Abstract
The apolipoprotein B editing complex 3 (APOBEC3) family of proteins is a group of intrinsic antiviral factors active against a number of retroviral pathogens, including HIV in humans and mouse mammary tumor virus (MMTV) in mice. APOBEC3 restricts its viral targets through cytidine deamination of viral DNA during reverse transcription or via deaminase-independent means. Here, we used virions from the mammary tissue of MMTV-infected inbred wild-type mice with different allelic APOBEC3 variants (APOBEC3BALB and APOBEC3BL/6) and knockout mice to determine whether cytidine deamination was important for APOBEC3's anti-MMTV activity. First, using anti-murine APOBEC3 antiserum, we showed that both APOBEC3 allelic variants are packaged into the cores of milk-borne virions produced in vivo. Next, using an in vitro deamination assay, we determined that virion-packaged APOBEC3 retains its deamination activity and that allelic differences in APOBEC3 affect the sequence specificity. In spite of this in vitro activity, cytidine deamination by virion-packaged APOBEC3 of MMTV early reverse transcription DNA occurred only at low levels. Instead, the major means by which in vivo virion-packaged APOBEC3 restricted virus was through inhibition of early reverse transcription in both cell-free virions and in vitro infection assays. Moreover, the different wild-type alleles varied in their ability to inhibit this step. Our data suggest that while APOBEC3-mediated cytidine deamination of MMTV may occur, it is not the major means by which APOBEC3 restricts MMTV infection in vivo. This may reflect the long-term coexistence of MMTV and APOBEC3 in mice.
INTRODUCTION
Organisms adapt to infectious agents by developing protective responses, and conversely, infectious agents develop adaptive countermeasures to these responses. Retroviruses are major causes of disease, such as cancer and acquired immunodeficiency in animals and humans, and are likely one of the infectious agents that puts selective pressure on host evolution. Because of the frequent encounter of vertebrates with retroviruses, selection for host antiviral defense systems is likely, and indeed, various host restriction factors have been identified (1). These include the TRIM proteins, Bst2/tetherin, and apolipoprotein B editing complex 3 (APOBEC3) proteins (2). Most of these antiviral intrinsic restriction factors were identified through the discovery of viral gene products that counteract their actions. In particular, the APOBEC3 proteins were discovered because human immunodeficiency virus (HIV), a retrovirus, encodes a protein termed viral infectivity factor (Vif) that blocks APOBEC3 antiviral activity (3).
Members of the APOBEC3 gene family encode DNA and RNA editing enzymes. The number of APOBEC3 genes varies from species to species, from 1 gene in rodents to 7 genes in humans (4). Two APOBEC3 proteins made in human cells, APOBEC3G and APOBEC3F, were first shown to inhibit HIV-1 lacking the vif gene (3, 5–8). In vif-deficient HIV-1 producer cells, APOBEC3G and APOBEC3F are packaged into progeny virions via interaction with the nucleocapsid (NC) protein and viral RNA. In cells infected with vif+ virus, Vif binds the APOBEC3 proteins and targets them for degradation or prevents their packaging through other means, thereby overcoming the antiviral activity (9).
Once packaged, APOBEC3 proteins inhibit HIV infection in target cells by deaminating deoxycytidine residues on the DNA minus strand following reverse transcription (RT), inducing hypermutation in newly synthesized retroviral DNA. The various human APOBEC3 proteins have different preferred target sequences; for example, APOBEC3G-mediated deamination occurs more frequently at CCC residues, whereas APOBEC3F preferentially deaminates TCC sites (5, 10, 11). APOBEC3 proteins also inhibit replication by undefined cytidine deaminase (CDA)-independent mechanisms (12). A number of in vitro studies have suggested that APOBEC3 proteins in particles can inhibit binding of the tRNALys3 primer to the viral RNA, RT-mediated elongation, and accumulation of HIV-1 reverse transcription products or integration into the host genome, at least in tissue culture cells (13–20). Although several studies have argued that CDA-independent inhibition is an artifact of APOBEC3 overexpression (21, 22), other studies have argued that it is biologically relevant (18, 20, 23), and recent work with mouse retroviruses indicates that CDA-independent inhibition occurs in vivo (see below).
Clearly, viruses that persist in their hosts cannot be totally restricted by antiviral host mechanisms. In support of this, we have shown that mouse APOBEC3 contributes to resistance to mouse mammary tumor virus (MMTV) infection but does not totally restrict infection. MMTV is an endemic milk-borne retrovirus that entered mice ∼10 to 20 million years ago and causes mammary carcinomas in female mice (24). Using APOBEC3 knockout mice, we provided the first demonstration that APOBEC3 proteins function in vivo by showing that APOBEC3−/− mice were more susceptible to MMTV infection than their wild-type (WT) littermates; virus spread and tumorigenesis were more rapid and extensive in the knockout mice (25). Moreover, we found no evidence of cytidine deamination of the MMTV genome in wild-type mice. Similarly, several studies have shown that APOBEC3 inhibits Moloney (M-MLV) and Friend (F-MLV) murine leukemia virus infection in vivo in the absence of mutations suggestive of cytidine deamination (26–29). However, mouse APOBEC3 retains deaminase activity on HIV substrates, with a preference for TCC targets (5), and has also been shown to have low activity on TTC residues in the endogenous murine leukemia virus AKV (AKV-MLV) (30).
Interestingly, there are allelic differences in mouse APOBEC3 among different inbred mouse strains that encode proteins with the ability to restrict MMTV, as well as F-MLV, to different extents (28, 31). In particular, C57BL/6 and BALB/c mice express different APOBEC3 variants, with 15 polymorphic amino acids in the proteins encoded in their genomes (see Fig. 2B). Moreover, the BALB/c allele (APOBEC3BALB) encodes a longer transcript and protein than that found in C57BL/6 mice (APOBEC3BL6) because it includes a fifth exon that is spliced out in the latter. Finally, APOBEC3BALB transcript and protein levels in BALB/c mice are significantly lower in various tissues than those of APOBEC3BL6 in C57BL/6 mice (28, 31–34). The APOBEC3BL6 allele encodes a more effective in vivo restriction factor of murine retroviruses. It is currently unclear as to whether the differential ability to restrict MLV or MMTV infection in vivo is due to the coding region changes that affect its antiviral activity or to expression level differences (31, 32, 34).
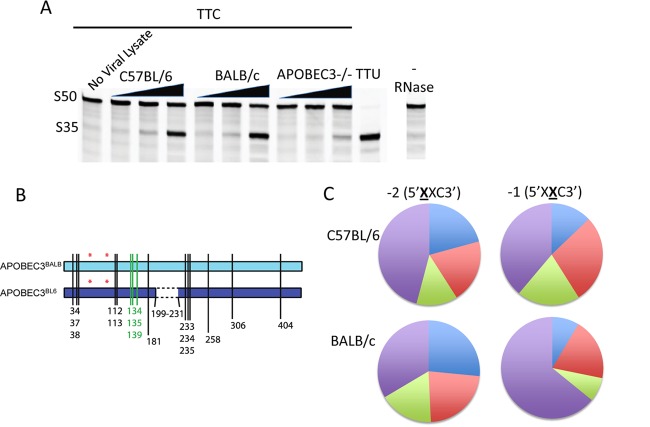
Fig 2.
