Abstract
Poxvirus infections have been found in 230 species of wild and domestic birds worldwide in both terrestrial and marine environments. This ubiquity raises the question of how infection has been transmitted and globally dispersed. We present a comprehensive global phylogeny of 111 novel poxvirus isolates in addition to all available sequences from GenBank. Phylogenetic analysis of the Avipoxvirus genus has traditionally relied on one gene region (4b core protein). In this study we expanded the analyses to include a second locus (DNA polymerase gene), allowing for a more robust phylogenetic framework, finer genetic resolution within specific groups, and the detection of potential recombination. Our phylogenetic results reveal several major features of avipoxvirus evolution and ecology and propose an updated avipoxvirus taxonomy, including three novel subclades. The characterization of poxviruses from 57 species of birds in this study extends the current knowledge of their host range and provides the first evidence of the phylogenetic effect of genetic recombination of avipoxviruses. The repeated occurrence of avian family or order-specific grouping within certain clades (e.g., starling poxvirus, falcon poxvirus, raptor poxvirus, etc.) indicates a marked role of host adaptation, while the sharing of poxvirus species within prey-predator systems emphasizes the capacity for cross-species infection and limited host adaptation. Our study provides a broad and comprehensive phylogenetic analysis of the Avipoxvirus genus, an ecologically and environmentally important viral group, to formulate a genome sequencing strategy that will clarify avipoxvirus taxonomy.
INTRODUCTION
Avian pox is a viral disease affecting more than 230 species in 23 orders of wild and domesticated birds (1). Poxviruses were identified as causative agents of pox lesions almost a century ago (2, 3), but understanding of their phylogenetics and epidemiology remains rudimentary. The genomes of only two well-diverged avian poxviruses (isolated from chicken and canaries) have thus far been sequenced. All avian poxviruses (avipoxviruses) are assigned to the genus Avipoxvirus in the subfamily Chordopoxvirinae of the Poxviridae family. Within the Avipoxvirus genus there are currently 10 recognized species (established primarily in the presequence era, with subsequent limited use of restriction fragment length polymorphism analysis): Fowlpox virus, Canarypox virus, Juncopox virus, Mynahpox virus, Psittacinepox virus, Sparrowpox virus, Starlingpox virus, Pigeonpox virus, Turkeypox virus, and Quailpox virus, according to the International Committee on Taxonomy of Viruses (www.ictvonline.org). The exact number of existing avipoxvirus species, strains, and variants is unknown, since new isolates continue to be identified from a wide variety of avian species, such as Berthelot's pipit (Anthus berthelotii) (4), lesser flamingos (Phoenicopterus minor) (5), or crested serpent eagle (Spilornis cheela) (6).
Avian pox infections cause significant economic losses in domestic poultry due to decreased egg production, reduced growth, blindness, and increased mortality (7). Effects of avian pox on wild bird species can also be severe. The infection may produce several negative effects including elevated predation among affected birds (8), secondary infections, trauma, reduced male mating success (9) and death (10). The lifestyle of wild birds allows avian poxviruses to reach new hosts through bird migration, species introductions, and habitat change. Avian pox has been identified as an important risk factor in the conservation of small and endangered populations, particularly in island bird species (4). The impact of the introduction of avian pox has been disastrous for the avifauna of various archipelagos (11). Poxvirus infection has been responsible for the population decline of native bird species on Hawaii (12), Galápagos (2, 13), and the Canary Islands (14). Avian pox has also been identified as a risk factor in the reintroduction programs of houbara bustard (Chlamydotis undulata macqueenii) in the Middle East, Floreana mockingbirds (Mimus trifasciatus) in Galapagos (15, 16), and peregrine falcons (Falco peregrinus) in Germany (17). The recent emergence of an epizootic of conspicuous and distinctive avian pox among great tits (Parus major) in the United Kingdom (18), and its penetrance of a historically well-studied population near Oxford, allowed detailed study of the epidemiology (19) and population-level impacts (20) of the disease in wild birds.
The currently available vaccines against fowlpox, canarypox, pigeon pox, and quail pox are each produced using virus strains isolated from the respective avian group. There is an increasing demand for new vaccines against avian poxvirus infections to help protect a wide range of birds, especially endangered species (21).
Fowlpox virus is the type species of the Avipoxvirus genus. The complete genomic sequences of Fowlpox virus (AF198100) (22) and Canarypox virus (AY318871) (23) are available. The two genomes are highly diverged, sharing only ca. 70% sequence identity. The 365-kbp genome of Canarypox virus is larger than that of Fowlpox virus (288 kbp) and shows significant differences in gene content, particularly in the expansion and diversification of some gene families that are already large in Fowlpox virus, notably the ankyrin repeat proteins (19). The phylogenetic relationships among avipoxviruses are only partially characterized. Comparative analysis of genomic sequences is the most informative and reliable method for comparing closely related viral genomes, so a definite phylogeny will have to await additional genome sequencing. The relationships of avian poxviruses isolated from free-ranging birds have been analyzed using DNA sequences of the 4b core protein coding genomic region (21, 24–27). Until recently, the significant divergence among avipoxviruses impeded the efforts to identify other pan-genus PCR primers. Jarmin et al. (25) and Manarolla et al. (21) sequenced the fpv140 locus (FPV140 gene; virion envelope protein, p35) of some avian poxvirus strains, while Thiel et al. (13) sequenced the intergenic region between CA.X (CNPV114 gene; HT motif protein), and TK (CNPV113 gene; thymidine kinase) genes. Unfortunately, these markers appeared to fail to identify some clades or subclades that were identified by the 4b core protein-based PCR system. These phylogenetic studies have concluded that the vast majority of avian poxvirus isolates clustered into three major clades, represented by the Fowlpox virus (clade A), the Canarypox virus (clade B), and the Psittacinepox virus (clade C). However, other pan-genus markers, similar to the 4b core protein coding genomic region, are needed in order to achieve a more robust phylogenetic classification of avian poxviruses.
This study was aimed at identifying another such pan-genus marker from the wider set of genomic core genes (the DNA polymerase gene) and combining it with sequences from the 4b region to provide a robust and global phylogenetic framework for the study and classification of avian poxviruses. Our analysis included partial 4b core protein and DNA polymerase gene sequences of virus strains isolated from natural pox infection cases occurring in 111 wild and captive birds from 57 different species sampled in North and South America, Europe, Asia, Antarctica, and the Pacific Ocean.
MATERIALS AND METHODS
Sample collection and preparation.
Samples were collected by biopsy or during postmortem examinations from a wide range of clinically ill or dead birds in the United States, Ecuador (Galapagos Islands), Argentina, Chile, Hungary, Spain, Netherlands, Belgium, United Kingdom, South Korea, and Antarctica (Table 1). Tissue samples were frozen at −20 or −80°C or fixed in 10% neutral buffered formalin and embedded in paraffin blocks.
Table 1.
List of samples with their information and GenBank accession numbers of derived sequences used in the study
| Subclade (clade)a | Sample code | GenBank ID |
English name | Latin name | Order | Family | Originb | Yr | Pox lesion category | Source for DNA extraction | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4b core protein gene sequence | DNA polymerase gene sequence | ||||||||||
| A1 | P1 | KC017960 | KC017850 | Domestic fowl | Gallus domesticus | Galliformes | Phasianidae | Hungary† | 2003 | Cutaneous | Skin lesion |
| A1 | P2 | KC017961 | KC017866 | Domestic turkey | Meleagris gallopavo | Galliformes | Phasianidae | Nevada (USA) | 2005 | Cutaneous-oral mucosa | Tissue culture |
| A1 | P3 | KC017962 | KC017867 | Domestic fowl | Gallus domesticus | Galliformes | Phasianidae | Hawaii† (USA) | 1996 | Cutaneous | Tissue culture |
| A1 | P4 | KC017963 | KC017883 | Superb parrot | Polytelis swainsonii | Psittaciformes | Psittacidae | Chile† | 2004 | Cutaneous | CAM |
| A1 | P5 | KC017964 | KC017851 | Blue-eared pheasant | Crossoptilon auritum | Galliformes | Phasianidae | Hungary† | 2005 | Cutaneous | Skin lesion |
| A2 | P6 | KC017965 | KC017868 | Rock dove | Columba livia | Columbiformes | Columbidae | Hawaii (USA) | 1994 | Cutaneous | Skin lesion |
