Abstract
Baculovirus (BV) is a promising gene therapy vector and typically requires readministration because BV mediates transient expression. However, how the prime-boost regimen triggers BV-specific adaptive responses and their impacts on BV readministration, transgene expression, and therapeutic/vaccine efficacy remain unknown. Here we unraveled that BV injection into BALB/c mice induced the production of BV-specific antibodies, including IgG1 and IgG2a, which could neutralize BV by antagonizing the envelope protein gp64 and impede BV-mediated transgene expression. Moreover, humans did not possess preexisting anti-BV antibodies. BV injection also elicited BV-specific Th1 and Th2 responses as well as CD4+ and CD8+ T cell responses. gp64 was a primary immunogen to activate the antibody and CD8+ T cell response, with its peptide at positions 457 to 465 (peptide 457-465) being the major histocompatibility complex (MHC) class I epitope to stimulate CD8+ T cell and cytotoxic responses. Nonetheless, a hybrid Sleeping Beauty-based BV enabled long-term expression for >1 year by a single injection, indicating that the T cell responses did not completely eradicate BV-transduced cells and implicating the potential of this hybrid BV vector for gene therapy. These data unveil that BV injection triggers adaptive immunity and benefit rational design of BV administration schemes for gene therapy and vaccination.
INTRODUCTION
Baculovirus (BV) is a DNA virus that infects insects as its natural hosts but also efficiently transduces a wide variety of mammalian cells, in which BV neither replicates nor is toxic (1, 2). BV is nonpathogenic to humans; thus, recombinant BV construction, propagation, and handling can be performed readily in biosafety level 1 facilities. These attributes have prompted the development of BV vectors for in vitro and in vivo gene delivery (3), RNA interference (4), cell-based assay development (5), production of viral vectors (6) and recombinant proteins (7), as well as cartilage and bone tissue engineering (8). Beyond these applications, BV is also used as an expression vector for the treatment of various cancers, including hepatoma (9), melanoma (10), colon cancer (11), brain cancer (12–16), prostate cancer (17, 18), and ovarian cancer (17). In addition, BV has been explosively developed as a vaccine expression/display vector against a variety of pathogens, including avian influenza virus (AIV) (19–22), avian reovirus (23), pseudorabies virus (24), enterovirus 71 (25), Plasmodium berghei (26), and many others (for a review, see reference 1).
When BV is used as a vaccine, the immunization scheme usually involves the primary injection of BV at a dose ranging from 107 to 1010 PFU, followed by a second or even a third booster injection at a certain time interval. For instance, intramuscular (i.m.) injection of BV (109 PFU) expressing the pseudorabies virus antigens into mice 3 times at 2-week intervals induces protective immunity against lethal virus challenge (24). i.m. immunization with a BV (109 PFU) expressing the hemagglutinin (HA) of H5N1 AIV at weeks 0 and 3 also confers complete protection from lethal virus challenge (21). In another study, mice receiving i.m. administration of a BV expressing/displaying the HA of H5N2 AIV (108, 109, and 1010 PFU) at weeks 0, 2, and 4 developed HA-specific humoral and cellular immune responses (19).
In the context of cancer therapy, repeated BV injection at a specific time interval is also common. Protective antitumor effects can be elicited in mice by i.m. injection of a BV (107, 108, or 109 PFU) expressing murine telomerase reverse transcriptase (a tumor antigen for cancer immunotherapy) twice, at days 0 and 7 (13). Injection of a recombinant BV (3 × 109 PFU) expressing an antiangiogenic fusion protein into a mouse bearing a prostate tumor every 3 days 3 times also imparts strong antiangiogenic and antitumor effects (18). Those studies collectively demonstrated that BV-mediated gene delivery can provoke antitransgene humoral and cellular immune responses and impart potent vaccine and antitumor effects on animals.
Although BV, as a vaccine vector or anticancer vehicle, is commonly injected into animals repeatedly at a certain time interval, how the repeated administrations induce adaptive immune responses and influence the ensuing transgene expression has yet to be explored. Since BV-induced innate responses have been well documented (27–29), this study focused on examining the transgene expression profile after repeated BV administrations and exploring the humoral and cell-mediated responses against the BV vector. The immunogenic component of BV and the specific epitopes contributing to adaptive immunity were identified. The consequences of the adaptive responses were unveiled, and implications of these findings for BV-mediated gene therapy are discussed.
MATERIALS AND METHODS
Preparation of recombinant BV vectors.
Bac-CE expressing enhanced green fluorescent protein (EGFP) as the reporter was constructed as described previously (30). To construct pBac-luc/w, the firefly luciferase gene was amplified from pGEM-luc (Promega) and cloned into pBac-CMV5 (whose polyhedrin promoter was replaced by the cytomegalovirus immediate-early [CMV-IE] promoter [31]). WPRE (woodchuck hepatitis virus posttranscriptional regulatory element) was then cloned at the 3′ end of the luciferase gene to yield pBac-luc/w. pBac-T2Fluc/w was constructed by subcloning the CMV-IE–luciferase–WPRE cassette into pBac-T2 (17) between the inverted repeat/direct repeat (IR/DR) elements for Sleeping Beauty (SB) transposase recognition. pBac-SB100X encoding the SB100X transposase under the control of the CMV-IE promoter was constructed as described previously (31). pBac-luc/w, pBac-T2Fluc/w, and pBac-SB100X were used to generate the recombinant BV vectors Bac-luc/w, Bac-T2Fluc/w, and Bac-SB100X according to the instructions provided with the Bac-to-Bac system (Invitrogen). The viruses were propagated in Sf-9 cells, concentrated by ultracentrifugation, and resuspended in phosphate-buffered saline (PBS) (pH 6.2), as described previously (17). The virus titer was determined by an endpoint dilution assay and is expressed as PFU/ml.
Mouse immunization and splenocyte preparation.
All animal experiments were performed in compliance with the Guide for the Care and Use of Laboratory Animals (National Science Council, Taiwan). Female BALB/c mice (National Laboratory Animal Center, Taiwan) aged 6 to 8 weeks were injected via the i.m. route with the adjuvant-free, concentrated BV vectors at a dose of 3 × 109 PFU in 50 μl of PBS. BV readministration was performed in the same manner. The spleens were harvested from mice at the indicated time points, crushed through iron mesh, and pressed through a 70-μm strainer (BD Biosciences) to yield suspended cells. The cells were incubated in ammonium chloride lysis (ACK) buffer for 5 min on ice to lyse red blood cells, washed with serum-free RPMI medium twice, and resuspended in complete RPMI medium.
In vivo bioluminescence imaging.