In vivo-packaged APOBEC3 variants are enzymatically active and target different substrates. (A) Ten-fold increasing concentrations of virions isolated from C57BL/6, BALB, and APOBEC3−/− mice were lysed with 0.1% Triton-X in the presence or absence of RNase A and incubated with a 50mer single-stranded oligonucleotide (S50) containing a single cytosine and uracil DNA glycosylase, followed by cleavage with NaOH. An oligonucleotide containing a uracil confirmed complete cleavage of uracilated products and acted as a size standard (uncleaved substrate at S50, cleavage product at S35). C57BL/6 virions were also lysed in the absence of RNase A and subjected to otherwise identical conditions. (B) Representation of the two murine APOBEC3 variants. *, active site residue; vertical lines, locations of amino acid differences; green, differences within the substrate recognition loop; dotted line, deletion of exon 5. Numbering is relative to the APOBEC3BALB variant. (C) Virions isolated from C57BL/6 and BALB mice were incubated with 16 different substrates containing a single cytosine with variations of nucleotides in the −1 and −2 positions to include each possible combination of A, G, T, or mC. Product-to-substrate ratios were calculated for 3 to 4 replicate experiments. Blue, A; red, mC; green, G; purple, T. t tests were used to verify significant differences between the APOBEC variants' preferences for A (higher preference by APOBEC3BALB; P < 0.05) and T (higher preference by APOBEC3BL6; P = 0.05) at the −2 position and T at the −1 position (higher preference by APOBEC3BALB; P < 0.05).
Here we show that APOBEC3 packaged into virions retains its deamination activity and deaminates MMTV reverse transcripts at a low level. Interestingly, the APOBEC3BL6 and APOBEC3BALB variants packaged in MMTV virions have altered substrate preferences for deamination. In spite of the deamination activity, however, the major means by which mouse APOBEC3 inhibits MMTV is by blocking early reverse transcription.
MATERIALS AND METHODS
Plasmids.
Plasmids expressing Flag-tagged APOBEC3 (pFLAG-cytomegalovirus [CMV] vector) cloned from primary DNA of C57BL/6 and BALB/c mice and hemagglutinin (HA)-tagged catalytically inactive APOBEC3BL6 (E73Q/E253Q; pCMVpA vector) have been described (26, 28).
Mice.
MMTV (RIII)-infected APOBEC−/− mice and APOBEC+/+ mice were previously described (25). APOBEC−/− and APOBEC+/+ mice were crossed onto the C57BL/6 background, and thus, wild-type mice encode the APOBEC3BL6 allele. For genetic consistency, experiments used the two matched strains; however, wild-type animals are referred to as C57BL/6 in the text to reflect their APOBEC3 allele. Mice were housed according to the policies of the Institutional Animal Care and Use Committee of the University of Pennsylvania. Heterozygous F1 mice were created by crossing APOBEC−/− males with MMTV (RIII)-infected C57BL/6 or BALB/c females. The MMTV-infected female F1 mice were then bred, and virus was isolated from milk collected from their 1- to 2-day-old pups as previously described (35).
Cells and viruses.
293T cells stably expressing mTfR1 were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml Geneticin and have been previously described (36). These cells were stably transfected with APOBEC3 plasmids. Expression was verified by SDS-PAGE and Western blotting using anti-murine APOBEC3 (37), anti-Flag (Cell Signaling), and anti-HA (Abcam) antibodies. MMTV virions were isolated from mammary tumors or breast milk of MMTV-infected mice and purified via ultracentrifugation over a sucrose gradient from 0 to 60%. RNA purified from these virions was measured by reverse transcription-quantitative PCR (qPCR) for normalization. Viral cores were isolated by ultracentrifugation through 10% sucrose with 5% Triton-X on a 30% sucrose cushion.
In vitro deamination.
6-carboxyfluorescein (FAM)-labeled 50mer oligonucleotide substrates with a TTC target site were incubated with serial dilutions of tumor-derived virions. Virus was first lysed in buffer containing 10 μg/ml RNase A, 50 mM Tris (pH 8.0), 40 mM KCl, 50 mM NaCl, 5 mM EDTA, and 0.1% Triton X-100. Virus was then incubated with 1× uracil-DNA glycosylase (UDG) buffer (NEB), 2.5 U UDG (NEB), 5 mM EDTA, and 100 nM substrate at 30°C for 4 h. Samples were heated to 95°C for 20 min before addition of formamide load buffer and 0.1 M NaOH to cleave abasic sites. Separation of products on a 20% acrylamide/Tris-borate-EDTA (TBE)/urea gel was imaged via direct fluorescence on a Typhoon 9410 molecular imager. Control deamination reactions were carried out with purified mouse APOBEC1 or APOBEC3 (38). For targeting preference experiments, 60mer oligonucleotide substrates were labeled with ddUTP-FAM at their 3′ end using terminal deoxynucleoside triphosphate (dNTP) transferase (NEB) as directed (substrate sequences available upon request). Determination of targeting preferences was carried out as previously described (39).
In vitro reverse transcription.
Virions isolated as described above were normalized to equal RNA levels and incubated with phosphate-buffered saline (PBS), 2.5 mM MgCl2, 0.01% NP-40, and 1 mM dNTPs at 37°C. Reverse transcription products were isolated as previously described (20). Intracellular reverse transcription products were produced by infecting 293T-mTfR1 cells with tumor-derived virions in the presence of 8 μg/ml Polybrene. At the designated time points, medium and virus were removed, cells were washed with PBS, and DNA was purified using the DNeasy kit from Qiagen. To measure the effect of intracellular APOBEC3, the same protocol was followed using the 293T-mTfR1 cells stably transfected with APOBEC3BL6, APOBEC3BALB, or mutant (E73Q/E253Q) expression vectors.
PCR.
PCRs to amplify edited reverse transcription products were performed using a gradient cycler. Equal volumes of viral DNA were added to GoTaq DNA polymerase (Promega), and either a 200-bp region of env or a 480-bp region of the 3′ long terminal repeat (LTR) was amplified with the following primers: Env_Forward, 5′-GCC TCG AGC TAA GTA ACA CAG-3′; Env_Reverse, 5′-TCA GGG GCC AAT ACA AAA CTG GT-3′; LTR_Forward, 5′-CGT GAA AGA CTC GCC AGA GCT A-3′; LTR_Reverse, 5′-GAA GAT CTT CAA GGG CAA TGC CTT AA-3′.
A temperature gradient of 81 to 95°C was applied during an initial 5-min denaturing step, as well as during the 1-min denaturing step of each subsequent cycle (35 total), followed by 1 min at 55°C and 1 min at 72°C. DNA was purified from a 1% agarose gel using a Qiagen QIAquick gel extraction kit. Quantitative PCR for strong-stop DNA was performed in triplicate. DNA was combined with Power SYBR green PCR master mix and amplified under standard conditions on an ABI Prism 7900HT model.
Sequencing.
Following PCR and DNA isolation, the env and LTR fragments were cloned into pCR2.1-TOPO vector as directed (Invitrogen, Inc.). Clones were then sequenced using a BigDye Terminator v3.1 cycle sequencing kit from Applied Biosystems. Sequences were aligned using the ClustalW program, and G-to-A mutations were annotated by Hypermut (www.hiv.lanl.gov/content/sequence/HYPERMUT/hypermut.html).
Statistical analysis.
Statistical analysis was performed using the GraphPad/PRIZM software.
RESULTS
Both APOBEC3 variants are packaged into virion cores.
We first considered the possibility that APOBEC3 allelic variants may differ in the levels of protein incorporated into virus particles or in their localization within virions. Most APOBEC3 proteins are incorporated into virus particles during virion assembly in infected producer cells. Indeed, APOBEC3G binds to the HIV NC (8) and mouse APOBEC3 binds to the MMTV NC (25) as well as viral RNA, suggesting that upon viral maturation, the restriction factor is concentrated within the viral core. However, APOBEC3A is packaged into virions yet remains outside the viral core, preventing it from restricting HIV infection (40). If APOBEC3 was excluded from the MMTV cores, this could result in an inability to access the reverse transcription complex and cause cytidine deamination. Moreover, the changes in protein sequence may differentially alter packaging of the full-length APOBEC3BALB, thereby diminishing its ability to restrict infection.
