Abstract
We report here that the acidic ribosomal protein P0 is a component of the membrane-associated Potato virus A (PVA) ribonucleoprotein complex. As a constituent of the ribosomal stalk, P0 functions in translation. Although the ribosomal stalk proteins P0, P1, P2, and P3 are all important for PVA infection, P0 appears to have a distinct role from those of the other stalk proteins in infection. Our results indicate that P0 also regulates viral RNA functions as an extraribosomal protein. We reported previously that PVA RNA can be targeted by VPg to a specific gene expression pathway that protects the viral RNA from degradation and facilitates its translation. Here, we show that P0 is essential for this activity of VPg, similar to eIF4E/eIF(iso)4E. We also demonstrate that VPg, P0, and eIF(iso)4E synergistically enhance viral translation. Interestingly, the positive effects of VPg and P0 on viral translation were negatively correlated with the cell-to-cell spread of infection, suggesting that these processes may compete for viral RNA.
INTRODUCTION
Viruses have acquired mechanisms by which they efficiently recruit the host translational machinery. Viral RNA translation is sometimes accompanied by the repression of host mRNA translation (1–4) by strategies that frequently involve the virus-induced modification of translation initiation factors. An alternative strategy is illustrated by a Cauliflower mosaic virus (CaMV) protein, which associates directly with the ribosome and regulates its function (5). Many host proteins that function in conventional translation also participate in viral RNA replication (6). For example, the replicase of bacteriophage Qβ contains four host proteins connected to translation, i.e., ribosome-associated HF-1, ribosomal S1, and elongation factors EF-Tu and EF-Ts (7). The eukaryotic homolog of EF-Tu is eEF1A, a protein frequently associated with viral replicases (8). Different subunits of eIF3 have also been shown to be functional components of both the Brome mosaic virus (BMV) and Tobacco mosaic virus (TMV) replicases (9–11).
Studies showing that only replicated viral RNAs are efficiently translated have indicated that positive-stranded RNA virus translation and replication are functionally coupled (12–14). The process of RNA replication is associated with virus-induced cellular membrane structures called viral replication complexes (RCs) (15). The model virus of this study, Potato virus A (PVA), is a positive-stranded RNA virus belonging to the genus Potyvirus. Several host proteins that function in translation have been associated with RCs of Turnip mosaic virus (TuMV) (genus Potyvirus), including eIF(iso)4E, PABP, and eEF1A (16–18). Since both PABP and eEF1A interact with the TuMV RNA-dependent RNA polymerase (RdRp), PABP and eEF1A may function in RNA replication (18, 19). Most recessive genes conferring resistance to infection by potyviruses are alleles of eIF4E and eIF(iso)4E, and successful infection commonly requires compatible interactions between viral VPg and eIF4E and eIF(iso)4E (20).
Ribosomes are essential for translation. In plants, they contain a structure referred to as a ribosomal stalk (21), which forms a lateral protrusion from the ribosome and is composed of the acidic proteins P0, P1, and P2 (22) and the plant-specific protein P3 (23, 24). P proteins are also present in non-ribosome-associated pools (25–27). P0 functions as a scaffold for the stalk structure by interacting with 28S rRNA, and the remaining P proteins assemble to form the extended stalk via interactions with P0. P0 is a protein conserved across kingdoms, as shown by the ability of P0 genes from worm, mammals, and protozoa to functionally complement Saccharomyces cerevisiae lacking endogenous P0 (28–30). P0 is the only essential P protein for both in vitro translational activity of yeast ribosomes and cell survival (31, 32). Ribosomal stalk proteins can affect several aspects of ribosome function, including translational capacity, polysome pattern, and ribosomal subunit joining (33, 34). P proteins are also regarded as having different effects on the translation of distinct mRNAs in yeast (31). In this study, we show that ribosomal P proteins are important for PVA infection of Nicotiana benthamiana. We found that P0, a viral RNP component, has functions distinct from those of the other P proteins in regulating PVA RNA expression.
MATERIALS AND METHODS
Plants.
Nicotiana benthamiana plants were grown in a greenhouse at 22°C for an 18-h day period and at 18°C for a 6-h night period and used for experiments at the 4- to 6-leaf stage.
Protein analysis.
Viral RNP complexes were purified from infected plants, and P0 was identified by proteomic tools as described previously (35). Ribosomes were isolated as described previously (36), except that the phosphatase inhibitors were omitted. P proteins were detected by Western blot analysis using a human autoimmune disease serum against ribosomal P antigen (catalog no. HP0-0100; Immunovision).
Viruses, plant overexpression, and gene silencing constructs.
PVA and firefly luciferase (FLUC) constructs were described previously (37). A P0 plant expression vector was constructed by generating a Gateway Cloning Technology (Invitrogen)-compatible cDNA of Arabidopsis thaliana 60S acidic ribosomal protein P0 (RPP0C) (GenBank accession no. NM_111960) by PCR. The cDNA was inserted into pMDC32 (38), pGWB17, and pGWB18 (39) via pDONR/Zeo (Invitrogen), using standard Gateway cloning. An eIF(iso)4E Gateway-compatible PCR product was recombined via pDONR/Zeo (Invitrogen) into pGWB18. The GUS and VPg plant expression constructs were described previously (40). P-protein-silencing vectors were constructed by generating Gateway-compatible N. benthamiana P-protein cDNA fragments, which were inserted into pHELLSGATE8 (pHG8) (41) via pDONR/Zeo; empty pHG8 was used as a control. The silencing constructs for eIF4E and eIF(iso)4E were described previously (40). All plant expression vectors were used to transform Agrobacterium tumefaciens strain C58C1/pGV2260.
Gene silencing by transient expression of hairpin RNA.
The method used for transient Agrobacterium-mediated silencing was described previously (42). Agrobacterium cells carrying hairpin vectors (pHG8) with gene-specific inserts were infiltrated into leaves. Hairpins were either cotransformed with wild-type (wt) PVA or pretransformed 4 days before inoculation of mutant PVAs. Gene silencing was verified by reverse transcription (RT)-PCR. Here, total RNA was extracted from plant leaves 4 days after infiltration of Agrobacterium cells carrying hairpin constructs. Total RNA was treated with DNase I, and cDNA was synthesized by using Moloney murine leukemia virus (M-MLV) reverse transcriptase and oligo(dT) primers. The same primers that were used to generate the sequences for cloning of cDNAs into pHG8 were used for PCR amplification.
Analysis of infection foci by fluorescence microscopy.
Agrobacterium cells carrying green fluorescent protein (GFP)-tagged PVA were infiltrated into plant leaves at an optical density at 600 nm (OD600) of 0.0005. Exogenous proteins or RNA hairpins were coexpressed by coinfiltrating Agrobacterium cells carrying the respective expression cassettes. Infection foci were examined by using a Zeiss Axio Scope.A1 microscope and a 2.5× or 10× objective. The area of individual infection foci and the percentage of infected tissue were determined by using microscope software. For each condition, data on infection foci were collected from a minimum of three individual plants and 50 foci.
RLUC- and qPCR-based PVA infection assay.
Luciferase quantitation of virus-derived Renilla luciferase (RLUC) and control FLUC and quantitative RT-PCR (qPCR) were described previously(37, 40). Details of Agrobacterium OD600s used to transform PVA and other expression cassettes, time points of data collection, and other relevant information are given in the respective figure legends and the text. Error bars represent the standard deviations.
Nucleotide sequence accession numbers.
Data have been deposited in the GenBank database under accession no. NM111960 (A. thaliana RPP0C); FN666434 [Nicotiana tabacum eIF(iso4)E]; and JN227614 (P0), JN227615 (P1), JN227616 (P2), and JN227617 (P3) (N. benthamiana silencing fragments).