BV vectors (Bac-luc/w alone or Bac-SB100X plus Bac-SB-T2Fluc/w) were injected into BALB/c mice via the i.m. route, and at the desired time points, d-luciferin (Caliper Life Sciences) was injected (150 μl/mouse) intraperitoneally. After 10 min, animals were anesthetized with a 2.5% isoflurane-air mixture, and luciferase expression was detected by the Xenogen IVIS spectrum imaging system (Caliper Life Sciences). To quantify luminescence, the region on captured images was selected, and the luminescence output was analyzed by using Living Image software and expressed as total flux (photons/second). The relative total fluxes were calculated with the maximum total flux defined as 100% and the background total flux defined as 0%.
Analyses of anti-BV IgG titers by ELISA.
Mouse blood samples (0.5 ml) were collected from the submandibular vein, inactivated at 56°C for 30 min, and then 2-fold serially diluted with diluent buffer (0.05% Tween 20 and 0.1% bovine serum albumin [BSA] in PBS) from 25- to 222-fold dilution. The BV-specific total IgG and IgG subtype titers were measured by an enzyme-linked immunosorbent assay (ELISA). Each well in the ELISA plates was coated with 100 μl (1.5 μg/ml in PBS) concentrated wild-type BV particles for 12 h at 4°C and blocked with 300 μl blocking buffer (0.05% Tween 20 and 1% BSA in PBS) for 1 h at 37°C. After washing with PBST buffer (0.05% Tween 20 in PBS), the serially diluted serum samples were added to the wells (100 μl/well) and incubated for 1 h at 37°C. After washing, the horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG(H+L), IgG1, or IgG2a monoclonal antibody (MAb) (1:1,000 dilution; Southern Biotech) was added to the wells (100 μl/well) and incubated for 1 h at 37°C. The subsequent color development and absorbance measurements were performed as described previously (19). The titers of anti-BV total IgG, IgG1, or IgG2a were determined as the highest dilutions at which the absorbance was 0.2 units higher than that in noninjected mice.
Human venous blood samples were collected from 20 healthy individuals with written consent. The sera were inactivated and 2-fold serially diluted. HRP-conjugated rabbit-anti human IgG(H+L) (1:1,000 dilution; Southern Biotech) was used for ELISAs, as mentioned above.
Analysis of neutralizing antibody titer and transducing titer.
The neutralizing antibody (NAb) titers were measured by an in vitro assay. The mouse or human inactivated serum samples were 2-fold serially diluted with PBS and mixed with an equal volume of EGFP-expressing Bac-CE (5 × 107 PFU in 100 μl). After 2 h of incubation at 4°C, the mixture containing the neutralized Bac-CE was used to transduce HeLa cells cultured in 6-well plates (5 × 105 cells/well). The cells were harvested 1 day later, and the total fluorescence intensity (FI) emitted by 10,000 cells was analyzed by using a flow cytometer (FACSCalibur; BD Biosciences). As the control, HeLa cells were transduced with Bac-CE, and the total fluorescence was measured. The neutralizing titer was defined as the dilution at which transduction was inhibited by >50% compared to transduction controls in the absence of serum (32). For gp64 antagonization, gp64 was first purified as described previously (33). Mouse sera were 100-fold diluted with PBS and mixed with an equal volume of Bac-CE and various concentrations of gp64. After incubation at 4°C for 2 h, the mixture was used to transduce HeLa cells. Bac-CE without mixing with the diluted sera and gp64 served as the control. The relative total fluorescence was calculated by dividing the total fluorescence of the experimental group by that of the control.
To measure the transducing titer, sera were diluted 20-fold with PBS and mixed with an equal volume of Bac-CE (5 × 107 PFU in 100 μl) at 4°C for 2 h. HeLa cells were transduced with the mixture containing the neutralized Bac-CE and cultured for 24 h. The transducing titer was calculated as described previously (30).
Peptide predication and synthesis.
The protein sequences of BV gp64 were obtained from GenBank (accession number NP_054158.1) and analyzed for mouse MHC class I binding motifs by using two computer-driven algorithms: SYPFEITHI and BIMAS. Four peptides in gp64 that were given the highest binding scores in both predication programs were chosen. These peptides, including the peptide at positions 457 to 465 (peptide 457-465) (KFGGVGTSL), 360-368 (TFLSDDTFL), 74-82 (AYAYNGGSL), and 428-436 (TYHDSWKDA), were synthesized (MDBio, Hsinchu, Taiwan), purified to >98% purity, dissolved in double-distilled water (ddH2O) at a concentration of 10 mg/ml, and stored at −20°C until use. MHC class I epitopes of the firefly luciferase (GFQSMYTFV) and nucleoprotein (NP) of avian influenza virus (IYSTVASSL) were synthesized and served as the positive and irrelevant controls, respectively.
ELISPOT assay and intracellular cytokine staining assay.
ELISPOT (enzyme-linked immunosorbent spot) assays of gamma interferon (IFN-γ) and interleukin-4 (IL-4) were performed by using mouse IFN-γ and IL-4 ELISPOT Ready-SET-Go! kits (eBioscience). The ELISPOT assay of granzyme B was performed by using a mouse ELISpot kit (R&D Systems). The spot-forming units (SFU) were measured by using the CTL-ImmunospotRS5 UV analyzer (Cellular Technology Ltd.). An intracellular cytokine assay coupled with flow cytometry was performed for the analysis of the frequency of CD4+ IFN-γ+ or CD8+ IFN-γ+ cells. All the experiments were performed according to the manufacturer's instructions.
Statistical analysis.
All quantitative data represent the average values measured for samples from 5 to 7 animals. The data were statistically analyzed by one-way analysis of variance (ANOVA) or Student's t test. P values of less than 0.05 were considered significant.
RESULTS
Transgene expression after repeated BV injection.
To examine the BV-mediated expression profile in vivo, a recombinant BV (Bac-luc/w) transiently expressing luciferase was constructed and i.m. injected into the right tibialis of BALB/c mice (3 × 109 PFU/mouse; n = 5) three times, at days 0, 14, and 28. Expression was monitored by bioluminescence imaging (Fig. 1A), and data for total flux (Fig. 1B) at different time points were normalized against that at day 1 (defined as 100%). After primary injection, luciferase expression was robust initially but decayed rapidly to ∼1.1% at day 7 and became barely detectable at day 14. Surprisingly, the booster injection at day 14 elevated the expression level only slightly at days 15 (∼3.9%) and 17 (∼1.6%). The third injection at day 28 only marginally stimulated expression at day 29 (∼2.5%). When Bac-luc/w was repeatedly injected at 3-day intervals (at days 0, 3, and 6), the second and third injections also failed to enhance the transgene expression level (data not shown). These data confirmed the transient expression kinetics in vivo and unraveled very poor expression mediated by repeated administration of BV.
Fig 1.