To test this, we isolated virions from the mammary glands of MMTV-infected C57BL/6, APOBEC3 knockout (APOBEC3−/−), and BALB/c mice. We used reverse-transcribed real-time quantitative PCR primers specific to viral RNA to normalize virion levels in the three different virion preparations. In addition, Western blot analysis for both MMTV and APOBEC3 proteins was performed to confirm equal protein loading and to determine the relative level of packaged APOBEC3. APOBEC3 was detected in virions isolated from C57BL/6 and BALB/c mice but not APOBEC3−/− mice (Fig. 1A). The presence or absence of the fifth exon was visible by a 4-kDa size difference. BALB/c-produced virions packaged primarily the full-length version of the protein but also packaged a small quantity of the shorter version. C57BL/6-isolated virions packaged solely the isotype lacking exon 5 and contained a significantly higher level of packaged protein than did BALB/c virions. The level of virion-packaged protein reflected the intracellular levels of APOBEC3 mRNA in C57BL/6 and BALB/c mouse tissues as previously described (37).
Fig 1.

APOBEC3 is packaged within MMTV viral cores in vivo. (A) Virions were isolated from the mammary tissue of MMTV-infected C57BL/6, BALB, and APOBEC3−/− mice. Virus levels were normalized to viral RNA via RT-qPCR, and equal amounts were subjected to Western blot analysis using rabbit anti-mouse APOBEC3 antiserum. The immunoblot was stripped and reprobed with goat anti-Env (gp52) antiserum. C57BL/6 mice express and package solely a shorter form of the protein lacking a fifth exon. BALB mice express predominantly a protein including all exons (4 kDa larger) but also express a small amount of protein lacking this exon. The anti-APOBEC3 antiserum also detects a cross-reacting protein (nonspecific [NS]) of approximately 50 kDa that is present in some but not all virion preparations, as previously reported (37). (B) Core fractions and whole virus were assessed for APOBEC3 as described above, and the immunoblot was serially stripped and reprobed for MMTV Env and p27 as controls for removal of envelope and retention of viral cores. The p27 blot was exposed for 5 s and 1 min to visualize protein in both cores and whole virus.
We next isolated viral cores by centrifugation of purified virus through 10% sucrose containing 5% Triton-X over 30% sucrose cushions. The detergent treatment removes the viral envelope and contaminating cellular membrane compartments, including exosomes, and allows viral cores to pellet. Purified viral cores were then used for Western blotting. Both APOBEC3BL6 and APOBEC3BALB were incorporated within viral cores. Importantly, there was substantially more APOBEC3BL6 than APOBEC3BALB in cores (Fig. 1B), suggesting that the overall higher levels of APOBEC3BL6 in virions are not associated with mislocalized APOBEC3.
Allelic differences in substrate specificity.
A second possible explanation for the lack of cytidine deamination was that the APOBEC3 packaged into MMTV virions is rendered enzymatically inactive, perhaps by a viral protein. To determine if MMTV-packaged APOBEC3 retains its deaminase activity, we isolated virions from the mammary glands of MMTV-infected C57BL/6, APOBEC3 knockout, and BALB/c mice and examined the purified viral lysates for in vitro deaminase activity against a fluorescently tagged oligonucleotide substrate bearing the reported mouse APOBEC3 consensus target sequence (5′-TTC-3′) (30). Although C57BL/6-isolated virions packaged substantially more APOBEC3 than BALB/c virions (Fig. 1A), the protein packaged in BALB/c virions had activity equivalent to that of the protein packaged in BL/6 virions (Fig. 2A). A densitometry analysis measuring packaged APOBEC3 protein (Fig. 1A) and deamination on this substrate (Fig. 2A) revealed a 1.9-fold increase in activity of APOBEC3BALB compared to activity of APOBEC3BL6 when normalized to protein content. In contrast, very little deamination activity was detected in particles isolated from knockout mice: the small amount of deamination (<5% of virions containing APOBEC3BL6 or APOBEC3BALB) may be attributed to cellular contamination with other known mouse cytidine deaminases, such as APOBEC1 (41). The deaminase activity required RNase A treatment, demonstrating that like human APOBEC3G, mouse APOBEC3 is not enzymatically active when bound to RNA within the virion (Fig. 2A). These data suggested that both APOBEC3BL6 and APOBEC3BALB are functional deaminases that retain enzymatic activity in virions.
Different cellular cytidine deaminases can be distinguished from one another by their preferred editing contexts based on the nucleotides surrounding the targeted cytosine. This preference has been mapped to a recognition loop of 9 to 11 amino acids that interacts with nucleotides upstream of the target site (39). The two APOBEC3 isoforms differ in the presence of exon 5 and, in addition, have several amino acid differences in their other exons (Fig. 2B), including three polymorphisms in the putative substrate recognition loop which may influence the editing context. We thus examined if the nucleotide contexts of cytidine deaminase activity differed between each isoform. The in vitro deaminase assay was carried out with fluorescently labeled substrates bearing each of the 16 possible 5′-XXC-3′ combinations. To make the assay specific for deamination at the target cytosine, 5-methylcytosine (mC) was used in place of cytosine at the two upstream positions (38). The assay was performed with APOBEC3 isolated from virions as described above, and substrate-to-product ratios were measured (Fig. 2C). While APOBEC3BL6 exhibited a higher selectivity at the −2 position for the canonical TTC substrate, APOBEC3BALB had stronger preferences at the −1 position, with ATC as a favored substrate. These results imply a difference in activity that is likely due to a change in substrate recognition based on the allelic amino acid differences, not simply based on different levels of packaged protein.
APOBEC3 deamination of natural MMTV reverse transcripts is low.
Although the results of the preceding sections demonstrate that APOBEC3 packaged into MMTV virions retains functional deaminase activity, we previously showed that integrated MMTV proviruses found in wild-type mice showed no mutagenic hallmarks of cytidine deamination compared to knockout mice (25). However, uracil-containing reverse-transcribed DNA is able to be degraded in cells prior to integration into the host genome or prevented from integration (42), which would account for the absence of G-to-A mutations in integrated MMTV proviruses. We thus next tested whether ex vivo reverse-transcribed MMTV DNA made from viruses containing packaged APOBEC3 underwent cytidine deamination. Purified virions from C57BL/6, knockout, and BALB/c mice were used in endogenous reverse transcription assays (EnRT), and DNA produced by these particles was subjected to differential DNA denaturation PCR (3DPCR) using primers to two different regions of MMTV (3′ long terminal repeat [LTR] and envelope [env]) (Fig. 3A). PCR was performed using a gradient range of denaturing temperatures from 81 to 95°C. Amplification of products from all three types of virions was similar at melting temperatures from 85 to 95°C (3′ LTR amplification shown in Fig. 3A). When the PCR was carried out at higher stringency (≤83°C melting temperature for 3′ LTR, ≤85°C for env), reverse-transcribed DNA from either BALB/c or BL/6 virions was more highly amplified than that from APOBEC3−/− virions, indicating a higher AT content in reverse-transcribed DNA produced in the presence of either allele of APOBEC3.
Fig 3.
Packaged APOBEC3 edits MMTV reverse transcription products. (A) 3DPCR amplification of MMTV viral DNA isolated from EnRT reactions using virions from C57BL/6, BALB, and APOBEC3−/− mice. A ∼200-bp region from the 3′ LTR was amplified using an increasing denaturing temperate gradient (Td) from 81° to 95°. Densitometry was performed, and the density of each band was normalized to 95° (labeled as 100%). (B) 3DPCR amplification of MMTV viral DNA isolated 6 h after infection of 293T-mTfR1 cells with virions isolated from C57BL/6, BALB, and APOBEC−/− mice. A 480-bp region of env was amplified using an increasing denaturing gradient from 83° to 95°. (C) 3′ LTR DNA isolated from low- and high-denaturation-temperature PCRs shown in panel A was cloned and sequenced. These sequences were aligned, and G-to-A mutations were annotated using Hypermut compared to a reference MMTV RIII NCBI GenBank sequence (AF136898). A representative portion of these sequences is displayed. (D) All G-to-A mutations found in clonal sequences isolated from 3′LTR sequences (A) and env sequences (B) were analyzed for sequence context at the −1 and −2 nucleotide positions for the minus strand targeted cytosine.
