Abstract
Tripartite motif (TRIM) protein superfamily members are emerging as important effectors of the innate immune response against viral infections. In particular, TRIM22 was reported to exert antiviral activity against RNA viruses, such as hepatitis B virus (HBV), encephalomyocarditis virus (ECMV), and human immunodeficiency virus type 1 (HIV-1). We demonstrate here, for the first time, that TRIM22 is upregulated by influenza A virus (IAV) infection at both mRNA and protein levels in human alveolar epithelial A549 cells. Conversely, TRIM22 potently restricted IAV replication, in that prevention of TRIM22 expression by means of short hairpin RNA led to a 10-fold enhancement of IAV replication in these cells. Depletion of TRIM22 also reduced the anti-IAV activity of alpha interferon (IFN-α), suggesting that TRIM22 is an important IFN-stimulated gene that is required for maximal suppression of IAV by type I IFN. Furthermore, the IAV infectious titer decreased up to 100-fold in MDCK cells expressing exogenous human TRIM22. Restriction of IAV replication was accounted for by the interaction between TRIM22 and the viral nucleoprotein (NP), resulting in its polyubiquitination and degradation in a proteasome-dependent manner. Thus, TRIM22 represents a novel restriction factor upregulated upon IAV infection that curtails its replicative capacity in epithelial cells.
INTRODUCTION
Influenza A viruses (IAV) are enveloped, segmented, negative-sense RNA viruses and represent an important global health problem. Influenza viruses cause yearly seasonal influenza, with more than 300,000 deaths worldwide (http://www.who.int/influenza/en). New influenza virus pandemics often occur when the human population has no humoral immunity to prevent infection by novel circulating viruses (1). An integral component of the innate immune response active against IAV is the production of type I interferons (IFNs), which play a critical role in rendering host cells resistant to infection and favor the establishment of adaptive immunity effector responses (2).
Shortly after IAV infection of epithelial cells, Toll-like receptor 3 (TLR3) (3) and TLR4 (4), or the retinoic inducible gene I (RIG-I) (5), sense the presence of viral nucleic acids and elicit the production of type I IFNs. Type I IFNs lead to the induction of more than 300 genes in target cells, which are believed to affect all stages of pathogen life cycle as well as its transmission and, ultimately, human health (6). Among IFN-inducible genes, there are members of the TRIM (for tripartite motif) family of proteins that have emerged as antiviral molecules involved in both innate and adaptive immunity (7). The TRIM family of RING (for really interesting new gene) domain-containing proteins comprises more than 70 human members, and their roles in immune signaling and antiviral functions are rapidly growing (7–9). In particular, TRIM5α is a potent restriction factor of retroviruses, including human immunodeficiency virus type 1 (HIV-1), as reviewed in reference 10. A broad antiviral activity has also been described for TRIM19, the defining component of promyelocytic leukemia (PML) bodies in the nucleus, including activity against vesicular stomatitis virus, human cytomegalovirus, herpes simplex virus type 1, Ebola virus, Lassa fever virus, lymphocytic choriomeningitis virus, human foamy virus, HIV-1, and IAV (7). TRIM28 restricts murine leukemia virus (MLV) as well as endogenous retroviral replication in cells of germ line origin (11). Recently, TRIM79α was shown to specifically restrict tick-borne encephalitis virus (12). The importance of TRIM molecules in host defense against IAV infection has been illustrated by the finding that the RING ubiquitin E3 ligase TRIM25 is crucial for RIG-I-mediated induction of type I IFNs (13), which can be directly counteracted by the IAV nonstructural protein 1 (NS1) (14).
Human TRIM22 lies on chromosome 11 within a group of closely related genes, encompassing TRIM5, TRIM6, and TRIM34, which have presumably arisen by gene duplication. TRIM5 and TRIM22 have been shown to have a dynamic history of gene expansion and loss during the evolution of mammals (15). Like TRIM5, TRIM22 has evolved under strong positive selection, suggesting direct interaction with pathogenic organisms. Overexpression of TRIM22 was reported to inhibit HIV-1 replication in certain cells, including primary human monocyte-derived macrophages (MDM) (16). In this regard, a few studies have reported that TRIM22 exerts antiviral effects on both HIV-1 transcription and virion production in a variety of cells, including primary human MDM (16–19). TRIM22 has also been implicated in the inhibition of hepatitis B virus (HBV) by acting as a transcriptional suppressor (20) and encephalomyocarditis virus (ECMV) by inducing ubiquitination of 3C protease (21). The E3 ubiquitin activity located in the RING domain was required for restriction of both HBV and ECMV (20, 21).
Regarding IAV infection, TRIM22 was identified in Shapira's high-throughput study that was aimed at determining the host-pathogen interaction of IAV-infected human bronchial epithelial cells (22). However, its role in IAV has not been further investigated. In addition, TRIM22 has been reported as a marker of activation of the type I IFN system in adenocarcinomic human alveolar basal epithelial A549 cells infected with a PB1-F2-containing IAV strain (23).
As IAV infection stimulates a strong IFN response and TRIM22 is an IFN-induced gene, we sought evidence for a potential restrictive role for TRIM22 in IAV infection. Here, we demonstrate that IAV stimulates TRIM22 expression in A549 epithelial cells, whereas suppression of IAV-induced TRIM22 expression by RNA interference led to significantly enhanced IAV replication in these cells. Concordantly, IAV infectious titers decreased by 100-fold in epithelial cells overexpressing TRIM22. Restriction relied on interaction between TRIM22 and the viral nucleoprotein (NP). In fact, TRIM22 catalyzed NP ubiquitination and degradation in a proteasome-dependent manner. Thus, our study provides the first evidence that IAV is a target of the restriction factor TRIM22 and that TRIM22 upregulation upon infection contributes to suppression of IAV replication in epithelial cells.
MATERIALS AND METHODS
Cells and virus.
Adenocarcinomic human alveolar basal epithelial A549, MDCK, and 293T human embryonic kidney cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with glutamine (2 mmol/liter), penicillin (100 U/ml), streptomycin (100 U/ml), and 10% fetal bovine serum (FBS) (complete DMEM; Thermo Fisher Scientific Inc., Erembodegem, Belgium).
A/New Caledonia/20/99 (H1N1) and A/Wisconsin/67/2005 (H3N2) were kindly provided by Nadia Naffakh, Pasteur Institute, Paris, France. A/Brisbane/59/2007 (H1N1) and NYMC X-181 (H1N1pdm) strains were obtained from the National Institutes for Biological Standards and Control (NIBSC), Potters Bar, United Kingdom.
Plasmids.
pLKO.1/TRIM22shRNA lentiviral vector contains a short hairpin RNA (shRNA) targeting TRIM22 (RHS3979-9574742; GenBank accession no. NM_006074; 5′-CGG AGC ACT CAT CTA CAA GTT CTC GAG AAC TTG TAG ATG AGT GCT CCG-3′; Open Biosystems, Huntsville, AL). pLKO.1/randomshRNA (nonsilencing control) was a kind gift of Davide Gabellini, San Raffaele Scientific Institute, Milan, Italy. pMD2.G is a cytomegalovirus (CMV)-driven expression plasmid that encodes vesicular stomatitis virus envelope protein G (VSV-G). psPax2 is the packaging vector expressing HIV Gag-Pol (19). pEXN and pEXN-TRIM22 are MLV vectors based on the retroviral expression vector pLNCX2 (Clontech, Mountain View, CA), which was modified such that the expressed protein had a double hemagglutinin (HA) epitope tag at its amino terminus, as described in reference 24. pEXN-TRIM22 contains the full-length human TRIM22 cDNA. 3×-Flag-TRIM22 wild type (WT) and 3×-Flag-TRIM22 RING deletion mutant (ΔRING) have been previously described (21). TRIM22-expressing plasmid was obtained from 3×-Flag-TRIM22 by cloning TRIM22 coding sequence between the NotI and XbaI sites of the pcDNA3.1(+) vector (Invitrogen, Carlsbad, CA). To obtain an NP-expressing vector, the NP coding sequence was amplified from total cDNA of MDCK cells infected with A/New Caledonia/20/99 (H1N1) and cloned between the NotI and XhoI sites of the pcDNA3.1(+) vector (Invitrogen). The full-length NP cDNA (fragment NotI-XhoI of the pcDNA3-NP plasmid) was cloned into the NotI and XhoI sites of p3XFlag-myc-CMV-24 expression vector (Sigma-Aldrich, St. Louis, MO) to generate Flag-NP fusion protein. The His-ubiquitin expression vectors (His-Ubi) were a generous gift from M. T. Burgering (Utrecht, The Netherlands).
