Abstract
Cytokine production by innate immunity is critical for shaping the adaptive immunity through regulation of T cell differentiation. In this report, we studied T cell immunoglobulin mucin domain protein 3 (Tim-3) expression on monocytes and its regulatory effect on interleukin-12 (IL-12)/IL-23 production by CD14+ monocytes, as well as IL-17 production by CD4+ T cells in individuals with chronic hepatitis C virus (HCV) infection. We found that Tim-3 and IL-23p19 are highly expressed and that IL-12p35 is inhibited in human CD14+ monocytes, while IL-17 expression is upregulated in CD4+ T cells, in chronically HCV-infected individuals compared to healthy subjects. Interestingly, Tim-3 expression is closely associated with the differential regulation of IL-12/IL-23 expression in CD14+ monocytes and correlated to IL-17 production by CD4+ T cells. These Tim-3-associated IL-12/IL-23/IL-17 dysregulations in HCV-infected individuals are also recapitulated in vitro by incubating healthy monocytes or peripheral blood mononuclear cells with Huh-7 hepatoma cells transfected with HCV RNA. Importantly, blocking Tim-3 signaling on monocytes restores the balance of IL-12/IL-23 through the intracellular STAT3 signaling, which in turn reverses the upregulated IL-17 expression both ex vivo and in vitro. Our findings suggest that Tim-3-mediated differential regulation of IL-12/IL-23 drives TH17 cell development, a milieu favoring viral persistence and autoimmune phenomenon during HCV infection.
INTRODUCTION
Hepatitis C virus (HCV) infection is characterized by a high rate of persistent infection that is associated with autoimmune disorders such as glomerulonephritis and mixed cryoglobulinemia (1, 2). The underlying mechanism(s) for HCV persistence and its associated autoimmune phenomenon is not fully elucidated, but virus-mediated dysregulation of host immunity seems to play a major role; i.e., this virus is able to modulate host innate to adaptive immune responses and by doing so facilitates chronic viral infection and its associated diseases (3, 4). The mechanisms by which HCV impairs antigen-presenting cell (APC) and T cell immunity include blunted monocyte activation and dendritic cell maturation, skewed T cell differentiation (TH1 deficiency or TH2 dominance) and proliferation, T cell anergy (antigen-specific hypo-responsiveness or exhaustion), T cell depletion (cell apoptosis or death), and the induction of regulatory T cells (Tregs) (3, 4). It has also been postulated that HCV-mediated dysregulation of cytokine production by APCs may be critical for shaping the adaptive immune response through inhibitory signaling pathways during viral infection.
Interleukin-12 (IL-12), a heterodimeric cytokine composed of p40 and p35 subunits, is considered essential for TH1-type immune response that is critical for clearance of pathogen infection (5–7). How IL-12 endows T cells with such pathogenic potential is unclear, but the discovery of IL-23, a heterodimer of IL-12p40 and a unique IL-23p19 subunit, offers new insights into the role of heterodimeric cytokines in the pathogenesis of chronicity and autoimmunity in the setting of infection (8–10). Although both are produced by APCs, IL-12 and IL-23 has different characteristics and functions in polarization of naive T cell differentiation (5–10). IL-12 induces tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) production that promotes polarization toward a TH1 phenotype. IL-23 is critical in differentiation of TH17 cells: a member of the TH cell family that is pivotal in the development of autoimmune diseases under pathogenic conditions (5–10). The conditions for differentiation of TH17 cells remain elusive to date, but the levels of IL-12 and IL-23 expression by APCs seems to promote differentiation of naive T cells into TH17 cells (11–13). Increases in IL-23-producing CD14+ monocytes or IL-17-producing CD4+ T cells have been found to be associated with immune-mediated diseases (14–19). Although IL-12 expression has been shown to be significantly suppressed during HCV infection (20–23), the mechanisms by which HCV mediates the regulation of IL-12 and its relationship to IL-23 expression, whether and how an altered IL-12/IL-23 balance affects TH17 cell development during HCV infection, remain to be investigated.
Tim-3, a member of T cell immunoglobulin and mucin domain proteins (Tim), serves as a maker of negative signals to ensure an appropriate immune response against pathogens and yet prevent overactivation of lymphocytes and thus immune injury or autoimmunity (24–26). Compelling evidence is emerging for the role of Tim-3 in antiviral immune evasion, raising the possibility that a therapeutic strategy targeting this inhibitory pathway might be of clinical benefit, especially for HCV/HIV infection (26–28). While Tim-3 has been identified as an inhibitory receptor preferably expressed on exhausted T cells (24–28), its role in monocyte/macrophage (M/Mϕ) modulation remains less well understood. We have previously demonstrated that Tim-3 is upregulated on M/Mϕ and negatively regulates IL-12 expression during HCV infection (20, 21). The role of Tim-3 expression on M/Mϕ in controlling the IL-12/IL-23 balance, which in turn may affect TH17 differentiation during HCV infection, remains largely unknown.
In the present study, we assessed Tim-3 expression on M/Mϕ and its regulatory effect on IL-12 and IL-23 expression, as well as T cell IL-17 expression, in individuals with HCV infection. We provide pilot evidence suggesting that Tim-3-mediated differential regulation of IL-12/IL-23 promotes TH17 cell development. Since deficiency of IL-12 is crucial to impaired T cell responses against intruding pathogens and may facilitate chronic infection, whereas excess IL-23 is associated with autoimmune disorders and contributes to TH17, as well as regulatory Treg development, the present study, demonstrating Tim-3-mediated differential regulation of inflammatory cytokines, suggests the formation of a milieu that favors the viral persistence and autoimmune phenomenon observed in the setting of chronic HCV infection.
MATERIALS AND METHODS
Subjects.
The study protocol was approved by an institutional review board at East Tennessee State University and James H. Quillen VA Medical Center (ETSU/VA IRB, Johnson City, TN), which has contributed to a database for the storage of blood samples from HCV-infected individuals for the purpose of viral immunology studies. The study subjects are composed of two populations: 60 HCV-infected subjects and 16 healthy subjects. Written informed consent was obtained from all participants. HCV genotype (70% type 1, 30% type 2 or 3) and viral load (ranging from 12,300 ∼ 500,000 IU/ml) were performed by Lexington VAMC, and all subjects were virologically and serologically positive for HCV, prior to the antiviral treatment. Healthy subjects are negative for HBV, HCV, and HIV infections. The majority of the study subjects are male. The mean age of HCV-infected individuals was comparable to healthy subjects (P > 0.05).
Cell isolation and culture.
Human peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of study subjects by Ficoll density centrifugation with Lympho-H (Atlanta Biological, Lawrenceville, GA). CD14+ monocytes and CD4+ T cells were purified from PBMCs by magnetic beads with positive selection according to the manufacturer's instructions (purity, >95%; Miltenyi Biotec, Inc., Auburn, CA). The purified cells were cultured with RPMI 1640, containing 10% fetal bovine serum (Life Technologies, Gaithersburg, MD), 100 mg of penicillin-streptomycin (Thermo Scientific, Logan, UT)/ml, and 2 mM l-glutamine (Thermo Scientific) at 37°C with 5% CO2 atmosphere for the following experiments.
Flow cytometry.