| A2 | P7 | KC017966 | KC017885 | Rock dove | Columba livia | Columbiformes | Columbidae | Georgia (USA) | 1995 | Cutaneous | Tissue culture |
| A2 | P8 | KC017967 | KC017852 | Eastern imperial eagle | Aquila heliaca | Accipitriformes | Accipitridae | Hungary | 2000 | Cutaneous | Skin lesion |
| A2 | P9 | KC017968 | KC017853 | Rock dove | Columba livia | Columbiformes | Columbidae | Hungary | 2003 | Cutaneous | Skin lesion |
| A2 | P10 | KC017969 | KC017854 | Rock dove | Columba livia | Columbiformes | Columbidae | Hungary | Unknown | Unknown | Unknown |
| A2 | P11 | KC017970 | KC017855 | Great bustard | Otis tarda | Gruiformes | Otidae | Hungary | 2003 | Cutaneous | Skin lesion |
| A2 | P12 | KC017971 | KC017856 | Rock dove | Columba livia | Columbiformes | Columbidae | Hungary | 2005 | Cutaneous | Skin lesion |
| A2 | P13 | KC017972 | KC017886 | Oriental turtle-dove | Streptopelia orientalis | Columbiformes | Columbidae | South Korea | Unknown | Cutaneous | Skin lesion |
| A2 | P14 | KC017973 | KC017887 | Oriental turtle-dove | Streptopelia orientalis | Columbiformes | Columbidae | South Korea | Unknown | Cutaneous | Skin lesion |
| A2 | P15 | KC017974 | KC017890 | Great bustard | Otis tarda | Gruiformes | Otidae | Spain | 2003 | Cutaneous | Skin lesion |
| A2 | P16 | KC017975 | KC017857 | Indian peafowl | Pavo cristatus | Galliformes | Phasianidae | Hungary† | 2003 | Cutaneous-oral mucosa | Skin lesion |
| A2 | P17 | KC017976 | KC017891 | Booted eagle | Hieraaetus pennatus | Accipitriformes | Accipitridae | Spain | 2000 | Cutaneous | CAM |
| A2 | P18 | KC017977 | KC017892 | Red-legged partridge | Alectoris rufa | Galliformes | Phasianidae | Spain | 2000 | Cutaneous | Skin lesion |
| A2 | P19 | KC017978 | KC017893 | Red kite | Milvus milvus | Accipitriformes | Accipitridae | Spain | 2003 | Cutaneous | CAM |
| A2 | P20 | KC017979 | KC017894 | Booted eagle | Hieraaetus pennatus | Accipitriformes | Accipitridae | Spain | 2003 | Cutaneous | CAM |
| A2 | P21 | KC017980 | KC017895 | Red-legged partridge | Alectoris rufa | Galliformes | Phasianidae | Spain | 2002 | Cutaneous | CAM |
| A3 | P22 | KC017981 | KC017898 | Southern giant petrel | Macronectes giganteus | Procellariiformes | Procellariidae | Antarctica | 2004 | Cutaneous-oral mucosa | CAM |
| A3 | P23 | KC017982 | KC017899 | Pelagic cormorant | Phalacrocorax pelagicus | Suliformes | Phalacrocoracidae | Alaska (USA) | 1989 | Cutaneous | CAM |
| A3 | P24 | KC017983 | KC017888 | Eurasian eagle owl | Bubo bubo | Strigiformes | Strigidae | South Korea | Unknown | Cutaneous | Skin lesion |
| A3 | P25 | KC017984 | KC017889 | Eurasian eagle owl | Bubo bubo | Strigiformes | Strigidae | South Korea | Unknown | Cutaneous | Skin lesion |
| A3 | P26 | KC017985 | KC017902 | Common murre | Uria aalge | Charadriiformes | Alcidae | Washington (USA) | 1991 | Cutaneous-oral mucosa | Tissue culture |
| A3 | P27 | KC017986 | KC017904 | Laysan albatross | Phoebastria immutabilis | Procellariiformes | Diomedeidae | Midway Islands (USA) | 1983 | Cutaneous | CAM |
| A3 | P28 | KC017987 | KC017905 | Magellanic penguin | Spheniscus magellanicus | Sphenisciformes | Spheniscidae | Argentina | 2007 | Cutaneous | Skin lesion |
| A4 | P29 | KC017988 | KC017858 | Peregrine falcon | Falco peregrinus | Falconiformes | Falconidae | Hungary | 2005 | Cutaneous | Skin lesion |
| A4 | P30 | KC017989 | KC017859 | Red-footed falcon | Falco vespertinus | Falconiformes | Falconidae | Hungary | 2007 | Cutaneous | Skin lesion |
| A5 | P31 | KC017990 | KC017906 | Trumpeter swan | Cygnus buccinator | Anseriformes | Anatidae | Wisconsin (USA) | 1991 | Cutaneous | Tissue culture |
| A5 | P32 | KC017991 | KC017920 | Mottled duck | Anas fulvigula | Anseriformes | Anatidae | Texas (USA) | 2005 | Cutaneous | Skin lesion |
| A5 | P33 | KC017992 | KC017907 | Blue-winged teal | Anas discors | Anseriformes | Anatidae | Wisconsin (USA) | 1991 | Cutaneous | Tissue culture |
| A5 | P34 | KC017993 | KC017908 | Redhead duck | Aythya americana | Anseriformes | Anatidae | Wisconsin (USA) | 1991 | Cutaneous | Tissue culture |
| A5 | P35 | KC017994 | KC017924 | Mallard duck | Anas platyrhynchos | Anseriformes | Anatidae | New York (USA) | 1994 | Cutaneous | Skin lesion |
| A5 | P36 | KC017995 | KC017909 | Trumpeter swan | Cygnus buccinator | Anseriformes | Anatidae | Wisconsin (USA) | 1989 | Unknown | CAM |
| A5 | P37 | KC017996 | KC017910 | Wood duck | Aix sponsa | Anseriformes | Anatidae | Wisconsin (USA) | 1991 | Cutaneous | Skin lesion |
| A6 | P38 | KC017997 | KC017926 | Mourning dove | Zenaida macroura | Columbiformes | Columbidae | Illinois (USA) | 1993 | Cutaneous | CAM |
| A6 | P39 | KC017998 | KC017928 | Mourning dove | Zenaida macroura | Columbiformes | Columbidae | California (USA) | 1993 | Oral mucosa | Tissue culture |
| A6 | P40 | KC017999 | KC017911 | Mourning dove | Zenaida macroura | Columbiformes | Columbidae | Wisconsin (USA) | 1994 | Cutaneous | Skin lesion |
| A6 | P41 | KC018000 | KC017912 | Mourning dove | Zenaida macroura | Columbiformes | Columbidae | Wisconsin (USA) | 1987 | Cutaneous | Tissue culture |
| A6 | P42 | KC018001 | KC017929 | Rock dove | Columba livia | Columbiformes | Columbidae | California (USA) | 1980 | Cutaneous | Skin lesion |
| A6 | P43 | KC018002 | KC017913 | Canada goose | Branta canadensis | Anseriformes | Anatidae | Wisconsin (USA) | 1992 | Cutaneous | Tissue culture |
| A7 | P44 | KC018003 | KC017932 | Bald eagle | Haliaeetus leucocephalus | Accipitriformes | Accipitridae | Florida (USA) | 1993 | Cutaneous | Tissue culture |
| A7 | P45 | KC018004 | KC017933 | Bald eagle | Haliaeetus leucocephalus | Accipitriformes | Accipitridae | Florida (USA) | 1992 | Cutaneous | Tissue culture |
| A7 | P46 | KC018005 | KC017935 | Bald eagle | Haliaeetus leucocephalus | Accipitriformes | Accipitridae | Minnesota (USA) | 1993 | Cutaneous | Skin lesion |
| A7 | P47 | KC018006 | KC017914 | Red-tailed hawk | Buteo jamaicensis | Accipitriformes | Accipitridae | Wisconsin (USA) | 1985 | Cutaneous | Skin lesion |
| A7 | P48 | KC018007 | KC017934 | Bald eagle | Haliaeetus leucocephalus | Accipitriformes | Accipitridae | Florida (USA) | 1989 | Cutaneous | Tissue culture |
| A7 | P49 | KC018008 | KC017860 | Northern goshawk | Accipiter gentilis | Accipitriformes | Accipitridae | Hungary | 2003 | Cutaneous | Skin lesion |
| A7 | P50 | KC018009 | KC017861 | Common buzzard | Buteo buteo | Accipitriformes | Accipitridae | Hungary | 2000 | Cutaneous | Skin lesion |
| A7 | P51 | KC018010 | KC017896 | Red kite | Milvus milvus | Accipitriformes | Accipitridae | Spain | 2000 | Cutaneous | Skin lesion |
| A7 | P52 | KC018011 | KC017900 | Bald eagle | Haliaeetus leucocephalus | Accipitriformes | Accipitridae | Alaska (USA) | 1981 | Cutaneous | CAM |
| A7 | P53 | KC018012 | KC017901 | Bald eagle | Haliaeetus leucocephalus | Accipitriformes | Accipitridae | Alaska (USA) | 1991 | Oral mucosa | Tissue culture |
| A7 | P54 | KC018013 | KC017915 | Mallard duck | Anas platyrhynchos | Anseriformes | Anatidae | Wisconsin (USA) | 1991 | Cutaneous | Skin lesion |
| B1 | P55 | KC018014 | KC017869 | Canary | Serinus canaria | Passeriformes | Fringillidae | Hawaii† (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P56 | KC018015 | KC017870 | Canary | Serinus canaria | Passeriformes | Fringillidae | Hawaii† (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P57 | KC018016 | KC017871 | Canary | Serinus canaria | Passeriformes | Fringillidae | Hawaii† (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P58 | KC018017 | KC017872 | Canary | Serinus canaria | Passeriformes | Fringillidae | Hawaii† (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P59 | KC018018 | KC017873 | Apapane | Himatione sanguinea | Passeriformes | Fringillidae | Hawaii (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P60 | KC018019 | KC017874 | Canary | Serinus canaria | Passeriformes | Fringillidae | Hawaii† (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P61 | KC018020 | KC017875 | Apapane | Himatione sanguinea | Passeriformes | Fringillidae | Hawaii (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P62 | KC018021 | KC017876 | Canary | Serinus canaria | Passeriformes | Fringillidae | Hawaii† (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P63 | KC018022 | KC017936 | Dark-eyed junco | Junco hyemalis hyemalis | Passeriformes | Emberizidae | Utah (USA) | 1986 | Cutaneous | CAM |
| B1 | P64 | KC018023 | KC017938 | House finch | Carpodacus mexicanus | Passeriformes | Fringillidae | Arizona (USA) | 1991 | Cutaneous | Tissue culture |
| B1 | P65 | KC018024 | KC017942 | House finch | Carpodacus mexicanus | Passeriformes | Fringillidae | Oregon (USA) | 1995 | Cutaneous | Tissue culture |
| B1 | P66 | KC018025 | KC017939 | House finch | Carpodacus mexicanus | Passeriformes | Fringillidae | Arizona (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P67 | KC018026 | KC017943 | House finch | Carpodacus mexicanus | Passeriformes | Fringillidae | Oregon (USA) | 1998 | Cutaneous | Tissue culture |
| B1 | P68 | KC018027 | KC017944 | House finch | Carpodacus mexicanus | Passeriformes | Fringillidae | Oregon (USA) | 1998 | Cutaneous | Tissue culture |