IAV propagation and titration.
Monolayers of MDCK cells were washed twice with phosphate-buffered saline (PBS) and infected with different IAV strains at a multiplicity of infection (MOI) of 0.001. After virus adsorption for 1 h at 35°C, cells were washed twice and incubated at 35°C with DMEM without serum that had been supplemented with tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (1 μg/ml; Worthington Biomedical Corporation, Lakewood, NJ). Supernatants were recovered 48 h postinfection (p.i.). For viral titration, plaque assays were performed as previously described (25). Briefly, MDCK monolayer cells, plated in 24-well plates at 2.5 × 105 cells/ml, were washed twice with DMEM without serum, and serial dilutions of virus were adsorbed onto cells for 1 h. Cells were covered with a DMEM 2×-Avicel (FMC Biopolymer, Philadelphia, PA) mix supplemented with TPCK-treated trypsin (1 μg/ml). Crystal violet staining was performed 48 h p.i., and visible plaques were counted.
Vector production.
Vectors were produced by transfection of 293T cells seeded at 6 × 105 cells/ml in 6-well plastic plates (Falcon, Becton, Dickinson Labware, Lincoln Park, NJ) by using Lipofectamine 2000 (Invitrogen). VSV-pLKO.1/TRIM22shRNA and VSV-pLKO.1/random shRNA vectors were produced by cotransfecting pMD2.G, psPax2, and pLKO.1/TRIM22shRNA (or pLKO.1/randomshRNA), respectively, at 1:3:4 ratios. For stable TRIM22 overexpression, a previously described MLV vector encoding full-length human TRIM22 cDNA (24) was VSV-G pseudotyped as described previously (26).
Knockdown (KD) and overexpression of TRIM22.
In order to deplete TRIM22, A549 cells were seeded in 6-well plates at 6 × 105 cells/ml in complete DMEM and transduced with VSV-pLKO.1/TRIM22shRNA or VSV-pLKO.1/randomshRNA vector-containing supernatants at a 1:1 ratio with complete DMEM. After 24 h, a half volume of culture medium was replaced with fresh vector-containing supernatant. Seventy-two h after the second transduction, cells were subjected to selection with puromycin (0.5 μg/ml; Sigma-Aldrich) for 1 week. The levels of TRIM22 expression were verified by Western blotting as described below.
In order to express TRIM22, MDCK cells were transduced twice with pEXN-TRIM22 or empty control vectors at 24-h intervals, replacing culture medium with vector-containing supernatant at a 1:1 ratio. Seventy-two h from the second transduction, cells were subjected to G418 (3 mg/ml; Sigma-Aldrich) selection. TRIM22 expression was verified by Western blotting as described below.
Quantification of TRIM22 RNA by real-time PCR.
Total RNA was extracted from mock and transduced A549 cells by a TRIzol Plus RNA purification kit followed by DNase I treatment (Invitrogen). cDNA was synthesized from 1 μg of total RNA using a SuperScript First-Strand Synthesis System (Invitrogen) with oligo(dT). Semiquantitative PCR was performed on 50 ng of cDNA with the primer pair previously described (19) and SYBR green PCR master mix (Applied Biosystems, Foster City, CA). To normalize mRNA expression, the human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was amplified. Relative standard curves for TRIM22 and the normalizer were obtained using serially diluted (from 50 to 0.016 ng) total cDNA obtained from nonpermissive U937 cells (19) stimulated with IFN-α (1,000 U/ml) for 24 h. The amounts of target and normalizer DNA were calculated from the standard curves as previously described (19).
Specific primer pairs were designed for relative quantification of TRIM5α, TRIM6, TRIM34, and TRIM25 expression in KD-TRIM22 and KD-CTRL A549 cells. The primer sequences are the following: TRIM5α forward, 5′-AGGAGTTAAATGTAGTTGCT-3′; TRIM5α reverse, 5′-ATAGATGAGAAATCCATGGT-3′; TRIM6 forward, 5′-TTCCCCTTCCCTGCTTCTCT-3′; TRIM6 reverse, 5′-AGTACCAGCCTCATAATCTAAGAAAACC-3′; TRIM34 forward, 5′-TGTTGACAGAACCCTTGAGTCTAGA-3′; TRIM34 reverse, 5′-TGGTCACTGCCTCCTTGTTG-3′; TRIM25 forward, 5′-GACCACGGCTTTGTCATCTTC-3′; and TRIM25 reverse, 5′-AAAGTCCACCCTGAACTTATACATCA-3′. Real-time PCR was performed as described above. The fold modulation in gene expression was analyzed with the 2−ΔΔCt method (27), where the levels of the target transcript in cells stimulated with IFN-α (100 U/ml) were related to those of untreated control cells.
Transfections.
293T cells were seeded in 6-well plates at 0.6 × 106/ml. The following day, cells were transfected with increasing amounts of pcDNA3.1-TRIM22-expressing plasmid (50 to 5,000 ng) with a fixed amount (50 ng) of NP plasmid. The p3X-Flag-TRIM22 or p3X-Flag-TRIM22-ΔRING plasmid (2,500 ng) was transfected with 50 ng of NP plasmid. Lipofectamine 2000 transfection reagent (Invitrogen) was used according to the manufacturer's instructions. The medium was replaced with fresh culture medium 24 h posttransfection (p.t.).
Coimmunoprecipitation assay.
293T cells were seeded in T75 tissue culture flasks at 0.7 × 106/ml. The following day, cells were transfected with 7.5 μg of Flag-TRIM22 plasmid together with 7.5 μg of NP plasmid. U1A-expressing plasmid was used as a negative control (21). Transfected cells were washed twice with cold PBS and lysed in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) with protease inhibitor cocktail (Roche, Basel, Switzerland) for 30 min and then centrifuged at 13,000 rpm at 4°C for 5 min. Coimmunoprecipitation was performed using anti-NP monoclonal antibody (Ab) (Southern Biotech, Birmingham, AL) cross-linked to Dynabeads Protein G (Invitrogen) according to the manufacturer's protocol. The bound proteins were eluted by boiling with loading buffer containing sodium dodecyl sulfate (SDS) for 5 min and subjected to Western blotting.
Ni-NTA pulldown.
293T cell were transfected in 100-mm cell culture dishes with a total of 10 μg DNA using the calcium phosphate method (2 μg of Flag-NP plus 4 μg of His-Ubi plus 2 or 4 μg of TRIM22). Forty-eight h after transfection, cells were treated with 10 μM MG132 for 3 h. The cells were washed twice in PBS, and whole-cell extracts (WCE) were prepared from a 10% fraction of the cells and analyzed by Western blotting for the expression of transfected proteins. The remaining 90% of cells were lysed in a denaturing buffer (6 M guanidine-HCl, 100 mM Na2HPO4-NaH2PO4 at pH 8.0, 10 mM Tris-HCl at pH 8.0, 0.2% Triton X-100), and ubiquitinated proteins were precipitated using nickel-nitrilotriacetic acid (Ni-NTA) agarose (Qiagen, Hilden, Germany).
Western blotting.
Whole-cell extract from A549, MDCK, and 293T cells were prepared as previously described (28). Samples were run in SDS-polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose membrane by electroblotting, and blotted with a rabbit polyclonal antibody (Ab) raised against TRIM22 (21), an anti-NP monoclonal Ab (Southern Biotech), an anti-FLAG monoclonal Ab (Sigma-Aldrich), an anti-HA Ab (Sigma-Aldrich), or an anti-GFP monoclonal Ab (Sigma-Aldrich). Anti-actin monoclonal Ab (Sigma-Aldrich) was used as a control.
Immunofluorescence.