RESULTS
Ribosomal P0 copurifies with viral replication proteins from infected plants.
To identify host proteins participating in PVA infection, two viral replication proteins, VPg and RdRp, were expressed as Strep III-tagged fusion proteins from their native positions in the viral genome and used to affinity purify membrane-associated viral protein complexes from infected Nicotiana benthamiana plants. The composition of these complexes was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS), which showed that HSP70, viral RdRp, NIa, and VPg were all found in both the VPg- and RdRp-tagged samples (35). In this study, we report the identification of a protein homologous to Arabidopsis thaliana 60S acidic ribosomal protein P0 (P0) (GenBank accession no. AAL07229). Western blot analysis using human anti-P autoimmune disease serum showed that P0 was specifically copurified with both Strep III-tagged VPg and RdRp but was not visible in the control sample derived from leaves infected with nontagged PVA (Fig. 1A).
Fig 1.
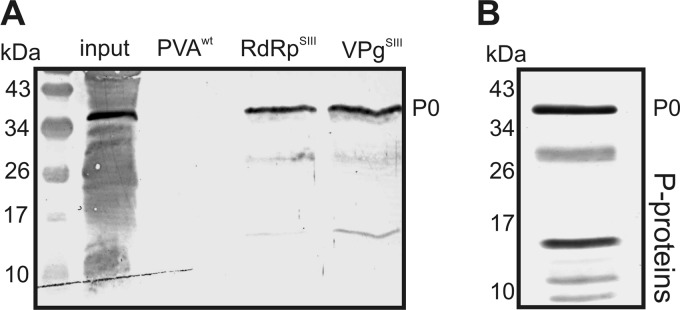
Ribosomal P0 is a component of membrane-associated viral RNPs. (A) Western blotting showing the presence of P proteins in the RNP complexes obtained by affinity purification, using Strep III-tagged PVA RdRp (RdRpSIII) and Strep III-tagged VPg (VPgSIII), from membrane fractions of infected N. benthamiana leaves. A sample purified from plants infected with wt PVA was used as a control. (B) Western blotting of P proteins associated with isolated ribosomes from N. benthamiana leaves. Human anti-P autoimmune disease serum was used to detect P proteins in panels A and B.
The serum used to detect P0 also recognizes the ribosomal stalk proteins P1, P2, and P3 via their homologous C termini (23, 43). These proteins are small (10 to 15 kDa) and may correspond to the weakly detected lower-molecular-weight proteins (Fig. 1A). The patterns of viral RNP- and ribosome-associated P proteins differed. A Western blot analysis with the human anti-P autoimmune disease serum of a ribosome-enriched fraction derived from noninfected N. benthamiana leaves yielded several proteins (Fig. 1B) with a pattern corresponding to that of maize ribosomal P proteins (44). In contrast, the viral RNP complex contained more P0 than the other P proteins. None of the other P proteins was detected by LC-MS/MS analysis, providing further evidence that they were not as abundant as P0 in the purified viral RNP.
Exogenous P0 promotes PVA infection.
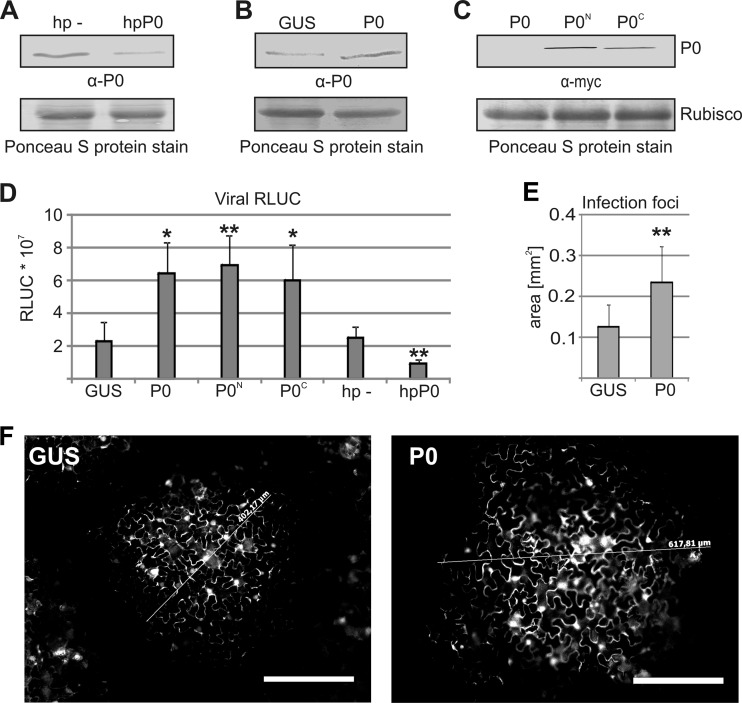
We inserted the Renilla luciferase (RLUC) reporter gene into PVA infectious cDNA to enable sensitive quantification of viral infection (Fig. 2) (37). To analyze the role of P0 in PVA infection, we constructed silencing and overexpression constructs for P0. P0 was transiently silenced by expressing a P0-targeting RNA hairpin in plant leaves using Agrobacterium infiltration, with the control being the empty hairpin vector. Western blot analysis using anti-P serum showed that silencing reduced the expression level of P0 (Fig. 3A). In contrast, when P0 was overexpressed, no obvious P0 accumulation could be detected (Fig. 3B), similar to findings in yeast cells, which do not accumulate P0 protein despite increased levels of P0 mRNA (45). We therefore verified ectopic P0 expression using N- and C-terminally fused myc tags and found that these proteins could be detected by using anti-myc IgGs (Fig. 3C). To address the role of P0 in infection, exogenous P0, with and without myc tags, was expressed along with RLUC-tagged wt PVA, and the effect on RLUC activity at 7 days after infection (DAI) was analyzed (Fig. 3D). GUS was expressed as a control for P0. Exogenous expression of both tagged and nontagged P0 increased viral RLUC activity by 3-fold, whereas silencing of P0 reduced viral RLUC activity by 3-fold (Fig. 3D).
Fig 2.
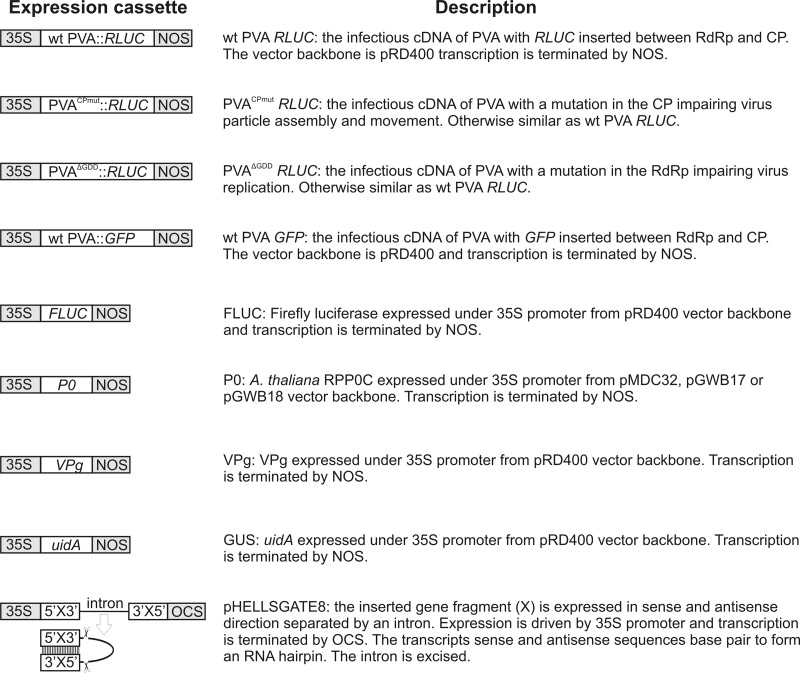
Schematic representation of the constructs used in this study. The transcription CaMV 35S promoter and the nopaline synthase (NOS) and octopine synthase (OCS) terminators are in gray, and cDNAs are in white. Viral constructs lacking either the PVA 5′- or 3′-UTR were derived from the PVAΔGDD construct. Both monocistronic RLUC and monocistronic GUS were expressed from similar constructs to FLUC and VPg. eIF(iso)4E was expressed from a similar construct as P0 in the pGWB18 vector backbone.
