Abstract
Many mucosal factors in the female genital tract (FGT) have been associated with HIV susceptibility, but little is known about their anatomical distribution in the FGT compartments. This study comprehensively characterized global immune factor expression in different tissue sites of the lower and upper FGT by using a systems biology approach. Tissue sections from the ectocervix, endocervix, and endometrium from seven women who underwent hysterectomy were analyzed by a combination of quantitative mass spectrometry and immunohistochemical staining. Of the >1,000 proteins identified, 281 were found to be differentially abundant in different tissue sites. Hierarchical clustering identified four major functional pathways distinguishing compartments, including innate immune pathways (acute-phase response, LXR/RXR) and development (RhoA signaling, gluconeogenesis), which were enriched in the ectocervix/endocervix and endometrium, respectively. Immune factors important for HIV susceptibility, including antiproteases, immunoglobulins, complement components, and antimicrobial factors, were most abundant in the ectocervix/endocervix, while the endometrium had a greater abundance of certain factors that promote HIV replication. Immune factor abundance is heterogeneous throughout the FGT and shows unique immune microenvironments for HIV based on the exposure site. This may have important implications for early events in HIV transmission and site-specific susceptibility to HIV in the FGT.
INTRODUCTION
The female genital tract (FGT) is the first site of contact for sexually transmitted infections such as HIV, and heterosexual transmission via the FGT remains the main route of infection worldwide (1). The FGT has both anatomical and biological innate defensive mechanisms to prevent infection with invading microbes. These include physical barriers such as a mucous epithelial layer and biological barriers that include immune cells stationed in the submucosa and/or epithelium. Mucosal secretions that cover the epithelium contain a plethora of innate and adaptive factors that can neutralize and kill invading microorganisms. Although these offer protection, HIV is still capable of bypassing these barriers. Portals of entry into the FGT may include the multilayered squamous epithelium of the ectocervix and vaginal surface, the single columnar epithelium of the endocervix, and potentially the endometrium, as infected semen can move upward through the endocervical canal. Therefore, the entire FGT is a potential target for HIV acquisition.
Many soluble factors secreted in the FGT have been implicated in playing important roles against HIV infection. These include proteins such as mucins, antileukoproteinase (secretory leukocyte protease inhibitor [SLPI]), elafin, lysozyme, defensins, thrombospondin, cathepsins, histones, and heat shock proteins (2, 3). Mucosal antibodies are important for the antiviral activity of the FGT (4) and are implicated in protective mucosal responses in animal and human vaccine trials (5, 6). Complement components also play important roles in the innate and adaptive immune systems (7) and are of particular importance, as they can either inhibit or facilitate HIV-1 infection (8). Studies of HIV-exposed seronegative (HESN) individuals have shown that specific adaptive and innate factors are associated with HIV resistance, including HIV-neutralizing IgA antibodies (9–11) and overexpression of serine/cysteine antiproteases such as serpins, elafin, cystatins, and A2ML1 (12–14). However, little is known about the anatomical sites, spatial expression, or cell types that express these factors within the FGT. A better characterization of immune factor expression in these tissues and their anatomical distribution would aid in our understanding of this mucosal surface that is at the forefront of exposure to HIV and other pathogens. This would also help us define the immune environments of the sites of first exposure to HIV.
In this study, we define for the first time the anatomical distribution of immune factors in the FGT. We used a systems biology approach to characterize tissue sites of the lower and upper FGT, including the ectocervix, endocervix, and the endometrium from healthy women. The application of mass spectrometry-based proteomic techniques has allowed for a more in-depth examination of mucosal environments (13, 15), and here we characterized individual expression patterns of >1,000 unique proteins, identifying anatomical differences in immune factor expression important for HIV pathogenesis.
MATERIALS AND METHODS
Study population and sample collection.
Genital tissue samples were obtained from seven women (mean age, 48 years; range, 42 to 57 years) who underwent hysterectomy for nonmalignant and noninflammatory conditions (heavy menstrual bleeding and/or benign myoma) at the St. Göran Hospital, Stockholm, Sweden. Inclusion criteria included being HIV IgG seronegative and having no clinical symptoms of sexually transmitted infections during the 3 months prior to surgery. The hysterectomy samples were immediately transported on ice to the pathology department, where a pathologist specializing in gynecological specimens processed endometrial, endocervical, and ectocervical biopsy specimens (at least 3 by 3 mm per sample). All samples were snap-frozen in liquid nitrogen within 30 min of surgical removal and stored at −80°C until mass spectrometry analysis. Informed consent was obtained from all study subjects, and ethical approval was obtained from the Regional Ethical Review Board in Stockholm.
Preparation of FGT tissue samples for mass spectrometry analysis.
FGT tissue samples were added to a lysis buffer (7 M urea, 2 M thiourea, 40 mM Tris [pH 7.6], 100 mM dithiothreitol) and placed into MACS M tubes. Tissues were mechanically homogenized by a MACS Dissociator (Milteny Biotech) on setting RNA-02-01M, which sonicated the samples for three 85-s intervals. Samples were then centrifuged at 9,000 rpm at 4°C for 20 min for clarification and centrifuged again at 15,000 rpm at 4°C for 30 min to remove cell debris. The protein concentration of the supernatants was determined by 2D-Quant assay (GE Healthcare). The same amount of total protein from each sample was then prepared for label-free tandem mass spectrometry analysis (data not shown). Statistical analysis of protein expression was performed by one-way analysis of variance (ANOVA). The complete protein expression data set and detailed mass spectrometry methods can be found at our public database (www.corefacility.ca/proteomics/data/burgener/pubs/2013jvi).
Hierarchical clustering and IPA.
Cluster analysis was performed using Cluster software, version 3.0, and data were visualized using TreeView software, version 1.1.5 (16). Clustering of proteins and tissues was generated by unsupervised centroid linkage hierarchical clustering using the Pearson correlation coefficient as the distance metric. The data set containing differentially abundant proteins (P < 1 × 104) was analyzed by Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, Mountain View, CA). The association between proteins in the data set and the canonical pathways in the Ingenuity Pathway Knowledge Base was measured as a ratio of the number of molecules from the data that map to a pathway divided by the total number of molecules that map to the canonical pathway. A right-tailed Fisher exact test (with Benjamini-Hochberg multiple testing correction) was used to calculate the P value of the probability that the association between each protein in the data set and canonical pathway is random. Pathways with P values of <0.05 and at least two proteins were selected as potential pathways associated with each branch in the cluster analysis. Biological function analysis was also performed by the IPA software, which compares protein expression patterns against the biological function database. A P value association and weighted z score are calculated, where the z score denotes the predicted increased activation (positive) or decreased activation (negative) of this pathway.
In situ detection of selected proteins by immunohistochemistry.