To confirm these results in infection, we performed a similar experiment in 293T-mTfR1 cells (293T cells expressing the MMTV entry receptor transferrin receptor 1). The cells were infected with viruses isolated from the three strains of mice, and total cellular and viral DNA was isolated 6 h after infection, at which time preintegration reverse transcription products will predominate. This DNA was then amplified using 3DPCR. Similar to the in vitro reverse-transcribed DNA, MMTV reverse transcription products produced in the presence of either APOBEC3BALB or APOBEC3BL6 were amplified using highly stringent (low-denaturation-temperature) conditions, whereas APOBEC3−/− reverse-transcribed DNA was not amplified under these conditions (env amplification shown in Fig. 3B).
The PCR products generated in both cell-free and tissue culture infection conditions were cloned and sequenced. When the PCR was carried out at high stringency (95°C melting temperature), there were few G-to-A mutations in any of the viruses and there was no statistical difference in the rate of G-to-A mutations found in reverse transcription products of viral particles or in infected cells with or without packaged APOBEC3 (Table 1) (P = 0.17). However, low levels of cytidine deamination were detected in the APOBEC3BL6 and APOBEC3BALB reverse-transcribed DNA produced in both the in vitro endogenous reverse transcription assay (EnRT) and the tissue culture infection when amplified at high stringency (Fig. 3C; Table 1). This rate of deamination was statistically higher than that seen in products amplified at 95°C (P < 0.05). Even in the more-stringent conditions, which should enhance detection of G-to-A transitions, the overall rate of G-to-A mutation (0.47% for APOBEC3BL6, 0.41% for APOBEC3BALB) was significantly lower than that seen in viruses known to be disabled by APOBEC3 editing, such as HIV, which averages 7 to 8% G-to-A mutations in vivo (43). There was no difference in the frequency of mutations seen in reverse transcription products isolated from APOBEC3BALB- or APOBEC3BL6-containing particles (P = 0.67). This further supports our hypothesis that hypermutation is not a significant source of viral restriction, as the more restrictive APOBEC3BL6 variant did not show higher rates of mutagenesis. Some G-to-A mutations were detected from DNA isolated from reactions using APOBEC3−/− viral particles at both low- and high-stringency PCR, but the very low number can be attributed to RT error (not statistically different from no G-to-A mutations; P = 0.42).
Table 1.
Mutation analysis of MMTV reverse transcription productsa
| Genotype and temp (°C) | No. of mutations |
No. of clones | Total no. of bp | G-to-A mutation frequency | |
|---|---|---|---|---|---|
| G to A | Other | ||||
| C57BL/6, 95 | 28 | 20 | 50 | 13,236 | 0.21 |
| C57BL/6, 81–83 | 73 | 24 | 44 | 15,652 | 0.47 |
| BALB/c, 95 | 2 | 2 | 18 | 4,050 | 0.05 |
| BALB/c, 81–83 | 52 | 15 | 34 | 12,690 | 0.41 |
| APOBEC3−/−, 95 | 2 | 7 | 17 | 4,726 | 0.04 |
| APOBEC3−/−, 81–83 | 7 | 9 | 30 | 9,720 | 0.07 |
Sequences cloned from 3DPCR amplification products in Fig. 3A and B were aligned to their respective reference sequence. Rate of mutation (per 100 bp) for each condition was calculated for each of 3 to 5 sequencing runs. Unpaired, two-tailed t tests were performed using the mean values for each run. Sequences from C57BL6 viral DNA isolated using a low denaturing temperature contained significantly more mutations than APOBEC3−/−-isolated DNA (P < 0.05) but not significantly more mutations than BALB-isolated viral DNA (P = 0.67). Deamination levels in viral DNA isolated from all three genotypes using a regular denaturing temperature were not statistically significantly different from one another.
When the target nucleotide context was analyzed, a majority of, but not all, mutations were found in the mouse APOBEC3-preferred TXC context (92% for APOBEC3BL6 and 67% for APOBEC3BALB) (Fig. 3D). As seen in the in vitro targeting assay, APOBEC3BALB had a stronger preference for ATC, with 24% of all mutations falling within this context, compared to 2% for APOBEC3BL6. APOBEC3BL6 maintained a stronger preference than APOBEC3BALB for T at the −2 position, further supporting our in vitro data (Fig. 2C). Within the env and LTR fragments sequenced, 22.9% of all cytosines were found in the preferred TXC context (2.6% TGC, 3.9% TAC, 9.2% TTC, 7.2% TCC). The most-highly favored TTC (APOBEC3BL6) and ATC (APOBEC3BALB) target sequences were present 14 and 15 times, respectively, in these genomic regions (of 152 total cytosines); thus, the low levels of deamination are not due to a lack of preferred substrate.
APOBEC3 inhibits MMTV reverse transcription.
These results suggested that while APOBEC3 packaged into MMTV virions retains its deaminase activity, this is unlikely to be the major mode of restriction since it occurs only at low levels. To determine if APOBEC3 restricts virus infection by other means, we next tested if the mouse protein restricted MMTV at an early step of reverse transcription. We utilized the DNA produced by EnRT reactions with virions isolated from C57BL/6, APOBEC3−/−, and BALB/c mice and performed RT-qPCR using primers specific to MMTV strong-stop DNA. We found that synthesis of this early reverse transcription product was strongly inhibited when the reaction was carried out with APOBEC3BL6-containing virions compared to APOBEC3−/− virions, while APOBEC3BALB virions showed an intermediate phenotype (Fig. 4A).
Fig 4.
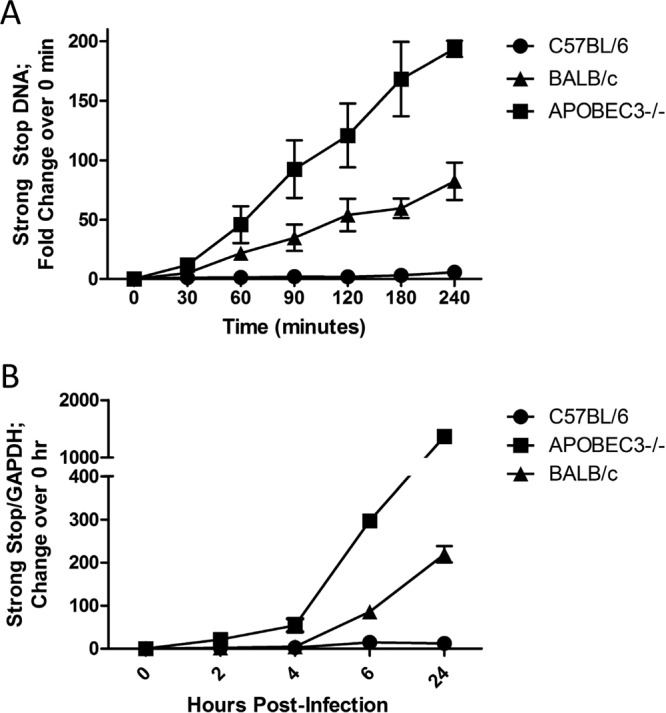
Packaged APOBEC3 restricts MMTV reverse transcription. (A) Equivalent amounts of MMTV virions isolated from C57BL/6, BALB, and APOBEC3−/− mice were subjected to EnRT reactions. DNA was harvested at the indicated time points, and strong-stop DNA was quantified via RT-qPCR. Shown is the average of three independent experiments. (B) 293T-mTfR1 target cells were infected with MMTV virions isolated from C57BL6, BALB, and APOBEC3−/− mice. Total DNA was isolated from cells at the indicated times after infection, and strong-stop DNA was quantified relative to the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) using RT-qPCR. Triplicates were performed for each time point and averaged; shown is a representative from three independent experiments.