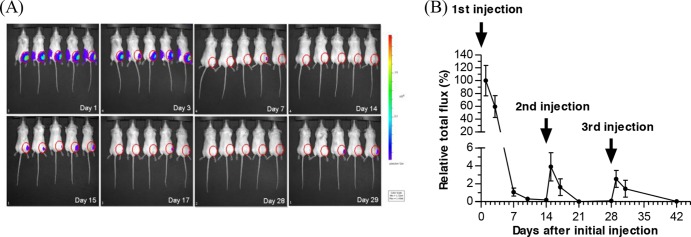
Inhibited transgene expression upon BV readministration. (A) Luciferase expression at different time points as detected by the Xenogen IVIS system. (B) Quantitative analysis of luciferase expression. A luciferase-expressing BV, Bac-luc/w, was i.m. injected into the right anterior muscle of BALB/c mice (n = 5; 3 × 109 PFU/mouse) three times, at days 0, 14, and 28. The total flux at different time points was normalized against that at day 1 and is expressed as relative total flux.
BV injection triggered neutralizing antibody responses.
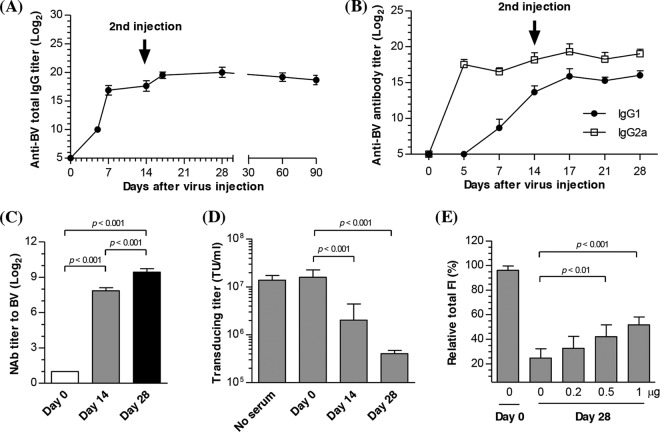
To elucidate whether BV triggered adaptive immune responses and their roles in the attenuated transgene expression, Bac-luc/w (3 × 109 PFU/animal; n = 7) was administered, as described in the legend of Fig. 1, twice, at days 0 and 14, and the anti-BV antibody responses were assessed by ELISA. Figure 2A shows that the primary injection elicited anti-BV IgG whose titers were detectable as early as day 5 (210) and continued to increase to 217 at day 14. After the second injection at day 14, the total IgG titers increased to ∼219 at day 17 and were maintained at ∼218 for at least 90 days. The anti-BV IgG response was dose dependent, because lowering the BV doses to 3 × 108 and 3 × 107 PFU/animal provoked lower but still evident anti-BV IgG titers (data not shown). The IgG subtype analysis (Fig. 2B) demonstrated a similar trend in the anti-BV IgG1 and IgG2a titers, yet IgG2a titers were significantly higher than IgG1 titers.
Fig 2.
Antibody responses to BV injection. (A) Anti-BV total IgG titers. (B) Anti-BV IgG1 and IgG2a titers. (C) BV-specific neutralizing antibody (NAb) titers. (D) Transducing titer of Bac-CE in the presence of mouse serum (20× dilution). (E) Effects of gp64-specific antibody on Bac-CE-mediated expression. BALB/c mice (n = 7) were injected i.m. with Bac-luc/w (3 × 109 PFU/mouse) twice, at days 0 and 14, and sera were collected at different time points for analyses. For NAb titer determination, serum samples taken at days 0, 14, and 28 were diluted and incubated with Bac-CE. HeLa cells were transduced with the neutralized Bac-CE, followed by total fluorescence measurement and calculation of NAb titers. For transducing titer measurement, Bac-CE was incubated with diluted sera (20× dilution), followed by determination of transducing titers. To evaluate the effect of anti-gp64 antibody on transduction, serum samples taken at day 28 were diluted 100-fold and mixed with increasing amounts of purified gp64 and Bac-CE. HeLa cells were transduced with the neutralized Bac-CE, and the relative total fluorescence was measured by flow cytometry. Statistical comparisons were performed by one-way ANOVA.
To confirm whether the antibody neutralized BV particles, an in vitro neutralization assay was performed, in which Bac-CE (a recombinant BV expressing EGFP) (30) was incubated with serially diluted sera, followed by transduction of HeLa cells, measurement of EGFP expression, and calculation of neutralizing antibody (NAb) titers (Fig. 2C). The NAb titer was at the background level (21) prior to injection and increased to ∼28 at day 14 after the primary injection. The second injection at day 14 elevated the NAb titer to 29 at day 28. The NAb titers were also dose dependent, as they became lower when the BV doses were reduced to 3 × 108 and 3 × 107 PFU/animal, and the second injection also significantly enhanced NAb titers (data not shown).
To quantify the effect of neutralizing antibodies on BV transduction, the transducing titer of Bac-CE, after mixing with serum, was measured as described previously (30) and is expressed as transducing units (TU)/ml (Fig. 2D). The transducing titer of Bac-CE (1.6 × 107 TU/ml) remained virtually unaffected when incubated with the serum samples withdrawn prior to BV injection (day 0) but decreased nearly 10-fold to 1.7 × 106 TU/ml when incubated with the serum samples taken at day 14. The transducing titer further dropped to 4.3 × 105 TU/ml when incubated with the serum samples taken at day 28, proving that the antibodies effectively neutralized BV and blocked the ability of BV to transduce mammalian cells.
gp64 is an envelope protein pivotal to BV transduction of mammalian cells (27). To assess whether the sera blocked transduction by antagonizing gp64, the serum samples taken at day 28 were treated with gp64 at various concentrations. Bac-CE was mixed with the treated sera and used for transduction of HeLa cells, followed by fluorescence intensity (FI) measurements. Figure 2E delineates that antagonizing the sera with increasing amounts of gp64 was able to restore transgene expression, suggesting that gp64 was a major immunogen and underscoring the importance of anti-gp64 neutralizing antibody in the blockade of transgene expression.
Absence of preexisting anti-BV antibody in humans.
The elicitation of anti-BV antibody and its ability to impede BV transduction raised a concern: do anti-BV antibodies preexist in humans? To address this question, sera were taken from 15 laboratory members who had more than 1 year of experience in handling BV and 5 non-laboratory members who had no prior contact with BV and were analyzed by BV-specific ELISA. Due to the lack of a compelling “negative control,” the ELISA data are expressed as optical density (OD) values. Figure 3A shows statistically similar (P > 0.1) OD values for sera from the laboratory and non-laboratory members. The sera were also mixed with Bac-CE for the neutralizing antibody assay, and Fig. 3B unravels comparable NAb titers (21 to 22) in the sera from the laboratory and non-laboratory members, which were very low compared with the background NAb titers for adeno-associated virus (AAV) (32). Therefore, these data indicated the absence of preexisting anti-BV antibodies in human sera.
Fig 3.