Immunohistochemistry was performed on 8-μm-thick sections of cryopreserved tissue samples. The sections were fixed in 2% formaldehyde prior to the use of the peroxidase-labeled streptavidin-biotin amplification method as previously described (17, 18). In brief, endogenous biotin was blocked with the Vector Laboratories biotin/avidin blocking kit (Vector Laboratories, Burlingame, CA); this was followed by the addition of primary antibodies polyclonal mouse anti-human A2ML1 IgG (ab72872; Abcam Inc., Cambridge, MA), monoclonal mouse anti-human anti-stefin B/cystatin B IgG2b (ab72353; Abcam), biotinylated goat anti-human IgA (2052-08; Southern Biotech, Birmingham, AL), serpin A1 IgG (ab9400; Abcam Inc.), and polyclonal goat anti-human elafin/trappin IgG (AF1747; R&D Systems Europe Ltd., Abingdon, United Kingdom). Thereafter, secondary biotinylated polyclonal rabbit anti-mouse Ig antibody (E0413; Dako Sweden AB, Stockholm, Sweden) and rabbit anti-goat Ig antibody (E0466; Dako Sweden AB) were added prior to the use of peroxidase-based Vectastain (Vectastain Elite Standard; Vector Laboratories). The staining reactions were developed brown by using diaminobenzidine tetrahydrochloride, and the sections were counterstained with hematoxylin. The negative control consisted of incubation in the presence of the secondary antibody alone. The immunohistochemically stained sections were examined with a DMR-X microscope (Leica, Wetzlar, Germany).
RESULTS
Hierarchical clustering analysis distinguishes FGT compartments by proteins involved in immune pathways.
Representative biopsy samples from different tissue compartments of the lower and upper FGT, including the ectocervix, endocervix, and endometrium, were obtained from seven healthy women donors who underwent hysterectomy for nonmalignant and noninflammatory conditions. The same amount of total protein extracted from each tissue sample, representing each compartment, was independently analyzed by mass spectrometry. More than 1,000 unique proteins were identified with high confidence. The relative abundance ratios of these proteins were calculated by dividing each by the average intensity across all of the samples. Detailed information on this data set can be found in Materials and Methods.
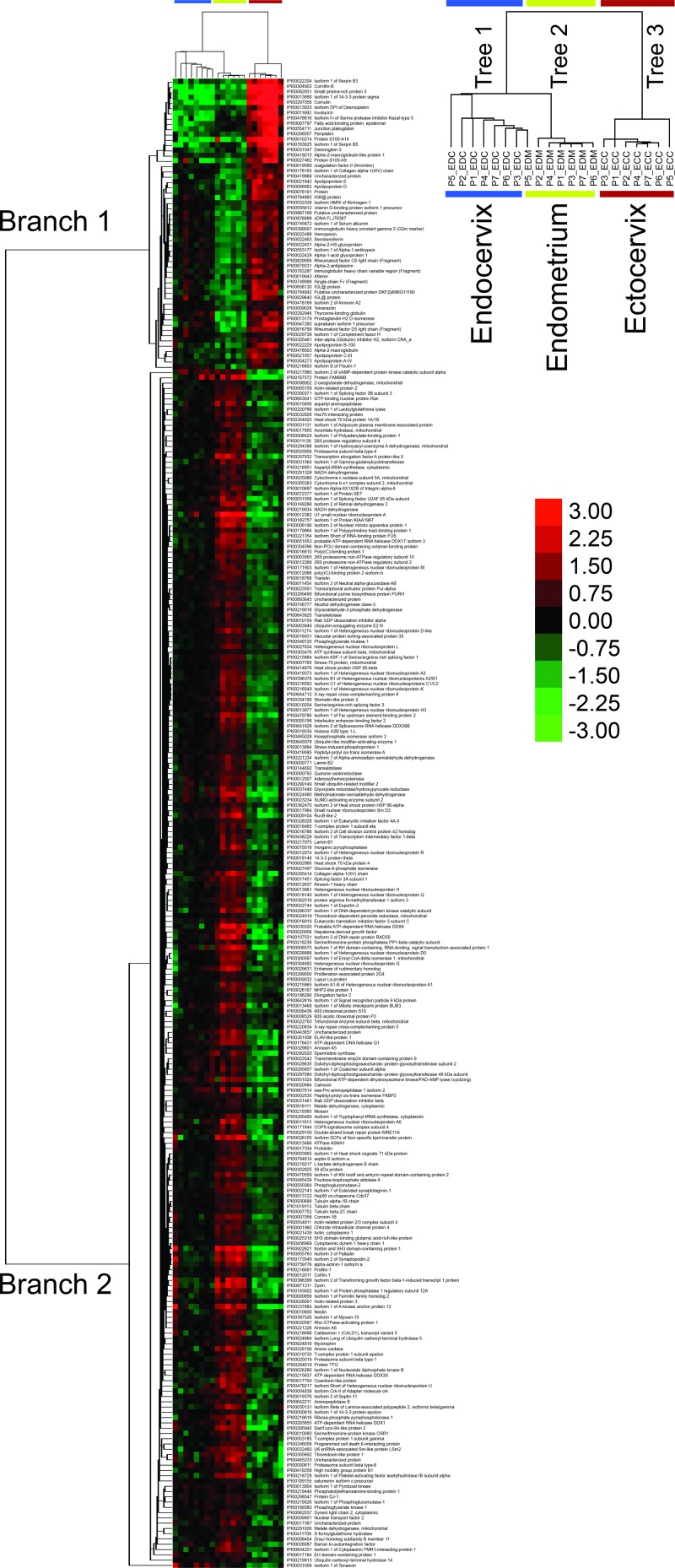
Hierarchical clustering was performed in order to visualize compartment-specific differences in protein expression (Fig. 1). A high threshold was set to distinguish differentially abundant proteins (P value of <1 × 10−4 based on a one-way ANOVA) to increase the stringency of selection, which represented a total of 281 proteins. Cluster analysis showed a clear discrimination of each compartment on the basis of protein abundance, grouping each compartment into three distinct trees (columns). One exception noted was sample P5-EDM, which showed a closer relationship with the endocervix group. Overall, a clear distinction of factor-specific expression by each tissue was identified.
Fig 1.
Protein abundance patterns differentiate anatomical sites of the lower and upper FGT. The heat map shown illustrates 281 proteins that were differentially abundant across tissue subsets according to one-way ANOVA (P < 1 × 10−4). Clustering of proteins and tissues was generated by unsupervised centroid linkage hierarchical clustering using the Pearson correlation coefficient as the distance metric. Protein abundance levels are shown in color, with red indicating overabundant proteins and green indicating underabundant proteins compared to the mean of all tissue samples. FGT compartment tissues (columns) are grouped together and distinguished by two distinct branches (left side) of protein abundance patterns (ECC, ectocervix; EDC, endocervix; EDM, endometrium). The heat map shows almost perfect clustering of tissue compartments based on protein abundance patterns (with the exception of EDM5, which clustered with the endocervical group).