| B1 | P69 | KC018028 | KC017937 | House finch | Carpodacus mexicanus | Passeriformes | Fringillidae | Utah (USA) | 2001 | Cutaneous | Tissue culture |
| B1 | P70 | KC018029 | KC017940 | House finch | Carpodacus mexicanus | Passeriformes | Fringillidae | Arizona (USA) | 2001 | Cutaneous | Tissue culture |
| B1 | P71 | KC018030 | KC017903 | House finch | Carpodacus mexicanus | Passeriformes | Fringillidae | Washington (USA) | 1988 | Cutaneous | Tissue culture |
| B1 | P72 | KC018031 | KC017945 | American crow | Corvus brachyrhynchos, | Passeriformes | Corvidae | Washington, DC (USA) | 1999 | Cutaneous | Tissue culture |
| B1 | P73 | KC018032 | KC017877 | House finch | Carpodacus mexicanus | Passeriformes | Fringillidae | Hawaii (USA) | 1987 | Cutaneous | Tissue culture |
| B1 | P74 | KC018033 | KC017946 | Medium ground finch | Geospiza fortis | Passeriformes | Emberizidae | Galapagos Islands (Ecuador) | 2008 | Cutaneous | Skin lesion |
| B1 | P75 | KC018034 | KC017947 | Galapagos mockingbird | Mimus parvulus | Passeriformes | Mimidae | Galapagos Islands (Ecuador) | 2008 | Cutaneous | Skin lesion |
| B1 | P76 | KC018035 | KC017941 | Northern (masked) bobwhite | Colinus virginianus ridgwayi | Galliformes | Odontophoridae | Arizona (USA) | 1993 | Conjunctiva, infraorbital sinus | Tissue culture |
| B1 | P77 | KC018036 | KC017950 | American crow | Corvus brachyrhynchos | Passeriformes | Corvidae | Massachusetts (USA) | 2008 | spleen | Spleen |
| B1 | P78 | KC018037 | KC017951 | Black-billed magpie | Pica hudsonia | Passeriformes | Corvidae | Colorado (USA) | 1997 | Lung | Lung |
| B1 | P79 | KC018038 | KC017952 | Black-hooded siskin | Carduelis atrata | Passeriformes | Fringillidae | The Netherlands† | 2003 | Cutaneous | Paraffin-embedded tissue |
| B1 | P80 | KC018039 | KC017930 | Common raven | Corvus corax | Passeriformes | Corvidae | California (USA) | 2004 | Cutaneous | Tissue culture |
| B1 | P81 | KC018040 | KC017953 | American crow | Corvus brachyrhynchos | Passeriformes | Corvidae | Maryland (USA) | 2005 | Cutaneous | Skin lesion |
| B1 | P82 | KC018041 | KC017931 | Common murre | Uria aalge | Charadriiformes | Alcidae | California (USA) | 1980 | Cutaneous | Skin lesion |
| B1 | P83 | KC018042 | KC017948 | Medium ground finch | Geospiza fortis | Passeriformes | Emberizidae | Galapagos Islands (Ecuador) | 2008 | Cutaneous | Skin lesion |
| B1 | P84 | KC018043 | KC017949 | Woodpecker finch | Camarhynchus pallidus | Passeriformes | Thraupidae | Galapagos Islands (Ecuador) | 2008 | Cutaneous | Skin lesion |
| B1 | P85 | KC018044 | KC017956 | American crow | Corvus brachyrhynchos | Passeriformes | Corvidae | Pennsylvania (USA) | 1999 | Cutaneous | Tissue culture |
| B1 | P86 | KC018045 | KC017897 | Northern (hen) harrier | Circus cyaneus | Accipitriformes | Accipitridae | Spain | 2000 | Cutaneous | Skin lesion |
| B1 | P87 | KC018046 | KC017957 | Common bullfinch | Pyrrhula pyrrhula | Passeriformes | Fringillidae | Belgium† | 2008 | Cutaneous | Skin lesion |
| B1 | P88 | KC018047 | KC017862 | Great tit | Parus major | Passeriformes | Paridae | Hungary | 2007 | Cutaneous | Skin lesion |
| B1 | P89 | KC018048 | KC017958 | Mississippi sandhill crane | Grus canadensis | Gruiformes | Gruidae | Mississippi (USA) | 1992 | Cutaneous | Tissue culture |
| B1 | P90 | KC018049 | KC017916 | Swainson's thrush | Catharus ustulatus | Passeriformes | Turdidae | Wisconsin (USA) | 1994 | Cutaneous | CAM |
| B1 | P91 | KC018050 | KC017959 | Gray-crowned rosy finch | Leucosticte tephrocotis | Passeriformes | Fringillidae | Montana (USA) | 1985 | Cutaneous | Skin lesion |
| B1 | P92 | KC018051 | KC017917 | Humboldt penguin | Spheniscus humboldti | Sphenisciformes | Spheniscidae | Wisconsin (USA)† | 2008 | Cutaneous | Tissue culture |
| B1 | P93 | KC018052 | KC017878 | Hawai'i amakihi | Hemignathus virens | Passeriformes | Fringillidae | Hawaii (USA) | 1987 | Cutaneous-oral mucosa | Tissue culture |
| B1 | P94 | KC018053 | KC017927 | Dark-eyed junco | Junco hyemalis hyemalis | Passeriformes | Emberizidae | Illinois (USA) | 1986 | Cutaneous | Tissue culture |
| B1 | P95 | KC018054 | KC017918 | Canada goose | Branta canadensis | Anseriformes | Anatidae | Wisconsin (USA) | 1989 | Cutaneous | CAM |
| B1 | P96 | KC018055 | KC017879 | Elepaio | Chasiempis sandwichensis | Passeriformes | Monarchidae | Hawaii (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P97 | KC018056 | KC017880 | Apapane | Himatione sanguinea | Passeriformes | Fringillidae | Hawaii (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P98 | KC018057 | KC017881 | Apapane | Himatione sanguinea | Passeriformes | Fringillidae | Hawaii (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P99 | KC018058 | KC017863 | Golden eagle | Aquila chrysaetos | Accipitriformes | Accipitridae | Spain | 2000 | Cutaneous | CAM |
| B1 | P100 | KC018059 | KC017882 | Apapane | Himatione sanguinea | Passeriformes | Fringillidae | Hawaii (USA) | 1996 | Cutaneous | Tissue culture |
| B1 | P101 | KC018060 | KC017884 | Canary | Serinus canaria | Passeriformes | Fringillidae | Chile† | 2008 | Cutaneous | CAM |
| B1 | P102 | KC018061 | KC017921 | Common grackle | Quiscalus quiscula | Passeriformes | Icteridae | Texas (USA) | 1993 | Cutaneous | Tissue culture |
| B1 | P103 | KC018062 | KC017922 | Boat-tailed grackle | Quiscalus major | Passeriformes | Icteridae | Texas (USA) | 1989 | Cutaneous | Skin lesion |
| B2 | P104 | KC018063 | KC017954 | European starling | Sturnus vulgaris | Passeriformes | Sturnidae | Maryland (USA) | 1984 | Cutaneous | Tissue culture |
| B2 | P105 | KC018064 | KC017919 | European starling | Sturnus vulgaris | Passeriformes | Sturnidae | Wisconsin (USA) | 1985 | Cutaneous | Skin lesion |
| B2 | P106 | KC018065 | KC017955 | European starling | Sturnus vulgaris | Passeriformes | Sturnidae | Maryland (USA) | 1985 | Cutaneous | CAM |
| B2 | P107 | KC018066 | KC017864 | Great bustard | Otis tarda | Gruiformes | Otidae | Hungary | 2005 | Cutaneous | Skin lesion |
| B2 | P108 | KC018067 | KC017865 | Common hill myna | Gracula religiosa | Passeriformes | Sturnidae | Hungary† | Unknown | Cutaneous | Skin lesion |
| B3 | P109 | KC018068 | KC017923 | American robin | Turdus migratorius | Passeriformes | Turdidae | Texas (USA) | 2005 | Cutaneous | Tissue culture |
| C | P110 | KC018069 | KC017925 | Yellow-crowned amazon | Amazona ochrocephala | Psittaciformes | Psittacidae | New York† (USA) | 1980 | Cutaneous | Tissue culture |
| C | P111 | AM050383 | KC017849 | Parrot | Undescribed | Psittaciformes | Psittacidae | UK† | Unknown | unknown | Tissue culture |
That is, subclades of fowlpox and canarypox clades and the Psittacinepox virus clade in Bayesian analysis of concatenated, 4b, and DNA polymerase gene sequences.
†, Samples collected from captive birds (aviaries, zoos, etc.).
Virus isolation on muscovy duck embryo fibroblasts (MSDEF) (28, 29) or the chorioallantoic membrane (CAM) of embryonated chicken eggs (28, 29) was carried out in several cases (Table 1). A lesion (ca. 1g) was homogenized for 2 min using a tissue grinder in 10 ml of Hanks' balanced salt solution (Gibco-Invitrogen, Carlsbad, CA) supplemented with 5% glycerin (Sigma-Aldrich, St. Louis, MO) and 5% gelatin (Difco-BD, Franklin Lakes, NJ). The tissue suspension was centrifuged at 800 × g at 4°C for 30 min. About 0.2 ml of supernatant was inoculated onto the CAM of 13-day-old embryonated chicken eggs after filtration through a 0.45-μm-pore-size filter. The eggs were incubated for 5 days at 37°C before harvesting. The CAM was excised under microscope and observed for generalized thickening or lesions. MSDEF cell culture was prepared and handled by the method of Docherty and Slota (28, 29). About 0.5 ml of supernatant, after filtration through a 0.45-μm-pore-size filter, was inoculated into a 7-day-old confluent T-75 flask of MSDEF. The flask was incubated at 37°C and 5% CO2 in a humidified air incubator and read on days 3 to 7 after inoculation to observe for cytopathic effect (CPE). The flask was freeze-thawed for blind passage 7 days after the original inoculation if no CPE was seen (28, 29).
DNA was extracted from frozen tissue samples, CAM homogenates, tissue cultures, and paraffin-embedded samples with a QIAamp DNA minikit (Qiagen, Inc., Valencia, CA) according to the manufacturer's recommendations.
Primers, PCR, and sequencing.
In order to amplify a fragment of the avian poxviruses DNA polymerase gene, a PCR system was designed based on the known Fowlpox virus DNA polymerase gene sequence (30) utilizing the primer pair: PoPr1, 5′-CGCCGCATCATCTACTTATC-3′; and PoPr2, 5′-CCACACAGCGCCATTCATTA-3′. Since this method was not able to detect all poxvirus strains, a pair of universal primers (PPolF [5′-GGCYAGTACKCTTATYAAAGG-3′] and PPolR [5′-CGTCTCTACGTGTTTCGCT-3′]) was designed from the consensus sequence of the aligned DNA polymerase gene sequences of Fowlpox and Canarypox virus. Alignments were generated with the web-based Multalin software (31), while PRIMER2 (Scientific and Educational Software, Cary, NC) and PrimerSelect from the Lasergene software package (DNASTAR, Inc., Madison, WI) were used for primer design. The PCR amplifying a sequence of the 4b core protein gene was used as described by Lee and Lee (32).