To determine which Toll-like receptor (TLR) is critical to regulate IL-12/IL-23 production and Th17 development during HCV infection, we detected intracellular IL-12 and IL-23 expression by CD14+ monocytes and IL-17 expression by CD4+ T cells stimulated with specific TLR ligands. Specifically, PBMCs isolated from HCV patients were stimulated (6 and 18 h) with 2 μg of peptidoglycan/ml (E. coli strain O111:B4 PGN; InvivoGen, San Diego, CA) for TLR2, 2 μg of poly(I·C) (Amersham Pharmacia, Minneapolis, NJ)/ml for TLR3, 1 μg of lipopolysaccharide (LPS; BD Pharmingen)/ml for TLR4, 20 ng of flagellin (recFLA-ST; InvivoGen)/ml for TLR5, 2.5 μg of R848 (InvivoGen)/ml for TLR7/8, or 20 μg of ODN2395 (InvivoGen)/ml for TLR9. PBMCs were also stimulated with 100 ng of phorbol 12-myristate 13-acetate (PMA)/ml and 1 μg of ionomycin mitogens (InvivoGen)/ml, followed by flow cytometry analysis. IL-12/IL-23 expression was detected in CD14+ monocytes with PBMCs stimulated with TLR4/7/8 and PMA/ionomycin (levels high at 6 h and low at 18 h), and IL-23 was also detected by TLR2 stimulation, whereas TH17 cells were only detectable with PBMCs stimulated with PMA/ionomycin at 6 h. Annexin V/PI apoptosis staining of the purified CD14+ monocytes and CD4+ T cells stimulated with LPS/R848 or PMA/ionomycin for 6 h exhibits slightly increased annexin v (Av) expression, but no significant dead cells within 6 h stimulation. Therefore, in the following experiments, PBMCs or purified CD14+ monocytes were stimulated by 1 μg of TLR4 ligand LPS/ml and 2.5 μg of TLR 7/8 ligand R848/ml for 6 h. Brefeldin A (BioLegend, San Diego, CA) was added 5 h prior to harvesting the cells, inhibiting cytokine secretion. PBMCs or CD4+ T cells were stimulated by 100 ng of PMA/ml and 1 μg of ionomycin/ml for 6 h, with brefeldin A added 5 h prior to harvest the cells. The use of specific antibody direct conjugates for cell surface staining was carried out using Tim-3-APC (R&D, Minneapolis, MN), CD4-APC or CD14-FITC (Miltenyi Biotec), followed by intracellular staining for IL-12p35-APC (R&D), IL-23p19-PE (eBioscience), IL-17A-PE (eBioscience), or pSTAT3-perCP (BD Pharmingen). The intracellular cytokine staining was carried out using Inside Stain kit (Miltenyi Biotec) according to the manufacturer's instructions. Isotype-matched control antibodies (eBioscience) and fluorescence minus one (FMO) controls were used to determine background levels of staining and adjust multicolor compensation as gating strategy. The cells were analyzed on a FACSCalibur flow cytometer (BD, Franklin Lakes, NJ) and FlowJo software.
Healthy CD14+ monocytes or PBMCs cocultured with HCV+/− Huh-7 hepatocytes.
Transfection of Huh-7 hepatocytes (kindly provided by T. J. Liang, Liver Section, National Institutes of Health [NIH]/National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK]) with HCV JFH-1 strain (kindly provided by T. Wakita) was carried out as described previously (20, 21). For coculture experiments, HCV+/− Huh-7 hepatocytes were serum starved for 18 h and then activated with rhIFN-γ (0.1 μg/ml; R&D Systems) for 48 h. Activated hepatocytes were removed from plates by 0.05% trypsin-EDTA and then plated at 5 × 105 cells/well in a 12-well plate. Purified healthy CD14+ monocytes, CD4+ T cells, or peripheral blood mononuclear cells (PBMCs) were added to the adherent hepatocytes in RPMI media, cocultured for another 72 h in the presence or absence of specific inhibitor or blockers, stimulated with LPS/R848 or PMA/ionomycin for 6 h, and then analyzed by flow cytometry or Western blotting.
Western blot analyses.
Purified CD14+ monocytes from patients or healthy monocytes cocultured with HCV+/− hepatocytes were treated as described above, and the expressions of phosphorylated and total STAT3 were measured by Western blotting. The cells were lysed in 1× radioimmunoprecipitation assay lysis buffer (Boston BioProducts, Inc., Ashland, MA) supplied with protease inhibitors/phosphorylase inhibitors (Thermo Scientific, Rockford, IL) and EDTA on ice. Cell lysates were centrifuged for 15 min at 4°C, and the protein concentrations were measured. Protein samples were thereafter combined with 4× Laemmli sample buffer (Boston BioProducts, Ashland, MA), denatured, and separated by SDS-PAGE. After transfer to an Amersham Hybond-P membrane (GE Heathcare, Piscataway, NJ), the membrane was blocked and probed with phospho-STAT3 (Tyr705) or total STAT3 antibody (Cell Signaling Technology, Inc., Danvers, MA) at 4°C overnight. Finally, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (Millipore, Temecula, CA) and developed by using Amersham ECL Plus Western blot detection reagents (GE Healthcare Biosciences, Pittsburgh, PA) on Kodak X-Omat-LS X-ray film (Sigma-Aldrich, St. Louis, MO).
Tim-3 blocker or STAT3 inhibitor.
Purified CD14+ cells or PBMCs were incubated with LEAF anti-human Tim-3 antibody (10 μg/ml; BioLegend) or control IgG for 72 h, followed by stimulation with LPS/R848 for 6 h or PMA/ionomycin for 6 h, and then subjected to flow cytometric analysis of IL-12p35, IL-23p19, IL-17A, or pSTAT3 expression as described above. CD14+ cells were also cocultured with HCV-expressing hepatocytes in complete RPMI medium, with or without 100 μM STAT3-specific inhibitor S3I-201 (NSC 74859; Santa Cruz Biotechnology, Santa Cruz, CA). After 48 h, cultured cells were stimulated with LPS/R848 for 6 h, followed by intracellular cytokine staining.
Statistical analysis.
Study results are summarized for each group, and results are expressed as means ± the standard deviations (SD). Comparison between two groups was performed by SPSS-18 software with the Student t test. A pairwise t test was used to compare the significance of changes in Tim-3 blocking experiments. Correlations between Tim-3 expression on monocytes and IL-12p35 or IL-23p19 expression, as well as IL-17A expression, were analyzed by using a Pearson correlation program. P values of <0.05 (*) or <0.01(**) were considered significant or very significant, respectively.
RESULTS
Tim-3 overexpressed on CD14+ monocyte is associated with downregulation of IL-12p35 expression and upregulation of IL-23p19 expression in patients with chronic HCV infection.
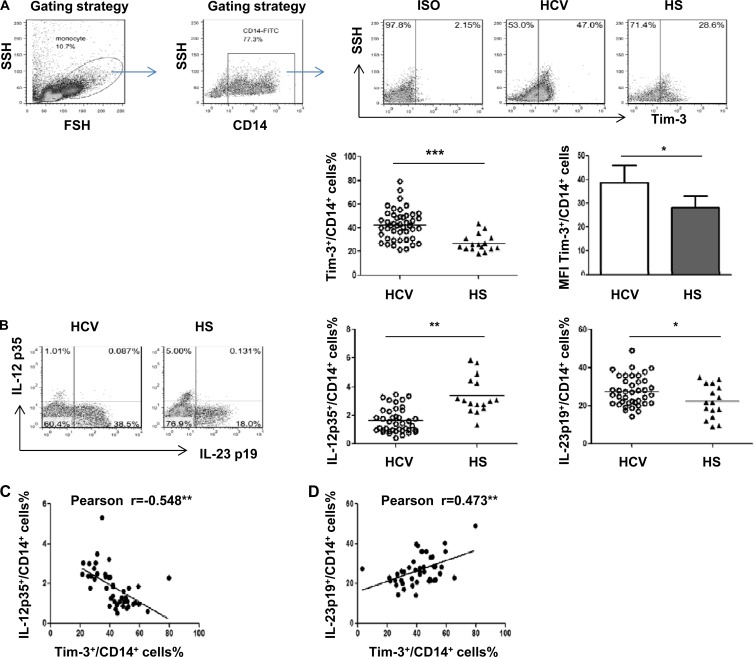
As an initial approach to investigate the role of Tim-3 in M/Mϕ regulation, Tim-3 expression, along with intracellular IL-12p35 and IL-23p19 production, in resting and TLR-stimulated monocytes derived from chronically HCV-infected patients and healthy subjects was examined by flow cytometry. The representative dot plots and summary data of Tim-3, IL-12p35, and IL-23p19 expression by CD14+ monocytes of healthy subjects (HS; n = 16) and HCV-infected individuals (HCV; n = 40) are shown in Fig. 1. Notably, chronically HCV-infected individuals exhibit significantly elevated Tim-3 expression, not only in the frequency of Tim-3 expression on CD14+ cells but also in the level of Tim-3 mean fluorescence intensity (MFI) per CD14+ cell, compared to those in HS (Fig. 1A). As shown previously (20, 21), with stimulation of purified CD14+ monocytes by LPS/R848 for 6 h, Tim-3 expression rapidly declined, accompanied by an increase of IL-12 and IL-23 expression by activated monocytes. Notably, IL-12p35 is downregulated, whereas IL-23p19 is upregulated, in response to LPS/R848 stimulation of CD14+ monocytes in individuals with chronic HCV infection compared to HS (Fig. 1B). The increase in IL-23p19-positive cells observed in HCV infection over HS holds true upon analysis of the MFI of IL-23 expression levels in CD14+ monocytes, whereas no significant difference in IL-12p35 MFI level is detected between HCV and HS (data not shown). With Pearson correlation analysis, Tim-3 expression was found to be inversely associated with monocyte IL-12p35 inhibition (Fig. 1C) and positively correlated with monocyte IL-23p19 expression (Fig. 1D), suggesting that Tim-3 may function as a marker or regulator for controlling IL-12/IL-23 expression.