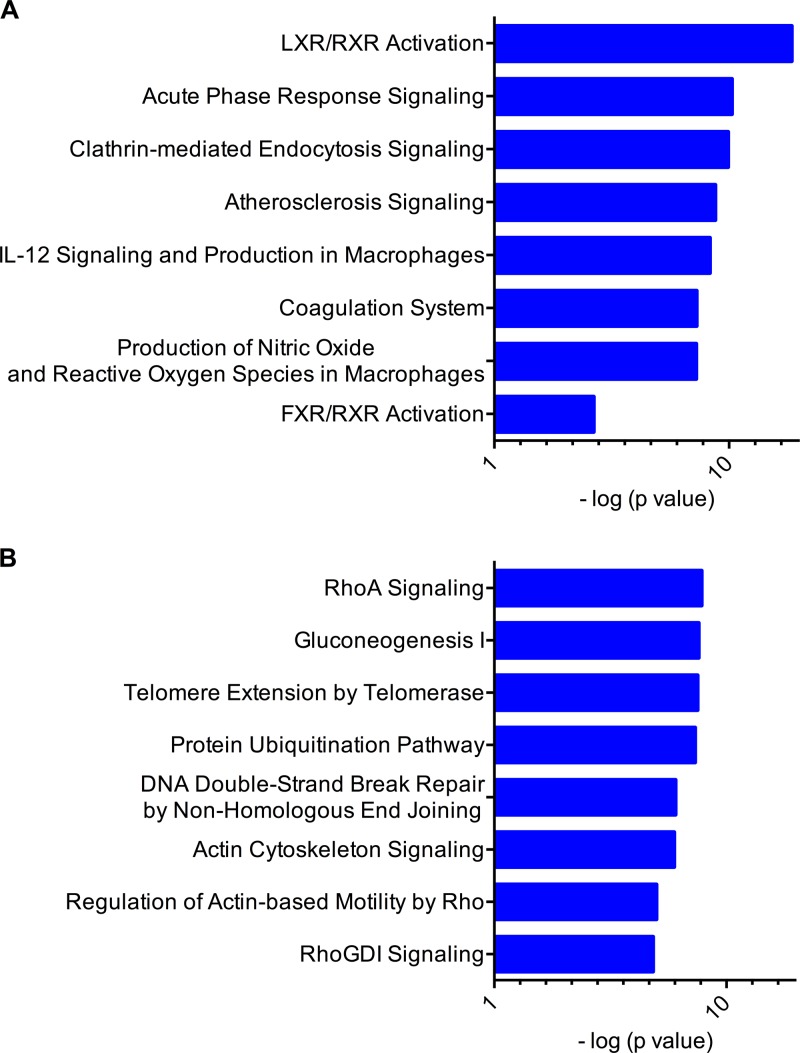
To determine if any of the differentially abundant proteins were enriched for any particular pathways, the IPA tool was used to analyze the data set. Two distinct branches were observed that showed a clear overabundance of specific proteins in ectocervical samples (branch 1) and many in endometrial samples (branch 2). The top two canonical pathways associated with branch 1 were critical immune response pathways, including LXR/RXR activation (P = 1.82 × 10−17, where 14 out of the 136 proteins involved in this pathway were found) and acute-phase signaling (P = 1.44 × 10−9, 10/178 proteins) (Fig. 2A). In contrast, the top two canonical pathways associated with branch 2 were RhoA signaling (P = 2.31 × 10−6, 12/114 proteins) and gluconeogenesis (P = 2.31 × 10−6, 7/50 proteins) (Fig. 2B). This unbiased approach shows the distinct biology of each compartment based on protein expression patterns.
Fig 2.
The ectocervix and endocervix are distinguished by increased activation of immune response pathways. The top two branches identified by hierarchical clustering were analyzed by comparative IPA software. The major canonical pathways represented in branches are shown (A, branch 1; B, branch 2), with the most statistically significantly associated pathways shown in decreasing order (top to bottom). Branch 1 identifies the LXR/RXR and acute-phase response pathways as the top pathways associated with proteins found to be overabundant in ectocervical tissue. The RhoA and gluconeogenesis pathways are identified as the top pathways associated with proteins found to be overabundant in endometrial tissue. Associations calculated by a right-tailed Fisher exact test (with Benjamini-Hochberg correction) (log P values are on the horizontal axis) were used to calculate the P value of the probability that the association between each protein appearing in the data set and a canonical pathway is random. Only the top eight associated pathways are shown for vertical sizing.
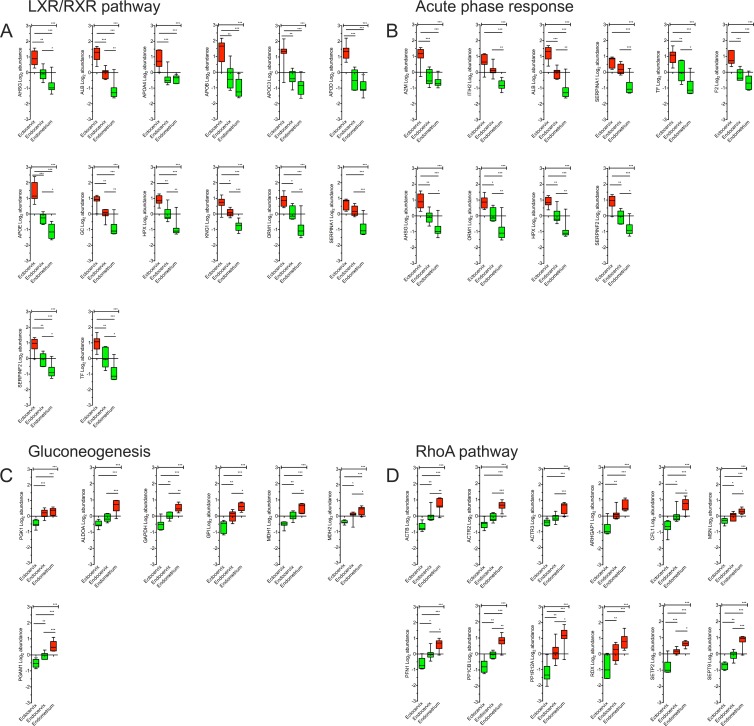
The abundance profiles of proteins involved with the LXR/RXR (14 proteins) and acute-phase response (10 proteins) pathways are shown in Fig. 3A and B, respectively. These pathways overlap significantly; seven of these proteins (AHSG, ALB, HPX, ORM1, SERPINA1, SERPINF2, and TF) are found in both pathways. These pathways are important in providing first-response innate protection from pathogens, as well as regulating immune responses (19, 20). The acute-phase response includes many antiproteases such as serpins, alpha-2-macroglobulin, and inter-alpha-trypsin inhibitor, whereas the LXR/RXR pathway includes these, as well as apolipoproteins, transferrin, and other mediators. A clear overabundance of these proteins was observed in ectocervix tissue compared to the other two compartments, which indicates that the ectocervix is the major contributor of these immune response proteins. The endometrium was enriched for proteins involved in gluconeogenesis and RhoA signaling (Fig. 3C and D, respectively). The RhoA pathway is important for cellular organization, regulation of intermediate filaments, and contraction (21). Many of these proteins included actins and actin-related proteins, Rho GTPase-activating proteins, septins, radixin, protein phosphatase 1, cofilin, moiesin, and profilin. Gluconeogenesis proteins included enzymes that process triose phosphates, citric acid cycle intermediates, and hexoses for storage, which is critical for the development of cells and tissue. Not surprisingly, this indicates that the endometrium is in a state of cellular growth, turnover, and development.