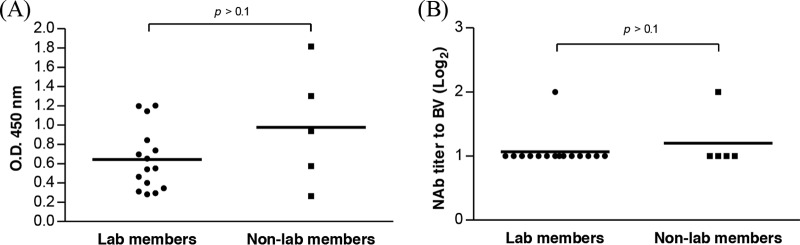
Detection of anti-BV antibody in human sera. (A) Anti-BV antibody titers analyzed by ELISA. (B) BV-specific NAb titers. Human sera were obtained from 20 healthy donors, 15 of whom were laboratory members and 5 of whom were not laboratory members who had no prior contact with BV. Statistical comparisons were performed by Student's t test.
BV-specific cellular immune responses.
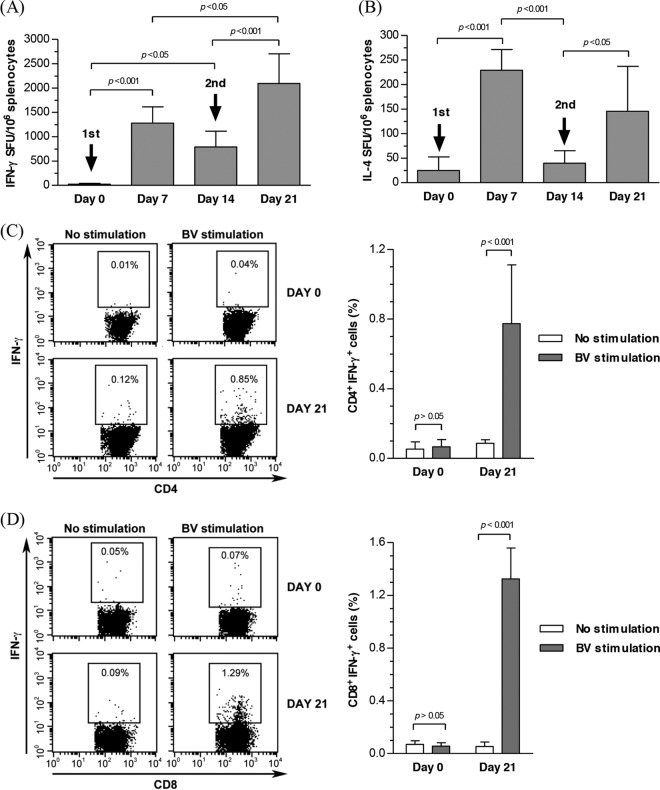
To further characterize the cellular responses evoked by BV, BALB/c mice were injected with Bac-luc/w twice, as described in the legend of Fig. 2, and splenocytes were harvested at different time points (n = 6 for each) for ELISPOT assays after pulsing with BV particles. Figure 4A and B delineates that BV significantly increased IFN-γ- and IL-4-expressing spot-forming units (SFU) at day 7, which declined at day 14. After the second injection at day 14, the IFN-γ and IL-4 responses were boosted again at day 21. Furthermore, intracellular cytokine staining, which measured the frequencies of BV-specific IFN-γ-expressing mouse CD4+ and CD8+ T cells at days 0 and 21, demonstrated the activation of CD4+ and CD8+ T cells after BV injection, as evidenced by the significant increase in the percentages of CD4+ IFN-γ+ (Fig. 4C) and CD8+ IFN-γ+ (Fig. 4D) T cells after BV stimulation.
Fig 4.
BV-specific cellular immune response. (A) Frequency of IFN-γ-expressing mouse splenocytes. (B) Frequency of IL-4-expressing mouse splenocytes. (C) Frequency of CD4+ IFN-γ+ T cells. (D) Frequency of CD8+ IFN-γ+ T cells. Mice were i.m. injected with Bac-luc/w as described in the legend of Fig. 2, and splenocytes were harvested at different time points and stimulated with BV particles for ELISPOT assays. Splenocytes harvested at days 0 and 21 were either not stimulated or stimulated with BV particles for intracellular cytokine staining/flow cytometry to detect the frequencies of CD4+ IFN-γ+ and CD8+ IFN-γ+ T cells. Statistical comparisons were performed by one-way ANOVA.
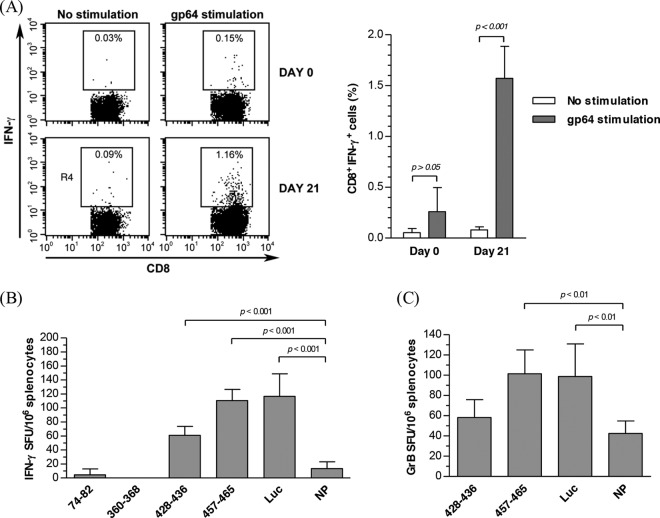
gp64 is a glycoprotein abundantly present on the BV envelope and may potentially induce immune responses in vivo. Indeed, by pulsing the mouse splenocytes with purified gp64 and intracellular cytokine staining, we demonstrated that gp64 stimulation significantly enhanced the frequency of CD8+ IFN-γ+ cells (Fig. 5A). To identify the gp64 peptides accounting for the CD8+ T cell activation, 4 putative major histocompatibility complex (MHC) class I epitope peptides with the highest binding scores were predicted by using the SYFPEITHI and BIMAS databases, synthesized, and used to pulse the cells harvested at day 21. The ELISPOT assay (Fig. 5B) showed that peptides 74-82 and 360-368 failed to induce an IFN-γ response compared with the negative control NP (nucleoprotein peptide of AIV), while peptides 428-436 and 457-465 pronouncedly increased the frequencies of IFN-γ-producing cells. In particular, peptide 457-465 elicited the strongest IFN-γ response, which was statistically comparable to that of the positive-control luciferase peptide. In accord with the elicitation of CD8+ T cell responses, peptide 457-465, but not peptide 428-436, significantly increased the frequencies of granzyme B-expressing splenocytes (Fig. 5C), suggesting the activation of cytotoxic T lymphocytes (CTL). These data altogether confirmed that peptide 457-465 (KFGGVGTSL) on gp64 is the primary MHC class I epitope to stimulate the CD8+ T cell response.