Fig 3.
Ribosomal P0 promotes PVA infection. (A) P0 was silenced by expressing an RNA hairpin (hp) that targets P0 transcripts (hpP0), followed by Western blotting using human anti-P autoimmune disease serum directed against the ribosomal P antigen. The empty hairpin vector was expressed as a control (hp −). (B) Exogenous P0 was expressed, and P0 levels were analyzed by Western blotting as described above for panel A. GUS was expressed as a control. (C) Exogenous expression of myc-tagged P0 followed by Western blotting. P0N and P0C have N- and C-terminal myc fusions, respectively. The membrane stained for total protein by Ponceau S is shown at the position of the RUBISCO large subunit as a loading control in panels A to C. (D) Exogenous P0 with or without myc tags or GUS (control) was coexpressed with wt PVA. In parallel, P0 was silenced, and the level of luciferase activity derived from RLUC-tagged wt PVA was determined 7 days after infection (DAI). The Agrobacterium OD600 values used for infiltration were as follows: 0.0005 for wt PVA and 0.5 for P0, GUS, hp −, and hpP0. (E) Fluorescence microscopy of the average area of infection foci formed by GFP-tagged wt PVA at 3 DAI. (F) Representative infection foci from leaves expressing GUS and P0. Scale bar, 200 μm. The number of analyzed foci was >100 for both GUS and P0. *, P < 0.05; **, P < 0.01 (determined by Student's t tests).
We also estimated the average area of individual infection foci formed by wt GFP-tagged PVA in the presence of exogenous P0 or GUS at 3 DAI (Fig. 3E). GFP fluorescence was detected in numerous cells within separate foci at this time, showing that infection had already spread by cell-to-cell movement (Fig. 3F). Expression of exogenous P0 increased the average area of infection foci compared with the control. It is important to emphasize that very low concentrations of Agrobacterium (OD600 = 0.0005) were used to transform RLUC/GFP-tagged wt PVA (Fig. 3D to F), resulting in the initiation of 1 infection focus per mm2 (e.g., see Fig. 4B). This low density reduced any possible effects due to the Agrobacterium-based inoculation method and left space for the infection to spread by cell-to-cell movement. However, it was necessary to measure the diameter of the infection foci at 3 DAI, as at 4 DAI, many foci will have coalesced (data not shown). Separate infection foci in which cell-to-cell movement had occurred by 3 DAI were also achieved with TuMV under similar inoculation conditions (46).
Fig 4.
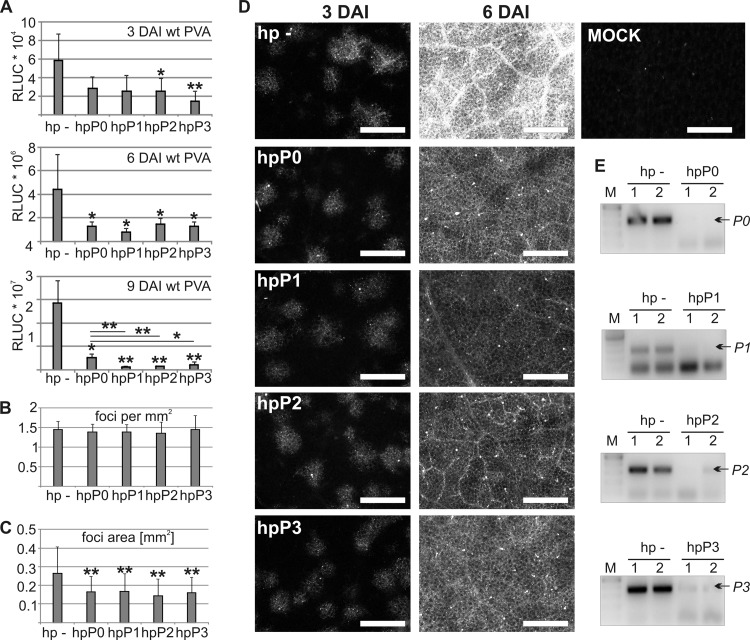
Ribosomal P proteins are important for PVA infection. (A) RLUC activity of wt PVA was determined at 3, 6, and 9 DAI during silencing of P proteins by using RNA hairpins (hp) targeting each P protein (hpP0, hpP1, hpP2, and hpP3). An empty hairpin vector was expressed as a control (hp −). The Agrobacterium OD600 values used for infiltration are described in the legend of Fig. 3D. (B) Average numbers of infection foci per mm2 3 days later. Twelve areas of 4 mm2 were analyzed under each condition by using fluorescence microscopy. (C) The average size of infection foci was determined. Over 100 foci were analyzed under each condition. *, P < 0.05; **, P < 0.01 (determined by Student's t tests). (D) Representative leaf tissue images of PVA-derived GFP fluorescence in infection foci during P-protein silencing at 3 and 6 DAI. Scale bar, 1 mm. (E) RT-PCR was used to verify silencing of P0 to P3 transcripts achieved by expression of RNA hairpins. The expected PCR products are indicated by arrows, and the size marker (M) is on the left.
Ribosomal stalk proteins are important for PVA infection.
The results described above showed that P0 is important for PVA infection. Since P0 is essential for cellular translation as part of the ribosomal stalk structure, altered P0 homeostasis may affect PVA infection through effects on either the ribosomal stalk or other P0 functions. To analyze the possible role of the ribosomal stalk in PVA infection, RNA hairpins were expressed to silence the other stalk P proteins (P1, P2, and P3) as well as P0, and wt PVA RLUC activities were analyzed at 3, 6, and 9 DAI (Fig. 4A). Although silencing of any P protein reduced viral RLUC activity, the latter was higher during P0 silencing than during P1, P2, or P3 silencing at 9 DAI.
The infection foci of GFP-tagged PVA were also analyzed by fluorescence microscopy. First, the number of GFP foci was calculated at 3 DAI (Fig. 4B), a time point when most foci were isolated clusters of cells showing PVA-derived GFP fluorescence (e.g., see Fig. 3F). Silencing of P proteins did not alter the amount of initially infected cells compared with the control, indicating that the reduction in viral RLUC activity during P-protein silencing was not due to reduced initiation but to reduced progression of infection. Moreover, silencing of the P proteins resulted in a slight reduction in the average areas of infection foci at 3 DAI (Fig. 4C). At 6 DAI, however, the leaves of both the control and P-protein-silenced plants were fully infected, as GFP fluorescence was uniform throughout the leaves (Fig. 4D). Fluorescence intensity, as well as viral RLUC activity, was strongest in the control (Fig. 4A). Together, these results show that the silencing of ribosomal stalk proteins affects PVA infection by reducing the cellular level of infection but has little effect on the cell-to-cell spread of infection. RT-PCR verified silencing of the target transcripts (Fig. 4E).
P0 has functions that are distinct from those of other P proteins in PVA infection.
Coordination of the potyvirus genome between translation, replication, and other cellular functions is complex, as these stages are coupled, and inhibition of virus infection due to the silencing of P proteins may result from disruption of any of those processes. We therefore utilized different RLUC-tagged PVA mutants to determine which viral process is affected under each experimental condition. PVACPmut is a virus with a coat protein (CP) mutation that undergoes replication in inoculated cells but is incapable of virus particle assembly and cell-to-cell and systemic movement (37). PVAΔGDD carries a mutation in the RdRp that inhibits replication (37); it therefore does not carry VPg at its 5′ end, differentiating it from authentic viral RNAs. The validity of the use of this construct for studying PVA translation is addressed in Discussion.