To determine if reverse transcription was also inhibited during virus infection, the virions were used to infect 293T-mTfR1 cells. DNA was isolated at various time points after infection and subjected to RT-qPCR. Similar to what was seen in the in vitro EnRT reactions, strong-stop DNA synthesis was greatly reduced in cells infected with APOBEC3-containing virions compared to cells infected with knockout virions, particularly at later time points. Infection with APOBEC3BALB virions again showed an intermediate phenotype, suggesting that these virions are restricted compared to those produced in the absence of APOBEC3, but to a lesser extent than those produced in BL/6 mice (Fig. 4B). These data suggest that inhibition of early reverse transcription is the major means by which virion-packaged APOBEC3 inhibits MMTV infection.
Restriction of reverse transcription is correlated to the level of packaged APOBEC3.
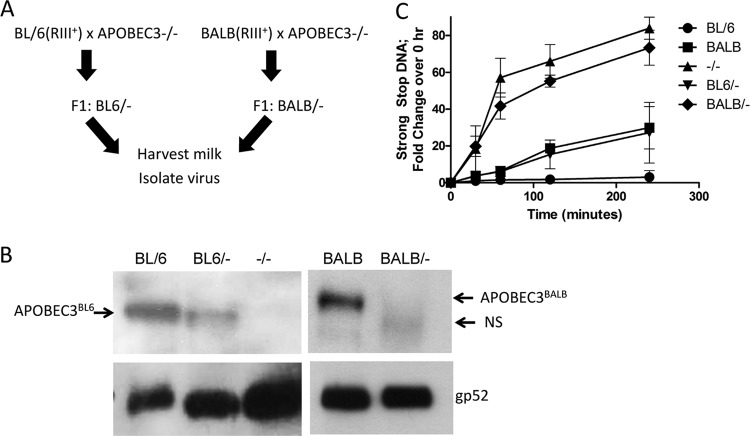
The differential abilities of APOBEC3BL6 and APOBEC3BALB to restrict MMTV infection may be due to the level of packaging or to the differences in the proteins. We used a genetic approach to determine if the level of restriction was affected by the level of packaged APOBEC3. We crossed MMTV-infected C57BL/6 or BALB/c females and APOBEC3−/− males and generated F1 heterozygotes; these heterozygotes acquired the virus from their infected mothers (Fig. 5A). Virus was then isolated from the milk of the heterozygotes at their first pregnancy. Virions derived from both APOBEC3BL6/− and APOBEC3BALB/− F1 mice packaged an intermediate amount of APOBEC3 compared to virions isolated from the parental homozygous strains of mice (Fig. 5B). Virions isolated from both heterozygous crosses were then used in EnRT assays, and the level of strong-stop DNA was compared to that produced by virus isolated from homozygous C57BL/6, BALB, and APOBEC3−/− mice; the level of virus in each reaction was normalized by RNA and viral protein levels. Virions isolated from homozygous C57BL/6 mice showed the strongest effects of APOBEC3, reflected by low levels of viral transcripts, and homozygous APOBEC3−/− virions produced the highest level of strong-stop DNA (Fig. 5C). Virions from heterozygous animals produced an intermediate level of strong-stop DNA between that seen with virus isolated from the parental strain and that seen with knockout animals (Fig. 5C). In addition, the amount of strong-stop DNA produced by the APOBEC3BALB/− virions was higher than that seen with the APOBEC3BL6/− virions and was not statistically different from that seen with virions from APOBEC3−/− mice (Fig. 5C). This suggests that while alterations in the protein sequences in APOBEC3BL6 and APOBEC3BALB affect the substrate specificity, the differential restriction by these proteins is due largely to the level of packaged protein.
Fig 5.
Restriction of reverse transcription correlates with the amount of packaged APOBEC3. (A) MMTV-infected heterozygous F1 mice were obtained by mating APOBEC3−/− male mice to MMTV-infected wild-type C57BL/6 or BALB females. The female F1 mice were bred, and MMTV was isolated from milk as described in Materials and Methods. (B) Virions obtained from homozygous, heterozygous, and APOBEC3−/− milk were subjected to Western blotting after normalization by RT-qPCR. Immunoblots were serially probed with rabbit anti-mouse APOBEC3 antiserum and goat anti-gp52 antiserum as a loading control. (C) Virions isolated from milk of the indicated mouse genotypes were used in endogenous reverse transcription reactions. Strong-stop DNA was quantified from total viral DNA harvested at the indicated time points. Three independent reactions were averaged.
Target cell expression of either APOBEC3 allele leads to virus restriction.
Virion packaging of APOBEC3 is thought to be critical for viral restriction. However, intracellular cytoplasmic APOBEC3 is also believed to restrict incoming HIV virions (23, 44–46). We also previously showed that cells transfected with APOBEC3 can restrict incoming MMTV viral particles and that APOBEC3 expression in target dendritic cells limits infection in vivo (47). To determine the intracellular effects of both natural variants of APOBEC3 on incoming in vivo-produced virions, 293T-mTfR1 cells were stably transfected with Flag epitope-tagged BL/6 or BALB/c variants of APOBEC3 (26). Western blot analysis using anti-Flag and anti-APOBEC3 antibodies demonstrated that the two variants were expressed at similar levels in each cell line (Fig. 6A).
Fig 6.
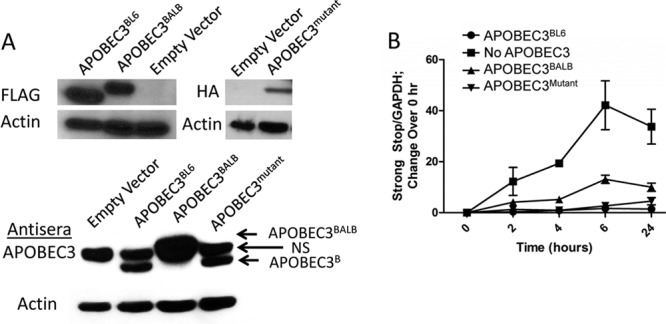
Target cell APOBEC3 can restrict reverse transcription of incoming virions and is independent of cytidine deamination. (A) 293T-TfR1 cells were stably transfected with plasmids encoding APOBEC3BL6, APOBEC3BALB, APOBEC3mutant, or an empty vector (no APOBEC3). (Top) Protein extracts were assessed for APOBEC3 content using their respective epitope tags (anti-Flag for APOBEC3BL6 and APOBEC3BALB; anti-HA for APOBEC3mutant). Blots were stripped and reprobed for β-actin as a loading control. (Bottom) Cell lysates were also probed using anti-APOBEC3 antiserum. A cross-reactive protein (NS) seen in all lysates is indicated. (B) Stably transfected cells were infected with MMTV isolated from APOBEC3−/− mice. Total cellular DNA was harvested at indicated time points after infection, and strong-stop DNA was quantified in relation to GAPDH. Data are representative of three independent experiments.
Virions produced from APOBEC3−/− mice were used to infect each of the cell lines, total cellular DNA was collected at various time points after infection, and early reverse transcription products were measured by quantitative PCR. Cells expressing either variant were similarly able to restrict early reverse transcription, with approximately 5- to 10-fold less DNA produced than in cells lacking APOBEC3 (Fig. 6B). The intermediate phenotype typical of virions packaging APOBEC3BALB was attenuated in cells expressing this variant compared to cells expressing APOBEC3BL6, suggesting that the two natural variants may share similar restriction capacities when expressed at similar levels.