TRIM22-expressing and control MDCK cells were plated at 2.5 × 105 cells/ml, infected with A/New Caledonia/20/99 (H1N1) at an MOI of 2 in LabTek chamber slides (Nunc, Thermo Fisher Scientific Inc., Erembodegem, Belgium), and fixed 6 h p.i. TRIM22 was stained using mouse monoclonal Ab to the HA tag (Sigma-Aldrich) followed by secondary Ab to mouse immunoglobulin conjugated to Alexa fluor 568 (Invitrogen). Viral NP was detected using a monoclonal Ab coupled to fluorescein isothiocyanate (FITC) (Acris Antibodies, Herford, Germany). The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). The images were acquired using a TCS SP2 laser-scanning Leica confocal microscope equipped with a 63× oil objective and processed using Leica confocal software (LCS) (Leica Microsystems, Wetzlar, Germany).
Statistical analysis.
All statistical analysis was performed using Prism GraphPad software, v. 4.0 (GraphPad Software).
RESULTS
Endogenous TRIM22 expression is upregulated by either IFN-α stimulation or IAV infection.
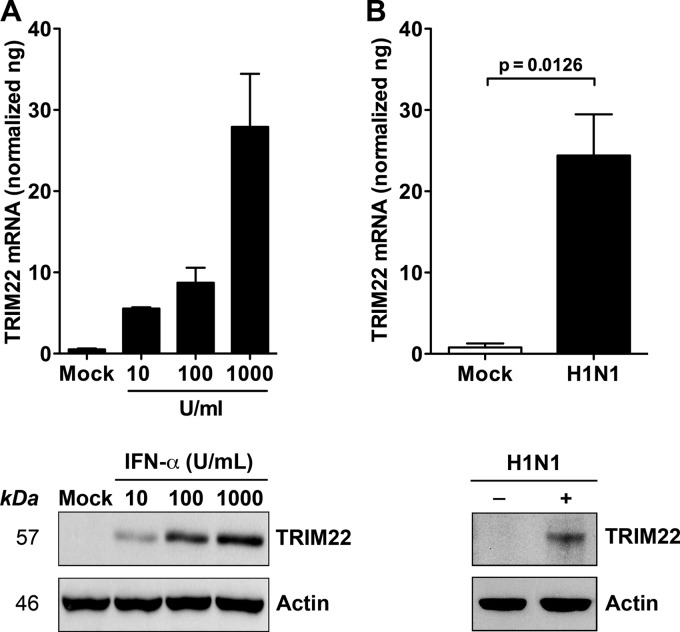
In order to investigate a potential role of TRIM22 in IAV infection, we first investigated whether TRIM22 was induced by IFN-α treatment or IAV infection of the human alveolar epithelial cell line A549. This cell line was selected because it typically expresses low levels of TRIM22 in uninfected or unstimulated conditions (Fig. 1A and B). Indeed, increasing concentrations of IFN-α induced TRIM22 mRNA expression up to 30-fold over the baseline, with a top concentration of 1,000 U/ml (Fig. 1A, upper). In parallel, 24 h of infection by IAV at an MOI of 5 induced TRIM22 mRNA expression to a level comparable to that observed after treatment with the highest dose of IFN-α (Fig. 1B, upper). TRIM22 protein levels measured by Western blotting reflected the changes in mRNA observed in both experimental conditions (Fig. 1A and B, lower). However, although the levels of induction of TRIM22 mRNA by either IFN-α treatment (1,000 U/ml) or IAV infection were similar, IAV-induced TRIM22 protein expression was lower than that induced by IFN-α treatment. These results are consistent with previous results demonstrating that influenza virus shuts off the host protein synthesis machinery rather than the cellular mRNAs (29).
Fig 1.
IFN-α exposure and IAV infection induce TRIM22 expression in A549 cells. (A) A549 cells were exposed to increasing doses of IFN-α for 20 h. (B) A549 cells were infected with A/NewCaledonia/20/99 (H1N1) at an MOI of 5. TRIM22 mRNA levels (upper) and protein expression (lower) were evaluated 24 h postexposure and were compared to those of mock-treated cells.
TRIM22 expression is required for IFN-α-mediated repression of IAV replication.
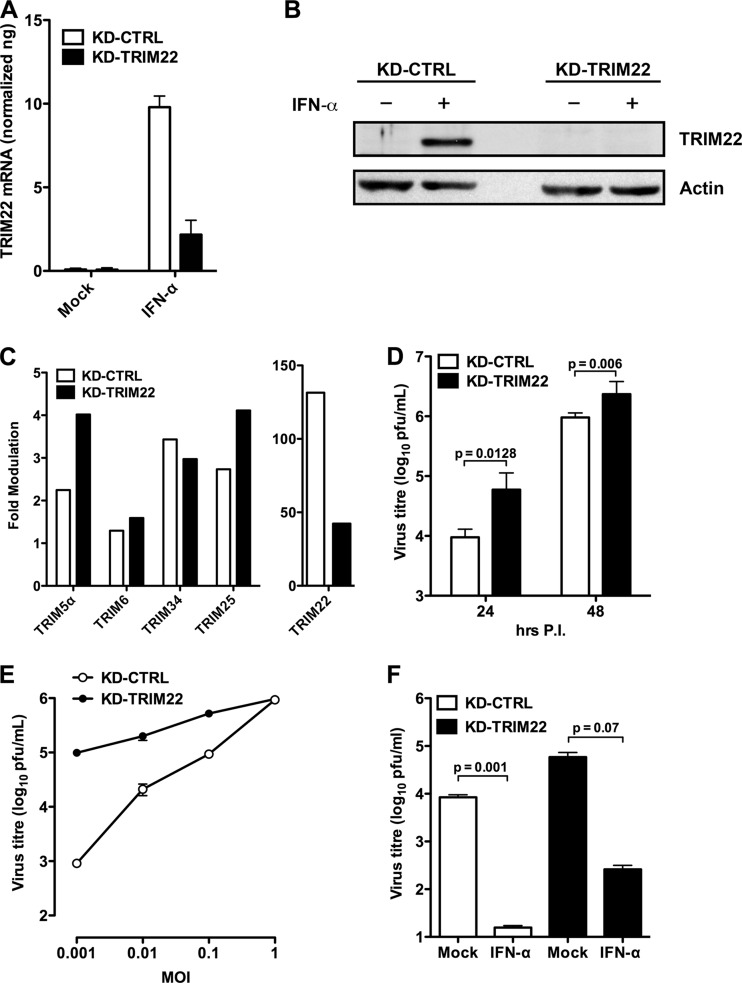
In order to investigate whether TRIM22 induction affected IAV replication, we generated stable A549 cell lines expressing an shRNA efficient in preventing RNA and protein TRIM22 accumulation after IFN-α stimulation. IFN-α stimulation (100 U/ml) increased TRIM22 mRNA levels in control transduced cells (Fig. 2A, KD-CTRL). In contrast, TRIM22 shRNA expression reduced TRIM22 mRNA levels after IFN-α stimulation (Fig. 2A, KD-TRIM22), whereas the expression of other TRIM members that lie on chromosome 11 adjacent to TRIM22, such as TRIM5α, TRIM6, TRIM34, and TRIM25 (the latter was shown to be a target of NS1 protein [14]), was not significantly affected by KD-TRIM22, validating the specificity of our TRIM22 shRNA (Fig. 2C). Furthermore, TRIM22 protein expression became undetectable by Western blotting in TRIM22-depleted cells after IFN-α stimulation, validating the efficacy of the depletion strategy (Fig. 2B).
Fig 2.
