favoreel HW, Van Minnebruggen G, Adriaensen D, Nauwynck HJ. 2005. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc. Natl. Acad. Sci. U. S. A. 102:8990–8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finnen RL, Roy BB, Zhang H, Banfield BW. 2010. Analysis of filamentous process induction and nuclear localization properties of the HSV-2 serine/threonine kinase Us3. Virology 397:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ladelfa MF, Kotsias F, Del Medico Zajac MP, Van den Broeke C, Favoreel H, Romera SA, Calamante G. 2011. Effect of the US3 protein of bovine herpesvirus 5 on the actin cytoskeleton and apoptosis. Vet. Microbiol. 153:361–366 [DOI] [PubMed] [Google Scholar]
- 5. Murata T, Goshima F, Daikoku T, Takakuwa H, Nishiyama Y. 2000. Expression of herpes simplex virus type 2 US3 affects the Cdc42/Rac pathway and attenuates c-Jun N-terminal kinase activation. Genes Cells 5:1017–1027 [DOI] [PubMed] [Google Scholar]
- 6. Schumacher D, Tischer BK, Trapp S, Osterrieder N. 2005. The protein encoded by the US3 orthologue of Marek's disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J. Virol. 79:3987–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Minnebruggen G, Favoreel HW, Jacobs L, Nauwynck HJ. 2003. Pseudorabies virus US3 protein kinase mediates actin stress fiber breakdown. J. Virol. 77:9074–9080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van den Broeke C, Radu M, Deruelle M, Nauwynck H, Hofmann C, Jaffer ZM, Chernoff J, Favoreel HW. 2009. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc. Natl. Acad. Sci. U. S. A. 106:8707–8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moriyama K, Iida K, Yahara I. 1996. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells 1:73–86 [DOI] [PubMed] [Google Scholar]
- 10. Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C. 2008. Ins and outs of ADF/cofilin activity and regulation. Eur. J. Cell Biol. 87:649–667 [DOI] [PubMed] [Google Scholar]
- 11. Berkova Z, Crawford SE, Blutt SE, Morris AP, Estes MK. 2007. Expression of rotavirus NSP4 alters the actin network organization through the actin remodeling protein cofilin. J. Virol. 81:3545–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han X, Yu R, Ji L, Zhen D, Tao S, Li S, Sun Y, Huang L, Feng Z, Li X, Han G, Schmidt M, Han L. 2011. InlB-mediated Listeria monocytogenes internalization requires a balanced phospholipase D activity maintained through phospho-cofilin. Mol. Microbiol. 81:860–880 [DOI] [PubMed] [Google Scholar]
- 13. Moffatt CE, Inaba H, Hirano T, Lamont RJ. 2012. Porphyromonas gingivalis SerB-mediated dephosphorylation of host cell cofilin modulates invasion efficiency. Cell Microbiol. 14:577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stolp B, Reichman-Fried M, Abraham L, Pan X, Giese SI, Hannemann S, Goulimari P, Raz E, Grosse R, Fackler OT. 2009. HIV-1 Nef interferes with host cell motility by deregulation of Cofilin. Cell Host Microbe 6:174–186 [DOI] [PubMed] [Google Scholar]
- 15. Xiang Y, Zheng K, Ju H, Wang S, Pei Y, Ding W, Chen Z, Wang Q, Qiu X, Zhong M, Zeng F, Ren Z, Qian C, Liu G, Kitazato K, Wang Y. 2012. Cofilin 1-mediated biphasic F-actin dynamics of neuronal cells affect herpes simplex virus 1 infection and replication. J. Virol. 86:8440–8451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pei Y, Xiang YF, Chen JN, Lu CH, Hao J, Du Q, Lai CC, Qu C, Li S, Ju HQ, Ren Z, Liu QY, Xiong S, Qian CW, Zeng FL, Zhang PZ, Yang CR, Zhang YJ, Xu J, Kitazato K, Wang YF. 2011. Pentagalloylglucose downregulates cofilin1 and inhibits HSV-1 infection. Antiviral Res. 89:98–108 [DOI] [PubMed] [Google Scholar]
- 17. Vorster PJ, Guo J, Yoder A, Wang W, Zheng Y, Xu X, Yu D, Spear M, Wu Y. 2011. LIM kinase 1 modulates cortical actin and CXCR4 cycling and is activated by HIV-1 to initiate viral infection. J. Biol. Chem. 286:12554–12564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jimenez-Baranda S, Gomez-Mouton C, Rojas A, Martinez-Prats L, Mira E, Ana Lacalle R, Valencia A, Dimitrov DS, Viola A, Delgado R, Martinez AC, Manes S. 2007. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat. Cell Biol. 9:838–846 [DOI] [PubMed] [Google Scholar]
- 19. Yoder A, Yu D, Dong L, Iyer SR, Xu X, Kelly J, Liu J, Wang W, Vorster PJ, Agulto L, Stephany DA, Cooper JN, Marsh JW, Wu Y. 2008. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell 134:782–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geenen K, Favoreel HW, Olsen L, Enquist LW, Nauwynck HJ. 2005. The pseudorabies virus US3 protein kinase possesses anti-apoptotic activity that protects cells from apoptosis during infection and after treatment with sorbitol or staurosporine. Virology 331:144–150 [DOI] [PubMed] [Google Scholar]
- 21. Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. 2008. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 15:322–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nauwynck HJ, Pensaert MB. 1995. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch. Virol. 140:1137–1146 [DOI] [PubMed] [Google Scholar]