Fig 1.
Tim-3 overexpression on CD14+ monocytes positively correlates with the upregulation of IL-23p19 and negatively correlates with the inhibition of IL-12p35 expression in chronically HCV-infected individuals. Peripheral blood mononuclear cells (PBMCs) from chronically HCV-infected patients (n = 40) and healthy subjects (n = 16) were stimulated with or without TLR ligands LPS and R848 for 6 h, followed by immunostaining with antibodies against CD14, Tim-3, IL-12p35, and IL-23p19. CD14+ monocytes were gated to measure Tim-3, IL-12p35, and IL-23p19 expression in unstimulated and LPS/R848-stimulated monocytes by flow cytometry. (A) Representative dot plots of gating strategy for Tim-3 expression on CD14+ monocytes from HCV-infected and HS are shown above. The percentage of positive cell frequency and the mean MFI values + the standard deviations (SD) for Tim-3 expression on gated CD14+ cells in HCV versus HS are shown below. Each symbol represents an individual subject, and the horizontal bars represent median values. *, P < 0.05; ***, P < 0.001 (as determined by Student t test). (B) Representative dot plots measuring IL-12p35 and IL-23p19 expression in CD14+ monocytes from HCV versus HS. Summary data of the percentage of IL-12p35+ cells and IL-23p19+ cells in CD14+ monocytes of HCV versus HS are shown on the right panels. *, P < 0.05; **, P < 0.01 (as determined by Student t test). (C) Correlation between Tim-3 expression and IL-12p35 or IL-23p19 production by CD14+ monocytes, as analyzed by Pearson correlation with two-tailed significance. **, P < 0.01.
Tim-3-associated differential regulation of IL-12/IL-23 expression by monocytes is correlated with an increase of IL-17-producing TH cells in HCV infection.
IL-12 and IL-23 share a chain, but their production in response to pathogens is differentially regulated, and their functions are distinct and often antithetical (6–9). IL-12 is involved in the induction or amplification of the TH1 response, whereas IL-23 has been associated with the generation of the TH17 response (5–10). To determine whether the observed differential regulation of IL-12/IL-23 may lead to the generation of IL-17-producing T helper cells (TH17) during HCV infection, PBMCs isolated from chronically HCV-infected individuals and HS were stimulated with PMA/ionomycin, and the prevalence of TH17+ CD4+ T cells was assessed by flow cytometry. As shown in Fig. 2A, individuals with chronic HCV infection exhibit significantly higher frequencies of IL-17+ cells in CD4+ T cell populations than HS. In addition, the IL-17 expression level (MFI) per CD4+ T cells is also higher in HCV-infected patients versus HS. Importantly, the increased frequency of IL-17+ CD4+ cells positively correlated with the ratio of IL-23/IL-12 productions (Fig. 2B), as well as Tim-3 expression by CD14+ monocytes (Fig. 2C), suggesting that Tim-3-associated differential regulation of IL-12/IL-23 might contribute to the development of TH17 cells during HCV infection.
Fig 2.
TH17 cells upregulation in HCV-infected individuals is associated with Tim-3 expression and differential regulation of IL-23/IL-12 production by monocytes. PBMCs from HCV-infected individuals and HS were stimulated with PMA/ionomycin for 6 h, stained with CD4 and IL-17A, followed by flow cytometric analysis. (A) Representative dot plots of IL-17A expression in CD4+ T cells. Summary data of percentages of the positive cell frequency and the mean MFI values + the SD of for IL-17A expression in CD4+ cells from HCV versus HS are shown. **, P < 0.01 (as determined by Student t test). (B) IL-17+ expression by CD4+ cells positively correlates to Tim-3 expression on CD14+ cells (*, P < 0.05. (C) IL-17+ expression by CD4+ cells positively correlates with the ratio of IL-23/IL-12 produced by CD14+ cells (*, P < 0.05).
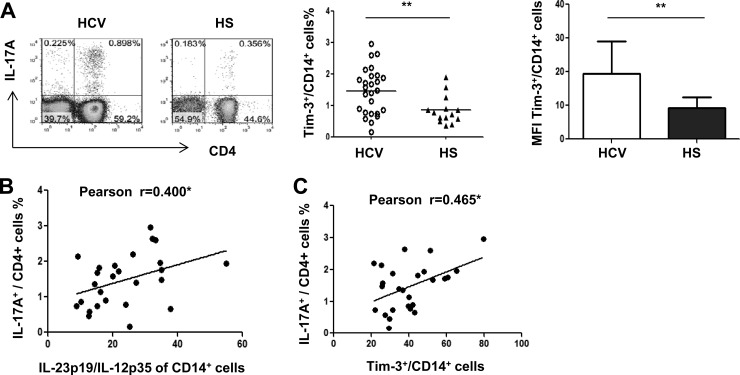
Tim-3 blockade on monocytes upregulates IL-12 expression, downregulates IL-23 expression, and inhibits IL-17 expression in HCV-infected individuals.
The observed Tim-3 expression and differential regulation of IL-12, IL-23, and IL-17 production might be a concurrent but unrelated phenomenon during HCV infection. To determine the role of Tim-3 on IL-12, IL-23, and IL-17 expression, we isolated CD14+ monocytes, CD4+ T cells, or PBMCs from 16 chronically HCV-infected individuals and 16 HS, incubated ex vivo with anti-Tim-3 (αTim-3) or a control antibody (IgG) for 72 h and then stimulated with LPS/R848 or PMA/ionomycin for 6 h, followed by detecting IL-12/IL-23 and IL-17 expression by flow cytometry. As shown in Fig. 3A, representative dot plots and summary data of IL-12p35 versus IL-23p19 expression in CD14+ monocytes, blocking Tim-3 signaling significantly improves IL-12 expression yet inhibits IL-23 expression by monocytes in HCV-infected individuals, but not in HS. We also examined Tim-3 expression and its blockade on IL-17 production in purified CD4+ T cells. As shown in Fig. 3B, IL-17 is primarily produced by Tim-3− TH cells, and blocking Tim-3 on CD4+ T cells enhances IL-17 production, suggesting a directly inhibitory role of Tim-3 expression on IL-17 expression in TH cells. Given its negative signaling on IL-17-producing CD4+ T cells, Tim-3 blockade on purified T cells would result in a decrease in Tim-3+ but increase in Tim-3− T cells, likely through regulating cell apoptosis and proliferation (as we have reported in T cell regulation [36]), thus leading to enhanced IL-17 secretion.
Fig 3.