Endogenous TRIM22 contributes to controlling IAV replication in A549 cells. (A) Stable knockdown of endogenous TRIM22 (KD-TRIM22) was obtained in A549 cells through lentiviral transduction, and levels of TRIM22 mRNA were evaluated in the presence or absence of IFN-α stimulation (100 U/ml for 20 h). (B) Protein expression levels in KD-TRIM22 A549 cells were undetectable by Western blotting upon stimulation with 100 U/ml of IFN-α for 20 h. Cells transduced with a lentiviral vector expressing a nonsilencing shRNA (KD-CTRL) were used as a control. (C) Real-time PCR of TRIM family members was performed on total cDNA from either KD-CTRL or KD-TRIM22 A549 cells. The fold modulation of the target transcript in cells stimulated with IFN-α (100 U/ml) was related to that of untreated control cells. Of note, TRIM22 was significantly more expressed than the other TRIMs upon IFN-α stimulation. One experiment of three performed is shown. (D) KD-CTRL and KD-TRIM22 cells were infected with A/NewCaledonia/20/99 (H1N1) at an MOI of 0.01. Viral replication was measured by titrating infectious particle production in culture supernatants 24 and 48 h p.i. (E) KD-CTRL and KD-TRIM22 cells were infected with A/NewCaledonia/20/99 (H1N1) at an increasing MOI. Viral replication was measured by titrating infectious particle production in culture supernatants 24 h p.i. (F) Viral titers were determined in the supernatant from KD-CTRL and KD-TRIM22 A549 cells that were either mock treated or treated with IFN-α at 100 U/ml 24 h prior to IAV infection at an MOI of 0.01. Twenty-four h p.i., culture supernatants were collected and virus titers were determined by plaque assay in MDCK cells. All results are the means ± SD of experiments performed in triplicate, and the Western blots are representative of one of three independently performed. P values were determined with paired t test.
To monitor TRIM22 activity on viral infection and spreading, we next infected KD-TRIM22 or KD-CTRL shRNA-expressing cells with a low dose of A/New Caledonia/20/99 H1N1 (MOI of 0.01), and the titers of the infectious output were measured in the culture supernatants collected 24 and 48 h p.i. Indeed, TRIM22 silencing increased production of infectious virus by 10-fold in A549 cells compared to cells transduced with KD-CTRL (Fig. 2D). This was evident both at 24 and 48 h p.i. The enhancement of infectious virus output was progressively lost with increasing MOI, eventually resulting in similar levels of virion production in both KD-TRIM22 and KD-CTRL A549 cells at an MOI of 1 (Fig. 2E). Such a saturation of restriction by high-dose infection is typical of TRIM proteins, as exemplified by TRIM5 restriction of retroviruses (30).
We next evaluated whether the depletion of TRIM22 decreased the antiviral actions of IFN-α. For this purpose, both KD-TRIM22 and KD-CTRL cells were incubated with 100 U/ml of IFN-α 24 h prior to IAV infection at an MOI of 0.01. In the absence of exogenously added IFN-α, TRIM22 depletion increased virus replication. Importantly, depletion of TRIM22 also reduced the anti-IAV activity of IFN-α, suggesting that TRIM22 is an important interferon-stimulated gene required for maximal suppression of IAV by IFN-α (Fig. 2F).
Thus, IAV infection upregulates the expression of endogenous TRIM22 in A549 cells, while TRIM22 is required both for basal levels of resistance as well as for the enhanced defenses elicited by exogenously added IFN-α. It is important to underscore that, as observed with TRIM5α-mediated restriction of retroviruses, TRIM22-dependent restriction of IAV was saturable and was overcome by increasing the infectious viral input (30).
TRIM22 overexpression leads to decreased IAV replication.
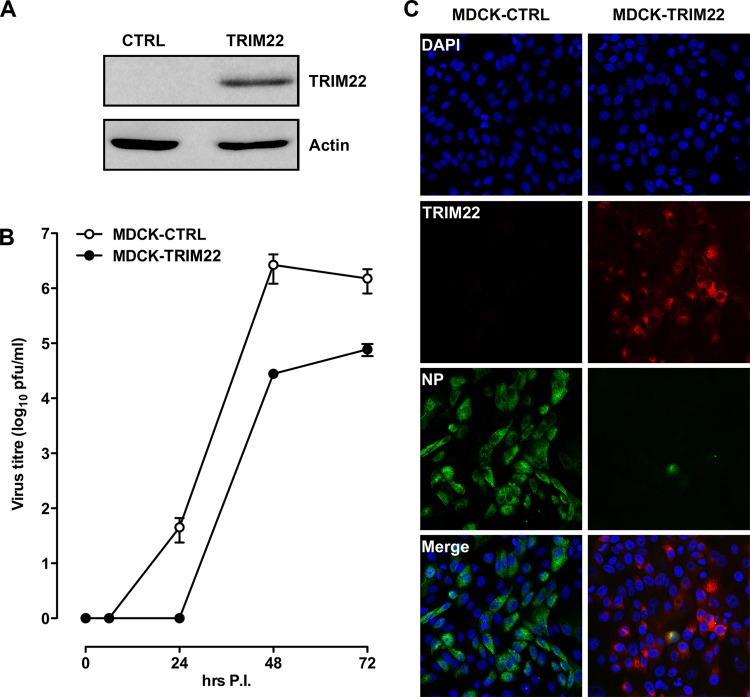
In order to test whether TRIM22 overexpression could prevent IAV infection or curtail its replication, we transduced MDCK cells with either a retroviral vector encoding HA-tagged TRIM22 or an empty vector as a control. TRIM22 expression was observed in TRIM22-transduced cells, whereas control transduced cells were devoid of its expression, as expected (Fig. 3A). Both control and TRIM22-overexpressing MDCK cells were infected with A/New Caledonia/20/99 (H1N1) at an MOI of 0.001, which is 10-fold lower than that used to infect A549 cells, as MDCK cells are more permissive than A549 cells for IAV replication. Culture supernatants were collected at different times after infection, and the titers of infectious virus were determined on fresh MDCK cells. As expected, TRIM22 overexpression led to a decrease in IAV titers of up to 100-fold at 48 h postinfection (Fig. 3B).
Fig 3.
Overexpression of TRIM22 significantly impairs IAV replication in MDCK cells. (A) MDCK cells were stably transduced with a retroviral vector expressing HA-tagged TRIM22 (MDCK-TRIM22) or an empty vector control (MDCK-CTRL). TRIM22 expression was evaluated by Western blotting in whole-cell extracts using an anti-HA Ab. Actin was detected as a control input. (B) Stably transduced MDCK cells were infected with A/NewCaledonia/20/99 (H1N1) at an MOI of 0.001, and the kinetics of viral replication were measured by titrating infectious particles produced at the time points shown. Viral titers are expressed as the means ± SD of one representative of three experiments performed in triplicate. (C) To assess the impact of TRIM22 during a single round of IAV replication, stably transduced MDCK cells were infected with A/NewCaledonia/20/99 (H1N1) at an MOI of 2. Cells were fixed and stained for TRIM22 (anti-HA) and viral NP at 6 h p.i. Images were acquired by confocal microscopy. The immunofluorescence images are representative of one of three independent experiments.
We next evaluated the impact of TRIM22 in a single cycle of IAV replication after infection of transduced MDCK cells (MOI of 2). We measured viral NP expression 6 h after infection. In control-transduced MDCK cells, viral NP expression was detected abundantly in the cytoplasm of infected cells after 6 h p.i. (Fig. 3C, left). Remarkably, little or no viral NP was detected in TRIM22-overexpressing MDCK cells (Fig. 3C, right). Thus, TRIM22 overexpression led to a significant decrease of IAV replication and NP expression in MDCK cells.
TRIM22 is active against major human-transmissible IAV strains.