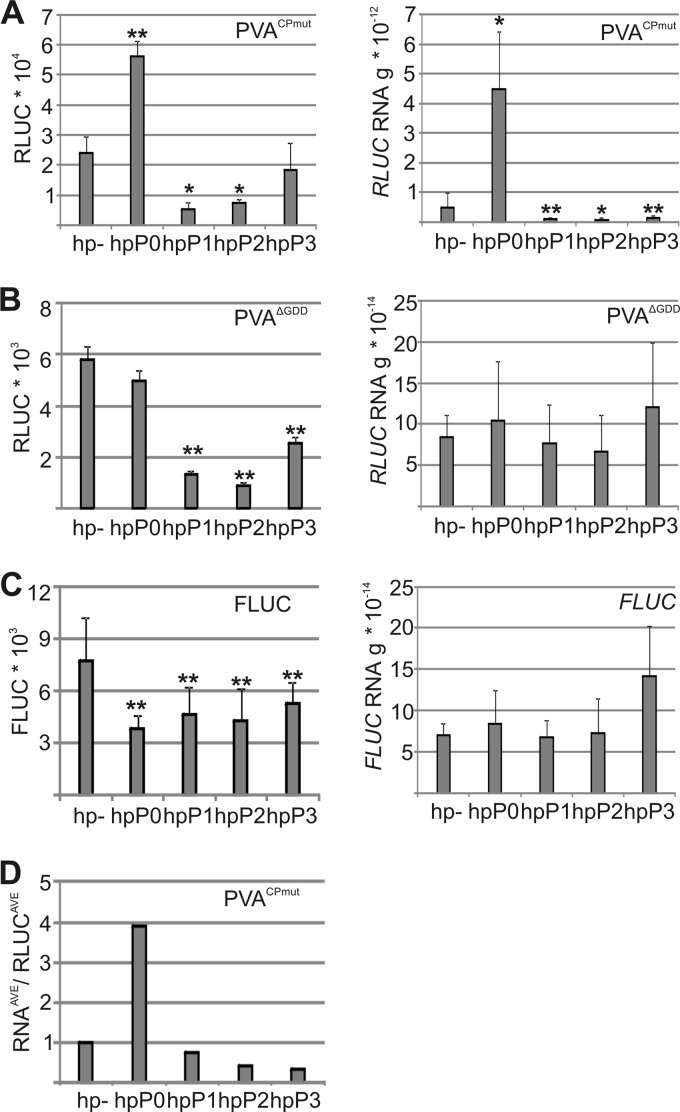
P proteins were silenced by transforming RNA hairpins 4 days prior to inoculation of mutant PVAs, along with firefly luciferase (FLUC) as an internal control for Agrobacterium-mediated nonviral expression. PVA RLUC activity and RNA accumulation were assayed at 3 DAI (Fig. 5A and B). Importantly, the RNA level was 10-fold higher for PVACPmut than for PVAΔGDD in the control plants, indicating that most of the PVACPmut RNA was derived from replication. PVACPmut RNA levels were markedly reduced by silencing of P1, P2, and P3, suggesting reduced replication. This may have been due to reduced translation, as both PVAΔGDD and FLUC RNA levels were unaffected, although the corresponding luciferase activities decreased (Fig. 5B and C). In contrast to the other P proteins, elevated PVACPmut RNA levels were detected after P0 silencing, suggesting that P0 can affect PVA RNA accumulation differently from the other stalk proteins and thus explaining why P0 was identified from membrane-associated viral RNPs (Fig. 1). The ratio of RNA to RLUC activity reflects the translation of accumulated RNA. The 4-fold increase in the ratio of PVACPmut RNA to RLUC during P0 silencing (Fig. 5D) suggests that despite the accumulation of PVACPmut RNA, it was not efficiently translated.
Fig 5.
P0 has functions distinct from those of other P proteins in PVA infection. (A to C) RLUC activity and RNA accumulation were determined for PVACPmut (A), PVAΔGDD (B), and FLUC (C) during P-protein silencing at 3 DAI. Hairpins were applied 4 days before PVAs. The Agrobacterium OD600 values used for infiltration were as follows: 0.05 for PVACPmut and PVAΔGDD, 0.005 for FLUC, and 0.4 for hairpins. Luciferase activities and RNA amounts were analyzed by using the same samples. (D) Ratio of RNA amount to viral RLUC activity for PVACPmut, calculated by dividing the average relative values calculated from data presented in panel A. *, P < 0.05; **, P < 0.01 (determined by Student's t tests).
P0 can increase viral translation.
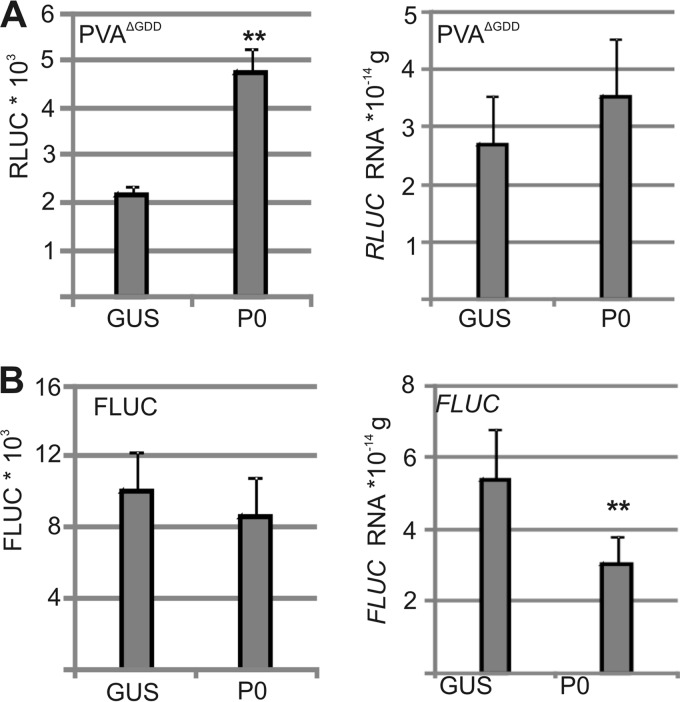
Since the expression of exogenous P0 increased wt PVA infection (Fig. 3D), we analyzed whether exogenous P0 expression affected RLUC-tagged PVAΔGDD and FLUC activities and RNA accumulation. Exogenous P0 increased RLUC activity but did not alter PVAΔGDD RNA levels (Fig. 6A), suggesting that P0 enhanced viral translation. FLUC activity and RNA levels did not respond to exogenous P0. The positive effect of exogenous P0 expression on RLUC accumulation was similar when derived from PVAΔGDD and wt PVA infection (Fig. 3D), indicating that P0 could promote wt infection through increased viral translation.
Fig 6.
P0 can increase viral translation. (A) Exogenous P0 was coexpressed with PVAΔGDD and FLUC, and luciferase activity and RNA amounts were assessed at 3 DAI. GUS was coexpressed as a control. The Agrobacterium OD600 values used for infiltration were as follows: 0.05 for PVAΔGDD, 0.005 for FLUC, and 0.5 for GUS and P0. **, P < 0.01 (determined by Student's t tests).
The 5′-UTR is an important part of the viral RNA response to P0.