Tim-3 blockade in PBMCs from HCV-infected individuals improves IL-12p35 and inhibits IL-23p19 productions by CD14+ monocytes, leading to a reduction of TH17 cells. (A) Purified CD14+ monocytes from HCV-infected individuals and HS were incubated with αTim-3 and control IgG antibody for 72 h and then stimulated with LPS/R848 for 6 h, followed by flow cytometric analysis of IL-12p35 and IL-23p19 expression. Representative dot plots and summary data measuring IL-12p35 and IL-23p19 production in CD14+ monocytes in HCV versus HS with the blockade of Tim-3 or IgG antibody are shown. Each symbol represents an individual subject, connected by a line meaning the same cells treated with IgG or aTim-3, the horizontal bars represent median values. *, P < 0.05; NS, no significance by paired t test. (B) Representative experiment of purified CD4+ T cells from an HCV-infected individual that were incubated with αTim-3 and IgG for 72 h, stimulated with PMA/ionomycin for 6 h, followed by flow cytometric analysis of IL-17 versus Tim-3 expression in CD4+ T cells. (C) Representative experiment of CD14+ monocyte-depleted PBMCs from an HCV-infected individual that were incubated with αTim-3 and IgG for 72 h and stimulated with PMA/ionomycin for 6 h, followed by flow cytometric analysis of IL-17 versus Tim-3 expression in CD4+ T cells. (D) PBMCs from HCV-infected individuals and HS were incubated with αTim-3 and control IgG antibody for 72 h and then stimulated with PMA/ionomycin for 6 h, followed by flow cytometric analysis of IL-17A expression in CD4+ T cells. Representative dot plots and summary data measuring IL-17 production in CD4+ T cells in HCV versus HS with the blockade of Tim-3 or IgG antibody are shown. Each symbol represents an individual subject, connected by a line meaning the same cells treated with IgG or aTim-3, the horizontal bars represent median values. *, P < 0.05; NS, no significance by paired t test.
We then sought to determine whether the Tim-3 signaling on monocytes that alters the IL-12/IL-23 balance may indirectly affect IL-17 expression by TH cells. To this end, we first depleted CD14+ monocytes from PBMCs and then carried out a Tim-3 blocking experiment in monocyte-free PBMCs, followed by the detection of TH17 cells. Interestingly, we observed enhanced IL-17-producing CD4+ T cells by Tim-3 blockade (Fig. 3C), similar to the results seen in purified CD4+ T cells upon Tim-3 blockade, suggesting that Tim-3 signaling on cell populations free of monocytes (including NK cells, B cells, and T cells) has an overall inhibitory effect on IL-17 production. To further address the role of Tim-3 on monocyte IL-12/IL-23 expression, which in turn, may affect TH17 cell differentiation, we next carried out the Tim-3 blocking experiment in purified CD14+ monocytes for 72 h and then changed the culture medium containing blocking antibody and secreted cytokines (such as IL-12/IL-23) and added homogeneous CD4+ T cells, followed by PMA/ionomycin stimulation for 6 h. We observed little IL-17-producing TH cells in this experimental setting (data not shown), supporting the notion that IL-23 might be necessary for TH17 cell development or survival.
We thus inhibited Tim-3 signaling in whole PBMCs from HCV-infected individuals (n = 16) or HS (n = 16) to determine the overall effect of blockade on IL-17 production by TH cells. As shown in Fig. 3D, representative dot plots and summary data of IL-17A expression in CD4+ T cells, blocking Tim-3 signaling in PBMCs ex vivo for 72 h, followed by PMA/ionomycin stimulation for 6 h significantly reduces the number of TH17 cells upregulated during chronic HCV infection, but not in HS. These results, in conjunction with the above data, further reinforce the role of Tim-3-mediated differential regulation of IL-12/IL-23 expression by monocytes affecting Th17 cell development in HCV infection, and it is feasible that Tim-3 signaling on monocytes overrides its effect on other types of cells in PBMCs, resulting in an overall stimulatory effect on IL-17 production or TH17 cell development, perhaps by an indirect effect through stimulating IL-23 production.
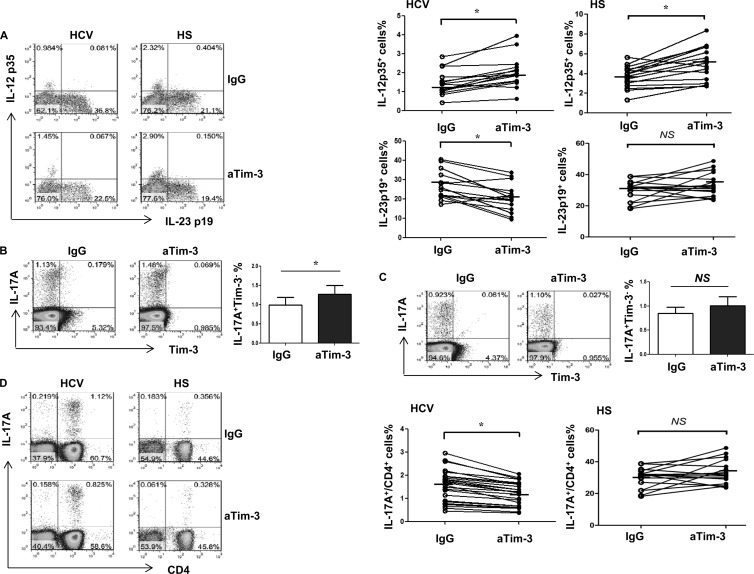
IL-12p35, IL-23p19, and IL-17A expression is differentially regulated in healthy CD14+ monocytes or PBMCs cocultured with Huh-7 hepatoma cells transfected with HCV RNA.
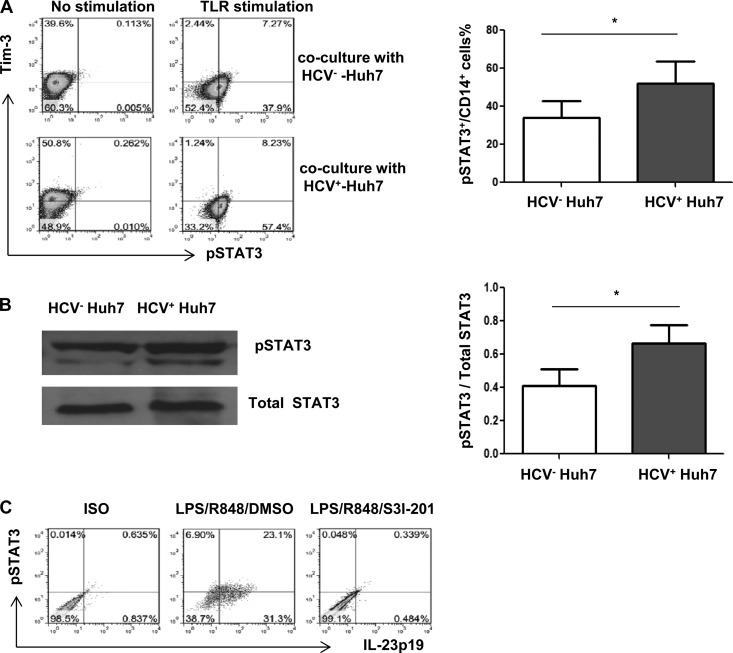
The Tim-3 expression observed above might be a result, rather than a cause, of differential regulation of IL-12/IL-23 expression and/or TH17 cell development during HCV infection. In addition, the driving force for Tim-3 upregulation during HCV infection remains to be determined. To further elucidate the role of HCV in the regulation of Tim-3 expression and cytokine production by inflammatory cells, we used a newly established cell culture system by transfecting Huh-7 hepatocytes with the HCV-JFH-1 strain in vitro to mimic the in vivo setting of early HCV infection (20, 21). To this end, healthy CD14+ monocytes or PBMCs were purified from HS and cocultured with HCV+/− Huh-7 hepatocytes for 48 h and then incubated with αTim-3 or IgG control for another 72 h, followed by LPS/R848 or PMA/ionomycin stimulation for 6 h. Tim-3 and IL-12/IL-23/IL-17 expression were detected by flow cytometry. As shown in Fig. 4 in a histogram, dot plots, and bar figures derived from multiple repeated experiments (n = 6), Tim-3 expression is upregulated on purified monocytes cocultured with Huh-7 hepatocytes expressing HCV compared to HCV− hepatocytes (Fig. 4A). These data are consistent with the results we have shown previously (20, 21). Notably, IL-12p35 and IL-23p19 expression is differently regulated by Huh-7 hepatoma cells transfected with HCV RNA, i.e., HCV+ Huh-7 significantly inhibits IL-12p35 expression and upregulates IL-23p19 expression by TLR-activated CD14+ monocytes compared to those cocultured with HCV− Huh-7 hepatocytes (Fig. 4B). Importantly, blocking Tim-3 signaling significantly corrects the imbalance of monocyte IL-12/IL-23 expression induced by Huh-7 hepatoma cells expressing HCV (Fig. 4B). These data indicate that HCV inhibits IL-12 expression and stimulates IL-23 expression by TLR-activated monocytes through Tim-3 signaling.