To further test the importance of cytidine deamination in restriction of MMTV, we performed a similar experiment using cells stably transfected with an HA-tagged APOBEC3BL6 cytidine deaminase mutant (E73Q/E253Q) (Fig. 6A). Cells expressing this variant were able to restrict reverse transcription of incoming MMTV equally as well as the WT APOBEC3BL6 (Fig. 6B). Restriction of reverse transcription is thereby independent of cytidine deamination, and these data further support this mechanism as the main method by which APOBEC3 mediates MMTV restriction in mice.
DISCUSSION
It is now well established that mouse APOBEC3 limits the pathogenesis of a number of murine retroviruses in vivo, including F-MLV, M-MLV, and MMTV (25, 28, 29, 48). Indeed, these studies, which compared infection and pathogenesis in wild-type and APOBEC3 knockout mice, provided the ultimate proof that APOBEC3 proteins functioned as in vivo restriction factors. APOBEC3 restriction of murine viruses in vivo has also provided a model for understanding the natural selection of host restriction factors, as the two natural allelic variants of murine APOBEC3 show differential restriction against a number of viruses, which also affects their pathogenicity in different inbred mouse strains (28, 31, 48).
However, the mechanism of APOBEC3-mediated restriction in vivo has been less well defined. The ability to deaminate cytidines within newly transcribed viral DNA certainly contributes to the effect of APOBEC3 against viruses such as HIV, creating an array of G-to-A mutations that can lead to missense and nonsense mutations and thereby preventing productive infection. Moreover, HIV DNA isolated from patients bears characteristic signatures of APOBEC3-mediated deamination (49–51). In contrast, while in vitro-transduced mouse APOBEC3 generates a high rate of G-to-A mutations on HIV in the presence or absence of Vif, in vivo, neither M-MLV nor MMTV shows evidence of APOBEC3-mediated mutations, although viral restriction still occurs (25, 28).
Here, we show that murine APOBEC3 packaged within MMTV virions is enzymatically active and is able to catalyze cytidine deamination. Moreover, although there is abundant evidence that APOBEC3BALB is less restrictive than APOBEC3BL6 in vivo, this is not due to diminished catalytic activity. We show that both allelic forms of the protein, produced and packaged in vivo, were capable of catalyzing this potentially antiviral reaction. In fact, APOBEC3BALB showed an increase in activity on the 5′-TTC-3′ substrate when normalized to protein input. However, when tested against a full array of deaminase substrates, APOBEC3BALB did not show an increase in activity on all substrates. Notably, while both variants showed relatively promiscuous targeting compared to APOBEC3G, the two alleles showed different patterns of preferred target sequences (10). Until this study, the mouse APOBEC3 preferred editing site was not well defined, as previous efforts have been inconsistent in APOBEC3 plasmid, allelic variant, and target substrate (5, 30, 52).
A difference in targeting between the allelic variants may be attributable to the presence of several polymorphisms localized to the substrate recognition loop that we have previously identified in the APOBEC enzyme family (39). However, as with HIV, for which altering the targeting preference of APOBEC3G did not impact retroviral restriction, we did not find evidence that the altered sequence preference contributed to differences in MMTV restriction in our efforts to sequence reverse transcription products (53).
Indeed, while native APOBEC3 is able to catalyze cytidine deamination, further investigation of early reverse transcription products showed very low levels of G-to-A mutation, similar to what has been seen in integrated proviruses in vivo (25). Utilization of 3DPCR, a technique that maximizes detection and amplification of DNA with increased A/T content, allowed us to uncover reverse transcription products with slightly more G-to-A mutations. However, even under these highly selective conditions, rates of mutation remained a fraction of that seen in HIV, which can vary from 7 to 8% for total G-to-A mutations in vivo (43, 54, 55).
Others have suggested that murine viruses may be able to block APOBEC3 deaminase activity. Browne and Littman showed that F-MLV was restricted by both forms of murine APOBEC3 in vitro but had only 1 G-to-A mutation per kb of genome. In comparison, both APOBEC3 alleles were also able to restrict HIV but produced 16 G-to-A mutations per kb of HIV genome (27). However, Petit et al. utilized 3DPCR to uncover a significantly higher rate of G-to-A mutations from F-MLV both in vitro and in vivo (41). Our MMTV data closely match the F-MLV data from these two groups, suggesting similarities between the two murine viruses. Both show G-to-A mutations at a very low level and may share a mechanism to block APOBEC3-induced deamination. Our data also showed that reverse transcription amplicons recovered from virions isolated from BALB/c or C57BL/6 mice had similar levels of editing. The large majority of the G-to-A mutations were found in the preferred deamination site 5′-GXA-3′, with preference for GG and GA dinucleotides within those parameters. Our analysis further supports a difference in target sequence preferences between the two APOBEC3 alleles, as targeting patterns between APOBEC3BL6 and APOBEC3BALB mirrored what was seen in the in vitro assay. However, if deamination were a critical component of restriction, we would expect these two alleles to show different levels of editing to mirror their different levels of overall restriction.
Since restriction of MMTV is clearly mediated by mouse APOBEC3, we also assessed whether reverse transcription was inhibited in the presence of the enzyme. This block to reverse transcription has been described in several systems, although primarily in viruses produced in vitro (13, 14, 17, 20). However, other studies suggest that this is an artifact of high levels of transfected protein and maintain that deamination is necessary for restriction (22). Our analysis of virions isolated in vivo allows an understanding of the activity of native protein packaged at endogenous levels. Our results clearly show that both alleles of APOBEC3 are able to prevent early reverse transcription when packaged within virions. The alleles show distinct differences in their ability to restrict in this manner, with packaged APOBEC3BALB showing less restriction than the APOBEC3BL6 counterpart. We have also shown that APOBEC3 expressed within target cells is able to restrict reverse transcription of incoming MMTV virions that do not contain packaged APOBEC3. This suggests a possible dual role for APOBEC3—both within the virion and within target cells. It also provides evidence that the MMTV core and the RT complex are accessible to intracellular factors during reverse transcription, supporting recent studies showing that uncoating and reverse transcription are linked (56, 57). However, unlike with the in vivo-packaged APOBEC3, we did not see as distinct a difference between the two allelic variants, a finding which is likely due to the similar levels of expression within our transfected cells. An APOBEC3 mutant that is unable to catalyze reverse transcription was also able to efficiently prevent reverse transcription, confirming that this arm of restriction is truly independent of deamination. Taken together, our data suggest that the mechanism by which APOBEC3 restricts infection by MMTV, namely, blocking reverse transcription, is the same when packaged or present in the target cell.
The two major APOBEC3 alleles differ in both sequence and level of expression in vivo, and it has been suggested that positive selection by murine retroviruses may have played a role in their acquisition. While we showed that the two alleles show altered sequence substrate specificity, our data support the idea that it is the levels of packaged APOBEC3 rather than the polymorphic amino acid differences in the APOBEC3BL6 and APOBEC3BALB alleles that affect the degree of reverse transcriptase inhibition. By creating heterozygous mice that express intermediate levels of APOBEC3, we generated MMTV virions that package half the parental levels of protein and showed that the amount of reverse transcription products seen correlated with the level of packaged protein. While several in vitro studies have suggested that coding sequence differences between the two alleles contribute to the more restrictive phenotype of the APOBEC3BL6 allele, in vivo studies using crosses of BL6 and BALB have led to conflicting results (28, 34, 48, 58, 59). Additionally, several studies suggested that a single copy of the APOBEC3BL6 allele is sufficient to confer full resistance to MLV infection in vivo, since there was no difference in F-MLV or AKV infection of APOBEC3+/+ and APOBEC3+/− mice (28, 30). These studies may be confounded by multiple genes in the different genetic backgrounds that affect both infectivity and immune response (e.g., levels of neutralizing antibodies) in whole-animal studies. Moreover, these studies examined late stages in infection, by which time restriction by the single copy of APOBEC3 in APOBEC3+/− mice may be dominant after many rounds of infection. Indeed, our studies with M-MLV indicated that at early times after infection, APOBEC+/− mice were intermediate in infection levels between knockouts and wild-type mice but succumbed to disease with the same kinetics as the knockouts (29). Our studies here, which used virions to infect the same target cells lacking APOBEC3 proteins (293T-mTfR1) in single-round infections, may more accurately reflect the direct effects of APOBEC3 on infection.