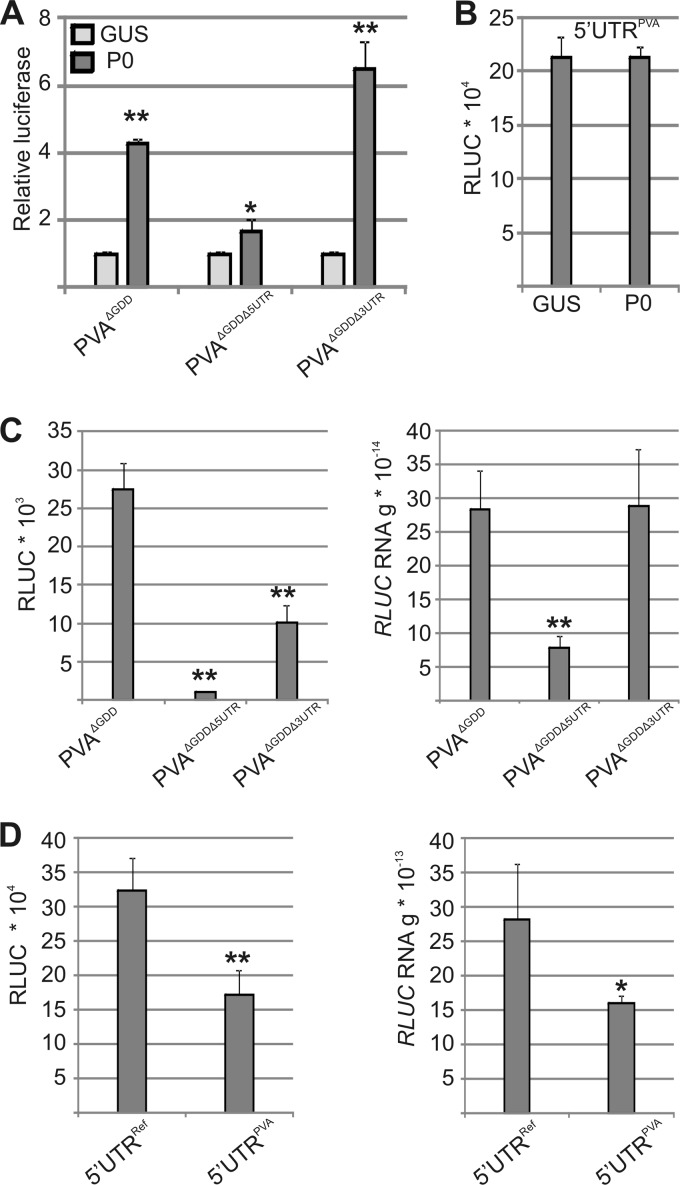
The viral untranslated regions (UTRs) appear to promote PVA RNA translation, as the removal of either UTR reduced viral protein accumulation (40). We analyzed the importance of viral UTRs in the P0 promotion of viral translation by expressing exogenous P0 with PVAΔGDD lacking either the 5′-UTR (PVAΔGDDΔ5′UTR) or the 3′-UTR (PVAΔGDDΔ3′UTR) and determined viral RLUC activity (Fig. 7A). RLUC activity hardly increased for PVAΔGDDΔ5′UTR, suggesting that the 5′-UTR is important for exogenous P0 to enhance viral translation. Exogenous P0 increased RLUC activity derived from PVAΔGDDΔ3′UTR more than that from PVAΔGDD. Hence, the 5′- and 3′-UTRs exerted opposite effects on viral RNA translation in the presence of exogenous P0. We further analyzed if monocistronic RLUC transcripts carrying the viral 5′-UTR could respond to exogenous P0 expression (Fig. 7B). Its RLUC activity was unaltered by exogenous P0 expression, suggesting that the viral RNA has additional properties required for P0 to increase its translation.
Fig 7.
The 5′-UTR is important for PVA RNA translation and the P0 response. (A) Exogenous P0 was expressed with PVAΔGDD or with PVAΔGDD lacking either its 5′-UTR (PVAΔGDDΔ5′UTR) or its 3′-UTR (PVAΔGDDΔ3′UTR), and RLUC activities were determined at 3 DAI. GUS was expressed as a control. Results are expressed as relative luciferase activity. The Agrobacterium OD600 values used for infiltration were as follows: 0.05 for PVAs and 0.5 for GUS and P0. (B) Exogenous P0 or GUS was expressed with a monocistronic RLUC transcript carrying the PVA 5′-UTR (5′UTRPVA), and RLUC activities were determined. The Agrobacterium OD600 values used for infiltration were as follows: 0.05 for the PVA 5′-UTR and 0.5 for GUS and P0. (C) Actual RLUC activities and RNA levels for PVAΔGDD, PVAΔGDDΔ5′UTR, and PVAΔGDDΔ3′UTR. (D) Monocistronic RLUC constructs with a reference leader (5′UTRRef) or the PVA 5′-UTR were transformed using an OD600 of 0.01, and the RLUC activity and RNA amounts were quantified. *, P < 0.05; **, P < 0.01 (determined by Student's t tests).
Next, we correlated RLUC activities with PVAΔGDD, PVAΔGDDΔ5′UTR, and PVAΔGDDΔ3′UTR RNA levels (Fig. 7C). Deletion of the 5′-UTR reduced RLUC activity more than RNA levels compared with PVAΔGDD, whereas an absence of the 3′-UTR reduced RLUC activity but not RNA levels, showing that both UTRs are important, especially for viral RNA translation. We also compared RLUC activities and RNA levels of monocistronic RLUC transcripts fused with two different 5′-UTRs, a cloning vector-derived random leader sequence (5′-UTRRef) and the PVA 5′-UTR (Fig. 7D). The viral 5′-UTR did not enhance either the accumulation or translation of the RLUC transcript. These findings indicate that the viral 5′-UTR is essential for efficient viral translation and is required for a translational response of PVAΔGDD to exogenous P0.
P0 is required for VPg-enhanced viral translation.
We reported previously that VPg requires the PVA 5′-UTR to increase viral translation when expressed in trans but that the PVA 5′-UTR alone is not sufficient to transfer this response to a monocistronic transcript (40), similarly to our results for P0. We therefore analyzed whether P0 and VPg were connected in promoting viral translation. As the expressions of exogenous VPg (40) and P0 increase RLUC activity from both wt PVA and PVAΔGDD, we used PVAΔGDD in this expression analysis to exclude any effects of virus replication and movement on viral protein accumulation. Either VPg or GUS (control) was coexpressed with PVAΔGDD during P-protein silencing (Fig. 8). VPg increased viral RLUC activity 3-fold in control and P1-, P2-, and P3-silenced leaves but not during P0 silencing, showing that of these P proteins, only P0 was required to increase viral translation.
Fig 8.
VPg requires P0 to enhance viral translation. VPg was coexpressed with PVAΔGDD during P-protein silencing, and the RLUC activities were quantitated at 3 DAI. Results are expressed as relative values. The Agrobacterium OD600 values used for infiltration were as follows: 0.05 for PVAΔGDD and 0.5 for GUS and VPg. *, P < 0.05; **, P < 0.01 (determined by Student's t tests).
PVA translation is increased further by P0 and VPg together.