Fig 3.
Expression profiles of proteins involved in innate immunity and tissue development pathways that distinguish FGT compartments. Shown are expression profiles of proteins identified in canonical pathways most significantly associated with each branch of the hierarchical clustering analysis that distinguished FGT compartments. These include the LXR/RXR (A), acute-phase response (B), gluconeogenesis (C), and RhoA (D) pathways. The y axis represents their log2 abundance profiles (mean ± standard error of the mean) based on the FGT compartment. Colors indicate whether the average abundance in each compartment (ectocervix, endocervix, endometrium) was above (red) or below (green) the mean. Significance values across all groups were calculated by one-way ANOVA, and intergroup variations were calculated by Tukey's multiple-comparison t test (P < 0.01, *; P < 0.001, **; P < 0.0001, ***).
Proteins differentially abundant in tissue compartments of the FGT are significantly associated with virus infectivity pathways.
Differentially abundant proteins were further compared against the biological function database in IPA to determine if these expression patterns overlap with specific biological functions. The IPA software calculates P values for the association of these proteins with specific functional categories and weighted z scores that denote increased expression (positive) or decreased expression (negative). Interestingly, the most statistically significant association was with proteins that have previously described involvement with viral infectivity (Table 1), where the ectocervix and endocervix showed decreased expression (negative z scores) and the endometrium showed increased expression (positive z score). The proteins involved in this pathway (67 molecules) are shown in Fig. 4, separated by cellular location (nucleus, cytoplasm, extracellular), with the average protein abundance in the ectocervix (A), the endocervix (B), and the endometrium (C) represented by color (red, overabundant; green, underabundant). A clear overexpression of these factors was observed in the endometrium, while both the ectocervix and endocervix showed lower expression. Many of the intracellular factors have roles in promoting HIV pathogenesis, including integrins (ITGA5, ITGB1, ITGAV), annexins (ANXA2, ANXA5, ANXA6) (22), CD63 (23), CD44 (24), filamin A (FLNA) (25), caveolin (CAV1) (26), ribosomal proteins (RPS10, RPS27A) (27), nuclear factor Sam68 (KHDRBS1) (28), and SAP145 (SF3B2) (29). Conversely, extracellular inflammatory and immune response factors such as serpins, immunoglobulins, and complement components were more abundant in the ectocervix than in the endocervix and endometrium, respectively. This demonstrates that proteins differentially abundant in different compartments are involved in viral infectivity pathways, many of which have known roles in promoting HIV infection.
Table 1.
Top biological functions associated with proteins differentially abundant in compartments of the FGT according to the IPA knowledge database
| Tissuea | Category | Biological function | P valueb | Predicted activation state | Activation z score | No. of proteins |
|---|---|---|---|---|---|---|
| ECC | Infectious disease | Viral infection | 1.71 × 10−5 | Decreased | −4.35 | 67 |
| EDC | Infectious disease | RNA virus replication | 5.97 × 10−4 | Decreased | −2.24 | 26 |
| EDM | Infectious disease | Viral infection | 1.71 × 10−5 | Increased | 4.35 | 67 |
ECC, ectocervix; EDC, endocervix; EDM, endometrium.
Each P value was calculated by Fischer's exact test, and multiple comparisons were corrected for by Benjamini-Hochberg correction.
Fig 4.
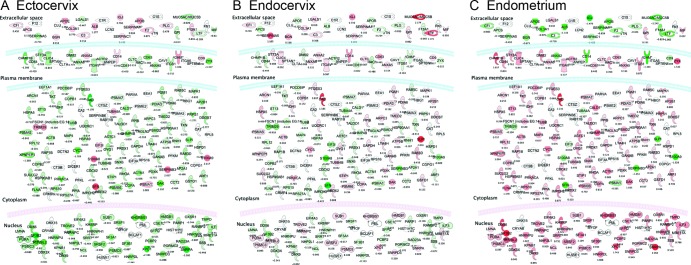
Cellular pathways involved in viral infectivity are differentially enriched in FGT tissue compartments. The highest-scoring immune pathways associated with differential protein abundance patterns in FGT compartments were those involved with viral infectivity according to the IPA biological function database. Proteins involved in the viral infectivity pathway are represented for each compartment (A, ectocervix; B, endocervix; C, endometrium), with the abundance log2 ratios indicated by intensity of color (green, underexpressed; red, overexpressed [compared to the average]), and their subcellular locations are shown. The endometrium shows a clear overabundance of intracellular proteins that are involved in this pathway, and the ectocervix/endocervix show a greater abundance of extracellular immune factors, such as immunoglobulins, complement components, and serpins. All of the proteins shown in color have a calculated P value of <0.05 between compartments by one-way ANOVA, and the effect of multiple comparisons was corrected for by Benjamini-Hochberg correction. Proteins in white were present in the data set but did not reach the statistical significance threshold.
Soluble mucosal immune factors are not uniformly abundant in compartments of the FGT.
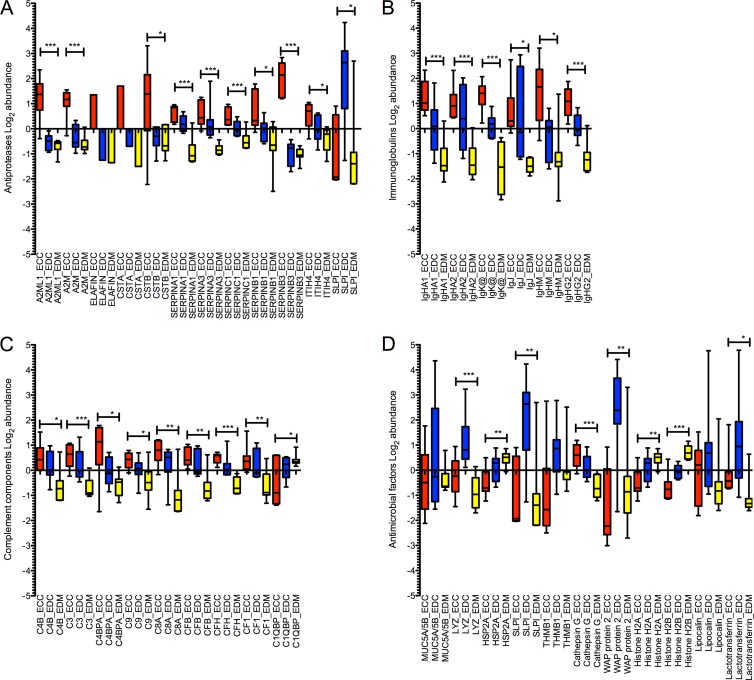
Soluble immune factors that have been previously reported to be involved in HIV pathogenesis were identified, separated by functional classes (antiproteases, immunoglobulins, complement components, antimicrobial factors), and graphed in Fig. 5 for each FGT compartment. Factors that have multiple functions are shown in more than one category.
