Abstract
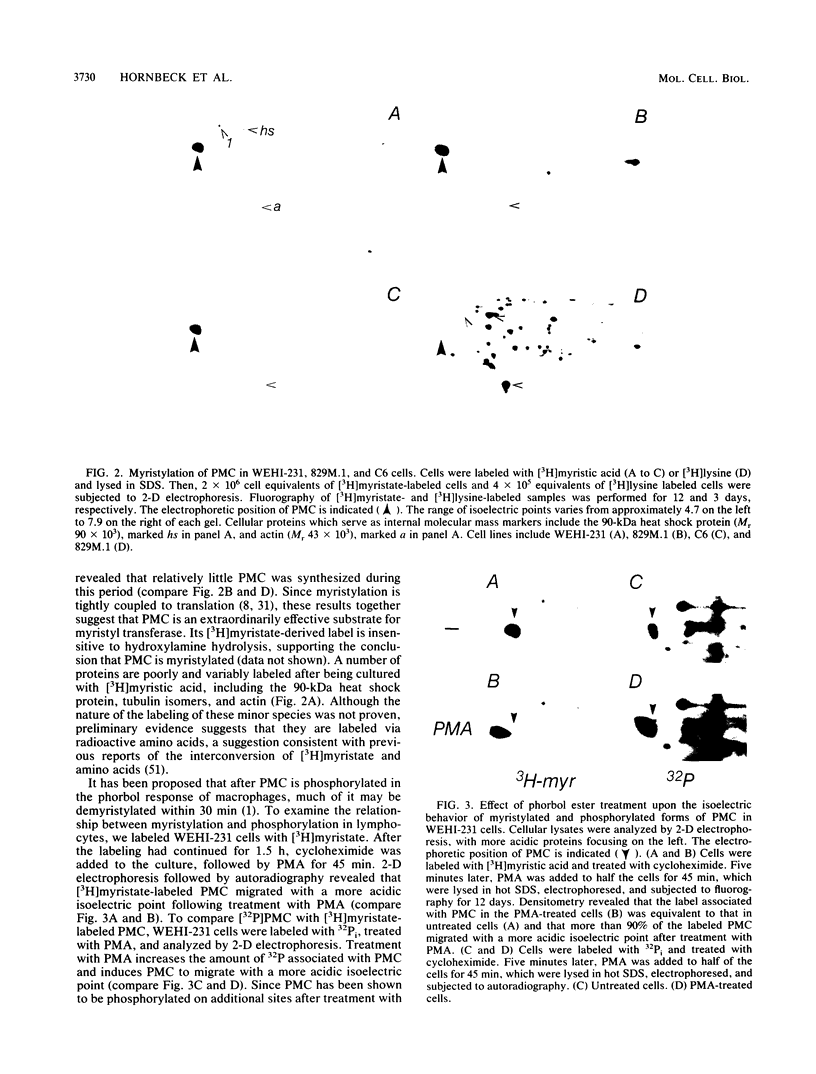
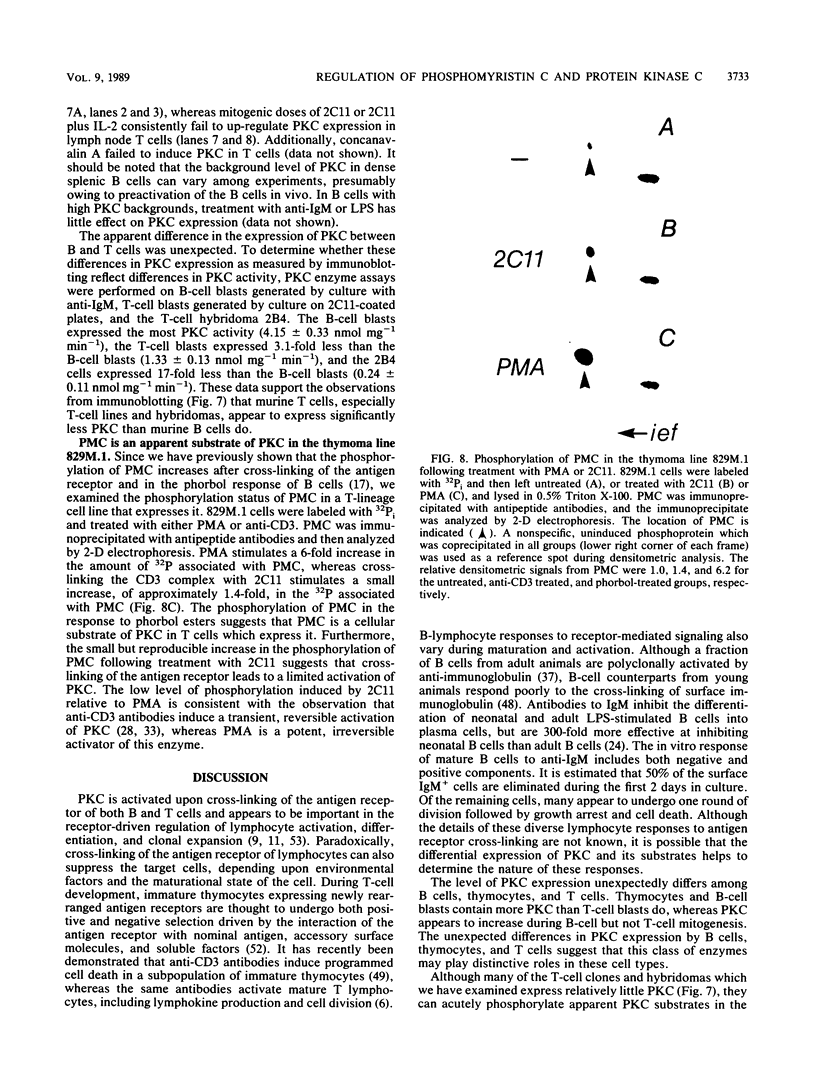
The regulation and expression of protein kinase C (PKC) and phosphomyristin C (PMC) (a principal substrate of PKC which is the major myristylated protein in lymphocyte and glioma lines that express it) in murine B and T lymphocytes were investigated. Both PMC and PKC are differentially regulated during T-cell development. The level of PMC expression is highest in CD4-8-, intermediate in CD4+8+, and lowest in J11d-, CD4, or CD8 single-positive thymocytes. PKC is equally expressed by all three thymic populations. In striking contrast to thymocytes, resting peripheral lymph node T cells and T-cell clones express little if any PMC and reduced levels of PKC. Neither PKC nor PMC is significantly induced upon the activation of lymph node T cells: treatment with anti-CD3 antibodies or anti-CD3 and interleukin-2 fails to induce PKC, whereas PMC is not induced by anti-CD3 alone and is only slightly induced by anti-CD3 and interleukin-2. In contrast to the situation with T cells, PMC and PKC are constitutively expressed at moderate levels in mature B cells. PMC is greatly increased in B-cell blasts generated by cross-linking the antigen receptor with anti-immunoglobulin. These results demonstrate that PMC and PKC are differentially regulated during the development and activation of B and T cells, suggesting that cellular events that rely upon PKC and PMC may differ during ontogeny and activation of different lymphocyte subsets.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Albert K. A., Keum M. M., Wang J. K., Greengard P., Cohn Z. A. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988 Mar 24;332(6162):362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- Albert K. A., Nairn A. C., Greengard P. The 87-kDa protein, a major specific substrate for protein kinase C: purification from bovine brain and characterization. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7046–7050. doi: 10.1073/pnas.84.20.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K. A., Walaas S. I., Wang J. K., Greengard P. Widespread occurrence of "87 kDa," a major specific substrate for protein kinase C. Proc Natl Acad Sci U S A. 1986 May;83(9):2822–2826. doi: 10.1073/pnas.83.9.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Bluestone J. A., Pardoll D., Sharrow S. O., Fowlkes B. J. Characterization of murine thymocytes with CD3-associated T-cell receptor structures. Nature. 1987 Mar 5;326(6108):82–84. doi: 10.1038/326082a0. [DOI] [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Gould K., Sefton B. M. The absence of myristic acid decreases membrane binding of p60src but does not affect tyrosine protein kinase activity. J Virol. 1986 May;58(2):468–474. doi: 10.1128/jvi.58.2.468-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C., Ransom J. T. Molecular mechanisms of transmembrane signaling in B lymphocytes. Annu Rev Immunol. 1987;5:175–199. doi: 10.1146/annurev.iy.05.040187.001135. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- DeFranco A. L. Molecular aspects of B-lymphocyte activation. Annu Rev Cell Biol. 1987;3:143–178. doi: 10.1146/annurev.cb.03.110187.001043. [DOI] [PubMed] [Google Scholar]