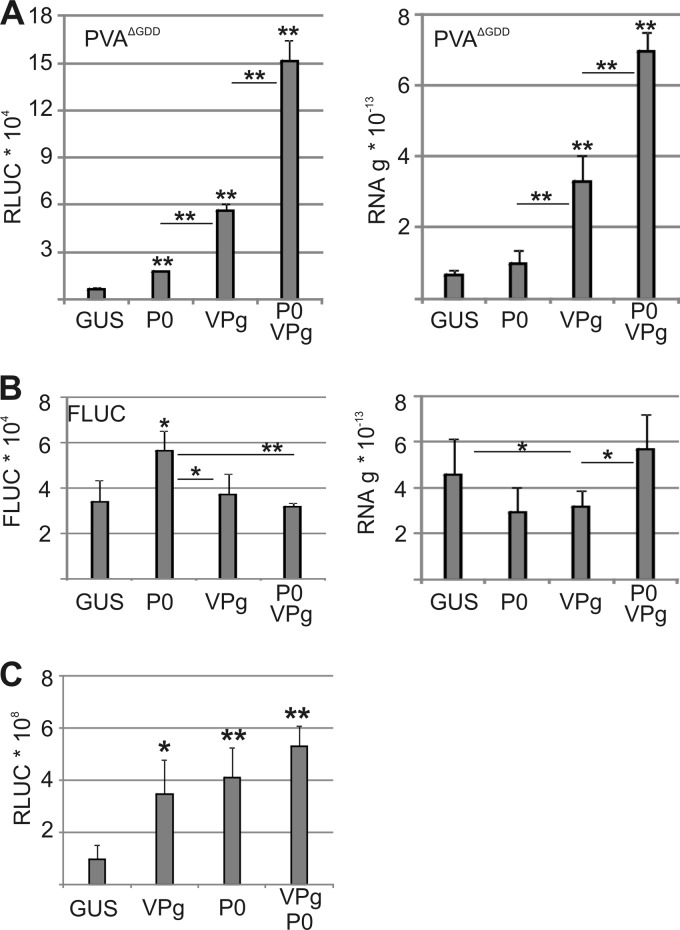
To further analyze the connection between P0 and VPg in viral translation, PVAΔGDD was coexpressed with P0 and/or VPg, and RLUC activity and RNA levels were determined at 3 DAI (Fig. 9A). As before, P0 increased RLUC activity but not the PVAΔGDD RNA level (Fig. 6A), whereas VPg increased both (40). Further increases in RLUC activity and viral RNA levels were observed when P0 and VPg were coexpressed. The levels of monocistronic FLUC RNA and FLUC activity remained essentially unaltered (Fig. 9B), showing that the response was specific for PVAΔGDD RNA.
Fig 9.
P0 and VPg act synergistically to increase PVA translation. (A and B) VPg and/or P0 was coexpressed with PVAΔGDD (A) and control FLUC (B), and luciferase activity and RNA levels were quantified at 3 DAI. GUS was expressed as a control. The Agrobacterium OD600 values used for infiltration were as follows: 0.05 for PVAΔGDD, 0.005 for FLUC, and 0.25 for P0 and VPg. Agrobacterium carrying the uidA gene (GUS) was used to adjust all infiltrated samples to the same final OD600. (C) GUS/P0/VPg coexpressions, as in panels A and B, in leaves systemically infected with RLUC-tagged wt PVA. The lower leaves were inoculated with PVA, and 7 days later, the systematically infected upper leaves were infiltrated with Agrobacterium carrying GUS/P0/VPg expression cassettes. Viral RLUC activity was determined 3 days later, corresponding to 10 days after PVA inoculation. *, P < 0.05; **, P < 0.01 (determined by Student's t tests).
To determine whether P0 and VPg together could affect natural infection, lower leaves of plants were inoculated with wt PVA, and after 7 days, P0 and VPg were expressed by Agrobacterium-mediated transformation in systemically infected upper leaves. Viral RLUC activity was determined 3 days after infiltrating P0 and VPg expression constructs into systemically infected leaves, equal to 10 days after inoculation of the plants with PVA (Fig. 9C). Expression of either exogenous VPg or P0 increased viral RLUC activity. Exogenous P0 increased systemic infection by wt PVA (Fig. 3D) and PVAΔGDD (Fig. 6A and 7A), further indicating that the P0 response functions in bona fide PVA infection. Expression of exogenous VPg increased viral RLUC activity in systemically infected leaves but less than that observed for PVAΔGDD (Fig. 9A). The synergistic effect of VPg and P0 on PVA gene expression could not be detected here.
Exogenous VPg increases viral translation together with eIF(iso)4E and P0 but reduces the spread of infection.
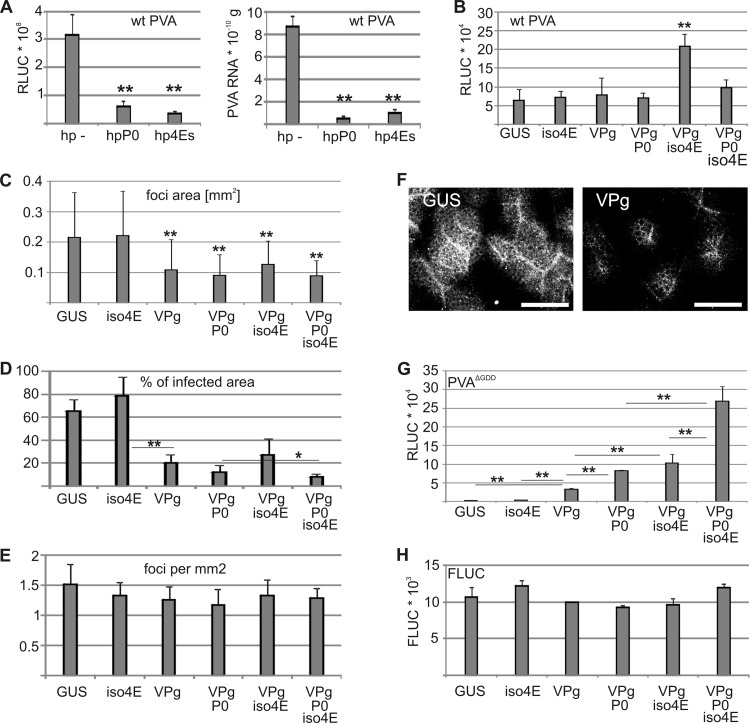
We showed previously that exogenously expressed VPg is unable to enhance viral translation when both eIF4E and eIF(iso)4E are silenced (40). To extend this finding, we cosilenced eIF4E and eIF(iso)4E and analyzed RLUC activities and RNA levels of RLUC-tagged wt PVA at 9 DAI (Fig. 10A). Both RLUC activity and viral RNA levels were reduced during eIF4E/eIF(iso)4E silencing and during P0 silencing (Fig. 10A). As both P0 and eIF4E/eIF(iso)4E are required for normal PVA infection and for a VPg-mediated boost in translation, the effects of different combinations of eIF(iso)4E, VPg, and P0 on wt PVA RLUC activity were determined at 3 DAI (Fig. 10B). To our surprise, RLUC activity was not increased by VPg or eIF(iso)4E and was increased only slightly by the combination of VPg, P0, and eIF(iso)4E but was markedly increased by VPg plus eIF(iso)4E. We therefore analyzed the effects of VPg, P0, and eIF(iso)4E expression on the development of infection foci by GFP-tagged wt PVA by determining the average area of infection foci at 3 DAI (Fig. 10C), at which time cell-to-cell movement had already occurred (see also Fig. 3F and 4D). Exogenous VPg expression reduced the intercellular spread of infection (Fig. 10C), an effect even more pronounced when the proportion of infected to noninfected tissue was determined at 4 DAI (Fig. 10D and F). Interestingly, coexpression of P0 with VPg reduced cell-to-cell infection spread even more than VPg alone (Fig. 10D), despite an increase when P0 was expressed alone (Fig. 3F). Also, the positive effect of VPg and eIF(iso)4E coexpression on PVA RLUC activity was overpowered when P0 was coexpressed. Importantly, the total number of infection foci remained similar under these conditions, showing that altering the PVA infection initiation rate was not responsible for the observed effects (Fig. 10E).
Fig 10.