Fig 5.
Soluble immune factors are predominant in the ectocervix/endocervix and lowest in the endometrium. Shown are log2 abundance values of antiproteases (A), immunoglobulins (B), complement components (C), and antimicrobial factors (D) in FGT compartments. Compartment-specific expression is shown by color as follows: red, ectocervix (ECC); blue, endocervix (EDC); yellow, endometrium (EDM). Significance values across all groups were calculated by one-way ANOVA (P < 0.01, *; P < 0.001, **; P < 0.0001, ***).
The majority of these factors were found to be more abundant in the ectocervical and endocervical compartments than in the endometrium. Antiproteases (Fig. 5A) were most predominant in the ectocervix, with average abundance levels of all serpins (serpins A1, A3, C1, B1, and B3) considerably higher, ranging from ∼0.5- to 1-log2-fold difference from the other compartments (serpins A1, A3, C1, and B1) and others with a >1-log2-fold difference (serpin B3). Other antiproteases (A2ML1, A2M, cystatins, elafin, and ITIH4) showed similar trends with an ∼1-log2-fold change between one or more compartments and the highest expression in the ectocervix. In contrast, SLPI was more abundant in the endocervix (∼3 log2-fold more abundant) than in the ectocervix and the endometrium. Immunoglobulins (Ig), including heavy chains of the IgG, IgM, IgJ, IgHA1, and IgHA2 subtypes and Igκ light chains (Fig. 5B) showed a clear trend of overabundance in ectocervix tissue and significantly lower levels in the endometrium. For example, extracellular IgHA1 and IgHA2 are shown to be ∼1- to 2-log2-fold more abundant in the ectocervix than in the endocervix and the endometrium, respectively. The highest expression of the majority of complement components (Fig. 5C) was observed in ectocervical tissue, with the exception of C1QBP (highest in the endometrium). The distribution range was generally within the 0.5-log2 range, with some exceptions, such as C4BPA, which was 1 log2 more abundant in the ectocervix than in the endocervix or the endometrium.
In contrast, specific antimicrobials trended toward greater abundance in endocervical tissue (Fig. 5D). Mucins, lysozyme, whey acidic proteins (including SLPI), thrombospondin, lipocalin, and lactoferrin were most abundant in the endocervix, with some (WAP protein 2 and SLPI) showing 3-log2-fold higher levels. Heat shock proteins and histones were most abundant in the endometrium, showing ∼0.5- and 1-log2-fold differences from the endocervix and ectocervix, respectively. Only cathepsin G and dermicidin had the highest expression in the ectocervix, ranging from 0.5- to ∼1-log2 greater abundance. However, as some antiproteases have antimicrobial activity and are overabundant in the ectocervix, this suggests that both the ectocervix and the endocervix are major sources of these antimicrobial proteins. These data show that many immune factors important for HIV pathogenesis are compartmentalized throughout the lower and upper FGT and are considerably heterogeneous between sites.
Tissue localization of factors associated with anti-HIV activity in different FGT compartments.
Several soluble mucosal proteins have been associated with altered susceptibility to HIV in the cervical mucosa of HESN individuals, including secretory IgA, serpins, cystatins, elafin, and A2ML1 (10, 12–14, 30). Many of these soluble factors may also inhibit HIV-1 replication in susceptible cell types (2, 30–32). Although they have been well characterized in mucosal secretions, their relative distribution in the compartments of the FGT and their cellular origin have not been elucidated. To further characterize these immune factors, immunohistochemical staining was performed to visualize the antiproteases serpin A1, elafin, cystatin B, A2ML1, and IgA in tissues of the ectocervix, endocervix, and endometrium, which are shown in Fig. 6.
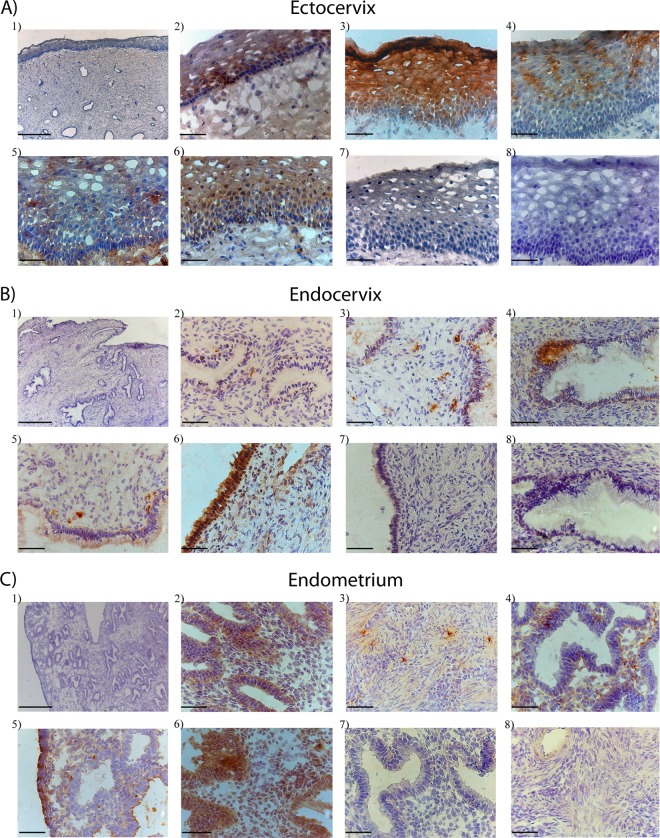
Fig 6.
Bright-field images of in situ staining of selected antiproteases and immunoglobulins in tissue sections from the ectocervix (A), endocervix (B), and endometrium (C). The tissue sections shown were stained with hematoxylin (blue) for visualization of cell nuclei. Images A1, B1, and C1 are ×5 overview pictures of representative ectocervix, endocervix, and endometrium tissue samples showing the epithelium and the submucosal layer. For visualization of immune proteins, tissue sections were stained (brown) for A2ML1 (A2, B2, and C2), cystatin B (A3, B3, and C3), elafin (A4, B4, and C4), IgA (A5, B5, and C5), serpin A1 (A6, B6, and C6), rabbit anti-mouse IgG (A7, B7, and C7), and rabbit anti-goat IgG (A8, B8, and C8) antibodies. The A1, B1, and C1 images were collected with a 5×/0.12 objective (scale bars, 500 μm), and all of the other images were collected with a 40×/0.65 objective (scale bars, 50 μm).