In sum, our data show that MMTV is susceptible to reverse transcriptase inhibition by APOBEC3 and is sensitive to levels of protein expressed and packaged within virions. Future work is required to determine the mechanism by which MMTV and other murine viruses avoid APOBEC3-mediated G-to-A mutations as well as the mechanism by which all APOBEC3 proteins are able to prevent reverse transcription.
ACKNOWLEDGMENTS
We thank Kristin Blouch for technical assistance, Masaaki Miyazawa and David Derse for the different APOBEC3 plasmids, and Sara Cherry for helpful suggestions.
This research was supported by PHS grant R01-AI-085015 (S.R.R.) and by the W. W. Smith Foundation (R.M.K). A.L.M. was supported by NIH T32-AI07324.
Footnotes
Published ahead of print 28 February 2013
REFERENCES
- 1. Emerman M, Malik HS. 2010. Paleovirology—modern consequences of ancient viruses. PLoS Biol. 8:e1000301 doi:10.1371/journal.pbio.1000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malim MH, Bieniasz PD. 2012. HIV restriction factors and mechanisms of evasion. Cold Spring Harb. Perspect. Med. 2:a006940 doi:10.1101/cshperspect.a006940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheehy AM, Gaddis NC, Choi JD, Malim MH. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646–650 [DOI] [PubMed] [Google Scholar]
- 4. LaRue RS, Andresdottir V, Blanchard Y, Conticello SG, Derse D, Emerman M, Greene WC, Jonsson SR, Landau NR, Lochelt M, Malik HS, Malim MH, Munk C, O'Brien SJ, Pathak VK, Strebel K, Wain-Hobson S, Yu XF, Yuhki N, Harris RS. 2009. Guidelines for naming nonprimate APOBEC3 genes and proteins. J. Virol. 83:494–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH. 2004. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr. Biol. 14:1392–1396 [DOI] [PubMed] [Google Scholar]
- 6. Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. 2004. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 23:2451–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liddament MT, Brown WL, Schumacher AJ, Harris RS. 2004. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr. Biol. 14:1385–1391 [DOI] [PubMed] [Google Scholar]
- 8. Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM. 2004. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J. Virol. 78:6073–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goila-Gaur R, Strebel K. 2008. HIV-1, APOBEC, and intrinsic immunity. Retrovirology 5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beale RC, Petersen-Mahrt SK, Watt IN, Harris RS, Rada C, Neuberger MS. 2004. Comparison of the differential context-dependence of DNA deamination by APOBEC enzymes: correlation with mutation spectra in vivo. J. Mol. Biol. 337:585–596 [DOI] [PubMed] [Google Scholar]
- 11. Langlois MA, Beale RC, Conticello SG, Neuberger MS. 2005. Mutational comparison of the single-domained APOBEC3C and double-domained APOBEC3F/G anti-retroviral cytidine deaminases provides insight into their DNA target site specificities. Nucleic Acids Res. 33:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newman EN, Holmes RK, Craig HM, Klein KC, Lingappa JR, Malim MH, Sheehy AM. 2005. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr. Biol. 15:166–170 [DOI] [PubMed] [Google Scholar]
- 13. Bishop KN, Holmes RK, Malim MH. 2006. Antiviral potency of APOBEC proteins does not correlate with cytidine deamination. J. Virol. 80:8450–8458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iwatani Y, Chan DS, Wang F, Maynard KS, Sugiura W, Gronenborn AM, Rouzina I, Williams MC, Musier-Forsyth K, Levin JG. 2007. Deaminase-independent inhibition of HIV-1 reverse transcription by APOBEC3G. Nucleic Acids Res. 35:7096–7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li XY, Guo F, Zhang L, Kleiman L, Cen S. 2007. APOBEC3G inhibits DNA strand transfer during HIV-1 reverse transcription. J. Biol. Chem. 282:32065–32074 [DOI] [PubMed] [Google Scholar]
- 16. Guo F, Cen S, Niu M, Yang Y, Gorelick RJ, Kleiman L. 2007. The interaction of APOBEC3G with human immunodeficiency virus type 1 nucleocapsid inhibits tRNA3Lys annealing to viral RNA. J. Virol. 81:11322–11331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holmes RK, Koning FA, Bishop KN, Malim MH. 2007. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation: comparisons with APOBEC3G. J. Biol. Chem. 282:2587–2595 [DOI] [PubMed] [Google Scholar]
- 18. Holmes RK, Malim MH, Bishop KN. 2007. APOBEC-mediated viral restriction: not simply editing? Trends Biochem. Sci. 32:118–128 [DOI] [PubMed] [Google Scholar]
- 19. Luo K, Wang T, Liu B, Tian C, Xiao Z, Kappes J, Yu XF. 2007. Cytidine deaminases APOBEC3G and APOBEC3F interact with human immunodeficiency virus type 1 integrase and inhibit proviral DNA formation. J. Virol. 81:7238–7248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH. 2008. APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS Pathog. 4:e1000231 doi:10.1371/journal.ppat.1000231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schumacher AJ, Haché G, MacDuff DA, Brown WL, Harris RS. 2008. The DNA deaminase activity of human APOBEC3G is required for Ty1, MusD and HIV-1 restriction. J. Virol. 82:2652–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyagi E, Opi S, Takeuchi H, Khan M, Goila-Gaur R, Kao S, Strebel K. 2007. Enzymatically active APOBEC3G is required for efficient inhibition of human immunodeficiency virus type 1. J. Virol. 81:13346–13353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gillick K, Pollpeter D, Phalora P, Kim EY, Wolinsky SM, Malim MH. 2013. Suppression of HIV-1 infection by APOBEC3 proteins in primary human CD4+ T cells is associated with the inhibition of processive reverse transcription as well as excessive cytidine deamination. J. Virol. 87:1508–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nandi S, McGrath CM. 1973. Mammary neoplasia in mice. Adv. Cancer Res. 17:353–414 [Google Scholar]
- 25. Okeoma CM, Lovsin N, Peterlin BM, Ross SR. 2007. APOBEC3 inhibits mouse mammary tumour virus replication in vivo. Nature 445:927–930 [DOI] [PubMed] [Google Scholar]
- 26. Rulli SJ, Jr, Mirro J, Hill SA, Lloyd P, Gorelick RJ, Coffin JM, Derse D, Rein A. 2008. Interactions of murine APOBEC3 and human APOBEC3G with murine leukemia viruses. J. Virol. 82:6566–6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Browne EP, Littman DR. 2008. Species-specific restriction of Apobec3-mediated hypermutation. J. Virol. 82:1305–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takeda E, Tsuji-Kawahara S, Sakamoto M, Langlois MA, Neuberger MS, Rada C, Miyazawa M. 2008. Mouse APOBEC3 restricts Friend leukemia virus infection and pathogenesis in vivo. J. Virol. 82:10998–11008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Low A, Okeoma CM, Lovsin N, de las Heras M, Taylor TH, Peterlin BM, Ross SR, Fan H. 2009. Enhanced replication and pathogenesis of Moloney murine leukemia virus in mice defective in the murine APOBEC3 gene. Virology 385:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langlois MA, Kemmerich K, Rada C, Neuberger MS. 2009. The AKV murine leukemia virus is restricted and hypermutated by mouse APOBEC3. J. Virol. 83:11550–11559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okeoma CM, Petersen J, Ross SR. 2009. Expression of murine APOBEC3 alleles in different mouse strains and their effect on mouse mammary tumor virus infection J. Virol. 83:3029–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyazawa M, Tsuji-Kawahara S, Kanari Y. 2008. Host genetic factors that control immune responses to retrovirus infections. Vaccine 26:2981–2996 [DOI] [PubMed] [Google Scholar]