- Defranco A. L., Raveche E. S., Asofsky R., Paul W. E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982 May 1;155(5):1523–1536. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I. Quantitative two-dimensional gel electrophoresis of proteins. Methods Enzymol. 1983;100:411–423. doi: 10.1016/0076-6879(83)00070-1. [DOI] [PubMed] [Google Scholar]
- Gindhart T. D., Stevens L., Copley M. P. Transformation and tumor promoter sensitive phosphoproteins in JB-6 mouse epidermal cells: one is also sensitive to heat stress. Carcinogenesis. 1984 Sep;5(9):1115–1121. doi: 10.1093/carcin/5.9.1115. [DOI] [PubMed] [Google Scholar]
- Hornbeck P., Huang K. P., Paul W. E. Lamin B is rapidly phosphorylated in lymphocytes after activation of protein kinase C. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2279–2283. doi: 10.1073/pnas.85.7.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck P., Paul W. E. Anti-immunoglobulin and phorbol ester induce phosphorylation of proteins associated with the plasma membrane and cytoskeleton in murine B lymphocytes. J Biol Chem. 1986 Nov 5;261(31):14817–14824. [PubMed] [Google Scholar]
- Hu-Li J., Shevach E. M., Mizuguchi J., Ohara J., Mosmann T., Paul W. E. B cell stimulatory factor 1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes. J Exp Med. 1987 Jan 1;165(1):157–172. doi: 10.1084/jem.165.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. P., Huang F. L. Immunochemical characterization of rat brain protein kinase C. J Biol Chem. 1986 Nov 5;261(31):14781–14787. [PubMed] [Google Scholar]
- Huang K. P., Huang F. L., Nakabayashi H., Yoshida Y. Biochemical characterization of rat brain protein kinase C isozymes. J Biol Chem. 1988 Oct 15;263(29):14839–14845. [PubMed] [Google Scholar]
- Huang K. P., Nakabayashi H., Huang F. L. Isozymic forms of rat brain Ca2+-activated and phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8535–8539. doi: 10.1073/pnas.83.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacke C. M., Meisenhelder J., Brown K. D., Gould K. L., Gould S. J., Hunter T. Early phosphorylation events following the treatment of Swiss 3T3 cells with bombesin and the mammalian bombesin-related peptide, gastrin-releasing peptide. EMBO J. 1986 Nov;5(11):2889–2898. doi: 10.1002/j.1460-2075.1986.tb04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Kearney J. F., Cooper M. D., Lawton A. R. B lymphocyte differentiation induced by lipopolysaccharide. III. Suppression of B cell maturation by anti-mouse immunoglobulin antibodies. J Immunol. 1976 Jun;116(6):1664–1668. [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Kligman D., Patel J. A protein modulator stimulates C kinase-dependent phosphorylation of a 90K substrate in synaptic membranes. J Neurochem. 1986 Jul;47(1):298–303. doi: 10.1111/j.1471-4159.1986.tb02862.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Gentry L. E., June C. H., Rabinovitch P. S., Purchio A. F. Stimulation of T cells through the CD3/T-cell receptor complex: role of cytoplasmic calcium, protein kinase C translocation, and phosphorylation of pp60c-src in the activation pathway. Mol Cell Biol. 1987 Feb;7(2):650–656. doi: 10.1128/mcb.7.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Courtneidge S. A. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J. 1985 May;4(5):1137–1144. doi: 10.1002/j.1460-2075.1985.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield R. B. Automated synthesis of peptides. Science. 1965 Oct 8;150(3693):178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- Nel A. E., Bouic P., Lattanze G. R., Stevenson H. C., Miller P., Dirienzo W., Stefanini G. F., Galbraith R. M. Reaction of T lymphocytes with anti-T3 induces translocation of C-kinase activity to the membrane and specific substrate phosphorylation. J Immunol. 1987 May 15;138(10):3519–3524. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Garra A., Rigley K. P., Holman M., McLaughlin J. B., Klaus G. G. B-cell-stimulatory factor 1 reverses Fc receptor-mediated inhibition of B-lymphocyte activation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6254–6258. doi: 10.1073/pnas.84.17.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. C. Stimulation of mouse lymphocytes by insoluble anti-mouse immunoglobulin. Nature. 1975 Nov 27;258(5533):361–363. doi: 10.1038/258361a0. [DOI] [PubMed] [Google Scholar]
- Patel J., Kligman D. Purification and characterization of an Mr 87,000 protein kinase C substrate from rat brain. J Biol Chem. 1987 Dec 5;262(34):16686–16691. [PubMed] [Google Scholar]
- Phillips N. E., Gravel K. A., Tumas K., Parker D. C. IL-4 (B cell stimulatory factor 1) overcomes Fc gamma receptor-mediated inhibition of mouse B lymphocyte proliferation without affecting inhibition of c-myc mRNA induction. J Immunol. 1988 Dec 15;141(12):4243–4249. [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Ralph P. Functional subsets of murine and human B lymphocyte cell lines. Immunol Rev. 1979;48:107–121. doi: 10.1111/j.1600-065x.1979.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Richie E. R., McEntire B., Crispe N., Kimura J., Lanier L. L., Allison J. P. Alpha/beta T-cell antigen receptor gene and protein expression occurs at early stages of thymocyte differentiation. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1174–1178. doi: 10.1073/pnas.85.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Schwartz R. H. T cell clone-specific alloantisera that inhibit or stimulate antigen-induced T cell activation. J Immunol. 1983 Dec;131(6):2645–2650. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Sato C., Nishizawa K., Nakayama T., Kobayashi T. Effect upon mitogenic stimulation of calcium-dependent phosphorylation of cytoskeleton-associated 350,000- and 80,000-mol-wt polypeptides in quiescent 3Y1 cells. J Cell Biol. 1985 Mar;100(3):748–753. doi: 10.1083/jcb.100.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieckmann D. G., Scher I., Asofsky R., Mosier D. E., Paul W. E. Activation of mouse lymphocytes by anti-immunoglobulin. II. A thymus-independent response by a mature subset of B lymphocytes. J Exp Med. 1978 Dec 1;148(6):1628–1643. doi: 10.1084/jem.148.6.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Kanagawa O., Bevan M. J. Hybrid antibodies can target sites for attack by T cells. Nature. 1985 Apr 18;314(6012):628–631. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. The developmental biology of T lymphocytes. Annu Rev Immunol. 1988;6:309–326. doi: 10.1146/annurev.iy.06.040188.001521. [DOI] [PubMed] [Google Scholar]