All proteins were found to be expressed within all three tissue types but with different distribution patterns. The highest expression was found in the ectocervix (Fig. 6A), followed by the endometrium and (Fig. 6C) and the endocervix (Fig. 6B). Within the ectocervix, A2ML1, serpin A1, and IgA expression was detected both in the basal layer of the epithelium and in the submucosal layer whereas cystatin B and elafin were detected only in the epithelium (Fig. 6A). Elafin and serpin A1 were localized to the columnar epithelium of the endocervix, whereas A2ML1, cystatin B, and IgA were detected in the submucosal layer of the endocervix (Fig. 6B). Within the endometrium, A2ML1, serpin A1, and IgA were found mainly at or adjacent to the columnar epithelium whereas cystatin B-positive cells were detected mainly in the myometrium (Fig. 6C). Thus, the in situ staining concurred with the mass spectrometry analysis: A2ML1, cystatin B, elafin, serpin A1, and IgA were expressed with various abundances in these three compartments of the FGT and the highest expression was found in ectocervical tissue. This demonstrates that these antiviral factors are positioned at the forefront of likely areas of HIV exposure, although their cellular and anatomical distribution is considerably heterogeneous.
DISCUSSION
This comprehensive proteomic analysis of the lower and upper FGT provides numerous insights into the biology of the ectocervical, endocervical, and endometrial compartments. This is the first study to comprehensively quantify immune factors in different compartments of the FGT and show that these environments are clearly distinguished by proteins involved in innate immune pathways. The ectocervix contains the highest abundance of soluble factors important for the immune response, including antiproteases, immunoglobulins, complement components, and other antiviral proteins, supporting a frontline role. In contrast, the endometrium expresses many proteins that are involved with supporting HIV infectivity. This is the first study that shows the distinct immunological differences of each tissue compartment in the lower and upper FGT by a systems biology approach and may have important implications for mucosally transmitted infections, such as HIV.
The LXR/RXR and acute-phase response pathways were found overabundant and predominantly in the ectocervix, which is consistent with its anatomical location. The lower FGT is populated with commensal microorganisms such as Lactobacillus that are kept separated from the body by a multilayered squamous (ectocervix and vaginal vault) or columnar (endocervix) epithelium. The maintenance of a balance of bacterial communities in the FGT is facilitated by both innate and adaptive immune mechanisms that are regulated in part by the LXR/RXR and acute-phase response pathways (19, 20). Therefore, the activation of these pathways in the ectocervix is expected and agrees with other studies showing that the ectocervix as a primary site for antigen-presenting cells and cytotoxic T-cell responses (33). The intermediate expression of acute-phase response/LXR/RXR pathways in the endocervix and their lowest expression in the endometrium also agree with the fact that the region from the endocervix to the upper portion of the FGT is less exposed to external pathogens. Activation of these pathways may be important for the inhibition of HIV infection, as acute-phase responses have been implicated as critical modulators that limit HIV replication during early stages of HIV viremia (34), with some proteins in these pathways, including serpins, capable of inhibiting HIV-1 replication (32). However, it is unknown how these pathways affect immune cell populations and these responses may be detrimental as they may increase target cell availability.
There is a paucity of information about the upper FGT and HIV infection, presumably because it is thought not to play a significant role in HIV pathogenesis. This systems analysis indicates that the endometrium harbors larger amounts of proteins involved with viral infectivity pathways and smaller amounts of proteins that could potentially inhibit HIV infection. The endometrium was distinguished by the increased expression of proteins involved in RhoA signaling and gluconeogenesis; although this is consistent with the biological function of this compartment in a reproductive capacity, it may also facilitate HIV infection. For example, RhoA pathway proteins, including actin, are utilized by HIV to facilitate entry into permissive cells, and the cellular proteins radixin, moesin, and cofilin further aid in HIV propagation (35). Gluconeogenic pathway activation may further support HIV replication by engaging enzymes that can enhance ATP production and thus support energy for the viral protein production machinery. Furthermore, biological function analysis indicated that the endometrium produces larger amounts of certain intracellular proteins involved with promoting virus and HIV infectivity. However, it is unknown what role these differences in protein expression have in tissue susceptibility to HIV infection. It is also unknown whether these expression patterns translate to HIV-susceptible cell types such as T cells. Certainly, this information would have to be balanced with both the availability of target cells and the number of virus particles exposed to each site (presumably far fewer than would be exposed at the ectocervix, for example), which are likely large contributing factors to in vivo infection events. However, the facts that an abundance of T cells and HIV-1 coreceptor expression has been described in all compartments, including the endometrium (18, 36, 37), and that endometrial tissue can support HIV-1 replication in vitro support it as a possible portal of entry (38, 39). At the very least, this finding warrants further studies of this compartment as a potential contributor to HIV transmission events and should not be excluded.
Many soluble mucosal factors have been described as important for the general immune response of the FGT and HIV pathogenesis, including antiproteases, immunoglobulins, complement components, and specific antimicrobial proteins with in vitro HIV-inhibitory activity. Antiproteases have diverse roles in nature, such as inhibition of protease activity, signal transduction pathways, regulation of inflammation (32, 40–42), and innate defense against microorganisms, including HIV (13, 32, 43, 44), as well as being associated with HIV resistance in HESN populations (10, 12–14). The role of complement components has been well studied (7), but for HIV infection they have been shown to be controversial as they can be involved in both inhibition and facilitation of HIV-1 infection (8). They can help control viremia but, on the other hand, act as a carrier of HIV infection and aid in its spread (8, 45–47). Mucosal antibodies such as IgG and IgM are important for anti-HIV responses (4, 48–50), although arguably the most important antibody is secretory IgA, as HIV-1 neutralizing IgA has been implicated in resistance to HIV infection in HESN cohorts (9–11, 51) and is functionally relevant in animal and anti-HIV vaccine trials (5, 6). Finally, many nonspecific innate antimicrobial proteins have in vitro HIV-inhibitory activity, such as mucins, whey acidic proteins, lysozyme, SLPI, lactotransferrin, and thrombospondin (2, 3), which can help to neutralize HIV. The predominance of all of these factors in the ectocervix and endocervix, both in the proteomic data set and by immunohistochemical analysis, suggests that these two sites are the major drivers and producers of soluble immune factors against HIV in the FGT.