Exogenous VPg increases viral translation with eIF(iso)4E and P0 but reduces spread of infection. (A) P0 and both eIF4E and eIF(iso)4E were silenced by expression of the corresponding RNA hairpins (hpP0 and hp4Es), and the levels of luciferase activity and RNA derived from RLUC-tagged wt PVA were determined at 9 DAI. The empty hairpin vector was expressed as a control (hp −). The Agrobacterium OD600 values used for infiltration were as follows: 0.0005 for wt PVA, 0.4 for hp − and hpP0, and 0.2 each for hp4Es [hpeIF4E and hpeIF(iso)4E]. (B to E) eIF(iso)4E was coexpressed with RLUC-tagged wt PVA in different combinations with VPg and/or P0. RLUC activity (B) and the area of infection foci (C) were determined at 3 DAI, and the percentage of GFP-tagged wt PVA-infected tissue at 4 DAI (D) and the number of established GFP-tagged PVA infection foci at 3 DAI (E) were determined. (F) Representative pictures of GFP-tagged wt PVA-infected tissue at 4 DAI during GUS and VPg coexpression. Scale bar, 1 mm. (G and H) Effects of the same coexpressions on PVAΔGDD (G) and FLUC (H) luciferase activities. eIF(iso)4E, P0, and VPg each used an Agrobacterium OD600 of 0.25 for infiltration, and GUS was used to adjust all infiltrated samples to the same final bacterial density. The other Agrobacterium OD600 values used for infiltration were as follows: 0.0005 for RLUC/GFP-tagged wt PVA, 0.05 for PVAΔGDD, and 0.005 for FLUC. *, P < 0.05; **, P < 0.01 (determined by Student's t tests).
When wt PVA is infiltrated at a high OD600, VPg increases viral RLUC activity (40). At an OD600 of 0.05, PVA RNA expression is often initiated from adjacent cells, leaving less space for cell-to-cell spread of infection (37). At a 100-fold-lower OD, the size of infection foci was severely reduced by exogenous VPg expression (Fig. 10D), with the lack of increased wt PVA RLUC activities (Fig. 10B) likely being due to impaired cell-to-cell spread of infection. Exogenous VPg increased viral RLUC activity when expressed in systemically infected leaves (Fig. 9C), showing that this mechanism operates during authentic infection. As the VPg-mediated increase of RLUC activity was less than that for PVAΔGDD, intercellular spread was likely hindered. The synergistic effect of VPg and P0 coexpression was not detected, which may also be explained by restricted cell-to-cell movement. Finally, we analyzed how the same combinatory expressions affected PVAΔGDD RLUC activity (Fig. 10G). Viral RLUC activity was higher when VPg was coexpressed with eIF(iso)4E than when either was expressed alone, indicating that eIF(iso)4E and VPg increased PVAΔGDD RLUC activity synergistically. The highest RLUC activity was achieved by coexpressing VPg with both eIF(iso)4E and P0. Control FLUC activity was unaffected under these conditions (Fig. 10H), indicating that viral RNA has specific properties by which it responds to ectopic expression of VPg, P0, and eIF(iso)4E.
DISCUSSION
Virus infection relies on mechanisms that protect viral RNA from being degraded and guarantee efficient production of viral proteins in a cellular environment. We have shown here that ribosomal P0 is important for PVA infection by promoting viral translation along with eIF(iso)4E and the viral protein VPg. P0 functions as part of the ribosomal stalk, with all the acidic P proteins that constitute the ribosomal stalk being important for PVA infection. Our findings also suggest that P0 functions as an extraribosomal protein in PVA infection and regulates viral RNA functions.
Ribosomal stalk proteins are important for PVA infection.
Few previous reports have linked ribosomal stalk proteins to virus infection. Lassa virus Z protein interacts with the nuclear fraction of P0 (47). Assembly of the 60S ribosomal subunit occurs in the nucleus, and upon export to the cytoplasm, the subunit matures through processes including association of the stalk (48). This suggests that Z protein interactions probably did not involve ribosomes. L-A virus has been found to propagate to higher levels in yeast strains lacking P1 and P2, with the viral Gag protein being associated with P0 (49). We found that silencing of all P proteins reduced PVA infection substantially. Because translation and replication correlate positively for some animal positive-stranded RNA viruses (50, 51), the reduction in PVA infection following P-protein silencing may be due to reduced translation, resulting in reduced replication. Genome-wide RNA interference (RNAi) screening identified 72% of all ribosomal proteins whose knockdown attenuated infection by Drosophila C virus (DCV), including P0 and P2, indicating the importance of ribosomal proteins for DCV infection (52). The sensitivity of DCV infection to knockdown of ribosomal proteins was shown to be connected to a cap-independent internal ribosomal entry site (IRES) translation initiation mechanism. Similar to DCV, PVA is an RNA virus that potentially employs IRES-dependent translation mechanisms, as do other potyviruses such as Tobacco etch virus (TEV) and TuMV (53–55). TuMV and TMV (genus Tobamovirus) infections were highly reduced by knockdown of several ribosomal proteins (56). The knockdown of RPS2, RPS6, RPL7, RPL13, and RPL19 reduced the number of TuMV infection foci, regardless of whether the plants were inoculated with virus particles or Agrobacterium. In contrast, P-protein silencing did not reduce the number of infection foci and affected the spread of PVA only slightly. The other ribosomal proteins may therefore have roles distinct from those of the stalk proteins in potyvirus infection. Clearly, ribosomal protein homeostasis is important for infection by potyvirus as well as other viruses.
Extraribosomal regulation of PVA RNA by P0.
Our findings suggest that compared with other P proteins, P0 has additional functions. For example, P0 was a component of viral RNP complexes purified via both VPg and RdRp, whereas the other P proteins were not, suggesting that the latter were not prominent components of purified viral RNPs (Fig. 1). These viral RNP complexes may have been derived from RCs, as they were purified from membrane fractions containing potyviral RNA synthesis activity (57, 58). HSP70 is associated with potyviral RCs and the RdRp (19) and was present in our purified samples, together with viral RNA, RdRp, NIa, and VPg (35). Therefore, P0 may associate with viral RNP complexes at the sites of viral RCs, possibly as an extraribosomal protein.
The abundant accumulation of PVACPmut RNA during P0 silencing (Fig. 5A) suggests a functional role for P0 in PVA RNA regulation. This accumulation occurred only during silencing of P0 and not during silencing of the other P proteins, suggesting that, independent of stalk function, P0 can affect PVA RNA accumulation. Although replicating PVACPmut RNA accumulated in single cells in the absence of P0, this RNA was not translated efficiently (Fig. 5). P0 was required for wt PVA to reach normal levels of infection (Fig. 3D), most likely due to the function of P0 in viral translation. In particular, we found that P0 was the only P protein required for a specific viral translation mechanism involving viral VPg and eIF(iso)4E (Fig. 8 to 10). Ribosomal proteins have been shown to have extraribosomal functions, e.g., plant L10 in geminivirus infection (59) and S1 associated with bacteriophage Qβ replicase (7). Extraribosomal P0 is part of the mRNA-regulating human IMP1 and STAU1 mRNP complexes (60, 61) and may function in nucleus-associated DNA repair (62). Taken together with our findings, P0 likely regulates viral RNA via extraribosomal mechanisms.
P0 and eIF(iso)4E are essential for VPg to promote PVA RNA translation.