Fig 5.
Identification of antigen and peptide epitopes responsible for the CD8+ T cell response. (A) Frequency of CD8+ IFN-γ+ T cells in response to gp64 stimulation. (B) Frequencies of IFN-γ+ T cells responding to different peptides. (C) Frequencies of granzyme B (GrB)-expressing cells responding to different peptides. Mice (n = 6 for each time point) were immunized as described in the legend of Fig. 4, and splenocytes were harvested at day 0 or 21 and stimulated with purified gp64 for intracellular cytokine staining analysis of the frequency of CD8+ IFN-γ+ T cells. Alternatively, splenocytes were stimulated with putative gp64 epitopes for ELISPOT analysis of IFN-γ and granzyme B-expressing cells. MHC class I epitopes of the firefly luciferase (Luc) and nucleoprotein (NP) of AIV served as the positive and irrelevant controls, respectively. Statistical comparisons were performed by one-way ANOVA.
Prolonged transgene expression by Sleeping Beauty-based hybrid BV vectors.
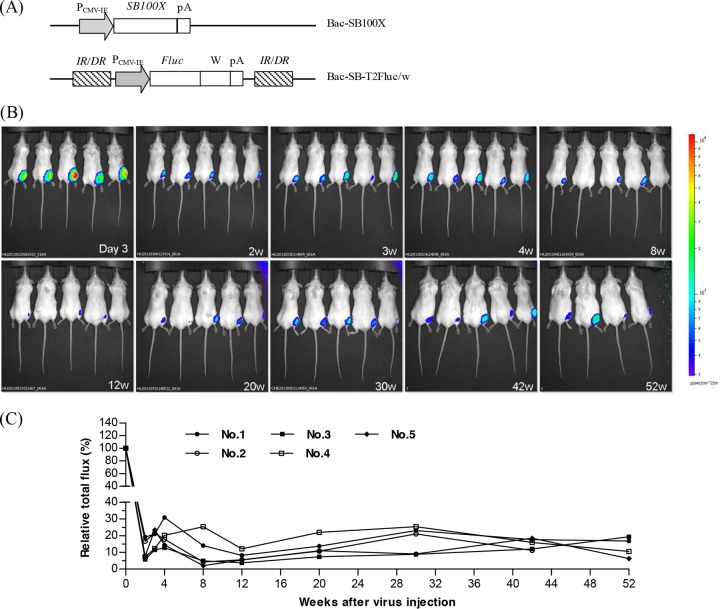
The data mentioned above collectively demonstrated that repeated administration of BV triggered humoral and cellular immune responses, thus mitigating transgene expression. To circumvent this problem, we developed a dual BV system exploiting the Sleeping Beauty (SB) transposon (Fig. 6A), in which one BV (Bac-SB100X) expressed the SB100X enzyme while the other BV (Bac-SB-T2Fluc/w) encoded the luciferase cassette flanked by IR/DR sequences, so that after cotransduction, SB100X was able to transpose the IR/DR-flanking cassette into the mouse chromosome. Coinjection of both Bac-SB100X and Bac-SB-T2Fluc/w into BALB/c mice led to robust luciferase expression at day 3 (defined as 100%), and the expression level declined at week 2 (Fig. 6B). Nonetheless, transgene expression persisted for at least 52 weeks (Fig. 6B and C), indicating that the SB transposon system was able to integrate the transgene into the mouse chromosome, partly circumvent the anti-BV immunity problems, and confer stable BV-mediated expression in vivo.
Fig 6.
Stable expression conferred by one injection of SB-based hybrid BV vectors. (A) Schematic illustration of Bac-SB100X and Bac-T2Fluc/w. (B) Bioluminescence imaging illustrating stable expression for up to 52 weeks. (C) Quantitative analysis of luciferase expression. Bac-SB100X and Bac-T2Fluc/w (109 PFU each) were coinjected into the right anterior muscle of 5 BALB/c mice, and luciferase expression was imaged by using the Xenogen IVIS system. The mice were labeled 1 to 5. Prior to week 52, 1 mouse died; thus, only 4 mice are shown in the bottom right panel. Both SB100X and the firefly luciferase gene were driven by the CMV-IE promoter. A WPRE element was added at the 3′ end of the firefly luciferase gene to enhance expression. IR/DR is an element for SB transposase recognition. Luminescence output is expressed as the relative total flux.
DISCUSSION
Adaptive immune responses against viruses such as AAV, adenovirus (Ad), and lentivirus (LV) remain a major obstacle to the success of virus-based gene therapy, as cellular responses affect viral clearance and humoral responses prevent vector readministration (34). Although the immune responses against Ad and AAV have been well characterized (34, 35), and there are similarities in immunity to different viruses, each vector contains its own set of activation signals (e.g., cytokine and costimulation molecules), resulting in distinct effector T and B cell responses (34). For instance, in vivo, Ad capsid induces inflammatory cytokines, activates dendritic cells (DCs) and natural killer (NK) cells, and results in IFN-γ expression, which are keys to stimulating Ad-specific adaptive responses responsible for extinguished transgene expression (36). In contrast, AAV is much less immunogenic than Ad and results in exceedingly long-term transgene expression in various preclinical animal models (35). The discrepancy in the responsiveness against AAV and Ad reinforces the importance of understanding how and whether viral vectors provoke adaptive immune responses.
Although BV-mediated in vivo transgene expression has been widely exploited in the vaccine and anticancer therapy settings, how the adaptive responses elicited by primary BV administration would impact the ensuing BV-mediated transgene expression and efficacy has yet to be explored. Here we uncovered that BV injection into the right tibialis conferred strong transgene expression but concomitantly triggered responses that impeded transgene expression when BV was readministered at the same site either 3 days (data not shown) or 14 days (Fig. 1) apart. It should be noted that luciferase expression was robust when BV was readministered at the contralateral (left) tibialis at day 3, but expression was considerably repressed when BV was readministered at the same site (data not shown). These observations suggested that local inflammation (e.g., elicitation of alpha/beta interferon [37, 38]) arose soon after BV administration into the muscle, which retarded the transgene expression mediated by BV injected into the same site at day 3, but the local inflammation failed to impede expression mediated by BV injected into the distant site at day 3 (data not shown). Conversely, the poor expression after readministration at the distant site at day 14 presumably stemmed from the emergence of systemic immune responses (data not shown).