- 33. Sanville B, Dolan MA, Wollenberg K, Yan Y, Martin C, Yeung ML, Strebel K, Buckler-White A, Kozak CA. 2010. Adaptive evolution of Mus Apobec3 includes retroviral insertion and positive selection at two clusters of residues flanking the substrate groove. PLoS Pathog. 6:e1000974 doi:10.1371/journal.ppat.1000974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Hakata Y, Takeda E, Liu Q, Iwatani Y, Kozak CA, Miyazawa M. 2012. Two genetic determinants acquired late in mus evolution regulate the inclusion of exon 5, which alters mouse APOBEC3 translation efficiency. PLoS Pathog. 8:e1002478 doi:10.1371/journal.ppat.1002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Golovkina TV, Chervonsky A, Dudley JP, Ross SR. 1992. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell 69:637–645 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Rassa JC, deObaldia EM, Albritton L, Ross SR. 2003. Identification of the mouse mammary tumor virus envelope receptor-binding domain. J. Virol. 77:10468–10478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Okeoma CM, Huegel AL, Lingappa J, Feldman MD, Ross SR. 2010. APOBEC3 proteins expressed in mammary epithelial cells are packaged into retroviruses and can restrict transmission of milk-borne virions. Cell Host Microbe 8:534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nabel CS, Jia H, Ye Y, Shen L, Goldschmidt HL, Stivers JT, Zhang Y, Kohli RM. 2012. AID/APOBEC deaminases disfavor modified cytosines implicated in DNA demethylation. Nat. Chem. Biol. 8:751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kohli RM, Abrams SR, Gajula KS, Maul RW, Gearhart PJ, Stivers JT. 2009. A portable hot spot recognition loop transfers sequence preferences from APOBEC family members to activation-induced cytidine deaminase. J. Biol. Chem. 284:22898–22904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aguiar RS, Lovsin N, Tanuri A, Peterlin BM. 2008. Vpr.A3A chimera inhibits HIV replication. J. Biol. Chem. 283:2518–2525 [DOI] [PubMed] [Google Scholar]
- 41. Petit V, Guetard D, Renard M, Keriel A, Sitbon M, Wain-Hobson S, Vartanian JP. 2009. Murine APOBEC1 is a powerful mutator of retroviral and cellular RNA in vitro and in vivo. J. Mol. Biol. 385:65–78 [DOI] [PubMed] [Google Scholar]
- 42. Russell RA, Moore MD, Hu WS, Pathak VK. 2009. APOBEC3G induces a hypermutation gradient: purifying selection at multiple steps during HIV-1 replication results in levels of G-to-A mutations that are high in DNA, intermediate in cellular viral RNA, and low in virion RNA. Retrovirology 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Armitage AE, Deforche K, Chang CH, Wee E, Kramer B, Welch JJ, Gerstoft J, Fugger L, McMichael A, Rambaut A, Iversen AK. 2012. APOBEC3G-induced hypermutation of human immunodeficiency virus type-1 is typically a discrete “all or nothing” phenomenon. PLoS Genet. 8:e1002550 doi:10.1371/journal.pgen.1002550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vetter ML, D'Aquila RT. 2009. Cytoplasmic APOBEC3G restricts incoming Vif-positive human immunodeficiency virus type 1 and increases two-long terminal repeat circle formation in activated T-helper-subtype cells. J. Virol. 83:8646–8654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berger G, Durand S, Fargier G, Nguyen XN, Cordeil S, Bouaziz S, Muriaux D, Darlix JL, Cimarelli A. 2011. APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 7:e1002221 doi:10.1371/journal.ppat.1002221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koning FA, Goujon C, Bauby H, Malim MH. 2011. Target cell-mediated editing of HIV-1 cDNA by APOBEC3 proteins in human macrophages. J. Virol. 85:13448–13452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okeoma CM, Low A, Bailis W, Fan HY, Peterlin BM, Ross SR. 2009. Induction of APOBEC3 in vivo causes increased restriction of retrovirus infection. J. Virol. 83:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Santiago ML, Montano M, Benitez R, Messer RJ, Yonemoto W, Chesebro B, Hasenkrug KJ, Greene WC. 2008. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science 321:1343–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Janini M, Rogers M, Birx DR, McCutchan FE. 2001. Human immunodeficiency virus type 1 DNA sequences genetically damaged by hypermutation are often abundant in patient peripheral blood mononuclear cells and may be generated during near-simultaneous infection and activation of CD4+ T cells. J. Virol. 75:7973–7986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kieffer TL, Kwon P, Nettles RE, Han Y, Ray SC, Siliciano RF. 2005. G→A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J. Virol. 79:1975–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pace C, Keller J, Nolan D, James I, Gaudieri S, Moore C, Mallal S. 2006. Population level analysis of human immunodeficiency virus type 1 hypermutation and its relationship with APOBEC3G and vif genetic variation. J. Virol. 80:9259–9269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Renard M, Henry M, Guetard D, Vartanian JP, Wain-Hobson S. 2010. APOBEC1 and APOBEC3 cytidine deaminases as restriction factors for hepadnaviral genomes in non-humans in vivo. J. Mol. Biol. 400:323–334 [DOI] [PubMed] [Google Scholar]
- 53. Kohli RM, Maul RW, Guminski AF, McClure RL, Gajula KS, Saribasak H, McMahon MA, Siliciano RF, Gearhart PJ, Stivers JT. 2010. Local sequence targeting in the AID/APOBEC family differentially impacts retroviral restriction and antibody diversification. J. Biol. Chem. 285:40956–40964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gandhi SK, Siliciano JD, Bailey JR, Siliciano RF, Blankson JN. 2008. Role of APOBEC3G/F-mediated hypermutation in the control of human immunodeficiency virus type 1 in elite suppressors. J. Virol. 82:3125–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Land AM, Ball TB, Luo M, Pilon R, Sandstrom P, Embree JE, Wachihi C, Kimani J, Plummer FA. 2008. Human immunodeficiency virus (HIV) type 1 proviral hypermutation correlates with CD4 count in HIV-infected women from Kenya. J. Virol. 82:8172–8182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hulme AE, Perez O, Hope TJ. 2011. Complementary assays reveal a relationship between HIV-1 uncoating and reverse transcription. Proc. Natl. Acad. Sci. U. S. A. 108:9975–9980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roa A, Hayashi F, Yang Y, Lienlaf M, Zhou J, Shi J, Watanabe S, Kigawa T, Yokoyama S, Aiken C, Diaz-Griffero F. 2012. RING domain mutations uncouple TRIM5α restriction of HIV-1 from inhibition of reverse transcription and acceleration of uncoating. J. Virol. 86:1717–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abudu A, Takaori-Kondo A, Izumi T, Shirakawa K, Kobayashi M, Sasada A, Fukunaga K, Uchiyama T. 2006. Murine retrovirus escapes from murine APOBEC3 via two distinct novel mechanisms. Curr. Biol. 16:1565–1570 [DOI] [PubMed] [Google Scholar]
- 59. Santiago ML, Benitez RL, Montano M, Hasenkrug KJ, Greene WC. 2010. Innate retroviral restriction by Apobec3 promotes antibody affinity maturation in vivo. J. Immunol. 185:1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]