Abstract
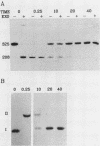
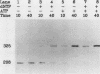
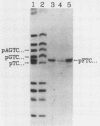
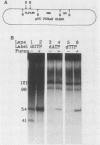
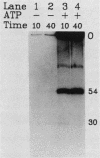
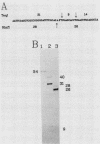
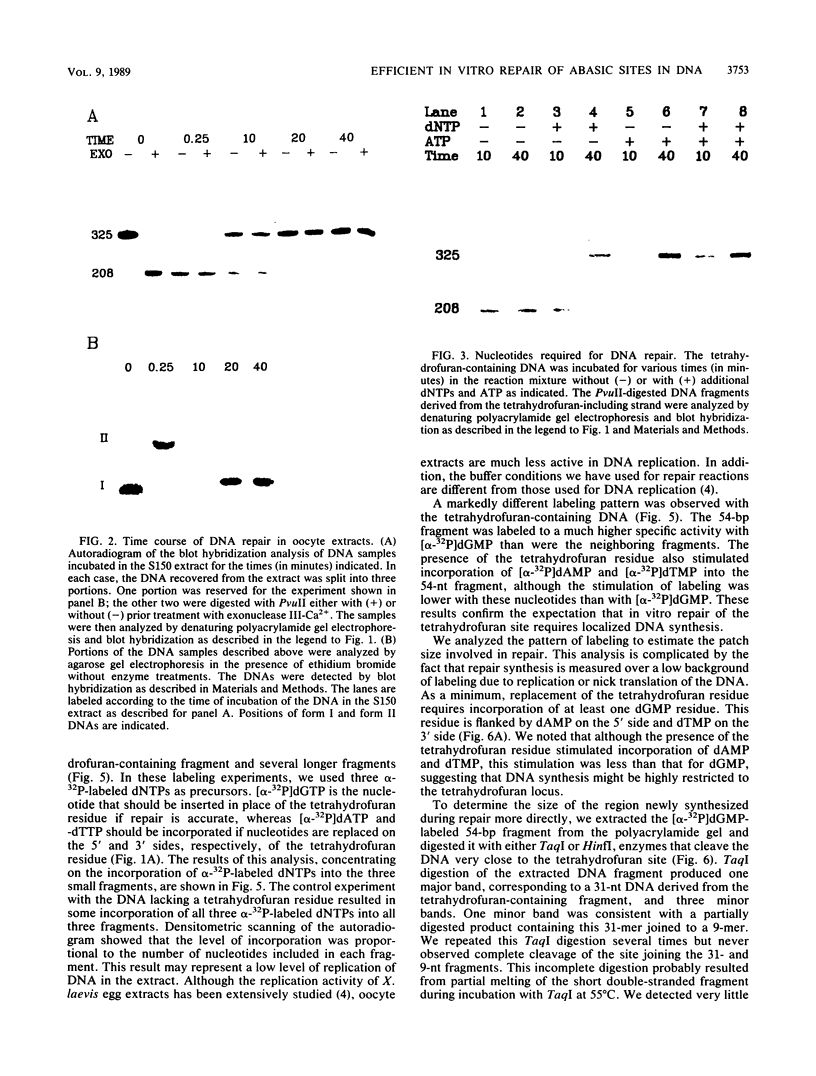
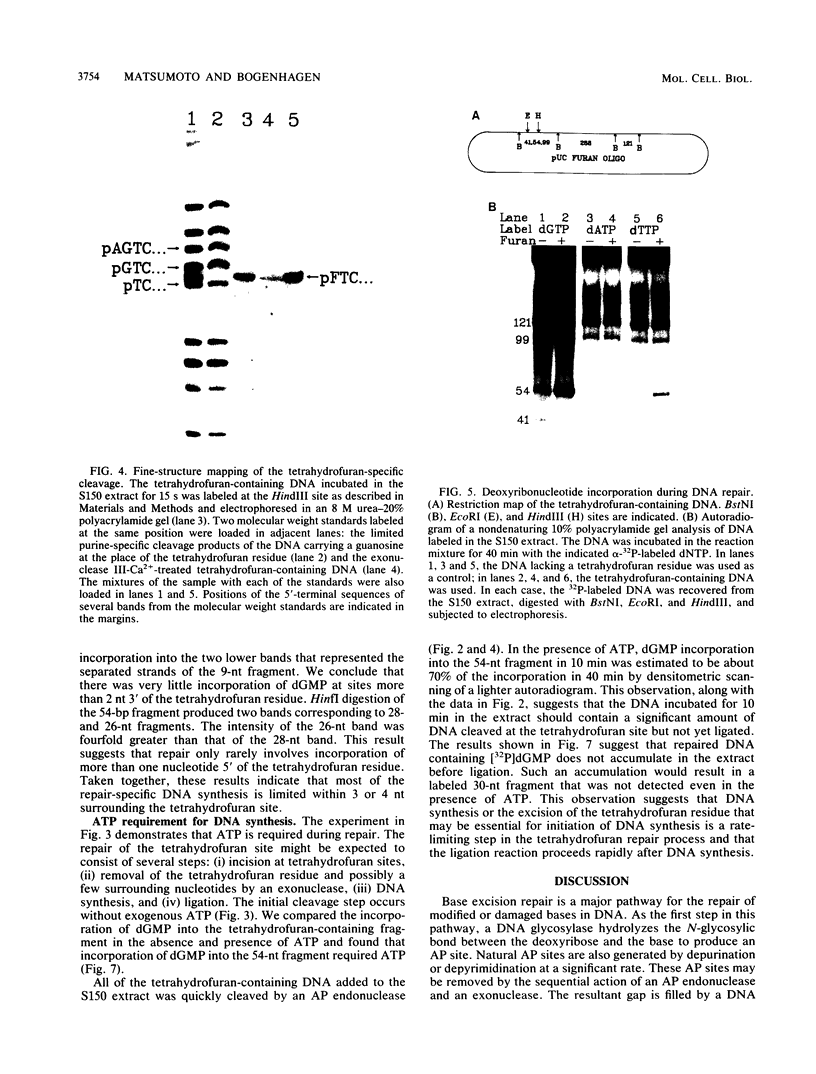
Covalently closed circular DNA containing a synthetic analog of an abasic site at a unique position was used as a substrate to study DNA repair. Incubation of this DNA in Xenopus laevis oocyte extracts resulted in rapid cleavage of the DNA at the abasic site by a class II apurinic-apyrimidinic endonuclease, followed by complete repair within 40 min. Nicked circular DNAs persisted for several minutes before repair by an ATP-dependent DNA synthesis reaction. The repair-related DNA synthesis was localized within 3 or 4 nucleotides surrounding the abasic site. These results are consistent with the short-patch repair reported for DNA damage at heterogeneous sites in human cells (J. D. Regan and R. B. Setlow, Cancer Res. 34:3318-3325, 1974).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly V., Verly W. G. Escherichia coli endonuclease III is not an endonuclease but a beta-elimination catalyst. Biochem J. 1987 Mar 1;242(2):565–572. doi: 10.1042/bj2420565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V., Verly W. G. Possible roles of beta-elimination and delta-elimination reactions in the repair of DNA containing AP (apurinic/apyrimidinic) sites in mammalian cells. Biochem J. 1988 Jul 15;253(2):553–559. doi: 10.1042/bj2530553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A. K., Essigmann J. M. Site-specifically modified oligodeoxynucleotides as probes for the structural and biological effects of DNA-damaging agents. Chem Res Toxicol. 1988 Jan-Feb;1(1):1–18. doi: 10.1021/tx00001a001. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Laskey R. A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986 Nov 21;47(4):577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Deutsch W. A., Linn S. DNA binding activity from cultured human fibrolasts that is specific for partially depurinated DNA and that inserts purines into apurinic sites. Proc Natl Acad Sci U S A. 1979 Jan;76(1):141–144. doi: 10.1073/pnas.76.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch W. A., Spiering A. L. Characterization of a depurinated-DNA purine-base-insertion activity from Drosophila. Biochem J. 1985 Nov 15;232(1):285–288. doi: 10.1042/bj2320285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., Lindahl T. DNA deoxyribophosphodiesterase. EMBO J. 1988 Nov;7(11):3617–3622. doi: 10.1002/j.1460-2075.1988.tb03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glikin G. C., Ruberti I., Worcel A. Chromatin assembly in Xenopus oocytes: in vitro studies. Cell. 1984 May;37(1):33–41. doi: 10.1016/0092-8674(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Penkala J. E., Peterson C. A., Wright D. A. Repair of UV-induced lesions in Xenopus laevis oocytes. Mol Cell Biol. 1987 Dec;7(12):4317–4323. doi: 10.1128/mcb.7.12.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T. Fractionation of DNA fragments by polyethylene glycol induced precipitation. Methods Enzymol. 1980;65(1):347–353. doi: 10.1016/s0076-6879(80)65044-7. [DOI] [PubMed] [Google Scholar]
- Livneh Z., Elad D., Sperling J. Enzymatic insertion of purine bases into depurinated DNA in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1089–1093. doi: 10.1073/pnas.76.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McConkey G. A., Bogenhagen D. F. Transition mutations within the Xenopus borealis somatic 5S RNA gene can have independent effects on transcription and TFIIIA binding. Mol Cell Biol. 1987 Jan;7(1):486–494. doi: 10.1128/mcb.7.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbaugh D. W., Linn S. Excision repair and DNA synthesis with a combination of HeLa DNA polymerase beta and DNase V. J Biol Chem. 1983 Jan 10;258(1):108–118. [PubMed] [Google Scholar]