Fig 4.
Differential regulations of IL-12/IL-23 expression and IL-17 expression in CD14+ monocytes or PBMCs cocultured with HCV+ hepatocytes occurs through Tim-3 signaling. (A) Purified CD14+ monocytes were incubated with HCV+/− Huh7 hepatocytes for 48 h, followed by flow cytometric analysis of Tim-3 expression on monocytes. Representative histogram and means + the SD from six repeated experiments for Tim-3 expression on monocytes incubated with HCV+ versus HCV− hepatocytes are shown. *, P < 0.05 (as determined by Student t test). (B) Purified CD14+ monocytes were incubated with HCV+/− Huh7 hepatocytes for 48 h, with αTim-3 or IgG antibody for another 72 h, and then stimulated with LPS/R848 for 6 h, followed by flow cytometric analysis for IL-12p35 and IL-23p19 expression. The data are shown as the results of a representative experiment (left) and means + the SD from six independent experiments (right). *, P < 0.05 (as determined by Student t test). (C) PBMCs were incubated with HCV+/− Huh7 hepatocytes for 48 h, with αTim-3 or IgG antibody for 72 h, and then stimulated with PMA/ionomycin for 6 h, followed by flow cytometric analysis for IL-17A expression by CD4+ T cells. The data are shown as the results of a representative experiment (left) and means + the SD from six independent experiments (right). *, P < 0.05 (as determined by Student t test).
To determine whether HCV may drive TH17 cell differentiation and Tim-3 may play a role in this process, we cocultured healthy PBMCs with HCV+/− Huh-7 cells for 48 h, in the presence of αTim-3 or IgG for another 72 h, and stimulated with PMA/ionomycin for 6 h, followed by detecting IL-17 expression by CD4+ T cells. Similar to what we observed in chronically HCV-infected individuals who have an increased number of TH17 cells over HS, TH17 cells are detected higher in PBMCs cocultured with HCV+ hepatocytes versus HCV− hepatocytes, and blocking Tim-3 signaling while coculturing these cells inhibits the HCV-induced IL-17 expression by TH cells (Fig. 4C). It should be noted that TH17 cells generated in this in vitro model, which mimics the early phase of HCV infection, recapitulated the phenomena observed in the setting of chronic HCV infection in vivo (Fig. 2A and Fig. 3D). This suggests that HCV triggers TH17 cell development, at least in part through Tim-3-mediated differential regulation of IL-12/IL-23 expression by monocytes.
HCV upregulates Tim-3 expression and induces IL-23 production in CD14+ monocytes through the STAT3 pathway.
Since HCV induces Tim-3 and IL-23 expression, whereas blockade of Tim-3 signaling inhibits HCV-mediated IL-23 expression in TLR-stimulated CD14+ monocytes, we next sought to explore the intracellular signaling pathway involved in this process. It has been reported that phosphorylation of signal transducer and activator of transcription 3 (STAT3) play a pivotal role in IL-12/IL-23 expression in macrophages infiltrated in the tumor microenvironment (29). We thus hypothesized that HCV-induced Tim-3 might modulate TLR-stimulated monocyte IL-23 expression through the Jak/STAT pathway. To test this hypothesis, we first determined the relationship of Tim-3 and STAT3 expression in monocytes cocultured with HCV+/− Huh-7 cells (48 h) in the presence or absence of TLR stimulation (6 h). As shown in Fig. 5A, phosphorylation of STAT3 is barely detectable in non-TLR-stimulated monocytes, regardless of coculturing of monocytes with HCV+ or HCV− hepatocytes. Nevertheless, Tim-3 expression is significantly upregulated in nonstimulated monocytes cocultured with HCV+ Huh7 compared to HCV− Huh7. After TLR stimulation, Tim-3 expression is significantly reduced, and STAT3 phosphorylation is upregulated, in monocytes cocultured with both HCV+ and HCV− hepatocytes. However, STAT3 phosphorylation is significantly increased in monocytes cocultured with HCV-expressing Huh-7 cells. We also measured the phosphorylation level of STAT3 protein in monocytes cocultured with HCV+/− Huh7 cells by Western blotting. As shown in Fig. 5B, the phosphorylation of STAT3 in monocytes is increased by HCV-expressing Huh-7 cells, whereas the levels of total STAT3 protein remained static between HCV+ and HCV− cultures. The densitometry ratio of phosphorylated STAT3 versus total STAT3 proteins from four independent experiments is summarized on the right. Notably, although IL-23p19 is also upregulated in monocytes cocultured with HCV+ Huh-7 versus HCV− Huh-7 (Fig. 4B), its expression is remarkably diminished in the presence of a STAT3-specific inhibitor (S3I-201, rather than a dimethyl sulfoxide control) when the cells are cocultured (48 h) after TLR stimulation (6 h) (Fig. 5C), supporting the notion that IL-23 expression is STAT3 dependent.
Fig 5.
Tim-3 regulates IL-23p19 expression through phosphorylation of STAT3 in CD14+ cells cocultured with HCV+ hepatocytes. (A) Purified CD14+ monocytes were incubated with HCV+/− Huh7 hepatocytes for 48 h, with or without TLR stimulation for 6 h, followed by flow cytometric analysis of Tim-3 and phosphorylated STAT3 expression in monocytes. Means + the SD from four repeated experiments are shown in the right panel. *, P < 0.05 (as determined by Student t test). (B) Western blot of STAT3 phosphorylation in purified monocytes incubated with HCV+/− hepatocytes after TLR stimulation. Total STAT3 is probed to serve as a protein load control. Representative imaging and the means + the SD from four experiments with densitometry ratios of phosphorylated STAT3/total STAT3 protein in monocytes cocultured with HCV+ versus HCV− hepatocytes are shown in the right panel. *, P < 0.05 (as determined by Student t test). (C) Purified CD14+ monocytes were incubated with HCV+ Huh7 cells with or without a STAT3-specific inhibitor for 48 h and stimulated with LPS/R848 for 6 h, followed by flow cytometric analysis. The results of representative experiments of IL-23p19 expression and phosphorylated STAT3 expression in monocytes, in the presence or absence of STAT3 inhibitor, are shown.