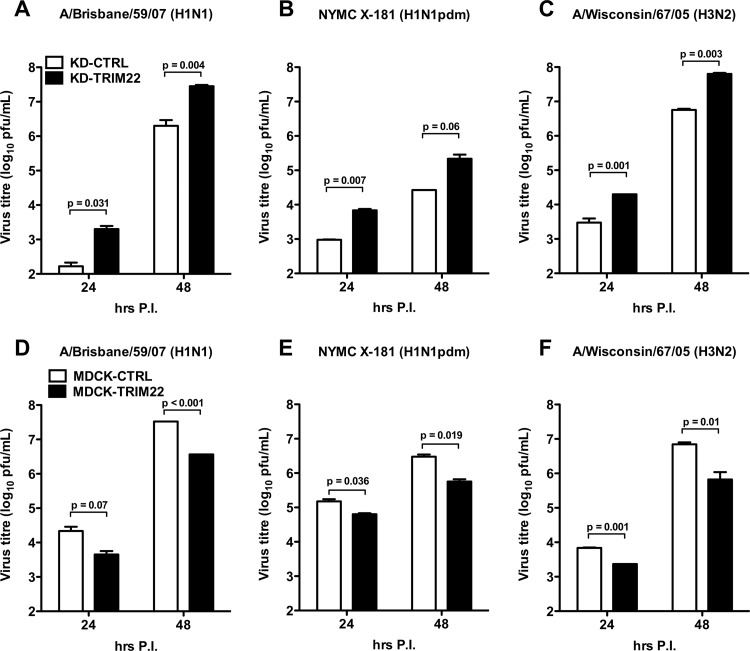
In order to examine whether TRIM22 restriction could be extended to IAV strains other than A/New Caledonia/20/99 (H1N1), either TRIM22-KD A549 cells or TRIM22-overexpressing MDCK cells were infected with two H1N1 strains, i.e., A/Brisbane/59/2007 and NYMC X-181 (H1N1pdm), and one H3N2 strain, i.e., A/Wisconsin/67/2005. Indeed, TRIM22 silencing significantly increased the production of both H1N1 and H3N2 strains in KD-TRIM22 cells compared to cells transduced with KD-CTRL (Fig. 4A, B, and C). As expected, TRIM22 overexpression led to a 10-fold decrease in infectious virus of the same strains in MDCK-TRIM22 compared to MDCK-CTRL cells (Fig. 4D, E, and F).
Fig 4.
TRIM22 is active against major human-transmissible IAV strains. (A) A549 cells depleted for TRIM22 (KD-TRIM22) or control cells (KD-CTRL) were infected with A/Brisbane/59/07 (H1N1), (B) NYMC X-181 (H1N1pdm), and (C) A/Wisconsin/67/05 at an MOI of 0.01. Viral replication was measured by titrating infectious particles produced at 24 and 48 h p.i. (D, E, and F). Stably transduced MDCK cells were infected with the same virus strains at an MOI of 0.001. The production of infectious virus was measured by titrating infectious particles produced at 24 and 48 h. Viral titers are expressed as the means ± SD of one representative of two experiments performed in triplicate.
TRIM22 inhibits NP expression.
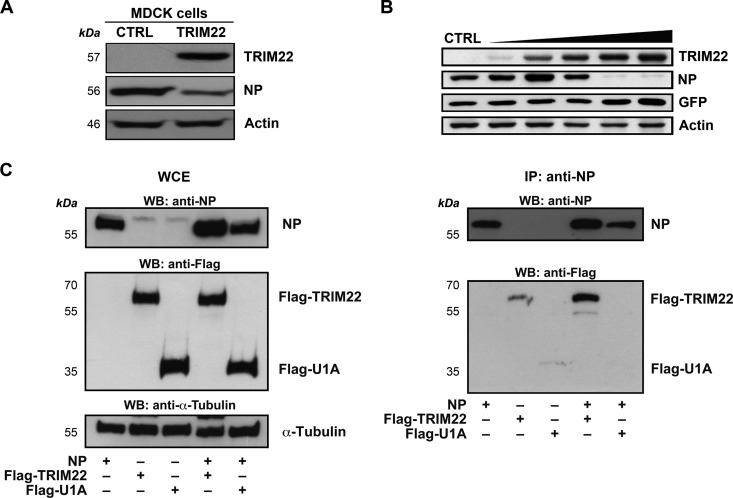
IAV NP is abundantly expressed during the virus life cycle. It has critical structural functions, as it binds to viral RNA segments along their entire lengths at regular intervals to form viral ribonuclear proteins (vRNPs), and it is also required for viral RNA synthesis (31, 32). Therefore, we investigated whether the TRIM22-mediated restriction could be consequent to an impaired expression of the IAV NP by determining viral NP protein levels in control and TRIM22-overexpressing MDCK cells 24 h after infection with A/New Caledonia/20/99 (H1N1). Indeed, a reduction in NP expression was observed in TRIM22-overexpressing cells (Fig. 5A).
Fig 5.
TRIM22 leads to impaired NP expression through protein-protein interaction. (A) Control and TRIM22-transduced MDCK cells (CTRL and TRIM22, respectively) were infected with A/NewCaledonia/20/99 (H1N1) at an MOI of 0.001, and the levels of viral NP expression were evaluated in WCE obtained 24 h p.i. Actin was used as a normalizer. (B) 293T cells were cotransfected with a fixed amount of both viral NP-expressing plasmid (50 ng) and GFP-expressing plasmid (50 ng) with increasing amounts of TRIM22-expressing plasmid (from 50 to 5,000 ng). WCE were prepared 24 h p.t., and TRIM22, NP, and GFP expression levels were evaluated by Western blotting. Actin was detected as a loading control. These results are representative of three independent experiments. (C) 293T cells were cotransfected with equal amounts (7.5 μg) of an NP-expressing and a Flag-TRIM22-expressing plasmid or a U1A-expressing plasmid as a control. WCE were prepared 48 h p.t., and immunoprecipitation (IP) with an anti-NP Ab was performed. Input (left) and pulldown product (right) were analyzed by Western blotting for the presence of NP, U1A, and TRIM22. α-Tubulin was detected as a loading control on input extracts.
We next tested whether TRIM22 could lead directly to impaired NP expression independently of IAV infection. For this purpose, we transfected 293T cells with a fixed amount (50 ng) of NP-expressing plasmid and increasing amounts of TRIM22-expressing plasmid (from 50 to 5,000 ng), equalizing the DNA doses with empty vector. NP expression was analyzed by Western blotting 24 h later in WCE. Remarkably, increasing amounts of TRIM22 resulted in the loss of NP expression, which became undetectable when at least a 50-fold or more excess of TRIM22 was cotransfected, whereas TRIM22 expression did not downregulate expression of an irrelevant protein (GFP) (Fig. 5B).
To verify whether the TRIM22-directed loss of NP expression is dependent on interaction between the two proteins, coimmunoprecipitation experiments were performed on extracts of 293T cells transfected with equivalent amounts (7.5 μg) of NP- and TRIM22-expressing plasmids. Cells cotransfected with the NP- and U1A-expressing plasmids were used as a control. Protein expression levels were analyzed in WCE by Western blotting using anti-Flag Ab (Fig. 5C, left). Indeed, Flag-TRIM22 and Flag-U1A were slightly retained on the beads when they were expressed alone (Fig. 5C, right); however, in the presence of NP the immunoprecipitation of Flag-TRIM22 was strongly increased, while no interaction between NP and U1A was observed in control cells cotransfected with the U1A-expressing plasmid. These results indicate that TRIM22 is able to interact with the viral NP and suggest that this interaction is followed by TRIM22-mediated downregulation of this viral protein.
TRIM22 RING domain mediates viral NP degradation.
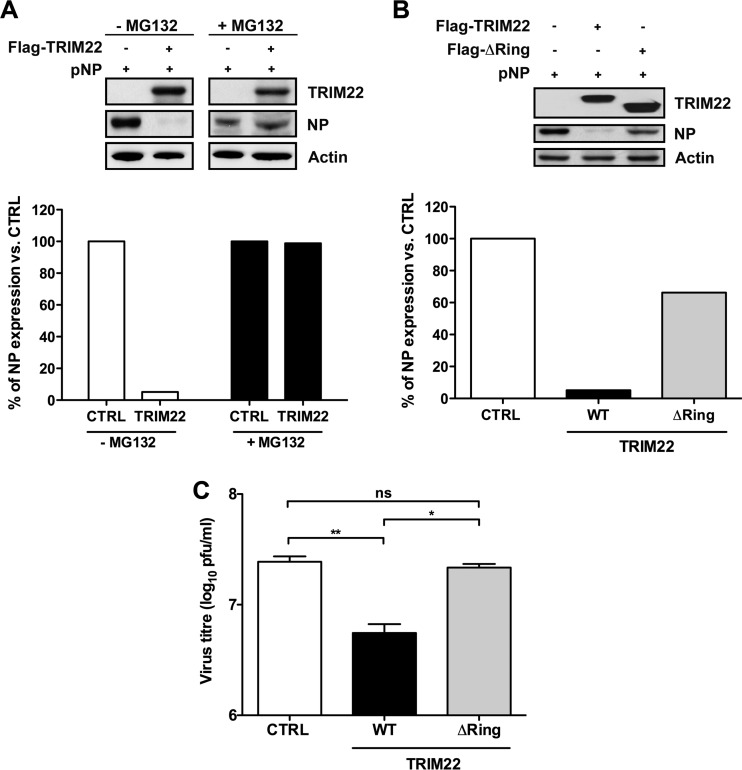
In the case of HBV (20) and ECMV (21), the antiviral effect of TRIM22 was shown to be dependent on its N-terminal RING finger domain, which is endowed with E3 ubiquitin-ligase activity (33). Seeking evidence for a role for the proteasome and/or ubiquitin in TRIM22-mediated restriction of IAV, we first investigated whether inhibiting the proteasome-dependent degradation pathway with MG132 could rescue NP expression from TRIM22. 293T cells were cotransfected with a fixed amount of NP-expressing plasmid and a 50-fold excess of either TRIM22-expressing plasmid or of an empty vector control in the presence or absence of the proteasome inhibitor MG132 (Fig. 6A). Twenty-four hours later, WCE were prepared and NP expression levels were assessed by Western blotting. Strikingly, incubation of cells with the proteasome inhibitor MG132 completely restored NP levels (Fig. 6A, upper). This suggests that the ubiquitin/proteasome system is required for degradation of NP. A semiquantitative analysis of signal intensity confirmed that TRIM22 expression led to an approximately 90% reduction in NP levels (Fig. 6A, lower, white bars), whereas addition of MG132 during transfection rescued NP expression in TRIM22-transfected cells to the levels observed in MG132-treated control cells (Fig. 6A, lower, black bars).