All PCRs were performed in a 25-μl total volume containing 10 to 100 ng of target DNA diluted in, 5 μl of 5× Green GoTaq Flexi Buffer (Promega, Inc., Madison, WI), 2 μl of MgCl2 (25 mM), 0.75 μl of deoxynucleoside triphosphates (10 mM; Qiagen), 2 μl of each primer (10 pmol/μl), and 0.2 μl of GoTaq DNA polymerase (5 U/μl; Promega). The PCR was performed in DNA Engine Thermal Cyclers PTC-0200 (Bio-Rad Laboratories Inc., Hercules, CA).
For the PCR amplifying the DNA polymerase gene segment with the PoPr1/2 primers the reaction consisted of initial denaturation for 5 min at 95°C, followed by 35 amplification cycles consisting of denaturation for 30 s at 95°C, primer annealing at 53°C for 30 s, and extension at 72°C for 1 min. The final extension step was performed for 5 min at 72°C. For the PPolF and PPolR primers, the annealing temperature was set to 50°C, with the rest of the protocol unaltered. In the PCR amplifying the 4b core protein sequence the amplification was extended to 45 cycles and consisted of 1 min of denaturation at 95°C, 1 min of annealing at 60°C, and 1 min of extension at 72°C.
After amplification, 5 μl of each reaction mixture was subjected to electrophoresis in 1% agarose gel, and the amplified gene products were visualized under UV light after ethidium bromide staining. PCR products were isolated from agarose gel (QIAquick gel extraction kit; Qiagen), and direct cycle sequencing was performed with the primers used for amplification on an ABI 373A or an ABI Prism 3100 automated DNA sequencer (Applied Biosystems, Foster City, CA).
Phylogenetic methods.
Nucleic acid databases were searched using BLASTN (33). Multiple alignments of the obtained DNA sequences were performed with CLUSTAL W in the DAMBE software package (34) using the translated amino acid sequence alignment as a template for the precise alignment of the DNA sequences. Alignments were edited and shaded with BioEdit software (35). The concatenated alignment containing the sections of both 4b core protein and DNA polymerase gene sequences was also produced in DAMBE.
Phylogenies were generated separately for the 4b gene and DNA polymerase gene sequences and for the concatenated sequences of these two genes. Trees were constructed using three methods: neighbor joining (NJ), maximum likelihood (ML), and a Bayesian approach. To determine the most likely model of evolution, jModelTest (36, 37) was performed. Based on Akaike's information criterion, the most likely model for the DNA polymerase gene and the concatenated sequences was a general time reversible model with a gamma distribution (GTR+G), while for the 4b gene, it was the transitional model TIM1+G. The gamma rates for the three gene sequences were as follows: concatenated = 0.2590, 4b = 0.3260, and polymerase = 0.2670. The model and parameter estimates for the closest matching model (see below) was entered using NJ in MEGA 5.0 (38, 39), ML analyses in PAUP* 4.0b (40), and Bayesian analysis in MrBayes 3.1 (41, 42). The LogDet model (43) with the estimated gamma rate was used for NJ analysis bootstrapped for 1,000 replicates. ML analyses utilized the PAUP block from jModelTest for each gene region in a heuristic search with tree bisection and reconnection (TBR) branch swapping, bootstrapped for 100 replicates. Bayesian analyses were run for 1 to 2.5 million generations, with sampling at every 100th generation, until model convergence was achieved. Four chains and a 25% burn-in that was then discarded for all analyses were used. A 50% majority rule consensus tree was built from the resulting trees. Initial phylogenies were generated with Molluscum contagiosum (NC001731) as the outgroup, according to the method of Jarmin et al. (25). Tree topologies within the avian poxviruses were unchanged when the following outgroups were used (Deerpox virus AY689437, Tanapox virus EF420157, and Yaba-like disease virus AJ293568) (44). Subsequent trees excluded these outgroup taxa, and the isolates clustered in the most basal group were used as an outgroup. The use of orthopoxviruses for outgroup(s) did not affect the tree topologies (data not shown).
The average evolutionary divergence between sequences was estimated with the MEGA 5.0 software (39) both between and within Avipoxvirus clades, subclades and Orthopoxvirus clades. Analyses were conducted using the Tamura-Nei model with standard error estimated through 1,000 bootstrap replicates. The rate variation among sites was modeled with a gamma distribution (shape parameter = 1) using all codon positions. Between group and within group analyses were performed on the partial (555 bp) alignment of avipoxvirus DNA polymerase sequences complemented with Orthopoxvirus type sequences available from GenBank (Old World clade: X94355, Coxpox virus; M35027, Vaccinia virus; L22579, Variola virus; AY009089, Camelpox virus; DQ437594, Taterapox virus; DQ792504, Horsepox virus; AY484669, Rabbitpox virus; HM172544, Monkeypox virus; and AF012825, Ectromelia virus; North American clade: FJ807738, Volepox virus; DQ066529, Skunkpox virus; DQ066531, Raccoonpox virus) (n = 121), while additional within group analyses were also conducted on concatenated (981 bp) avipoxvirus DNA polymerase and 4b core protein sequences (n = 109). Potential recombinant sequences were excluded from the analysis.
A recombination analysis was performed on the concatenated sequence alignment using the RDP 3 software (45) in order to detect potential recombination events resulting in incongruent topology of the two single gene trees. The analysis focused on identifying events involving large sequence segments, or indeed the whole of the partial 4b core and DNA polymerase sequences (426 and 555 bp, respectively). The default selection of detection methods (RDP, GeneConv, and MaxChi) and general settings were used to perform the analyses but sequences were treated as linear, the power of detection was set to 0.01, the number of permutations to 100 with the shuffle column option.
The relationship between the phylogeny of avian hosts and avipoxvirus isolates were analyzed based on the most basic fowlpox virus (clade A) and canarypox virus (clade B) groupings. First, an alignment of cytochrome b sequences was generated for all available avipoxvirus hosts from GenBank; this gene contained the largest number of comparable and phylogenetically informative sequences across these species. When a sequence was not available for the specific host, the taxonomically closest available species was chosen. The taxa in the analyses were pared down to a single representative for each host species to avoid bias due to highly sampled taxa with the same poxvirus genotype. The final data set contained 61 sequences; 29 from canarypox virus hosts and 32 from fowlpox virus hosts (Table 2). Sequences were trimmed to 589 bp shared among all of the taxa in Sequencher 4.10 (Gene Codes, Ann Arbor, MI). A maximum-likelihood phylogeny of the hosts was generated to visualize the distribution of different poxvirus groupings. Evolutionary divergence was estimated between and within the canarypox virus and fowlpox virus group. In order to estimate the evolutionary divergence between sequences, pairwise genetic distances, a measure of the genetic similarity between two groups based on shared nucleotides, were calculated with the MEGA 5.0 software (39). Both analyses estimated differences over sequence pairs using a maximum composite likelihood model with standard error estimated through 100 bootstrap replicates.
Table 2.
List of cytochrome b sequences for avipoxvirus hosts from GenBank
| Pox type | Host (English name) | Host (Latin name) | Alternate host sequence | GenBank no. |
|---|---|---|---|---|
| Canarypox | Yellow-crowned amazon | Amazona ochrocephala | AY194411.1 | |
| Golden eagle | Aquila chrysaetos | EU345512.1 | ||
| Canada goose | Branta canadensis | NC_007011.1 | ||
| Woodpecker finch | Cactospiza pallida | AF108793.1 | ||
| Black-hooded siskin | Carduelis atrata | L76385.1 | ||
| House finch | Carpodacus mexicanus | AF447364.1 | ||
| Swainson's thrush | Catharus ustulatus | EU619788.1 | ||
| Elepaio | Chasiempis sandwichensis | Eiao monarch Pomarea iphis fluxa | AY262704.1 | |
| Northern (hen) harrier | Circus cyaneus | Western marsh-harrier Circus aeruginosus | AY987305.1 | |
| Northern (masked) bobwhite | Colinus virginianus | EU372675.1 | ||
| American crow | Corvus brachyrhynchos | AY509619.1 | ||
| Common raven | Corvus corax | AY527266.1 | ||
| Medium Ground finch | Geospiza fortis | AF108773.1 | ||
| Common hill myna | Gracula religiosa | Common myna Sturnus tristis | NC_015195.1 | |
| Mississippi sandhill crane | Grus canadensis | FJ769855.1 | ||
| Hawai'i amakihi | Hemignathus virens | AF015755.1 | ||
| Apapane | Himatione sanguinea | AF015754.1 | ||
| Dark-eyed junco | Junco hyemalis hyemalis | AF290161.1 | ||
| Gray-crowned rosy finch | Leucosticte tephrocotis | AY156380.1 | ||
| Galapagos mockingbird | Mimus parvulus | Le Conte's thrasher Toxostoma lecontei | AY329478.1 | |
| Great tit | Parus major | EU167009.1 | ||
| Black-billed magpie | Pica hudsonia | AY030114.1 | ||
| Common bullfinch | Pyrrhula pyrrhula | HQ284613.1 | ||
| Boat-tailed grackle | Quiscalus major | AF089055.2 | ||
| Common grackle | Quiscalus quiscula | AF089058.2 | ||
| Canary | Serinus canaria | AY914127.1 | ||
| Humboldt penguin | Spheniscus humboldti | DQ137220.1 | ||
| European starling | Sturnus vulgaris | AF285790.1 | ||
| American robin | Turdus migratorius | EU619827.1 | ||
| Fowlpox | Northern goshawk | Accipiter gentilis | NC_011818.1 | |
| Wood duck | Aix sponsa | EU585605.1 | ||
| Red-legged partridge | Alectoris rufa | AM850840.1 | ||
| Blue-winged teal | Anas discors | EU914146.1 | ||
| Mottled duck | Anas fulvigula | Mallard duck Anas platyrhynchos, alt. haplotype | EU755252.1 | |
| Mallard duck | Anas platyrhynchos | EU755253.1 | ||
| Eastern imperial eagle | Aquila heliaca | Z73465.1 | ||
| Redhead duck | Aythya americana | NC_000877.1 | ||
| Canada goose | Branta canadensis | NC_007011.1 | ||
| Eurasian eagle owl | Bubo bubo | AJ003961.1 | ||
| Common buzzard | Buteo buteo | NC_003128.3 | ||
| Red tailed hawk | Buteo jamaicensis | GQ264785.1 | ||
| Rock dove | Columba livia | NC_013978.1 | ||
| Blue-eared pheasant | Crossoptilon auritum | AF534552.1 | ||
| Trumpeter swan | Cygnus buccinator | Tundra swan Cygnus columbianus | DQ083161.1 | |
| Peregrine falcon | Falco peregrinus | EU233100.1 | ||
| Red-footed falcon | Falco vespertinus | EU233132.1 | ||
| Domestic fowl | Gallus domesticus | Red junglefowl Gallus gallus | NC_007236.1 | |
| Bald eagle | Haliaeetus leucocephalus | GQ264818.1 | ||
| Booted eagle | Hieraaetus pennatus | Y15760.1 | ||
| Southern giant petrel | Macronectes giganteus | AF076060.1 | ||
| Domestic turkey | Meleagris gallopavo | NC_010195.2 | ||
| Red kite | Milvus milvus | AY987312.1 | ||
| Great bustard | Otis tarda | NC_014046.1 | ||
| Indian peafowl | Pavo cristatus | DQ010648.1 | ||
| Pelagic cormorant | Phalacrocorax pelagicus | EU167011.1 | ||
| Laysan albatross | Phoebastria immutabilis | AB276050.1 | ||
| Superb parrot | Polytelis swainsonii | Red-winged parrot Aprosmictus erythropterus | AB177959.1 | |
| Magellanic penguin | Spheniscus magellanicus | DQ137218.1 | ||
| Oriental turtle-dove | Streptopelia orientalis | Spotted dove Streptopelia chinensis | AF483341.1 | |
| Common murre | Uria aalge | DQ485892.1 | ||
| Mourning dove | Zenaida macroura | Eared dove Zenaida auriculata | NC_015203.1 |
RESULTS
Molecular phylogeny of the avipoxvirus sequences.