Indeed, we unraveled that primary BV injection induced systemic IgG1 and IgG2a antibodies (Fig. 2A and B). These antibodies were able to neutralize BV and hence impede BV-mediated transduction and transgene expression by antagonizing gp64 (Fig. 2C to E). Moreover, BV injection elicited cellular immune responses, as manifested by the induction of BV-specific IFN-γ- and IL-4-expressing splenocytes (Fig. 4A and B). Since IFN-γ is indicative of Th1 responses, while IL-4 is indicative of Th2 responses, these results demonstrated that BV induced mixed Th1 and Th2 responses, but the responses were Th1 biased, as judged by the higher IgG2a titers than IgG1 titers (Fig. 2B). Furthermore, BV injection triggered BV-specific CD4+ and CD8+ T cell responses (Fig. 4C and D) due to the gp64 protein on the BV envelope (Fig. 5A). Specifically, peptide 457-465 on gp64 was identified to be the major MHC class I epitope responsible for CD8+ T cell stimulation (Fig. 5B) and CTL activities (Fig. 5C).
Innate responses, including the induction of IFN-α/β, are crucial to bridge innate and adaptive immunity (39). BV transduction in vitro and in vivo elicits the production of IFN-α/β, IL-6, IL-8, tumor necrosis factor alpha (TNF-α), and IL-1β (27–29, 33, 40) and activates DCs (27), as evidenced by IFN-γ secretion and upregulation of MHC class I and II and costimulatory molecules (e.g., CD40, CD80, and CD86) (41). In vivo BV administration activates macrophages (27), DCs (42), and NK cells (9). Furthermore, BV administration in vivo can initiate the complement cascade pathway (43), which, acting in concert with IFN-α/β, can provoke NK cells and substantiate the uptake of BV particles, resulting in the further release of cytokines. These events are pivotal to the priming and enhancement of adaptive immunity.
As such, it is envisaged that after the primary i.m. injection, BV-induced innate immunity potentiates BV uptake by the resident antigen-presenting cells (APCs), including DCs and macrophages, and stimulates the maturation of DCs. These antigen-loaded DCs migrate to lymphoid organs (e.g., lymph node and spleen) and activate naive B and T cells via classical presentation or cross-presentation of the BV antigenic peptides, hence triggering antibody secretion and CD4+ (including Th1 and Th2) and CD8+ T cell responses specific for BV. The antibodies not only can neutralize the virus but also in turn can activate the classical, antibody-dependent complement pathway to substantiate the uptake of BV by macrophages (34), thus further potentiating the immune responses against BV. Consequently, BV readministration at day 14 or 35 gave rise to only poor transgene expression (data not shown), which explained why repeated BV immunization did not significantly enhance the antigen-specific antibody titers in a previous study (24). Note, however, that the adaptive responses are not the only factors that restrict transgene expression upon readministration, because BV readministration to nude mice defective in T cell maturation also gave rise to impaired transgene expression compared with the primary injection (data not shown). Since BV-mediated expression can be suppressed by IFN-α/β and complement (43), innate immunity may also play a role in the poor transgene expression upon BV readministration.
These humoral and cellular responses elicited against BV contributed to the ineffective BV readministration and posed a challenge for BV-mediated gene transfer. Nonetheless, this problem can be partly circumvented by the use of the SB-based hybrid BV vector that enables transgene integration and long-term expression for >1 year by a single injection (Fig. 6). The stable expression implicates the potential of the hybrid BV in therapies necessitating persistent expression, such as cancer, diabetes, and muscular dystrophy. More importantly, this proved that, despite the activation of CD8+ T cells and even CTL, the T cell response elicited by a single BV administration does not completely eradicate BV-transduced cells. A similar phenomenon was also observed for AAV-mediated gene therapy, whereby CTL triggered by AAV capsid failed to eliminate AAV-transduced cells in mice (44). In stark contrast, Ad-mediated expression is transient due to the clearance of Ad-transduced cells by CTL. The disparity in the clearance of BV- and Ad-transduced cells supported the notion that the magnitude of the anti-BV T cell response is lower than that of the anti-Ad response (42), which might be attributed to the differential ability to transduce APCs. Unlike Ad, which can transduce DCs at efficiencies approaching 100% (45) and give rise to potent immunogenicity, BV poorly transduces DCs (13, 46) and macrophages (27), thus leading to a lower immunogenicity than that of Ad.
Taken together, this study demonstrated that i.m. injection of BV triggers BV-specific humoral and cellular responses that could neutralize BV particles and hinder subsequent BV administration and transgene expression. Therefore, adaptive immunity, in addition to innate immunity, should be taken into consideration when designing the BV administration scheme. Fortunately, humans possess neither preexisting anti-BV antibodies (Fig. 3) nor BV-specific T cells (46), which circumvents the preexisting immunity problems encountered with Ad and AAV vectors. The SB-based hybrid BV that confers sustained expression by a single injection thus holds great promise for human gene therapy (e.g., cancer therapy). In the context of vaccine applications that require prime-boost administrations, the BV-specific immune responses can still compromise vaccine efficacy upon booster injections, which may be alleviated by several approaches. (i) The antigen expression level may be augmented (e.g., by using a strong promoter) so as to lower the required BV dose or by using a different vector (e.g., AAV) for the prime-boost regimen. (ii) Since gp64 is a major immunogen eliciting both humoral and cellular responses, escape of immune surveillance may be achieved by swapping the immunodominant epitopes (e.g., peptides 457-465 and 428-436) on gp64 with immunotolerant peptides or by pseudotyping BV with an envelope protein functionally analogous to gp64, such as F protein derived from a heterologous baculovirus (47) or G protein derived from vesicular stomatitis virus (13). (iii) BV genomic DNA contains abundant unmethylated CpG motifs (27) that can trigger innate immunity and, hence, subsequent adaptive immunity. Engineering of the viral genome to remove unnecessary genes and unmethylated CpG motifs may help diminish the innate/adaptive responses (37). Coupling Ad particles with polyethylene glycol (PEG) attenuates immunogenicity owing to reduced uptake by DCs (48). As such, conjugating BV with PEG or pseudotyping BV envelope with a decay-accelerating factor (43, 49) that confers complement resistance may help mitigate the BV-specific immune response. Aside from gp64, other major BV capsid proteins (e.g., vp39) may also be immunogenic, and their roles in anti-BV immunity await further examination. Additionally, several BV genes (e.g., orf149, ie0, p35, and gp64) are weakly expressed in transduced mammalian cells (50) despite the absence of viral replication. Determination of whether the BV-associated gene products also contribute to adaptive responses is worthy of further investigation.
ACKNOWLEDGMENTS
We thank Perry. B. Hackett of the University of Minnesota, Zsuzsanna Izsvak of the Max-Delbrück Center for Molecular Medicine Berlin, and Shu Wang of the National University of Singapore for providing plasmids for BV construction.
We also acknowledge financial support from the National Tsing Hua University (Toward World-Class University Project grants 100N2050E1 and 102N2051E1) and the National Science Council, Taiwan (NSC) (grants 99-2221-E-007-025-MY3, 101-2628-E-007-009-MY3, and 101-2923-E-007-002-MY3).
We declare no conflicts of interest.