Fig 6.
TRIM22 inhibits IAV replication by degrading the viral nucleoprotein. (A) 293T cells were cotransfected with a fixed amount (50 ng) of NP-expressing plasmid and a 50-fold excess of either TRIM22-expressing or empty vector in the presence or absence of MG132 (2 μM). WCE were prepared 24 h p.t. and analyzed for TRIM22 and NP expression by Western blotting. Actin was used as a normalizer, and relative quantification of detected signal was performed using Image J software (lower panel). (B) 293T cells were transfected with a fixed amount (50 ng) of NP-expressing plasmid and a 50-fold excess of either wild-type (WT) TRIM22 or mutant TRIM22 lacking the N-terminal RING domain (ΔRING). WCE were prepared 24 h p.t., and expression of TRIM22 (anti-FLAG) and NP (anti-NP) was analyzed by Western blotting. Actin was used as a normalizer. Relative quantification of signal was performed using Image J software (lower panel). (C) MDCK cells were transfected with WT or ΔRING mutant TRIM22-expressing plasmid followed by infection with A/NewCaledonia/20/99 (H1N1) at an MOI of 0.001. Infectious particle production was evaluated from culture supernatants collected at 48 h p.i. Results are expressed as the means ± SD of one representative out of three experiments performed in triplicate. P values were determined using one-way analysis of variance. Western blots shown in A and B are representative of 2 independent experiments.
To investigate whether TRIM22-mediated inhibition of IAV NP expression was dependent on the RING domain, 293T cells were cotransfected with a fixed amount of NP-expressing plasmid in the presence of a 50-fold excess of vectors expressing either WT TRIM22 or a TRIM22 mutant lacking the N-terminal RING domain (ΔRING) (19, 21). Twenty-four hours later, WCE were prepared and NP expression levels were assessed by Western blotting. Again, while NP expression was significantly diminished in cells transfected with WT TRIM22, cells transfected with the ΔRING mutant failed to promote NP degradation (Fig. 6B). Semiquantitative analysis of signal intensity was performed using Image J software. Degradation of the viral NP was indeed observed in cells transfected with WT TRIM22 but not in cells transfected with the ΔRING mutant (Fig. 6B, bottom). Thus, RING-mediated E3 ubiquitin ligation likely leads to the degradation of NP.
To confirm that the RING domain is required for restriction of IAV infectivity, MDCK cells were transfected with plasmids expressing either the WT TRIM22 or ΔRING mutant 24 h prior to infection with A/New Caledonia/20/99 (H1N1) at an MOI of 0.001. Viral production was evaluated in culture supernatants collected at 48 h p.i. Transient transfection of MDCK cells with WT vector led to a significant decrease in viral production compared to that of cells transfected with an empty control vector (Fig. 6C, WT versus CTRL). In contrast, the restriction of IAV replication was lost in MDCK transfected with the ΔRING mutant (Fig. 6C, ΔRING versus CTRL). Together, these results strongly suggest that TRIM22 controls IAV replication by degrading the viral NP in a RING- and ubiquitin/proteasome-dependent manner, likely through its E3 ubiquitin ligase activity.
TRIM22 induces ubiquitination of NP.
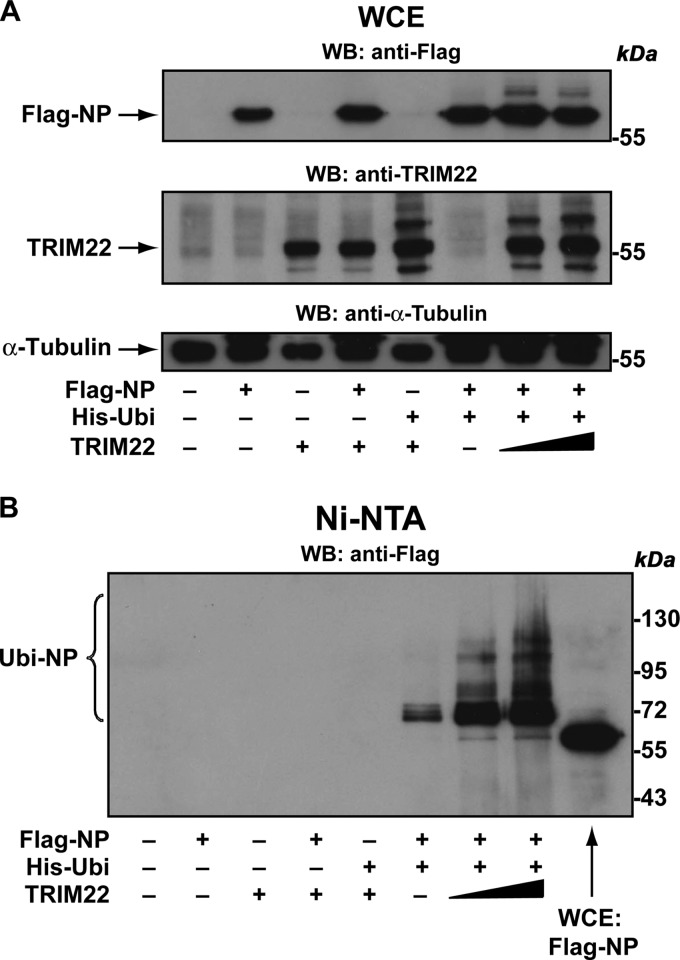
We finally evaluated whether TRIM22 could induce concentration-dependent NP ubiquitination. To do this, we cotransfected 293T cells with His-ubiquitin expression vector (His-Ubi), Flag-tagged NP expression vector, and an increasing amount of TRIM22 expression vector. Forty-eight h later, cells were treated with the proteasome inhibitor MG132 (10 μM for 3 h) to ensure accumulation of polyubiquitinated forms of NP. Ten percent of the cells were collected, and WCE were analyzed by Western blotting using either anti-Flag or anti-TRIM22 Ab. In the absence of TRIM22 expression, NP showed as a predominant band of 56 kDa; however, an additional band with a higher molecular mass appeared when TRIM22 was coexpressed with His-tagged ubiquitin (Fig. 7A, upper), suggesting that TRIM22 induces NP monoubiquitination. Notably, the anti-TRIM22 Ab revealed additional high-molecular-mass bands of TRIM22 in the presence of ubiquitin (Fig. 7A, middle), confirming previous results showing that TRIM22 is self ubiquitinated (21).
Fig 7.