- 23. Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. U. S. A. 99:7877–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coller KE, Smith GA. 2008. Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus. Traffic 9:1458–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van den Broeke C, Deruelle M, Nauwynck HJ, Coller KE, Smith GA, Van Doorsselaere J, Favoreel HW. 2009. The kinase activity of pseudorabies virus US3 is required for modulation of the actin cytoskeleton. Virology 385:155–160 [DOI] [PubMed] [Google Scholar]
- 26. Clemens MJ. 2005. Translational control in virus-infected cells: models for cellular stress responses. Semin. Cell Dev. Biol. 16:13–20 [DOI] [PubMed] [Google Scholar]
- 27. Jindal S, Malkovsky M. 1994. Stress responses to viral infection. Trends Microbiol. 2:89–91 [DOI] [PubMed] [Google Scholar]
- 28. Santoro MG. 1996. Viral infection. EXS 77:337–357 [DOI] [PubMed] [Google Scholar]
- 29. Simard JP, Reynolds DN, Kraguljac AP, Smith GS, Mosser DD. 2011. Overexpression of HSP70 inhibits cofilin phosphorylation and promotes lymphocyte migration in heat-stressed cells. J. Cell Sci. 124:2367–2374 [DOI] [PubMed] [Google Scholar]
- 30. Fu Q, Wu C, Shen Y, Zheng S, Chen R. 2008. Effect of LIMK2 RNAi on reorganization of the actin cytoskeleton in osteoblasts induced by fluid shear stress. J. Biomech. 41:3225–3228 [DOI] [PubMed] [Google Scholar]
- 31. Liu YH, Li YR, Shao MF, Zhang XJ, Fu Q. 2010. Effects of cofilin phosphorylation on the actin cytoskeleton reorganization induced by shear stress. Zhonghua Kou Qiang Yi Xue Za Zhi 45:763–766 [PubMed] [Google Scholar]
- 32. Popova EN, Pletjushkina OY, Dugina VB, Domnina LV, Ivanova OY, Izyumov DS, Skulachev VP, Chernyak BV. 2010. Scavenging of reactive oxygen species in mitochondria induces myofibroblast differentiation. Antioxid. Redox Signal. 13:1297–1307 [DOI] [PubMed] [Google Scholar]
- 33. Pontrello CG, Sun MY, Lin A, Fiacco TA, DeFea KA, Ethell IM. 2012. Cofilin under control of beta-arrestin-2 in NMDA-dependent dendritic spine plasticity, long-term depression (LTD), and learning. Proc. Natl. Acad. Sci. U. S. A. 109:E442–E451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leyman S, Sidani M, Ritsma L, Waterschoot D, Eddy R, Dewitte D, Debeir O, Decaestecker C, Vandekerckhove J, van Rheenen J, Ampe C, Condeelis J, Van Troys M. 2009. Unbalancing the phosphatidylinositol-4,5-bisphosphate-cofilin interaction impairs cell steering. Mol. Biol. Cell 20:4509–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takuma T, Ichida T, Yokoyama N, Tamura S, Obinata T. 1996. Dephosphorylation of cofilin in parotid acinar cells. J. Biochem. 120:35–41 [DOI] [PubMed] [Google Scholar]
- 36. Stolp B, Abraham L, Rudolph JM, Fackler OT. 2010. Lentiviral Nef proteins utilize PAK2-mediated deregulation of cofilin as a general strategy to interfere with actin remodeling. J. Virol. 84:3935–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. 2006. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 25:713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li R, Soosairajah J, Harari D, Citri A, Price J, Ng HL, Morton CJ, Parker MW, Yarden Y, Bernard O. 2006. Hsp90 increases LIM kinase activity by promoting its homo-dimerization. FASEB J. 20:1218–1220 [DOI] [PubMed] [Google Scholar]
- 39. Scott RW, Olson MF. 2007. LIM kinases: function, regulation and association with human disease. J. Mol. Med. (Berl.) 85:555–568 [DOI] [PubMed] [Google Scholar]
- 40. Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. 2007. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J. Biol. Chem. 282:20634–20646 [DOI] [PubMed] [Google Scholar]
- 41. Coniglio SJ, Zavarella S, Symons MH. 2008. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol. Cell. Biol. 28:4162–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. 2004. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ. Res. 94:194–200 [DOI] [PubMed] [Google Scholar]
- 43. Nagata-Ohashi K, Ohta Y, Goto K, Chiba S, Mori R, Nishita M, Ohashi K, Kousaka K, Iwamatsu A, Niwa R, Uemura T, Mizuno K. 2004. A pathway of neuregulin-induced activation of cofilin-phosphatase Slingshot and cofilin in lamellipodia. J. Cell Biol. 165:465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oleinik NV, Krupenko NI, Krupenko SA. 2010. ALDH1L1 inhibits cell motility via dephosphorylation of cofilin by PP1 and PP2A. Oncogene 29:6233–6244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Davidson MM, Haslam RJ. 1994. Dephosphorylation of cofilin in stimulated platelets: roles for a GTP-binding protein and Ca2+. Biochem. J. 301(Pt 1):41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Okada K, Takano-Ohmuro H, Obinata T, Abe H. 1996. Dephosphorylation of cofilin in polymorphonuclear leukocytes derived from peripheral blood. Exp. Cell Res. 227:116–122 [DOI] [PubMed] [Google Scholar]
- 47. Samstag Y, Dreizler EM, Ambach A, Sczakiel G, Meuer SC. 1996. Inhibition of constitutive serine phosphatase activity in T lymphoma cells results in phosphorylation of pp19/cofilin and induces apoptosis. J. Immunol. 156:4167–4173 [PubMed] [Google Scholar]
- 48. Samstag Y, Eckerskorn C, Wesselborg S, Henning S, Wallich R, Meuer SC. 1994. Costimulatory signals for human T-cell activation induce nuclear translocation of pp19/cofilin. Proc. Natl. Acad. Sci. U. S. A. 91:4494–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]