Footnotes
Published ahead of print 13 February 2013
REFERENCES
- 1. Chen C-Y, Lin C-Y, Chen G-Y, Hu Y-C. 2011. Baculovirus as a gene delivery vector: recent understandings of molecular alterations in transduced cells and latest applications. Biotechnol. Adv. 29:618–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kost TA, Condreay JP, Jarvis DL. 2005. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 23:567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Airenne KJ, Makkonen K-E, Mähönen AJ, Ylä-Herttuala S. 2010. In vivo application and tracking of baculovirus. Curr. Gene Ther. 10:187–194 [DOI] [PubMed] [Google Scholar]
- 4. Ong S-T, Li F, Du J, Tan Y-W, Wang S. 2005. Hybrid cytomegalovirus enhancer-H1 promoter-based plasmid and baculovirus vectors mediate effective RNA interference. Hum. Gene Ther. 16:1404–1412 [DOI] [PubMed] [Google Scholar]
- 5. Kost TA, Condreay JP, Ames RS. 2010. Baculovirus gene delivery: a flexible assay development tool. Curr. Gene Ther. 10:168–173 [DOI] [PubMed] [Google Scholar]
- 6. Lesch HP, Laitinen A, Peixoto C, Vicente T, Makkonen KE, Laitinen L, Pikkarainen JT, Samaranayake H, Alves PM, Carrondo MJ, Yla-Herttuala S, Airenne KJ. 2011. Production and purification of lentiviral vectors generated in 293T suspension cells with baculoviral vectors. Gene Ther. 18:531–538 [DOI] [PubMed] [Google Scholar]
- 7. Liu CY-Y, Chen H-Z, Chao Y-C. 2010. Maximizing baculovirus-mediated foreign proteins expression in mammalian cells. Curr. Gene Ther. 10:232–241 [DOI] [PubMed] [Google Scholar]
- 8. Lin C-Y, Lu C-H, Luo W-Y, Chang Y-H, Sung L-Y, Chiu H-Y, Hu Y-C. 2010. Baculovirus as a gene delivery vector for cartilage and bone tissue engineering. Curr. Gene Ther. 10:242–254 [DOI] [PubMed] [Google Scholar]
- 9. Kitajima M, Abe T, Miyano-Kurosaki N, Taniguchi M, Nakayama T, Takaku H. 2008. Induction of natural killer cell-dependent antitumor immunity by the Autographa californica multiple nuclear polyhedrosis virus. Mol. Ther. 16:261–268 [DOI] [PubMed] [Google Scholar]
- 10. Suzuki T, Oo Chang M, Kitajima M, Takaku H. 2010. Induction of antitumor immunity against mouse carcinoma by baculovirus-infected dendritic cells. Cell. Mol. Immunol. 7:440–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yin H-Y, Zhou X-A, Wu H-F, Li B-A, Zhang Y-F. 2010. Baculovirus vector-mediated transfer of NIS gene into colon tumor cells for radionuclide therapy. World J. Gastroenterol. 16:5367–5374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bak XY, Lam DH, Yang JY, Ye K, Wei ELX, Lim SK, Wang S. 2011. Human embryonic stem cell-derived mesenchymal stem cells as cellular delivery vehicles for prodrug gene therapy of glioblastoma. Hum. Gene Ther. 22:1365–1377 [DOI] [PubMed] [Google Scholar]
- 13. Kim C-H, Yoon J-S, Sohn H-J, Kim C-K, Paik S-Y, Hong Y-K, Kim T-G. 2007. Direct vaccination with pseudotype baculovirus expressing murine telomerase induces anti-tumor immunity comparable with RNA-electroporated dendritic cells in a murine glioma model. Cancer Lett. 250:276–283 [DOI] [PubMed] [Google Scholar]
- 14. Lee EX, Lam DH, Wu C, Yang J, Tham CK, Ng WH, Wang S. 2011. Glioma gene therapy using induced pluripotent stem cell derived neural stem cells. Mol. Pharm. 8:1515–1524 [DOI] [PubMed] [Google Scholar]
- 15. Wu C, Lin J, Hong M, Choudhury Y, Balani P, Leung D, Dang L-H, Zhao Y, Zeng J, Wang S. 2009. Combinatorial control of suicide gene expression by tissue-specific promoter and microRNA regulation for cancer therapy. Mol. Ther. 17:2058–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao Y, Lam DH, Yang J, Lin J, Tham CK, Ng WH, Wang S. 2012. Targeted suicide gene therapy for glioma using human embryonic stem cell-derived neural stem cells genetically modified by baculoviral vectors. Gene Ther. 19:189–200 [DOI] [PubMed] [Google Scholar]
- 17. Luo WY, Shih YS, Hung CL, Lo KW, Chiang CS, Lo WH, Huang SF, Wang SC, Yu CF, Chien CH, Hu YC. 2012. Development of the hybrid Sleeping Beauty-baculovirus vector for sustained gene expression and cancer therapy. Gene Ther. 19:844–851 [DOI] [PubMed] [Google Scholar]
- 18. Luo WY, Shih YS, Lo WH, Chen HR, Wang SC, Wang CH, Chien CH, Chiang CS, Chuang YJ, Hu YC. 2011. Baculovirus vectors for antiangiogenesis-based cancer gene therapy. Cancer Gene Ther. 18:637–645 [DOI] [PubMed] [Google Scholar]
- 19. Chen C-Y, Liu H-J, Tsai C-P, Chung C-Y, Shih Y-S, Chang P-C, Chiu Y-T, Hu Y-C. 2010. Baculovirus as an avian influenza vaccine vector: differential immune responses elicited by different vector forms. Vaccine 28:7644–7651 [DOI] [PubMed] [Google Scholar]
- 20. Prabakaran M, Madhan S, Prabhu N, Qiang J, Kwang J. 2010. Gastrointestinal delivery of baculovirus displaying influenza virus hemagglutinin protects mice against heterologous H5N1 infection. J. Virol. 84:3201–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu Q-F, Fang L-R, Wu X-B, Li B, Luo R, Yu Z-J, Jin M-L, Chen H-C, Xiao S-B. 2009. A pseudotype baculovirus-mediated vaccine confers protective immunity against lethal challenge with H5N1 avian influenza virus in mice and chickens. Mol. Immunol. 46:2210–2217 [DOI] [PubMed] [Google Scholar]
- 22. Wu Q, Xiao S, Fan H, Li Y, Xu J, Li Z, Lu W, Su X, Zou W, Jin M, Chen H, Fang L. 2011. Protective immunity elicited by a pseudotyped baculovirus-mediated bivalent H5N1 influenza vaccine. Antiviral Res. 92:493–496 [DOI] [PubMed] [Google Scholar]
- 23. Lin Y-H, Lee L-H, Shih W-L, Hu Y-C, Liu H-J. 2008. Baculovirus surface display of σC and σB proteins of avian reovirus and immunogenicity of the displayed proteins in a mouse model. Vaccine 26:6361–6367 [DOI] [PubMed] [Google Scholar]
- 24. Grabowska AK, Lipinska AD, Rohde J, Szewczyk B, Bienkowska-Szewczyk K, Rziha HJ. 2009. New baculovirus recombinants expressing pseudorabies virus (PRV) glycoproteins protect mice against lethal challenge infection. Vaccine 27:3584–3591 [DOI] [PubMed] [Google Scholar]