The primers PPolF and PPolR for DNA polymerase gene were successfully used to amplify sequences from all tested isolates which encompassed all previously known clades. These primers yielded products of ∼900 bp. However, only a 555-bp length part was included in the phylogenetic analysis since older samples were examined only with the PoPr1/2 primers, which produced a smaller PCR product. A 426-bp long sequence of the 4b core protein gene was used to prepare an additional alignment. Thus, the concatenated sequences of both genes were 981 bp long.
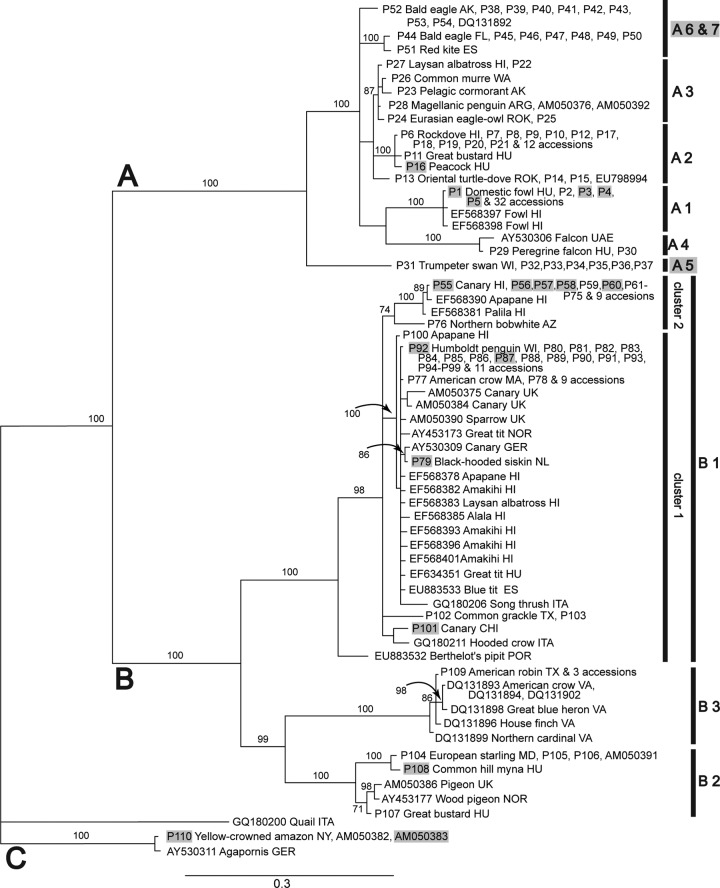
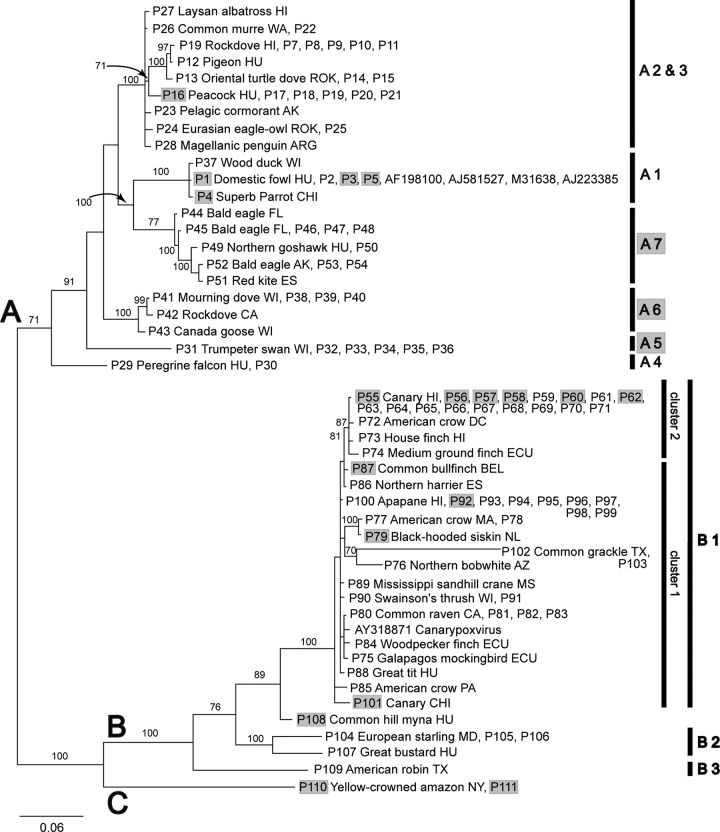
Partial sequences of both DNA polymerase and 4b core protein genes were amplified successfully from 111 avian pox lesion samples and virus isolates. The topologies of the phylogenetic trees created with different methods (neighbor joining [NJ], maximum likelihood [ML], and Bayesian) from the concatenated (Fig. 1), 4b core protein gene (Fig. 2), and DNA polymerase gene (Fig. 3) sequence alignments were very similar. Based on the posterior probability values and most consistent tree topology, the Bayesian trees were considered the most reliable, followed by the NJ analysis, while the ML trees had the lowest bootstrap values and poorest resolution. Based on these results we primarily used the topology of the concatenated Bayesian tree through our analysis. Avipoxviruses form two major clades (A and B) with strong support (Fig. 1), while the placement of the third major clade (C) is less certain.
Fig 1.
Bayesian phylogeny of concatenated DNA sequences from genes encoding 4b core and DNA polymerase proteins of avipoxviruses. Posterior probability values of the Bayesian trees (1,000 replicates) and neighbor-joining and maximum likelihood bootstrap values (1,000 replicates) of >70 are indicated (MB/NJ/ML). Symbols: <, lower than 70; ¤, branch does not exist with that method. Avipoxvirus clades A to C, subclades, and clusters are labeled according to the nomenclature of Jarmin et al. (25) and Jarvi et al. (46). Novel subgroups described in the present study are highlighted by gray. Isolate origins are given either as U.S. state abbreviations or using the following location codes: Antarctica (ANT), Argentina (ARG), Belgium (BEL), Chile (CHI), Ecuador (ECU), Germany (GER), Hungary (HU), Italy (ITA), Netherlands (NL), Norway (NOR), Portugal (POR), Spain (ES), South Korea (ROK), United Arab Emirates (UAE), and United Kingdom (UK). Avian poxviruses which were isolated from captive birds (aviaries, zoos, etc.) are highlighted by gray, isolates containing potential recombinations are set in a box. The scale represents the number of substitutions per site.
Fig 2.
Bayesian phylogram of DNA sequences from genes encoding 4b core proteins of avipoxviruses. Posterior probability values of >70 are shown. Avipoxvirus clades A to C, subclades, and clusters are labeled according to the nomenclature of Jarmin et al. (25) and Jarvi et al. (46). Novel subgroups described in the present study are highlighted by gray. Isolate origins are given either as U.S. state abbreviations or using the following location codes: Antarctica (ANT), Argentina (ARG), Belgium (BEL), Chile (CHI), Ecuador (ECU), Germany (GER), Hungary (HU), Italy (ITA), Netherlands (NL), Norway (NOR), Portugal (POR), Spain (ES), South Korea (ROK), United Arab Emirates (UAE), and United Kingdom (UK). Avian poxviruses that were isolated from captive birds (aviaries, zoos, etc.) are highlighted by gray. The scale represents the number of substitutions per site. Due to the large number of avian poxvirus isolates in the 4b gene analyses (n = 226), we abbreviated the names for isolates with identical sequences from GenBank accessions as follows: (i) P1 genotype, AB292647, AF198100, AJ005164, AJ581527, AM050377, AM050378, AM050379, AM050380, AY453171, AY453172, AY530302, AY530304, AY530307, DQ873808, EF568377, EF634347, EF634348, M25781, GU108500, GU108501, GU108502, GU108503, GU108504, GU108505, GU108506, GU108507, GQ221269, GQ180212, GQ180207, GQ180201, GU108509, and GU108508; (ii) P6 genotype, AM050385, AM050387, AM050388, AY530303, AY530305, DQ873809, DQ873810, DQ873811, EF016108, GQ180210, GQ180208, and GQ180204; (iii) P55 genotype, EF568379, EF568384, EF568386, EF568387, EF568388, EF568389, EF568391, EF568399, and EF568400; (iv) P77 genotype, AM050381, AM050389, AY530308, GQ487567, GU108510, GQ180202, GQ180203, GQ180205, and GQ180209; (v) P92 genotype, AY530310, AY318871, AY453174, AY453175, EF568380, EF568392, EF568394, EF568395, EF634349, EF634350, and GQ180213; and (vi) P109 genotype, DQ131895, DQ131897, DQ131900, and DQ131901.
Fig 3.