TRIM22 induces ubiquitination of NP. (A) Flag-NP was coexpressed with His-Ubi and increasing amounts of TRIM22 in 293T cells. Forty-eight h after transfection, the cells were treated with 10 μM MG132 for 3 h. Subsequently, WCE were prepared from a 10% fraction of the cells and analyzed by Western blotting using anti-Flag (upper), anti-TRIM22 (middle), and anti-tubulin (lower) antibodies. (B) The remaining 90% of the cells were lysed in denaturing conditions, and ubiquitinated proteins were precipitated using Ni-NTA agarose. Purified ubiquitinated proteins were analyzed by Western blotting for the presence of ubiquitinated forms of Flag-NP (Ubi-NP) using anti-Flag antibodies. WCE from Flag-NP-expressing 293T cells (WCE Flag-NP) was loaded on the gel for size comparison.
Subsequently, the remaining 90% of the cells were lysed in a denaturing buffer containing 6 M guanidine-hydrochloride to dissociate potential ubiquitinated proteins bound to Flag-NP. The ubiquitinated proteins then were purified using Ni-NTA beads and analyzed by 10% SDS-PAGE to resolve high-molecular-mass ubiquitin-conjugated NP products. Western blot detection using an anti-Flag Ab showed that TRIM22 expression induced a strong concentration-dependent mono- and polyubiquitination of Flag-NP (Fig. 7B). Indeed, we observed one prominent band with a relative molecular mass that corresponded to that of NP plus one ubiquitin molecule, consistent with TRIM22-induced NP monoubiquitination. However, the presence of a high-molecular-mass smear of His-ubiquitin-conjugated NP suggests that TRIM22 also induced NP polyubiquitination, which drives recruitment to the proteasome followed by degradation.
DISCUSSION
TRIM proteins constitute a family of proteins that are involved in a broad range of biological processes, including cell cycle progression and antiviral responses (7–9). In particular, several TRIM members are endowed with a potent capacity to restrict viral infectivity (11–13, 19–21, 34–37). Our current study demonstrates that TRIM22 extends its antiviral effects to IAV. Of interest, IAV infection, as well as IFNs, upregulated the expression of endogenous TRIM22 in A549 cells, whereas TRIM22 was required for basal levels of resistance and contributed to the antiviral effect of exogenously added IFN-α.
Restriction of IAV by TRIM22 appears mechanistically similar to the anti-HIV effect exerted by simian TRIM5α against HIV-1 infection (34). In this regard, TRIM22 and TRIM5 genes are adjacent on chromosome 11 in humans and probably represent paralogues (15). Both appear to involve the ubiquitin-proteasome system and RING-dependent ubiquitin ligation. However, other aspects of their antiviral effects are less clear, in that TRIM5 RING mutants still restrict HIV-1 infection and MG132 does not rescue infectivity (38–40). In the case of TRIM22, while its E3 ubiquitin ligase activity is required for anti-HBV and anti-EMCV effects (20, 21), this was not the case for its anti-HIV-1 effect (19). Concerning IAV infection, we observed a direct interaction of NP and TRIM22 resembling the recruitment of incoming retroviral capsid by TRIM5α. Restriction by both TRIM22 and TRIM5α was saturable by high viral MOIs (41, 42). However, we cannot exclude that TRIM22's effect is also exerted on NP produced in the cell during viral replication. Theoretically, both should be saturable by infection with high MOIs.
IAV infection and replication must deal with the antiviral effects triggered by pattern recognition receptor (PRR)-dependent pathways and the subsequent IFN production and signaling leading to gene expression changes and an increased antiviral state (43). This is evidenced by viral mutants, particularly in the NS1 gene, that were unable to replicate in the presence of IFN both in vitro and in vivo (44). The mechanisms by which IAV evades these innate responses are gradually being characterized. Viral RNP sequesters RNA away from PRR, and NS1 directly disrupts RIG-I activation by targeting TRIM25 via its coiled-coil domain, thereby preventing multimerization and complex formation (14, 45). Whether additional host cell mechanisms that suppress IAV replication exist is less well understood (46). In this regard, the membrane protein IFITM3 has been shown to play a key role in this process by preventing emergence of viral genomes from the endosomal pathway (47), but this is unlikely to be the sole restriction of IAV induced by IFN-α or IFN-β. In the present study, we demonstrate that TRIM22 was able to directly target the viral NP and cause its degradation in conjunction with the inhibition of IAV replication.
NP was previously shown to be a monoubiquitinated protein, and its deubiquitination by USP11 inhibits viral RNA replication (48). In this report, we demonstrate that TRIM22 induces both NP mono- and polyubiquitination, and as expected, the latter modification leads to its proteasomal degradation. However, we cannot exclude that TRIM22's ability to catalyze the monoubiquitination of NP plays in favor of IAV replication. The mono- and polyubiquitination of NP could reflect the ability of TRIM22 to differentially affect NP activity depending on the stage of the viral life cycle. TRIM22 ubiquitination was mediated by its E3 ubiquitin ligase activity of the RING domain that was also reported to be essential in restricting HBV and ECMV. Concerning HBV, TRIM22 was shown to act as a transcriptional suppressor by targeting the HBV core promoter (CP) in the nucleus of infected cells, and the RING domain of TRIM22 was important for the inhibition of CP activity (20). Regarding ECMV, the E3 ubiquitin ligase activity catalyzes the ubiquitination of the viral 3C protease (21). In this report, a new substrate, i.e., IAV NP, was identified to be the target of the E3 ubiquitin ligase activity of TRIM22.
Low, barely detectable levels of TRIM22 were present in the cell lines investigated here, perhaps reflecting their common use as IAV-permissive cell lines. Of interest, IAV infection led to a strong increase in TRIM22 expression, to an extent that was comparable to that induced by IFN-α, and virus-induced TRIM22 expression led to a partial containment of IAV spreading. This paradoxical observation is, however, consistent with the hypothesis that very high levels of virus replication trigger a proinflammatory cytokine storm, particularly in the airway, leading to respiratory and multiorgan diseases (49). Thus, induction of endogenous TRIM22 by IAV infection may represent a servomechanism limiting its damage to the host, thereby ultimately favoring its replication and transmission. In addition, we also provided evidence that TRIM22 is a significant, although dispensable, component of the IFN response against IAV infection.
In conclusion, we have provided evidence that TRIM22 constitutes an important mediator at the cross-roads between IAV infection and the IFN response against it. Finally, investigation of the degree of susceptibility of different IAV strains to TRIM22 restriction/escape could ultimately contribute to our understanding of IAV pathogenicity in humans and of its pandemic potential.
ACKNOWLEDGMENTS
This work has been partially supported by a UNIFLUVAC grant from the Ministry of Health, Rome, Italy, and Fondazione Cariplo Vaccine Program, grant no. 2009-3594. G.J.T. was supported by a Wellcome Trust Senior Fellowship, the Medical Research Council, and the National Institutes of Health Research UCL/UCLH Biomedical Research Centre.
A.D.P. conducted this study as partial fulfillment of his Ph.D. in Molecular Medicine, Program in Basic and Applied Immunology, International Ph.D. School, Vita-Salute San Raffaele University, Milan, Italy. We thank Paul Bieniasz for the TRIM22-encoding EXN MLV vector and Guido Poli for helpful discussions and critical reading of the manuscript.