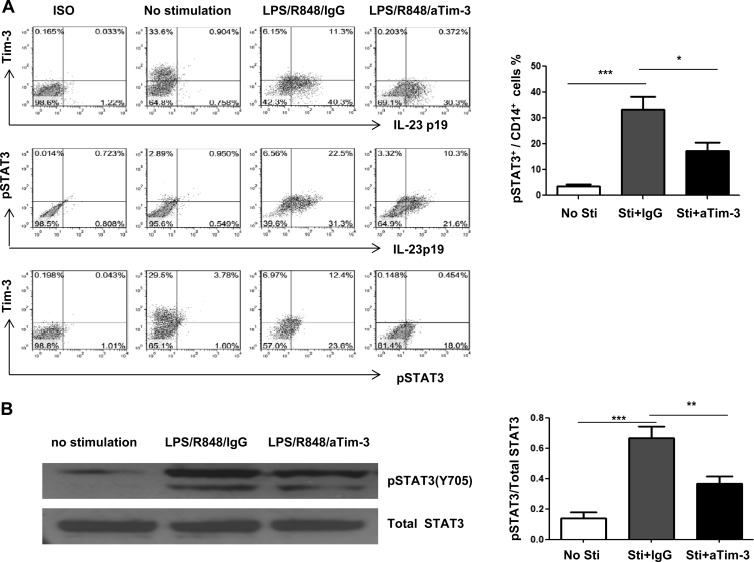
We further characterized the role of Tim-3 on IL-23 production through regulating TLR-mediated STAT3 activation in monocytes isolated from HCV-infected individuals. To this end, purified CD14+ cells from chronically HCV-infected patient were preincubated with αTim-3 or IgG for 72 h and then stimulated with or without LPS/R848 for 6 h, followed by flow cytometric analysis of Tim-3, IL-23, and STAT3 expression. As shown in Fig. 6A in representative dot plots, Tim-3 is constitutively expressed on unstimulated monocytes; its expression is significantly reduced with TLR stimulation and further decreased by Tim-3 blockade. IL-23 is barely detectable in unstimulated monocytes; its expression is significantly increased by TLR stimulation. Importantly, blocking Tim-3 signaling inhibits IL-23 expression, suggesting that Tim-3 stimulates TLR signaling to positively upregulate IL-23 expression in monocytes. Similarly, phosphorylation of STAT3 is only observed in TLR-activated monocytes, and Tim-3 blockade significantly inhibits STAT3 phosphorylation. To summarize these findings, the percentages of cell frequency of phosphorylated STAT3 in unstimulated, or TLR/IgG-stimulated, and TLR/αTim-3-stimulated CD14+ monocytes from six subjects are shown as a bar figure in the right panel.
Fig 6.
Tim-3 regulates IL-23p19 expression through STAT3 signaling in CD14+ cells of HCV-infected individuals. (A) CD14+ monocytes were purified from HCV-infected individuals, incubated with αTim-3 or IgG antibody for 72 h, and then stimulated with or without LPS/R848 for 6 h, followed by flow cytometric analysis for Tim-3, IL-23p19, and pSTAT3 expression. Representative dot plots and summary data under from six subjects with their cells under various treatments are shown. ***, P < 0.001; *, P < 0.05 (as determined by Student t test). (B) Western blot of STAT3 phosphorylation in purified monocytes from HCV-infected individuals following TLR stimulation. Total STAT3 is probed to serve as a protein load control. The results of representative imaging and the means + the SD from four experiments with the densitometry ratios of phosphorylated STAT3/total STAT3 protein in monocytes are summarized on the right panel. ***, P < 0.001; **, P < 0.01 (as determined by Student t test).
In addition, lysates from cells treated the same as described above were also immunoblotted to detect phosphorylated STAT3 protein, and total STAT-3 levels were measured to serve as loading controls. As shown in Fig. 6B, pSTAT3 protein is significantly upregulated in TLR-activated monocytes from HCV-infected patients, whereas Tim-3 blocking significantly downregulates pSTAT3 expression in monocytes compared to cells treated with control IgG. The statistical analysis of densitometry data from multiple experiments (n = 4) is significant and is summarized in the bar figures shown in the right panel. Taken together, these results suggest that HCV-induced Tim-3 expression positively regulates IL-23 production, likely through synergistic stimulation of TLR-mediated STAT3 phosphorylation in monocytes in the setting of viral infection. This is an extension of our recent finding that HCV-induced Tim-3 expression negatively regulates IL-12 production, though inhibiting TLR-mediated STAT1 phosphorylation in monocytes during HCV infection (20).
DISCUSSION
Chronic HCV infection is a worldwide infectious disease that can lead to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Although the pathogenesis of HCV infection remains only partly elucidated, it has become evident that the dysregulation of innate to adaptive immune responses plays a major role in viral persistence and disease progression. In this study, we demonstrate that (i) Tim-3 is upregulated on monocytes in HCV-infected individuals or by incubation with HCV-expressing hepatocytes, (ii) that this HCV-induced Tim-3 expression can inhibit IL-12 and stimulate IL-23 production through regulating Jak/STAT signaling pathways in monocytes in response to TLR stimulation, and (iii) that HCV-induced, Tim-3-mediated differential regulation of IL-12/IL-23 might play a pivotal role in TH17 cell development during viral infection. To our knowledge, this is the first report to demonstrate that HCV-induced Tim-3 expression differentially regulates IL-12/IL-23 production through modulating TLR signaling pathways in monocytes, leading to TH17 cell development during HCV infection.
Pathogen-associated molecule patterns are recognized by TLRs expressed on APCs to secret IL-12 and IL-23. IL-12 is important in stimulating TH1 responses that are essential for host defense and viral clearance, whereas IL-23 is a proinflammatory cytokine that plays an important role in inflammatory diseases, infection, and tumor environments (29). IL-23 signals through an IL-23 receptor complex consisting of the IL-12 receptor β1 chain and a specific IL-23 receptor subunit. Despite their similarities in ligand structure and receptor complexes, it has become evident that IL-12 and IL-23 have common but divergent activities (30). Recent studies demonstrated that mice deficient in IL-12p40 (thus lacking both IL-12 and IL-23) or IL-23p19 (lacking IL-23 but not IL-12) were resistant to experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA), whereas IL-12p35-deficinet mice (lacking IL-12 but not IL-23) were more susceptible to disease, indicating that IL-23, but not IL-12, is critical in the development of autoimmune inflammation (31, 32). Further studies revealed that IL-23-deficient mice had normal TH1 responses but did not produce IL-17A, whereas IL-12p35-deficient mice exhibit an increased number of IL-17-producing CD4+ T cells in inflamed tissues (31, 32). Functionally, TH17 cells contribute to host defense as a new effector TH cell subset with a role in protection against pathogens through activities on immune and non-immune epithelial cells. Their activities, however, are also pivotal in the development of autoimmune diseases under pathological conditions. For example, mice deficient in IL-17 were found to be resistant to EAE or CIA, compared to wild-type (WT) mice, IL-17A-deficient mice had significantly suppressed EAE as indicated by delayed disease onset, reduced maximum severity scores, attenuated histological changes, and early recovery from the disease, whereas overexpression of IL-17 exacerbated these autoimmune diseases (33, 34). Therefore, IL-12 and IL-23 both appear to have distinct roles in vivo in promoting antimicrobial immune responses and diseases. IL-12 suppresses IL-23 and IL-17 production and vice versa, IL-23 inhibits IL-12 and IFN-γ production, indicating cross-regulation between the IL-23/Th17 and IL-12/Th1 pathways (35, 36). It appears that IL-12 and IL-23/IL-17 may be paradoxically regulated, but the precise mechanism for their differential expression remains unclear.
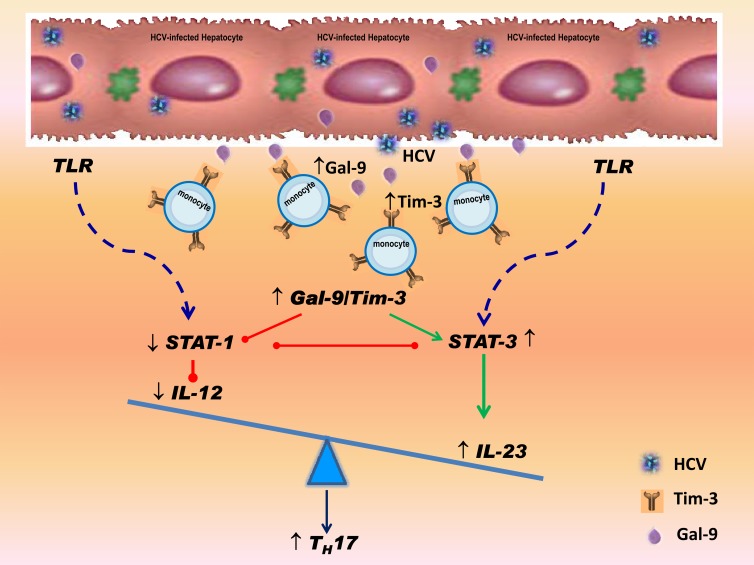
We have recently shown that HCV-induced Tim-3 expression inhibits IL-12 production through the delivery of negative signaling to TLR-mediated STAT1 activation in monocytes (20). Here, we further demonstrated that HCV-induced Tim-3 expression upregulates IL-23 expression by stimulating TLR-mediated STAT3 phosphorylation. Based on these data and our previous studies, we propose a model in which HCV-induced Tim-3 controls the balance of IL-12/IL-23 through differential regulation of STAT1/STAT3, promoting either differentiation or expansion of TH17 cells during viral infection (Fig. 7). This novel model is plausible and provides an insight into understanding the pathogenesis of HCV persistence.