There were some limitations to this study that warrant discussion. Because of the sensitivity limitations of this mass spectrometry analysis, the expression patterns of lower-abundance proteins, such as cytokines, chemokines, and other important immune regulators, could not be evaluated. Analysis of these factors is critically needed and is an important next step. Also, although tissue samples were taken from women who underwent hysterectomy for nonmalignant and noninflammatory conditions, they represented an older age group. Therefore, these findings agree with this specific age group and it is possible that age has an impact on the immunological environment, which may be different in younger women. However, none of these women were on hormonal contraceptives or had reached menopause. In any case, the homogeneity of compartment-specific results still supports the findings of different immune factor environments throughout the FGT.
This systems biology analysis provides an in-depth characterization of the distinctive biological compartments of the FGT and the production of soluble factors important for immune defense and HIV pathogenesis. The ectocervix contains the largest amounts of antiproteases, complement components, and immunoglobulins, while the endocervix has the largest amount of antimicrobials implicated as key players in HIV infection. While the biological significance of this in HIV transmission in different FGT compartments remains unknown, it does indicate a vastly different immunological environment for the virus, depending on the site of first exposure. The contributions of these factors are likely a complex interplay of activating and inhibiting components, a balance of which may influence viral replication, target cell availability, or the development of the initial adaptive immune response. This information should be an important consideration in the evaluation of these immune factors in the FGT during HIV vaccine and/or microbicide trials and studies that evaluate early transmission events in the FGT.
ACKNOWLEDGMENTS
We thank the study participants; Anette Hofmann for technical help; and Agnes Lagerstedt, Harry Flam, and colleagues at the St. Göran Hospital, Stockholm, Sweden, for the collection of clinical samples. We also thank staff at the National Laboratory for HIV Immunology and University of Manitoba.
This work was funded through the HIV Mucosal Immunology Group and HIV Trials Network (B.B.), the Swedish Research Council and SIDA (K.B.), the Swedish Society for Medical Research (A.T.), and the Public Health Agency of Canada (B.B.).
A.B. performed proteomic analysis, analyzed the data, and wrote the manuscript. A.T. performed immmunohistochemical analysis and helped write the manuscript. T.K. coordinated the sample collection and prepared the clinical data. M.A. and E.A. prepared samples for proteomic analysis. S.M. and G.R.W. performed mass spectrometry analysis, and K.M. aided in proteomic data analysis. B.B. and K.B. contributed equally to the scientific guidance of this study and the editing of the manuscript.
Footnotes
Published ahead of print 28 February 2013
REFERENCES
- 1. UNAIDS 2011. Global report: UNAIDS report on the global AIDS epidemic, 2010. Joint United Nations Programme on HIV/AIDS, New York, NY: http://www.unaids.org/documents/20101123_globalreport_em.pdf [Google Scholar]
- 2. Valore EV, Park CH, Igreti SL, Ganz T. 2002. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 187:561–568 [DOI] [PubMed] [Google Scholar]
- 3. Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. 2011. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am. J. Reprod. Immunol. 65:196–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, Mayer K, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. 2010. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One 5:e11366 doi:10.1371/journal.pone.0011366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hur EM, Patel SN, Shimizu S, Rao DS, Gnanapragasam PN, An DS, Yang L, Baltimore D. 2012. Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood 120:4571–4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 [DOI] [PubMed] [Google Scholar]
- 7. Carroll MC. 2004. The complement system in regulation of adaptive immunity. Nat. Immunol. 5:981–986 [DOI] [PubMed] [Google Scholar]
- 8. Yu Q, Yu R, Qin X. 2010. The good and evil of complement activation in HIV-1 infection. Cell. Mol. Immunol. 7:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Devito C, Broliden K, Kaul R, Svensson L, Johansen K, Kiama P, Kimani J, Lopalco L, Piconi S, Bwayo JJ, Plummer F, Clerici M, Hinkula J. 2000. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 165:5170–5176 [DOI] [PubMed] [Google Scholar]
- 10. Hirbod T, Kaul R, Reichard C, Kimani J, Ngugi E, Bwayo JJ, Nagelkerke N, Hasselrot K, Li B, Moses S, MacDonald KS, Broliden K. 2008. HIV-neutralizing immunoglobulin A and HIV-specific proliferation are independently associated with reduced HIV acquisition in Kenyan sex workers. AIDS 22:727–735 [DOI] [PubMed] [Google Scholar]
- 11. Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, Reynes JM, Lopalco L, Bomsel M. 2009. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2:412–426 [DOI] [PubMed] [Google Scholar]
- 12. Burgener A, Boutilier J, Wachihi C, Kimani J, Carpenter M, Westmacott G, Cheng K, Ball TB, Plummer F. 2008. Identification of differentially expressed proteins in the cervical mucosa of HIV-1-resistant sex workers. J. Proteome Res. 7:4446–4454 [DOI] [PubMed] [Google Scholar]
- 13. Burgener A, Rahman S, Ahmad R, Lajoie J, Ramdahin S, Mesa C, Brunet S, Wachihi C, Kimani J, Fowke K, Carr S, Plummer F, Ball TB. 2011. Comprehensive proteomic study identifies serpin and cystatin antiproteases as novel correlates of HIV-1 resistance in the cervicovaginal mucosa of female sex workers. J. Proteome Res. 10:5139–5149 [DOI] [PubMed] [Google Scholar]
- 14. Iqbal SM, Ball TB, Levinson P, Maranan L, Jaoko W, Wachihi C, Pak BJ, Podust VN, Broliden K, Hirbod T, Kaul R, Plummer FA. 2009. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS 23:1669–1677 [DOI] [PubMed] [Google Scholar]
- 15. Burgener A, Mogk K, Westmacott G, Plummer F, Ball B, Broliden K, Hasselrot K. 2012. Salivary basic proline-rich proteins are elevated in HIV-exposed seronegative men who have sex with men. AIDS 26:1857–1867 [DOI] [PubMed] [Google Scholar]
- 16. Eisen MB, Spellman PT, Brown PO, Botstein D. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U. S. A. 95:14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirbod T, Bailey RC, Agot K, Moses S, Ndinya-Achola J, Murugu R, Andersson J, Nilsson J, Broliden K. 2010. Abundant expression of HIV target cells and C-type lectin receptors in the foreskin tissue of young Kenyan men. Am. J. Pathol. 176:2798–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirbod T, Kaldensjo T, Lopalco L, Klareskog E, Andersson S, Uberti-Foppa C, Ferrari D, Manghi M, Andersson J, Lore K, Broliden K. 2009. Abundant and superficial expression of C-type lectin receptors in ectocervix of women at risk of HIV infection. J. Acquir. Immune Defic. Syndr. 51:239–247 [DOI] [PubMed] [Google Scholar]