Footnotes
Published ahead of print 13 February 2013
REFERENCES
- 1. Capua I, Kajaste-Rudnitski A, Bertoli E, Vicenzi E. 2009. Pandemic vaccine preparedness–have we left something behind? PLoS Pathog. 5:e1000482 doi:10.1371/journal.ppat.1000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katze MG, He Y, Gale M., Jr 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675–687 [DOI] [PubMed] [Google Scholar]
- 3. Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. 2005. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571–5580 [DOI] [PubMed] [Google Scholar]
- 4. Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 101:5598–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730–737 [DOI] [PubMed] [Google Scholar]
- 6. Der SD, Zhou A, Williams BR, Silverman RH. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. U. S. A. 95:15623–15628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nisole S, Stoye JP, Saib A. 2005. TRIM family proteins: retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 3:799–808 [DOI] [PubMed] [Google Scholar]
- 8. Meroni G, Diez-Roux G. 2005. TRIM/RBCC, a novel class of “single protein RING finger” E3 ubiquitin ligases. Bioessays 27:1147–1157 [DOI] [PubMed] [Google Scholar]
- 9. Ozato K, Shin DM, Chang TH, Morse HC., III 2008. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 8:849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Towers GJ. 2007. The control of viral infection by tripartite motif proteins and cyclophilin A. Retrovirology 4:40 doi:10.1186/1742-4690-4-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolf D, Goff SP. 2007. TRIM28 mediates primer binding site-targeted silencing of murine leukemia virus in embryonic cells. Cell 131:46–57 [DOI] [PubMed] [Google Scholar]
- 12. Taylor RT, Lubick KJ, Robertson SJ, Broughton JP, Bloom ME, Bresnahan WA, Best SM. 2011. TRIM79alpha, an interferon-stimulated gene product, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe 10:185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. 2007. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446:916–920 [DOI] [PubMed] [Google Scholar]
- 14. Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A. 2009. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5:439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sawyer SL, Emerman M, Malik HS. 2007. Discordant evolution of the adjacent antiretroviral genes TRIM22 and TRIM5 in mammals. PLoS Pathog. 3:e197 doi:10.1371/journal.ppat.0030197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bouazzaoui A, Kreutz M, Eisert V, Dinauer N, Heinzelmann A, Hallenberger S, Strayle J, Walker R, Rubsamen-Waigmann H, Andreesen R, von Briesen H. 2006. Stimulated trans-acting factor of 50 kDa (Staf50) inhibits HIV-1 replication in human monocyte-derived macrophages. Virology 356:79–94 [DOI] [PubMed] [Google Scholar]
- 17. Tissot C, Mechti N. 1995. Molecular cloning of a new interferon-induced factor that represses human immunodeficiency virus type 1 long terminal repeat expression. J. Biol. Chem. 270:14891–14898 [DOI] [PubMed] [Google Scholar]
- 18. Barr SD, Smiley JR, Bushman FD. 2008. The interferon response inhibits HIV particle production by induction of TRIM22. PLoS Pathog. 4:e1000007 doi:10.1371/journal.ppat.1000007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kajaste-Rudnitski A, Marelli SS, Pultrone C, Pertel T, Uchil PD, Mechti N, Mothes W, Poli G, Luban J, Vicenzi E. 2011. TRIM22 inhibits HIV-1 transcription independently of its E3 ubiquitin ligase activity, Tat, and NF-kappaB-responsive long terminal repeat elements. J. Virol. 85:5183–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao B, Duan Z, Xu W, Xiong S. 2009. Tripartite motif-containing 22 inhibits the activity of hepatitis B virus core promoter, which is dependent on nuclear-located RING domain. Hepatology 50:424–433 [DOI] [PubMed] [Google Scholar]
- 21. Eldin P, Papon L, Oteiza A, Brocchi E, Lawson TG, Mechti N. 2009. TRIM22 E3 ubiquitin ligase activity is required to mediate antiviral activity against encephalomyocarditis virus. J. Gen. Virol. 90:536–545 [DOI] [PubMed] [Google Scholar]
- 22. Shapira SD, Gat-Viks I, Shum BO, Dricot A, de Grace MM, Wu L, Gupta PB, Hao T, Silver SJ, Root DE, Hill DE, Regev A, Hacohen N. 2009. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell 139:1255–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Goffic R, Bouguyon E, Chevalier C, Vidic J, Da Costa B, Leymarie O, Bourdieu C, Decamps L, Dhorne-Pollet S, Delmas B. 2010. Influenza A virus protein PB1-F2 exacerbates IFN-beta expression of human respiratory epithelial cells. J. Immunol. 185:4812–4823 [DOI] [PubMed] [Google Scholar]
- 24. Zhang F, Hatziioannou T, Perez-Caballero D, Derse D, Bieniasz PD. 2006. Antiretroviral potential of human tripartite motif-5 and related proteins. Virology 353:396–409 [DOI] [PubMed] [Google Scholar]
- 25. Matrosovich M, Matrosovich T, Garten W, Klenk HD. 2006. New low-viscosity overlay medium for viral plaque assays. Virol. J. 3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schaller T, Hue S, Towers GJ. 2007. An active TRIM5 protein in rabbits indicates a common antiviral ancestor for mammalian TRIM5 proteins. J. Virol. 81:11713–11721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 28. Kajaste-Rudnitski A, Mashimo T, Frenkiel MP, Guenet JL, Lucas M, Despres P. 2006. The 2′,5′-oligoadenylate synthetase 1b is a potent inhibitor of West Nile virus replication inside infected cells. J. Biol. Chem. 281:4624–4637 [DOI] [PubMed] [Google Scholar]
- 29. Katze MG, Krug RM. 1984. Metabolism and expression of RNA polymerase II transcripts in influenza virus-infected cells. Mol. Cell. Biol. 4:2198–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Towers G, Collins M, Takeuchi Y. 2002. Abrogation of Ref1 retrovirus restriction in human cells. J. Virol. 76:2548–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Portela A, Digard P. 2002. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J. Gen. Virol. 83:723–734 [DOI] [PubMed] [Google Scholar]
- 32. Marklund JK, Ye Q, Dong J, Tao YJ, Krug RM. 2012. Sequence in the influenza A virus nucleoprotein required for viral polymerase binding and RNA synthesis. J. Virol. 86:7292–7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duan Z, Gao B, Xu W, Xiong S. 2008. Identification of TRIM22 as a RING finger E3 ubiquitin ligase. Biochem. Biophys. Res. Commun. 374:502–506 [DOI] [PubMed] [Google Scholar]
- 34. Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848–853 [DOI] [PubMed] [Google Scholar]
- 35. Rowe HM, Jakobsson J, Mesnard D, Rougemont J, Reynard S, Aktas T, Maillard PV, Layard-Liesching H, Verp S, Marquis J, Spitz F, Constam DB, Trono D. 2010. KAP1 controls endogenous retroviruses in embryonic stem cells. Nature 463:237–240 [DOI] [PubMed] [Google Scholar]
- 36. Cuchet D, Sykes A, Nicolas A, Orr A, Murray J, Sirma H, Heeren J, Bartelt A, Everett RD. 2011. PML isoforms I and II participate in PML-dependent restriction of HSV-1 replication. J. Cell Sci. 124:280–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. 2010. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc. Natl. Acad. Sci. U. S. A. 107:19985–19990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diaz-Griffero F, Li X, Javanbakht H, Song B, Welikala S, Stremlau M, Sodroski J. 2006. Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology 349:300–315 [DOI] [PubMed] [Google Scholar]
- 39. Perron MJ, Stremlau M, Lee M, Javanbakht H, Song B, Sodroski J. 2007. The human TRIM5alpha restriction factor mediates accelerated uncoating of the N-tropic murine leukemia virus capsid. J. Virol. 81:2138–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu X, Anderson JL, Campbell EM, Joseph AM, Hope TJ. 2006. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. U. S. A. 103:7465–7470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Besnier C, Takeuchi Y, Towers G. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. U. S. A. 99:11920–11925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cowan S, Hatziioannou T, Cunningham T, Muesing MA, Gottlinger HG, Bieniasz PD. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. U. S. A. 99:11914–11919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garcia-Sastre A. 2011. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 162:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hale BG, Randall RE, Ortin J, Jackson D. 2008. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 89:2359–2376 [DOI] [PubMed] [Google Scholar]
- 45. Rehwinkel J, Tan CP, Goubau D, Schulz O, Pichlmair A, Bier K, Robb N, Vreede F, Barclay W, Fodor E, Reis e Sousa C. 2010. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell 140:397–408 [DOI] [PubMed] [Google Scholar]
- 46. Stertz S, Shaw ML. 2011. Uncovering the global host cell requirements for influenza virus replication via RNAi screening. Microbes Infect. 13:516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feeley EM, Sims JS, John SP, Chin CR, Pertel T, Chen LM, Gaiha GD, Ryan BJ, Donis RO, Elledge SJ, Brass AL. 2011. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PLoS Pathog. 7:e1002337 doi:10.1371/journal.ppat.1002337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liao TL, Wu CY, Su WC, Jeng KS, Lai MM. 2010. Ubiquitination and deubiquitination of NP protein regulates influenza A virus RNA replication. EMBO J. 29:3879–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. 2012. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 76:16–32 [DOI] [PMC free article] [PubMed] [Google Scholar]