- Mosbaugh D. W., Meyer R. R. Interaction of mammalian deoxyribonuclease V, a double strand 3' to 5' and 5' to 3' exonuclease, with deoxyribonucleic acid polymerase-beta from the Novikoff hepatoma. J Biol Chem. 1980 Nov 10;255(21):10239–10247. [PubMed] [Google Scholar]
- Nelson E. M., Stowers D. J., Bayne M. L., Benbow R. M. Classification of DNA polymerase activities from ovaries of the frog, Xenopus laevis. Dev Biol. 1983 Mar;96(1):11–22. doi: 10.1016/0012-1606(83)90306-8. [DOI] [PubMed] [Google Scholar]
- Randahl H., Elliott G. C., Linn S. DNA-repair reactions by purified HeLa DNA polymerases and exonucleases. J Biol Chem. 1988 Sep 5;263(25):12228–12234. [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Rogers S. G., Weiss B. Exonuclease III of Escherichia coli K-12, an AP endonuclease. Methods Enzymol. 1980;65(1):201–211. doi: 10.1016/s0076-6879(80)65028-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sanderson B. J., Chang C. N., Grollman A. P., Henner W. D. Mechanism of DNA cleavage and substrate recognition by a bovine apurinic endonuclease. Biochemistry. 1989 May 2;28(9):3894–3901. doi: 10.1021/bi00435a040. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Chang C. N., Johnson F., Will S., Grollman A. P. Oligodeoxynucleotides containing synthetic abasic sites. Model substrates for DNA polymerases and apurinic/apyrimidinic endonucleases. J Biol Chem. 1987 Jul 25;262(21):10171–10179. [PubMed] [Google Scholar]
- Weiss B., Grossman L. Phosphodiesterases involved in DNA repair. Adv Enzymol Relat Areas Mol Biol. 1987;60:1–34. doi: 10.1002/9780470123065.ch1. [DOI] [PubMed] [Google Scholar]