- 19. Valledor AF. 2005. The innate immune response under the control of the LXR pathway. Immunobiology 210:127–132 [DOI] [PubMed] [Google Scholar]
- 20. Yoo JY, Desiderio S. 2003. Innate and acquired immunity intersect in a global view of the acute-phase response. Proc. Natl. Acad. Sci. U. S. A. 100:1157–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Etienne-Manneville S, Hall A. 2002. Rho GTPases in cell biology. Nature 420:629–635 [DOI] [PubMed] [Google Scholar]
- 22. Rai T, Mosoian A, Resh MD. 2010. Annexin 2 is not required for human immunodeficiency virus type 1 particle production but plays a cell type-dependent role in regulating infectivity. J. Virol. 84:9783–9792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li G, Dziuba N, Friedrich B, Murray JL, Ferguson MR. 2011. A post-entry role for CD63 in early HIV-1 replication. Virology 412:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shattock RJ, Rizzardi GP, Hayes P, Griffin GE. 1996. Engagement of adhesion molecules (CD18, CD11a, CD45, CD44, and CD58) enhances human immunodeficiency virus type 1 replication in monocytic cells through a tumor necrosis factor-modulated pathway. J. Infect. Dis. 174:54–62 [DOI] [PubMed] [Google Scholar]
- 25. Cooper J, Liu L, Woodruff EA, Taylor HE, Goodwin JS, D'Aquila RT, Spearman P, Hildreth JE, Dong X. 2011. Filamin A protein interacts with human immunodeficiency virus type 1 Gag protein and contributes to productive particle assembly. J. Biol. Chem. 286:28498–28510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang XM, Nadeau PE, Lin S, Abbott JR, Mergia A. 2011. Caveolin 1 inhibits HIV replication by transcriptional repression mediated through NF-kappaB. J. Virol. 85:5483–5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abbas W, Dichamp I, Herbein G. 2012. The HIV-1 Nef protein interacts with two components of the 40S small ribosomal subunit, the RPS10 protein and the 18S rRNA. Virol. J. 9:103 doi:10.1186/1743-422X-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reddy TR, Suhasini M, Xu W, Yeh LY, Yang JP, Wu J, Artzt K, Wong-Staal F. 2002. A role for KH domain proteins (Sam68-like mammalian proteins and quaking proteins) in the post-transcriptional regulation of HIV replication. J. Biol. Chem. 277:5778–5784 [DOI] [PubMed] [Google Scholar]
- 29. Terada Y, Yasuda Y. 2006. Human immunodeficiency virus type 1 Vpr induces G2 checkpoint activation by interacting with the splicing factor SAP145. Mol. Cell. Biol. 26:8149–8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Devito C, Hinkula J, Kaul R, Lopalco L, Bwayo JJ, Plummer F, Clerici M, Broliden K. 2000. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS 14:1917–1920 [DOI] [PubMed] [Google Scholar]
- 31. Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. 2010. Trappin-2/elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology 129:207–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shapiro L, Pott GB, Ralston AH. 2001. Alpha-1-antitrypsin inhibits human immunodeficiency virus type 1. FASEB J. 15:115–122 [DOI] [PubMed] [Google Scholar]
- 33. Pudney J, Quayle AJ, Anderson DJ. 2005. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol. Reprod. 73:1253–1263 [DOI] [PubMed] [Google Scholar]
- 34. Kramer HB, Lavender KJ, Qin L, Stacey AR, Liu MK, di Gleria K, Simmons A, Gasper-Smith N, Haynes BF, McMichael AJ, Borrow P, Kessler BM. 2010. Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog. 6:e1000893 doi:10.1371/journal.ppat.1000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu Y, Belkina NV, Shaw S. 2009. HIV infection of T cells: actin-in and actin-out. Sci. Signal. 2:pe23 doi:10.1126/scisignal.266pe23 [DOI] [PubMed] [Google Scholar]
- 36. Hirbod T, Kaldensjo T, Broliden K. 2011. In situ distribution of HIV-binding CCR5 and C-type lectin receptors in the human endocervical mucosa. PLoS One 6:e25551 doi:10.1371/journal.pone.0025551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaldensjo T, Petersson P, Tolf A, Morgan G, Broliden K, Hirbod T. 2011. Detection of intraepithelial and stromal Langerin and CCR5 positive cells in the human endometrium: potential targets for HIV infection. PLoS One 6:e21344 doi:10.1371/journal.pone.0021344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Howell AL, Edkins RD, Rier SE, Yeaman GR, Stern JE, Fanger MW, Wira CR. 1997. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J. Virol. 71:3498–3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeaman GR, Asin S, Weldon S, Demian DJ, Collins JE, Gonzalez JL, Wira CR, Fanger MW, Howell AL. 2004. Chemokine receptor expression in the human ectocervix: implications for infection by the human immunodeficiency virus-type I. Immunology 113:524–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kopitar-Jerala N. 2006. The role of cystatins in cells of the immune system. FEBS Lett. 580:6295–6301 [DOI] [PubMed] [Google Scholar]
- 41. Mangan MS, Kaiserman D, Bird PI. 2008. The role of serpins in vertebrate immunity. Tissue Antigens 72:1–10 [DOI] [PubMed] [Google Scholar]
- 42. Pott GB, Chan ED, Dinarello CA, Shapiro L. 2009. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J. Leukoc. Biol. 85:886–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Challacombe SJ, Sweet SP. 2002. Oral mucosal immunity and HIV infection: current status. Oral Dis. 8(Suppl 2):55–62 [DOI] [PubMed] [Google Scholar]
- 44. Whitney JB, Asmal M, Geiben-Lynn R. 2011. Serpin induced antiviral activity of prostaglandin synthetase-2 against HIV-1 replication. PLoS One 6:e18589 doi:10.1371/journal.pone.0018589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huber M, Fischer M, Misselwitz B, Manrique A, Kuster H, Niederost B, Weber R, von Wyl V, Gunthard HF, Trkola A. 2006. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 3:e441 doi:10.1371/journal.pmed.0030441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stoiber H, Pruenster M, Ammann CG, Dierich MP. 2005. Complement-opsonized HIV: the free rider on its way to infection. Mol. Immunol. 42:153–160 [DOI] [PubMed] [Google Scholar]
- 47. Tjomsland V, Ellegard R, Che K, Hinkula J, Lifson JD, Larsson M. 2011. Complement opsonization of HIV-1 enhances the uptake by dendritic cells and involves the endocytic lectin and integrin receptor families. PLoS One 6:e23542 doi:10.1371/journal.pone.0023542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 [DOI] [PubMed] [Google Scholar]
- 49. Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, Devillier G, Cong Z, Wei Q, Gao H, Qin C, Yang GB, Zurbriggen R, Lopalco L, Fleury S. 2011. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 34:269–280 [DOI] [PubMed] [Google Scholar]
- 50. Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449–12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choi R, Levinson P, Guthrie B, Lohman-Payne B, Bosire R, Liu A, Hirbod T, Kiarie J, Overbaugh J, John-Stewart G, Broliden K, Farquhar C. 2012. Cervicovaginal HIV-1 neutralizing immunoglobulin A detected among HIV-1-exposed seronegative female partners in HIV-1-discordant couples. AIDS 26:2155–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]