- 25. Premanand B, Kiener TK, Meng T, Tan YR, Jia Q, Chow VTK, Kwang J. 2012. Induction of protective immune responses against EV71 in mice by baculovirus encoding a novel expression cassette for capsid protein VP1. Antiviral Res. 95:311–315 [DOI] [PubMed] [Google Scholar]
- 26. Yoshida S, Araki H, Yokomine T. 2010. Baculovirus-based nasal drop vaccine confers complete protection against malaria by natural boosting of vaccine-induced antibodies in mice. Infect. Immun. 78:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abe T, Hemmi H, Miyamoto H, Moriishi K, Tamura S, Takaku H, Akira S, Matsuura Y. 2005. Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J. Virol. 79:2847–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abe T, Kaname Y, Wen X, Tani H, Moriishi K, Uematsu S, Takeuchi O, Ishii KJ, Kawai T, Akira S, Matsuura Y. 2009. Baculovirus induces type I interferon production through Toll-like receptor-dependent and -independent pathways in a cell-type-specific manner. J. Virol. 83:7629–7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abe T, Matsuura Y. 2010. Host innate immune responses induced by baculovirus in mammals. Curr. Gene Ther. 10:226–231 [DOI] [PubMed] [Google Scholar]
- 30. Chan Z-R, Lai C-W, Lee H-P, Chen H-C, Hu Y-C. 2006. Determination of the baculovirus transducing titer in mammalian cells. Biotechnol. Bioeng. 93:564–571 [DOI] [PubMed] [Google Scholar]
- 31. Chen C-L, Luo W-Y, Lo W-H, Lin K-J, Sung L-Y, Shih Y-S, Chang Y-H, Hu Y-C. 2011. Development of hybrid baculovirus vectors for artificial microRNA delivery and prolonged gene suppression. Biotechnol. Bioeng. 108:2958–2967 [DOI] [PubMed] [Google Scholar]
- 32. Rapti K, Louis-Jeune V, Kohlbrenner E, Ishikawa K, Ladage D, Zolotukhin S, Hajjar RJ, Weber T. 2012. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol. Ther. 20:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen G-Y, Shiah H-C, Su H-J, Chen C-Y, Chuang Y-J, Lo W-H, Huang J-L, Chuang C-K, Hwang S-M, Hu Y-C. 2009. Baculovirus transduction of mesenchymal stem cells triggers the Toll-like receptor 3 (TLR3) pathway. J. Virol. 83:10548–10556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nayak S, Herzog RW. 2010. Progress and prospects: immune responses to viral vectors. Gene Ther. 17:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mingozzi F, High KA. 2011. Immune responses to AAV in clinical trials. Curr. Gene Ther. 11:321–330 [DOI] [PubMed] [Google Scholar]
- 36. Muruve DA. 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther. 15:1157–1166 [DOI] [PubMed] [Google Scholar]
- 37. Abe T, Takahashi H, Hamazaki H, Miyano-Kurosaki N, Matsuura Y, Takaku H. 2003. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 171:1133–1139 [DOI] [PubMed] [Google Scholar]
- 38. Lee H-P, Matsuura Y, Chen H-C, Chen Y-L, Chuang C-K, Abe T, Hwang S-M, Shiah H-C, Hu Y-C. 2009. Baculovirus transduction of chondrocytes elicits interferon-α/β and suppresses transgene expression. J. Gene Med. 11:302–312 [DOI] [PubMed] [Google Scholar]
- 39. Somanathan S, Breous E, Bell P, Wilson JM. 2010. AAV vectors avoid inflammatory signals necessary to render transduced hepatocyte targets for destructive T cells. Mol. Ther. 18:977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chuang C-K, Wong T-H, Hwang S-M, Chang Y-H, Chen Y-H, Chiu Y-C, Huang S-F, Hu Y-C. 2009. Baculovirus transduction of mesenchymal stem cells: in vitro responses and in vivo immune responses after cell transplantation. Mol. Ther. 17:889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suzuki T, Chang MO, Kitajima M, Takaku H. 2010. Baculovirus activates murine dendritic cells and induces non-specific NK cell and T cell immune responses. Cell. Immunol. 262:35–43 [DOI] [PubMed] [Google Scholar]
- 42. Hervas-Stubbs S, Rueda P, Lopez L, Leclerc C. 2007. Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J. Immunol. 178:2361–2369 [DOI] [PubMed] [Google Scholar]
- 43. Kaikkonen MU, Maatta AI, Yla-Herttuala S, Airenne KJ. 2010. Screening of complement inhibitors: shielded baculoviruses increase the safety and efficacy of gene delivery. Mol. Ther. 18:987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li H, Murphy SL, Giles-Davis W, Edmonson S, Xiang Z, Li Y. 2007. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol. Ther. 15:792–800 [DOI] [PubMed] [Google Scholar]
- 45. Roth MD, Cheng Q, Harui A, Basak SK, Mitani K, Low TA, Kiertscher SM. 2002. Helper-dependent adenoviral vectors efficiently express transgenes in human dendritic cells but still stimulate antiviral immune responses. J. Immunol. 169:4651–4656 [DOI] [PubMed] [Google Scholar]
- 46. Strauss R, Huser A, Ni S, Tuve S, Kiviat N, Sow PS, Hofmann C, Lieber A. 2007. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against Plasmodium falciparum circumsporozoite protein. Mol. Ther. 15:193–202 [DOI] [PubMed] [Google Scholar]
- 47. Lung O, Westenberg M, Vlak JM, Zuidema D, Blissard GW. 2002. Pseudotyping Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV): F proteins from group II NPVs are functionally analogous to AcMNPV GP64. J. Virol. 76:5729–5736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Croyle MA, Chirmule N, Zhang Y, Wilson JM. 2001. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J. Virol. 75:4792–4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaname Y, Tani H, Kataoka C, Shiokawa M, Taguwa S, Abe T, Moriishi K, Kinoshita T, Matsuura Y. 2010. Acquisition of complement resistance through incorporation of CD55/decay-accelerating factor into viral particles bearing baculovirus GP64. J. Virol. 84:3210–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fujita R, Matsuyama T, Yamagishi J, Sahara K, Asano S, Bando H. 2006. Expression of Autographa californica multiple nucleopolyhedrovirus genes in mammalian cells and upregulation of the host β-actin gene. J. Virol. 80:2390–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]