Bayesian phylogeny of DNA sequences from gene encoding DNA polymerase protein of avipoxviruses. Posterior probability values of >70 are shown. Avipoxvirus clades A to C, subclades, and clusters are labeled according to the nomenclature of Jarmin et al. (25) and Jarvi et al. (46). Novel subgroups described in the present study are highlighted by gray. Isolate origins are given either as U.S. state abbreviations or using the following location codes: Antarctica (ANT), Argentina (ARG), Belgium (BEL), Chile (CHI), Ecuador (ECU), Germany (GER), Hungary (HU), Italy (ITA), Netherlands (NL), Norway (NOR), Portugal (POR), Spain (ES), South Korea (ROK), United Arab Emirates (UAE), and United Kingdom (UK). Avian poxviruses which were isolated from captive birds (aviaries, zoos, etc.) are highlighted by gray. The scale represents the number of substitutions per site.
Clade A represents seven subclades (A1 to A7). Subclade A1 comprises viruses isolated from birds of the order Galliformes (domestic fowl, blue-eared pheasant) with a wide geographic distribution. A poxvirus isolated from a superb parrot originating from Chile also clustered in subclade A1. Subclade A2 consists of viruses originating from birds of the order Columbiformes (rock doves, oriental turtle doves) from North America, Europe, and the Republic of Korea, with additional samples from a peacock, raptors, red-legged partridges, and great bustards from Europe. Subclade A3 formerly consisted of only an albatross virus and a falcon virus, but it has been expanded by isolates from other seabirds (southern giant petrel, pelagic cormorant, common murre, Laysan albatross, Magellanic penguin) from the coasts of the Pacific and Atlantic Ocean and Eurasian eagle-owls from Korea. Subclade A4 still forms an outlier and contains viruses from peregrine falcon and red-footed falcon from Hungary and a United Arab Emirates falcon isolate. A new subclade, A5, sharing a common ancestor with subclade A1, comprises isolates from Anseriformes (trumpeter swans, mottled duck, blue-winged teal, redhead duck, wood duck, mallard duck) originating from the United States. New subclades A6 and A7 share a ancestor with subclades A2 and A3. Subclade A6 comprises viruses from Columbiformes (mourning doves, rock doves) and a Canada goose from North America. Isolates from Accipitriformes (bald eagles, red tailed hawk, common buzzard, northern goshawk, red kite) from the United States and Europe and a mallard duck group under subclade A7.
Clade B is comprised of three subclades (B1 to B3). Previously reported subclade B1 comprises viruses isolated from a wide range of passerine species (Passeriformes) of worldwide distribution, although several nonpasserine hosts (e.g., northern harrier, Mississippi sandhill crane, Humboldt penguin, etc.) are represented as well. This subclade further diversifies into three outliers and a main branch consisting of two clusters. Nine house finch isolates from our study and further two from a previous work (46) with a diverse range of isolation dates and geographic origins were analyzed and found to group within cluster 2 of subclade B1. The three outliers were formed by two strains from grackles, a virus from a Chilean canary and a strain described from Berthelot's pipit (Fig. 2). Previously reported subclade B2 consisted of isolates from starlings and mynahs. It was found that starlings in Europe and North America host the same virus strain. Viruses isolated from a great bustard in Hungary and a rock and wood pigeon from Europe also clustered into subclade B2. Isolates from a wide range of different bird species presented to a wildlife center in Virginia in 2003 and 2004 form a new subclade, B3 (Fig. 2). From our samples, only a 2003 American robin isolate from Texas clustered into this subclade. Clade C consists exclusively of isolates from psittacine species. The location of this clade is ambiguous. It formed either a separate clade, or it was a weakly supported member of clade B.
The within-group mean genetic distances of the concatenated avian poxvirus sequences were 0.087 ± 0.007 standard error (SE) in clade A and 0.059 ± 0.005 SE in clade B, while the sequences were identical (genetic distance = 0.000) in clade C. Mean genetic distances of the concatenated sequences within avipoxvirus subclades ranged from 0.000 to 0.035 (Table 3). The results of the partial DNA polymerase sequence analysis allowing a comparison with orthopoxviruses (Old World and North American clade) are summarized in Table 3.
Table 3.
Estimates of average evolutionary divergence of sequence pairs between and within avipoxvirus subclades and orthopox virus cladesa
| Clade | Avg evolutionary divergence (SE) |
Distance (SE) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avipox |
Orthopox |
||||||||||||||
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | B1 | B2 | B3 | C | OW | NA | 1 | 2 | |
| Avipox A1 | (0.016) | (0.016) | (0.024) | (0.021) | (0.019) | (0.016) | (0.043) | (0.046) | (0.048) | (0.050) | (0.097) | (0.102) | 0.001 (0.000) | 0.000 (0.000) | |
| Avipox A2 | 0.107 | (0.004) | (0.024) | (0.021) | (0.016) | (0.013) | (0.042) | (0.049) | (0.049) | (0.047) | (0.104) | (0.111) | 0.016 (0.004) | 0.014 (0.003) | |
| Avipox A3 | 0.098 | 0.020 | (0.024) | (0.020) | (0.014) | (0.013) | (0.042) | (0.050) | (0.048) | (0.046) | (0.105) | (0.110) | 0.005 (0.002) | 0.006 (0.001) | |
| Avipox A4 | 0.169 | 0.165 | 0.158 | (0.027) | (0.022) | (0.024) | (0.044) | (0.043) | (0.051) | (0.054) | (0.099) | (0.104) | 0.000 (0.000) | 0.000 (0.000) | |
| Avipox A5 | 0.151 | 0.142 | 0.132 | 0.190 | (0.022) | (0.024) | (0.051) | (0.054) | (0.050) | (0.060) | (0.087) | (0.094) | 0.000 (0.000) | 0.000 (0.003) | |
| Avipox A6 | 0.120 | 0.099 | 0.078 | 0.149 | 0.140 | (0.016) | (0.039) | (0.049) | (0.044) | (0.052) | (0.088) | (0.089) | 0.005 (0.002) | 0.003 (0.001) | |
| Avipox A7 | 0.104 | 0.086 | 0.079 | 0.161 | 0.171 | 0.103 | (0.038) | (0.052) | (0.051) | (0.043) | (0.105) | (0.110) | 0.010 (0.003) | 0.007 (0.003) | |
| Avipox B1 | 0.354 | 0.356 | 0.359 | 0.345 | 0.386 | 0.312 | 0.327 | (0.026) | (0.034) | (0.048) | (0.120) | (0.124) | 0.017 (0.003) | 0.024 (0.003) | |
| Avipox B2 | 0.391 | 0.392 | 0.402 | 0.335 | 0.419 | 0.390 | 0.436 | 0.206 | (0.033) | (0.054) | (0.115) | (0.131) | 0.050 (0.008) | 0.036 (0.006) | |
| Avipox B3 | 0.385 | 0.401 | 0.400 | 0.378 | 0.391 | 0.369 | 0.426 | 0.262 | 0.226 | (0.059) | (0.114) | (0.116) | n/c | n/c | |
| Avipox C | 0.421 | 0.406 | 0.401 | 0.435 | 0.477 | 0.423 | 0.366 | 0.409 | 0.45 | 0.481 | (0.107) | (0.119) | 0.000 (0.000) | 0.000 (0.000) | |
| Orthopox OW | 0.778 | 0.827 | 0.83 | 0.775 | 0.751 | 0.745 | 0.819 | 0.942 | 0.917 | 0.907 | 0.871 | (0.019) | 0.013 (0.003) | 0.016 (0.003) | |
| Orthopox NA | 0.804 | 0.864 | 0.854 | 0.823 | 0.789 | 0.758 | 0.849 | 0.975 | 0.973 | 0.882 | 0.933 | 0.148 | 0.076 (0.011) | ||
Estimates of average evolutionary divergence of sequence pairs between and within avipoxvirus subclades (A1 to A7, B1 to B3, and C) and orthopoxvirus clades (Old World [OW] and North American [NA]). The number of base substitutions per site from averaging over all sequence pairs between (matrix) and within (columns) groups is shown. Standard error estimates are shown in parentheses. The results of within-group analyses are presented in the last two columns: the within-group analysis for column 1 was performed on a partial DNA polymerase sequence (555 bp) alignment (n = 121), while the within-group analysis for column 2 was conducted on concatenated (981-bp) DNA polymerase and 4b core protein sequences (n = 109). Potential recombinants were excluded from the analysis. Evolutionary analyses were conducted in MEGA5 (39). n/c, not calculated.
Possible recombination between 4b and DNA polymerase loci.
Apparent recombination breakpoints were located and confirmed with multiple analysis methods available in the RDP 3 software in five of the concatenated sequences at the junction of the 4b core protein and DNA polymerase (nucleotide [nt] 426). Isolates P52, P53, and P54 (from two bald eagles and a mallard) were identified by the RDP method (P = 8.719 × 10−07) as apparent interlocus recombinants of isolate P41 (mourning dove) as minor parent (4b core protein sequence) and P51 (red kite) as major parent (DNA polymerase sequence). It should be noted that in the concatenated sequence tree (Fig. 1), the apparent recombinants (P52, P53, and P54) are basal to the apparent parents P51 and P41. It is therefore possible that the apparent recombinant carries the ancestral sequences, whereas the apparent parents carry recombined loci. However, the topology of the three trees (shown in Fig. 1, 2, and 3) is ambiguous for these isolates, so it would be premature to speculate on the actual nature of the event.
Isolate P37 (from a wood duck) was also identified (P = 1.613 × 10−13) as an apparent interlocus recombinant, with P32 (mottled duck) as minor parent (4b core protein sequence) and P2 (domestic turkey) as major parent (DNA polymerase sequence).
A fifth apparent recombination event, in this case intralocus, affecting only a part of the DNA polymerase gene, was detected in a common hill mynah isolate P108 (P = 9.469 × 10−13) with one breakpoint identified at the sequence junction (nt 426) and an additional ending breakpoint at nt 763 of the alignment. The minor parent was identified as the dark-eyed junco isolate P94 (DNA polymerase gene sequence), while the major parent was a European starling isolate P104. All of the above apparent recombination events were confirmed with similarly significant P values by the GeneConv, BootScan, MaxChi, Chimaera, SiScan, and 3Seq methods.
Concordance of host and virus phylogeny.