Fig 7.
Proposed model for Tim-3-mediated differential regulation of IL-12/IL-23 by monocytes promote TH17 development. HCV-driven upregulation of Tim-3 expression on monocytes and interaction with upregulated galectin-9 (Gal-9) induces STAT-3 but inhibits STAT-1 signaling, leading to increased IL-23 and decreased IL-12 expression, respectively. This HCV-driven, Tim-3-mediated IL-12/IL-23 balance alteration in innate immunity would lead to a milieu conducive for the upregulation of TH17 cells within the adaptive immune response. This would counter immunopathology but favor viral persistence and autoimmune disorders in HCV infection.
Based on this model, interactions between HCV-infected hepatocytes and infiltrating immune cells might determine viral clearance or persistence and disease progression. We have recently demonstrated that HCV not only upregulates Tim-3 expression on activated T cells, but also a Tim-3 ligand, galectin-9 (Gal-9), on the surfaces of infected hepatocytes (37). The HCV-mediated Tim-3/Gal-9 interactions drive naive CD4+ T cells differentiation into CD4+CD25+Foxp3+ T regulatory cells (Tregs) but inhibit CD4+ CD25+ Foxp3− T effector cells (Teffs), indicating an immunomodulatory role of HCV-infected hepatocytes (37, 38). We show here that HCV also promotes TLR-mediated STAT3 signaling within the virally infected microenvironment and induces a heterodimeric cytokine, IL-23, while inhibiting its counterpart cytokine, IL-12, thereby shifting the balance of antiviral immunity toward a condition favoring viral persistence and autoimmunity. STAT3 appears to induce expression of IL-23 via direct transcriptional activation of the IL-23/p19 gene (29); furthermore, STAT3 inhibits NF-κB/c-Rel-dependent IL-12/p35 gene expression in tumor-associated macrophages (29), perhaps consistent with our observations that HCV infection leads to opposite effects in terms of IL-23 and IL-12 expression. In addition, tumor-associated Tregs express the IL-23 receptor, which activates STAT3 in this cell type and leads to upregulation of the Treg-specific transcription factor, Foxp3, and the immunosuppressive cytokine, IL-10 (29). Therefore, our proposed model does fit with the current observation that Tregs and TH17 cells accumulate in the setting of chronic viral infections.
The adaptive immune system is crucial for the elimination of viral infections, but dysregulation of adaptive immune responses can also lead to the development of inflammatory and autoimmune diseases. The TH1-TH2 paradigm of CD4+ T cell lineage was described by Mosmann et al. based upon distinct cytokine profiles and characteristic functions (39). TH1 cells develop in the presence of IL-12, produce primarily IL-2/IFN-γ, and are involved in cell-mediated immunity. TH2 cells differentiate in the presence of IL-4, produce IL-4/IL-5/IL-13, and are critical for humoral immunity. TH17 cells are newer members of TH cell family and are distinct from TH1 and TH2, being characterized by their ability to produce specific cytokines such as IL-17A, IL-17F, IL-22, and CCL20. The conditions for the differentiation of TH17 cells remains unclear, but the ratio of IL-12/IL-23 produced by monocytes may play a pivotal role in several disease models (40). IL-23 was essential for development of pathogenic TH17 cells in autoimmune inflammation as indicated by undetectable TH17 cells in IL-23p19 deficient mice (41, 42). Moreover, IL-23 and TH17 cells were found to be critical for the induction, but not the effector phase, of EAE, an animal model of human multiple sclerosis (42). In addition, IL-23 and TH17 cells were found to be linked to the development of multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus, chronic immune thrombocytopenia, psoriasis, asthma, atopic dermatitis, and chronic hepatitis B in humans (14–19, 30–32). In accordance with these findings, cytokines such as IL-17, IL-17F, IL-22, and IL-6 produced by Th17 cells are found to be elevated in several human inflammatory diseases, and both IL-23 and the Th17 pathway correlate with disease severity and immunopathology in diverse infections (33, 34).
In the present study, we provide pilot evidence indicating that TH17 cells accumulate in the peripheral blood of HCV-infected individuals. Increased TH17 cells are also observed in healthy PBMCs cocultured with hepatocytes expressing HCV. In conjunction with monocyte depletion or Tim-3 blockade, which reverses TH17 cell development both in vitro and ex vivo, these data suggest that HCV-induced, Tim-3-mediated differential regulation of IL-12/IL-23 expression by APCs may be critical to TH17 cell development. It would follow that the STAT3/IL-23/IL-17 pathway, rather than or, more likely, in addition to the STAT1/IL-12/IFN-γ axis, may be crucial to viral persistence and the autoimmune phenomena observed during HCV infection.
The mechanisms, by which HCV-mediated Tim-3 expression regulates innate to adaptive immune responses leading to viral persistence and autoimmune disorders during HCV infection, are likely multiple. We and others have shown that HCV-induced negative signaling molecules, including program death-1 (PD-1), suppressor of cytokine signaling-1 (SOCS-1), and Tim-3, play pivotal role in inhibiting several intracellular signaling pathways, including MAPK, Jak/STAT, and Akt/PI3K, and lead to the inhibition of monocyte IL-12 production, the suppression of virus-specific T cell responses, the promotion of B cell activation and proliferation, and the induction of CD4+ CD25+ Foxp3+ regulatory T cells during HCV infection (20, 21, 37, 38, 43). Notably, it has been reported that HCV-specific TH17 cells were suppressed by production of anti-inflammatory cytokines IL-10 and transforming growth factor β (TGF-β) (44). Since Foxp3+ Tregs suppress inflammatory effector T cells (Teffs) through the secretion of IL-10 and TGF-β (37, 38), Th17 cells would also be suppressed by anti-inflammatory cytokines IL-10 and TGF-β. Importantly, Tim-3 controls the ratio of Tregs/Teffs by regulating cell proliferation and apoptosis and fine-tunes the balance of T-cell-mediated antiviral immunity and self-injury pathology in the setting of chronic HCV infection (38). Nevertheless, the present study, demonstrating HCV-induced Tim-3 expression differentially regulates IL-12/IL-23 expression by monocytes that leads to TH17 cell development, adds new insight to the larger picture underlying HCV pathogenesis, and underscores the potential importance of immunotherapy in conjunction with antiviral treatment in the management of HCV infection.
ACKNOWLEDGMENTS
This study was supported by an NIH/National Institute of Allergy and Infectious Disease (NIAID) grant to Z.Q.Y. and J.P.M. (R15A1084057) and an NIH/NIDDK grant to Z.Q.Y. and J.P.M. (R01DK093526). L.S. is a visiting scholar, partially supported by the Guanghua Foundation of Xian Jiaotong University, China. X.J.J. is a visiting scholar, partially supported by Beijing Friendship Hospital, Capital Medical University, Beijing, China. R.S.Y., a visiting scholar, holds a grant for viral hepatitis research from Guangzhou Municipal Health Bureau, China. This publication is the result of work supported with resources and the use of facilities at the James H. Quillen Veterans Affairs Medical Center.
We are greatly appreciated support from T. Wakita, Department of Virology II, NIH of Japan, for transferring HCV JFH-1 strain through a MTA and from T. J. Liang, Liver Section, NIH/NIDDK, for sending Huh-7 cells.