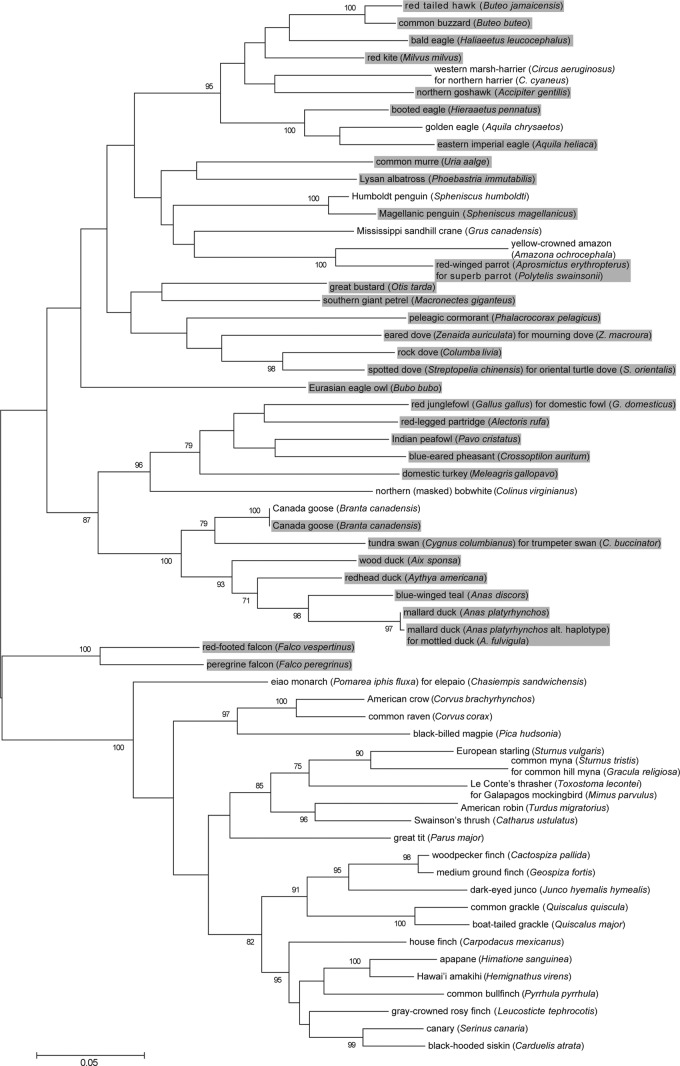
When assessing genetic diversity between the avian hosts, the mean between-group genetic distance for the hosts of canarypox viruses (clade B) and fowlpox viruses (clade A) was 0.209 ± 0.011 SE. The mean within-group genetic distances were 0.175 ± 0.009 SE for hosts of clade B viruses and 0.186 ± 0.107 SE for hosts of clade A viruses. Within-group distances were not significantly different based on the overlap of the 95% confidence intervals with the means. Overall, there was significantly greater between- than within-group genetic diversity, indicating two distinct groups of hosts. Nonetheless, although the phylogenetic distribution of hosts shows overall grouping congruent with the clade of virus, it has been possible to isolate clade A and B viruses from some closely related hosts (Fig. 4).
Fig 4.
Maximum-likelihood phylogeny of the hosts generated from the cytochrome b sequences from GenBank. Hosts of fowlpox viruses are highlighted by gray, and canarypox viruses are without highlight. Bootstrap values of >70 are shown. The scale represents the number of substitutions per site.
DISCUSSION
The phylogenetics and epidemiology of avian poxviruses is only partially understood. This study contributed to our understanding of this group of viruses by studying a broad range of isolates collected from around the world. Until now, the highly conserved 4b core protein gene was used as the sole pan-genus marker both in diagnostics and phylogeography (21, 24–27, 47). We show that the DNA polymerase gene is useful as another pan-genus marker, and the results of phylogenetic analyses are comparable to those based on the 4b core protein gene while the use of this additional gene provided the first opportunity to study the potential role of recombination in the evolution of avipoxviruses.
The updated classification of avian poxviruses, based on our concatenated Bayesian phylogeny and described below, primarily follows the nomenclature of Jarmin et al. (25). Three main clades (A to C) are differentiated within avipoxviruses (Fig. 1). Clade A appears to be the fowlpox clade, clade B the canarypox clade, and clade C the Psittacinepox virus clade. Clade A further differentiates into seven subclades. Subclades A1 to A4 were previously described (25). Subclade A1 is formed by Fowlpox virus in the narrowest sense. Subclade A2 was identified as Turkeypox virus, but it now appears to be more representative of a subset of pigeonpox viruses, as a large number of geographically diverse viruses isolated from the order Columbiformes are grouped here. When initially described by Jarmin et al. (25), subclades A3 and A4 contained only two and one sequences, respectively. Our study contributed a large number of novel sequences to these groups. It is now apparent that subclade A3 represents poxviruses of marine birds and subclade A4 those of falcons. Novel subclades A5 to A7 were identified in the present study. Subclade A5 appears to represent poxviruses of waterfowl, subclade A6 as a second, distinct group of pigeonpox viruses, and subclade A7 as poxviruses of raptors. Clade B was found to have three subclades as described earlier (25). Subclade B1 comprises the strict canarypox viruses. Mynahpox and Starlingpox viruses grouped together in subclade B2 and thus the use of the term “Sturnidaepox virus” is proposed. Considering that the isolates of subclade B3 originate from a narrow temporal and geographic range, we suggest it should be known as “Virginian epidemic avipoxvirus.” The finding of Jarvi et al. (46) establishing that subclade B1 has two main clusters was confirmed by our study. The outlier containing the Berthelot's pipit isolate from Macronesia was described earlier (4), but two further outliers, including one from grackles, were identified here.
The mean genetic distance within clades A and B of avian poxviruses appears to be similar to that of the North American clade of orthopoxviruses, but it is about four to five times the mean distance between Old World orthopoxviruses. However, since the average divergence values within Avipoxvirus subclades are generally quite similar to those calculated for orthopoxvirus clades, we may equally consider the option that the current subclades could eventually be viewed as equivalent taxonomical units. This relatively large genetic divergence among avian poxviruses, as well as the topology of the phylogenetic trees, indicates that the Avipoxvirus genus is one of the more widely diverged genera of the Chordopoxvirinae subfamily.
There is some evidence in our data for recombination events in the evolutionary history of the studied avipoxviruses. Although the limited number of loci (n = 2) examined, their length, and their genomic separation (103 kbp in fowlpox virus AF198100) constrains the possible conclusions, it seems likely that the detected events occurred in relatively well defined ecological and phylogenetic frameworks. These events primarily involved viruses (within subclades A5, A6, or A7) circulating in closely interacting hosts, providing a natural interface for potential virus exchange and coinfection (e.g., between and within Accipitriformes, Columbiformes, and Anseriformes), while the case involving Sturnidae (subclade B2) additionally highlights the potential of virus diversification and adaptation linked to extensive, primarily anthropogenic changes in the geographic distribution and concomitant “unnatural” contacts between species (in zoo collections or between alien, invasive, and native resident species in the wild). The confirmation of the nature of these events and elucidation of their role in the evolution and function (e.g., pathogenicity, adaptation, etc.) of avian poxviruses would, however, require the study of complete genomic sequences.
The range of hosts infected by fowlpox viruses (clade A), as estimated by within-group genetic diversity, was not significantly greater than that for those infected by canarypox viruses (clade B), indicating that each clade infects a similarly diverse range of bird hosts. One caveat is that the effect of sampling bias on the phylogenetic results is unknown. Sampling for avipoxviruses is not systematic across hosts and some taxa, e.g., poultry and songbirds are more intensively sampled than other groups. Several isolates originated from quarantine facilities, aviaries, or zoos where unusual transmissions may occur (particularly between already stressed or diseased birds), resulting in lesions but probably representing “dead-end” events that would rarely occur in the wild and would not lead to sustained epornitics. Such phenomena could have occurred, for example, in the cases of the fowlpox virus-infected superb parrot in subclade A1, the canarypox virus-infected Humboldt penguin in cluster 1 of subclade B1, or the isolates of subclade B3, which were isolated from a wide range of different bird species within a short time range during hospitalization in a wildlife center in Virginia.
In general, avian poxviruses tend to be host family or order specific, but ecological niche, habitat, and geography may modulate this pattern. A clear example of host family/order specificity is the European starling, which harbors the same virus strain both in Europe and North America and is a close relative of mynahs, infected with a closely related virus. The viruses isolated from and largely specific to falcons and raptors are other good examples.
The circulation of certain poxviruses within a prey-predator system can be recognized in several subclades (e.g., subclades A2 and B1). For example, we hypothesize that eastern imperial eagles may acquire pox infection from their dove prey (subclade A2) and northern harriers from a passerine species (subclade B1).
Another example of the role of the ecological niche and/or habitat lies with the poxviruses of marine birds (subclade A3), where evolutionarily distinct avian species with similar lifestyles harbor related viruses. In this case, although the isolates showed wide spatial separation, the effect of geography could not be excluded completely since these hosts migrate widely and share breeding sites where poxvirus infections could be transmitted and sustained. Except for this situation, geography seems to have only a minor effect on the avipoxvirus phylogeny, but it should not be dismissed, as in the case of the “Virginian epidemic avipoxvirus,” where the hospitalized birds infected each other.
An interesting phenomenon can be observed in cluster 2 of subclade B1. Viruses of this cluster infect different passerines, including all of the analyzed house finch isolates, with diverse retrieval dates and geographic origins. The timeline of sample collection indicates that the ancestor of cluster 2 might have been a house finch virus (see samples P64 to P71 and P73 in Fig. 1 to 3), the variants of which were subsequently dispersed around the Western Hemisphere and infected other bird species.
The data presented here provide novel insights into the complex relationship between avian poxviruses and their hosts. Generation of a significant number of whole-genome sequences of viruses from key points in the tree presented here would help to solve emerging problems in the conservation of endemic bird species and decrease pox-related economic losses in the poultry industry.
ACKNOWLEDGMENTS
M.G. and this project were supported in part by the Lendület program (grant number LP2012-22) of the Hungarian Academy of Sciences. K.E. was supported by the Bolyai János Research Scholarship of the Hungarian Academy of Sciences. Work at the USGS National Wildlife Health Center was funded by the U.S. Department of the Interior. Work in Spain was funded in part by the Junta de Comunidades of Castilla–La Mancha.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Use of trade or product names does not imply endorsement by the U.S. government.
We thank Carter Atkinson, Benjamin Lucio-Martinez, and Virginia Rago for providing samples for this study.
Footnotes
Published ahead of print 13 February 2013
REFERENCES
- 1. Bolte AL, Meurer J, Kaleta EF. 1999. Avian host spectrum of avipoxviruses. Avian Pathol. 28:415–432 [DOI] [PubMed] [Google Scholar]
- 2. Parker PG, Buckles EL, Farrington H, Petren K, Whiteman NK, Ricklefs RE, Bollmer JL, Jiménez-Uzcátequi G. 2011. 110 years of avipoxvirus in the Galapagos Islands. PLoS One 6:e15989 doi:10.1371/journal.pone.0015989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skinner MA, Laidlaw SM, Eldaghayes I, Kaiser P, Cottingham MG. 2005. Fowlpox virus as a recombinant vaccine vector for use in mammals and poultry. Expert Rev. Vaccines 4:63–76 [DOI] [PubMed] [Google Scholar]
- 4. Illera JC, Emerson BC, Richardson DS. 2008. Genetic characterization, distribution and prevalence of avian pox and avian malaria in the Berthelot's pipit (Anthus berthelotii) in Macaronesia. Parasitol. Res. 103:1435–1443 [DOI] [PubMed] [Google Scholar]
- 5. Zimmermann D, Anderson MD, Lane E, van Wilpe E, Caiulei O, Douglass N, Williamson AL, Kotze A. 2011. Avian poxvirus epizootic in a breeding population of lesser flamingos (Phoenicopterus minor) at Kamfers Dam, Kimberley, South Africa. J. Wildl. Dis. 47:989–993 [DOI] [PubMed] [Google Scholar]
- 6. Chen CC, Pei KJ, Lee FR, Tzeng MP, Chang TC. 2011. Avian pox infection in a free-living crested serpent eagle (Spilornis cheela) in southern Taiwan. Avian Dis. 55:143–146 [DOI] [PubMed] [Google Scholar]
- 7. Tripathy DN, Reed WM. 2008. Pox, p 291–309 In Saif Y-M, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE. (ed), Diseases of poultry, 12th ed Wiley-Blackwell, Ames, IA [Google Scholar]
- 8. Laiolo P, Serrano D, Tella JL, Carrete M, López G, Navarro C. 2007. Distress calls reflects poxvirus infection in lesser short-toed lark Calandrella rufescens. Behav. Ecol. 18:507–512 [Google Scholar]
- 9. Kleindorfer S, Dudaniec RY. 2006. Increasing prevalence of avian poxvirus in Darwin's finches and its effect on male pairing success. J. Avian Biol. 37:69–76 [Google Scholar]
- 10. Kane OJ, Uhart MM, Rago V, Pereda AJ, Smith JR, Van Buren A, Clark JA, Boersma PD. 2012. Avian pox in Magellanic penguins (Spheniscus magellanicus). J. Wildl. Dis. 48:790–794 [DOI] [PubMed] [Google Scholar]
- 11. van Riper C, van Riper SG, III, Hansen WR. 2002. Epizootiology and effect of avian pox on Hawaiian forest birds. Auk. 119:929–942 [Google Scholar]
- 12. Tripathy DN, Schnitzlein WM, Morris PJ, Janssen DL, Zuba JK, Massey G, Atkinson CT. 2000. Characterization of poxviruses from forest birds in Hawaii. J. Wildl. Dis. 36:225–230 [DOI] [PubMed] [Google Scholar]
- 13. Thiel T, Whiteman NK, Tirape A, Baquero MI, Cedeno V, Walsh T, Uzcátequi GJ, Parker PG. 2005. Characterization of canarypox-like viruses infecting endemic birds in the Galapagos Islands. J. Wildl. Dis. 41:342–353 [DOI] [PubMed] [Google Scholar]
- 14. Smits JE, Tella JL, Carrete M, Serrano D, Lopez G. 2005. An epizootic of avian pox in endemic short-toed larks (Calandrella rufescens) and Berthelot's pipits (Anthus berthelotti) in the Canary Islands, Spain. Vet. Pathol. 42:59–65 [DOI] [PubMed] [Google Scholar]
- 15. Bailey TA, Silvanose C, Manvell R, Gough RE, Kinne J, Combreau O, Launay F. 2002. Medical dilemmas associated with rehabilitating confiscated houbara bustards (Chlamydotis undulata macqueenii) after avian pox and paramyxovirus type 1 infection. J. Wildl. Dis. 38:518–532 [DOI] [PubMed] [Google Scholar]
- 16. Deem SL, Cruz MB, Higashiguchi JM, Parker PG. 2012. Diseases of poultry and endemic birds in Galapagos: implications for the reintroduction of native species. Anim. Conserv. 15:73–82 [Google Scholar]
- 17. Krone O, Essbauer S, Wibbelt G, Isa G, Rudolph M, Gough RE. 2004. Avipoxvirus infection in peregrine falcons (Falco peregrinus) from a reintroduction programme in Germany. Vet. Rec. 154:110–113 [DOI] [PubMed] [Google Scholar]
- 18. Lawson B, Lachish S, Colvile KM, Durrant C, Peck KM, Toms MP, Sheldon BC, Cunningham AA. 2012. Emergence of a novel avian pox disease in British tit species. PLoS One 7:e40176 doi:10.1371/journal.pone.0040176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lachish S, Lawson B, Cunningham AA, Sheldon BC. 2012. Epidemiology of the emergent disease Paridae pox in an intensively studied wild bird population. PLoS One 7:e38316 doi:10.1371/journal.pone.0038316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lachish S, Bonsall MB, Lawson B, Cunningham AA, Sheldon BC. 2012. Individual and population-level impacts of an emerging poxvirus disease in a wild population of great tits. PLoS One 7:e48545 doi:10.1371/journal.pone.0048545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manarolla G, Pisoni G, Sironi G, Rampin T. 2010. Molecular biological characterization of avian poxvirus strains isolated from different avian species. Vet. Microbiol. 140:1–8 [DOI] [PubMed] [Google Scholar]
- 22. Afonso CL, Tulman ER, Lu Z, Zsak L, Kutish GF, Rock DL. 2000. The genome of fowlpox virus. J. Virol. 74:3815–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tulman ER, Afonso CL, Lu Z, Zsak L, Kutish GF, Rock DL. 2004. The genome of canarypox virus. J. Virol. 78:353–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams CJ, Feldman SH, Sleeman JM. 2005. Phylogenetic analysis of avian poxviruses among free-ranging birds of Virginia. Avian Dis. 49:601–605 [DOI] [PubMed] [Google Scholar]
- 25. Jarmin S, Manvell R, Gough RE, Laidlaw SM, Skinner MA. 2006. Avipoxvirus phylogenetics: identification of a PCR length polymorphism that discriminates between the two major clades. J. Gen. Virol. 87:2191–2201 [DOI] [PubMed] [Google Scholar]
- 26. Luschow D, Hoffmann T, Hafez HM. 2004. Differentiation of avian poxvirus strains on the basis of nucleotide sequences of 4b gene fragment. Avian Dis. 48:453–462 [DOI] [PubMed] [Google Scholar]
- 27. Weli SC, Traavik T, Tryland M, Coucheron DH, Nilssen O. 2004. Analysis and comparison of the 4b core protein gene of avipoxviruses from wild birds: evidence for interspecies spatial phylogenetic variation. Arch. Virol. 149:2035–2046 [DOI] [PubMed] [Google Scholar]
- 28. Docherty DE, Slota PG. 1988. Use of muscovy duck embryo fibroblasts for the isolation of viruses from wild birds. J. Tissue Cult. Methods 11:165–170 [Google Scholar]
- 29. OIE 2008. Fowlpox. In OIE manual of diagnostic tests and vaccines for terrestrial animals, 6th ed OIE, Paris, France: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.10_FOWLPOX.pdf [Google Scholar]
- 30. Binns MM, Boursnell ME, Tomley FM, Campbell J. 1989. Analysis of the fowlpoxvirus gene encoding the 4b core polypeptide and demonstration that it possesses efficient promoter sequences. Virology 170:288–291 [DOI] [PubMed] [Google Scholar]
- 31. Corpet F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee LH, Lee KH. 1997. Application of the polymerase chain reaction for the diagnosis of fowl poxvirus infection. J. Virol. Methods 63:113–119 [DOI] [PubMed] [Google Scholar]
- 33. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 34. Xia X. 2001. Data analysis in molecular biology and evolution. Kluwer Academic Publishers, Boston, MA [Google Scholar]
- 35. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 36. Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696–704 [DOI] [PubMed] [Google Scholar]
- 37. Posada D. 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25:1253–1256 [DOI] [PubMed] [Google Scholar]
- 38. Saitou N, Nei M. 1987. The neighbor joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 39. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum-parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swofford DL. 2002. PAUP*. Phylogenetic analysis using parsimony (* and other methods), version 4.0. Sinauer Associates, Sunderland, MA [Google Scholar]
- 41. Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755 [DOI] [PubMed] [Google Scholar]
- 42. Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- 43. Lockhart PJ, Steel MA, Hendy MD, Penny D. 1994. Recovering evolutionary trees under a more realistic model of sequence evolution. Mol. Biol. Evol. 11:605–612 [DOI] [PubMed] [Google Scholar]
- 44. Emerson GL, Li Y, Frace MA, Olsen-Rasmussen MA, Khristova ML, Govil D, Sammons SA, Regnery RL, Karem KL, Damon IK, Carroll DS. 2009. The phylogenetics and ecology of the orthopoxviruses endemic to North America. PLoS One 4:e7666 doi:10.1371/journal.pone.0007666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heath L, van der Walt E, Varsani A, Martin DP. 2006. Recombination patterns in aphthoviruses mirror those found in other picornaviruses. J. Virol. 80:11827–11832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jarvi SI, Triglia D, Giannoulis A, Farias M, Bianchi K, Atkinson CT. 2008. Diversity, origins, and virulence of avipoxviruses in Hawaiian forest birds. Cons. Genet. 9:339–348 [Google Scholar]
- 47. Farias ME, LaPointe DA, Atkinson CT, Czerwonka C, Shrestha R, Jarvi SI. 2010. Taqman real-time PCR detects avipoxvirus DNA in blood of Hawai'i'amakihi (Hemignathus virens). PLoS One 5:e10745 doi:10.1371/journal.pone.0010745 [DOI] [PMC free article] [PubMed] [Google Scholar]