The contents in this publication do not represent the views of the Department of Veterans Affairs or the U.S. Government. The authors disclose no conflicts of interest.
Footnotes
Published ahead of print 6 February 2013
REFERENCES
- 1. Iannuzzella F, Vaglio A, Garini G. 2010. Management of hepatitis C virus-related mixed cryoglobulinemia. Am. J. Med. 123:400–408 [DOI] [PubMed] [Google Scholar]
- 2. Pipili C, Ilonidis G, Cholongitas E. 2011. Hepatitis C virus and kidney: a strong association with different clinical aspects. Liver Int. 31:1071–1080 [DOI] [PubMed] [Google Scholar]
- 3. Szabo G, Dolganiuc A. 2008. Hepatitis C and innate immunity: recent advances. Clin. Liver Dis. 12:675–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rehermann B. 2009. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J. Clin. Invest. 119:1745–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hikens CM, Kalinski P, de Boer M, Kapsenberg ML. 1997. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood 90:1920–1926 [PubMed] [Google Scholar]
- 6. Feili-Hariri M, Falkner DH, Morel PA. 2005. Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy. J. Leukoc. Biol. 78:656–664 [DOI] [PubMed] [Google Scholar]
- 7. Vasconcellos R, Carter NA, Rosser EC, Mauri C. 2011. IL-12p35 subunit contributes to autoimmunity by limiting IL-27-driven regulatory responses. J. Immunol. 187:3402–3412 [DOI] [PubMed] [Google Scholar]
- 8. Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715–725 [DOI] [PubMed] [Google Scholar]
- 9. Hunter CA. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5:521–531 [DOI] [PubMed] [Google Scholar]
- 10. Duvallet E, Semerano L, Assier E, Falgarone G, Boissier MC. 2011. Interleukin-23: a key cytokine in inflammatory diseases. Ann. Med. 43:503–511 [DOI] [PubMed] [Google Scholar]
- 11. Louten J, Boniface K, Rene de, Malefyt W. 2009. Development and function of TH17 cells in health and disease. J. Allergy Clin. Immunol. 123:1004–1011 [DOI] [PubMed] [Google Scholar]
- 12. Khayrullina T, Yen JH, Jing H, Ganea D. 2008. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J. Immunol. 181:721–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. 2008. Regulation of interleukin-12/ interleukine-23 production and the T-helper 17 response in human. Immunol. Rev. 226:112–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stamp LK, Easson A, Pettersson L, Highton J, Hessian PA. 2009. Monocyte derived interleukin (IL)-23 is an important determinant of synovial IL-17A expression in rheumatoid arthritis. J. Rheumatol. 36:2403–2408 [DOI] [PubMed] [Google Scholar]
- 15. Oosting M, ter Hofstede H, van de Veerdonk FL, Sturm P, Kullberg BJ, van der Meer JW, Netea MG, Joosten LA. 2011. Role of interleukin-23 (IL-23) receptor signaling for IL-17 responses in human Lyme disease. Infect. Immun. 79:4681–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, Fick L. 2011. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One 6:e23185 doi:10.1371/journal.pone.0023185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mok MY, Wu HJ, Lo Y, Lau CS. 2010. The relation of interleukin 17 (IL-17) and IL-23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus. J. Rheumatol. 37:2046–2052 [DOI] [PubMed] [Google Scholar]
- 18. Rocha AM, Souza C, Rocha GA, de Melo FF, Clementino NC, Marino MC, Bozzi A. 2011. The levels of IL-17A and of the cytokines involved in Th17 cell commitment are increased in patients with chronic immune thrombocytopenia. Haematologicae 96:1560–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, Fu JL. 2010. Interleukin-17-producing CD4+ T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology 51:81–91 [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Jia ZS, Moorman JP, Yao ZQ. 2011. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PLoS One 6:e19664 doi:10.1371/journal.pone.0019664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Ma CJ, Ni L, Zhang CL, Wu XY, Kumaraguru U, Li CF, Moorman JP, Yao ZQ. 2011. Cross-talk between programmed death-1 and suppressor of cytokine signaling-1 in inhibition of IL-12 production by monocytes/macrophages in hepatitis C virus infection. J. Immunol. 186:3093–3103 [DOI] [PubMed] [Google Scholar]
- 22. Eisen-Vandervelde AL, Waggoner SN, Yao ZQ, Cale EM, Hahn CS, Hahn YS. 2004. Hepatitis C virus core selectively suppresses interleukin-12 synthesis in human macrophages by interfering with AP-1 activation. J. Biol. Chem. 279:43479–43486 [DOI] [PubMed] [Google Scholar]
- 23. Waggoner SN, Hall CH, Hahn YS. 2007. HCV core protein interaction with gC1q receptor inhibits Th1 differentiation of CD4+ T cells via suppression of dendritic cell IL-12 production. J. Leukoc. Biol. 82:1407–1419 [DOI] [PubMed] [Google Scholar]
- 24. Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, Gutiérrez-Ramos JC, Coyle AJ, Strom TB. 2003. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4:1093–1110 [DOI] [PubMed] [Google Scholar]
- 25. Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ. 2003. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 4:1102–1110 [DOI] [PubMed] [Google Scholar]
- 26. Jin HT, Anderson AC, Tan WG, West RE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. 2010. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 107:14733–14738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 205:2763–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. 2009. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 83:9122–9130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. 2009. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell 15:114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kastelein RA, Hunter CA, Cua DJ. 2007. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25:221–242 [DOI] [PubMed] [Google Scholar]
- 31. Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744–748 [DOI] [PubMed] [Google Scholar]
- 32. Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. 2003. Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Komiyama Y, Nakae S, Matuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177:566–573 [DOI] [PubMed] [Google Scholar]
- 34. Nakae S, Nambu A, Sudo K, Iwakura Y. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171:6173–6177 [DOI] [PubMed] [Google Scholar]
- 35. Zhang J, Huang Z, Sun R, Tian Z, Wei H. 2012. IFN-γ induced by IL-12 administration prevents diabetes by inhibiting pathogenic IL-17 production in NOD mice. J. Autoimmun. 38:20–28 [DOI] [PubMed] [Google Scholar]
- 36. Espinosa V, Rivera A. 2012. Cytokines and the regulation of fungus-specific CD4 T cell differentiation. Cytokine 58:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ji XJ, Ma CJ, Wang JM, Wu XY, Niki T, Hirashima M, Moorman JP, Yao ZQ. 2013. Immunomodulatory role of the hepatocyte during HCV infection: driving CD4+ CD25+ Foxp3+ regulatory T cell development through the Tim-3/Gal-9 pathway. Eur. J. Immunol. 43:458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moorman JP, Wang JM, Zhang Y, Ji XJ, Ma CJ, Jia ZS, Wang KS, Yao ZQ. 2012. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J. Immunol. 189:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348–2357 [PubMed] [Google Scholar]
- 40. Zhou W, Dowell DR, Huckabee MM, Newcomb DC, Boswell MG, Goleniewska K, Lotz MT, Toki S, Yin H, Yao S, Natarajan C, Wu P, Sriram S, Breyer RM, Fitzgerald GA, Peebles RS., Jr 2012. Prostaglandin I2 signaling drives Th17 differentiation and exacerbates experimental autoimmune encephalomyelitis. PLoS One 7:e33518 doi:10.1371/journal.pone.0033518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanhan T, Kastelein RA, Cua DJ. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thakker P, Leach MW, Kuang W, Benoit SE, Leonard JP, Marusic S. 2007. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 178:2589–2598 [DOI] [PubMed] [Google Scholar]
- 43. Shen T, Zheng J, Liang H, Xu C, Chen X, Zhang T, Xu Q, Lu F. 2011. Characteristics and PD-1 expression of peripheral CD4+ CD127lo CD25hi FoxP3+ Treg cells in chronic HCV-infected patients. Virol. J. 8:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rowan AG, Fletcher JM, Ryan EJ, Moran B, Hegarty JE, O'Farrelly C, Mills KH. 2008. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-beta. J. Immunol. 181:4485–4494 [DOI] [PubMed] [Google Scholar]