Several potential translational components are associated with RCs of TuMV, including eIF(iso)4E, PABP, eEF1A, and AtRH8 (16–18, 63), but their roles have remained elusive. They may function to regulate viral translation (64), as translation is coupled with replication (35) and RCs (65) during potyvirus infection. P0 is found in membrane-associated viral RNP complexes (Fig. 1) and promotes PVA translation, suggesting that it may be an additional potyviral RC-associated host protein involved in translation. Exogenous P0 expression increased both wt PVA infection and PVAΔGDD translation (Fig. 3, 7, 9, and 10). We reported previously that exogenous expression of VPg increases both the stability and translation of wt PVA and PVAΔGDD RNA and that simultaneous silencing of eIF4E and eIF(iso)4E did not decrease basal PVAΔGDD translation but was essential for increased VPg translation (40). Similarly, we showed that P0 is required for VPg-mediated enhancement of PVA RNA translation (Fig. 8) and that PVAΔGDD translation (Fig. 5B) was less sensitive to reduced P0 amounts than wt PVA infection (Fig. 3D). Higher RNA and VPg levels of replicating viruses may require more P0 and eIF4E/eIF(iso)4E, making their translation more sensitive to silencing of these proteins. P0 transcription is induced early in PVA infection (66), and TuMV infection upregulates eIF4E (67), suggesting that the demand for these proteins is increased during natural potyvirus infection. This was supported by results showing that the silencing of both of these host factors reduced PVA infection similarly (Fig. 10A).
During infection, VPg-linked viral RNA is produced within membrane-associated RCs. Our results using PVAΔGDD show that viral RNA does not have to be produced via replication in authentic RCs for VPg, P0, and eIF(iso)4E to efficiently promote its translation. The viral 5′-UTR is an important RNA element by which P0 and VPg increase PVA translation (Fig. 7A) (40). Potyvirus TEV has an IRES element in the 5′-UTR that can recruit ribosomes independently of eIF4Es via direct interactions with eIF4G (54). TEV VPg has been shown to be associated with the eIF4F protein complex consisting of eIF4E and eIF4G, reducing the affinity of eIF4F for the conventional cap structure found in the 5′-UTR of cellular mRNAs and facilitating the translation of transcripts carrying the TEV 5′-UTR (68). The presence or absence of a 5′ cap did not alter the ability of VPg to facilitate the translation of TEV 5′-UTR transcripts. By analogy, the capped 5′-UTR of PVAΔGDD RNA may qualify as a substrate for IRES translation. Furthermore, as wt PVA RNA responds to exogenous VPg by increased translation in systematically infected leaves (Fig. 9C), it can be concluded that 5′ VPg-linked PVA RNA also qualifies for VPg-mediated enhancement of PVA RNA translation, suggesting that this mechanism can operate during natural infection. Therefore, the mechanism of the VPg response may involve PVA 5′-UTR-driven IRES translation. Its significance may be reflected by the reduced infection observed during eIF4E/eIF(iso)4E and P0 silencing. eIF4E and eIF(iso)4E are critical host components by which potyviruses establish infection, by interacting with VPg (20). Although the mechanisms by which eIF4E and eIF(iso)4E act during potyvirus infection have been hard to determine, our results show that they are essential for VPg to increase PVA translation (40).
Coordination of viral RNA functions.
Although VPg enhanced wt PVA gene expression (Fig. 9C) (40), it restricted the spread of infection (Fig. 10D). Reduced viral spread likely masked the increase in viral gene expression levels during active cell-to-cell movement. A comparison of areas of infection foci (Fig. 10D) with RLUC activity (Fig. 10B) indicated that VPg also enhanced translation under these conditions. Our experiments assessing the VPg-mediated effect on viral translation disturbed the natural stoichiometry of VPg production. Thus, the more PVA RNA is allocated to translation via VPg, the less is available for assembly and movement. Obviously, the correct stoichiometry of viral protein products is a prerequisite for a balanced natural infection. P0 and VPg coexpression increased the steady-state amount of PVAΔGDD RNA (Fig. 9A), indicating that replication was not necessary for RNA accumulation. Because the transcriptions of control FLUC and PVAΔGDD are both driven by the 35S promoter, the specific effects of P0 and VPg on PVAΔGDD RNA translation and accumulation are likely posttranscriptional. The 20- to 100-fold increase in the PVAΔGDD expression level is an intriguing example of how extensively posttranscriptional mRNA regulation can affect gene expression. VPg may reroute PVAΔGDD RNA from degradation pathways to translation. Although there have been other examples of the interdependence between mRNA translation and degradation (69, 70), it needs to be considered whether our results with PVAΔGDD reflect the mechanisms operating during natural PVA infection. This RNA is derived by nuclear transcription and carries a 5′-cap structure instead of 5′-linked VPg present in the authentic viral RNA. The specific effects observed for PVAΔGDD compared with our nonviral FLUC control (Fig. 10G and H) suggest that PVAΔGDD RNA has virus-specific features that mediate gene expression responses that are at least somewhat similar to those observed with authentic viral RNA (Fig. 9C and 10B) (40). Interestingly, we observed that PVA RNA induces the formation of P0-containing granule structures (A. Hafrén and K. Mäkinen, unpublished results). Similar to wt PVA, PVAΔGDD RNA also induced P0- but not P1- or P2-containing granule-like structures in plant cells, whereas the nonviral control FLUC did not. These granules may represent structures associated with viral RNA metabolism, similar to the P bodies and stress granules described, for example, for virus-infected animal cells (71).
Finally, it is noteworthy that silencing of P0 caused abundant accumulation of PVACPmut RNA. We are presently unable to distinguish between the contributions of RNA synthesis via replication and RNA degradation to the steady-state PVA RNA level. Moreover, mutant CP may contribute to the different outcomes of P0 silencing for PVACPmut and wt PVA. For example, P0 may be an essential host factor required for coordinating PVA RNA functions among translation, replication, degradation, and assembly/movement pathways. In summary, we hypothesize that (i) P0 is an essential host component required to achieve a normal strength of infection and (ii) P0 regulates PVA RNA translation together with VPg and eIF(iso)4E.
ACKNOWLEDGMENTS
We thank the CSIRO for kindly providing the pHELLSGATE8 silencing plasmid.
We gratefully acknowledge financial support from the Academy of Finland (grant no. 115922 and 1138329 to K.M. and grant no. 127969 to K.E.).
Footnotes
Published ahead of print 30 January 2013
REFERENCES
- 1. Bushell M, Sarnow P. 2002. Hijacking the translation apparatus by RNA viruses. J. Cell Biol. 158:395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dreher TW, Miller WA. 2006. Translational control in positive strand RNA plant viruses. Virology 344:185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lloyd RE. 2006. Translational control by viral proteinases. Virus Res. 119:76–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson SR, Sarnow P. 2003. Enterovirus 71 contains a type I IRES element that functions when eukaryotic initiation factor eIF4G is cleaved. Virology 315:259–266 [DOI] [PubMed] [Google Scholar]
- 5. Park HS, Himmelbach A, Browning KS, Hohn T, Ryabova LA. 2001. A plant viral “reinitiation” factor interacts with the host translational machinery. Cell 106:723–733 [DOI] [PubMed] [Google Scholar]
- 6. Ahlquist P, Noueiry AO, Lee WM, Kushner DB, Dye BT. 2003. Host factors in positive-strand RNA virus genome replication. J. Virol. 77:8181–8186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blumenthal T, Carmichael GG. 1979. RNA replication: function and structure of Qbeta-replicase. Annu. Rev. Biochem. 48:525–548 [DOI] [PubMed] [Google Scholar]
- 8. Li Z, Pogany J, Tupman S, Esposito AM, Kinzy TG, Nagy PD. 2010. Translation elongation factor 1A facilitates the assembly of the tombusvirus replicase and stimulates minus-strand synthesis. PLoS Pathog. 6:e1001175 doi:10.1371/journal.ppat.1001175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osman TA, Buck KW. 1997. The tobacco mosaic virus RNA polymerase complex contains a plant protein related to the RNA-binding subunit of yeast eIF-3. J. Virol. 71:6075–6082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quadt R, Kao CC, Browning KS, Hershberger RP, Ahlquist P. 1993. Characterization of a host protein associated with brome mosaic virus RNA-dependent RNA polymerase. Proc. Natl. Acad. Sci. U. S. A. 90:1498–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor DN, Carr JP. 2000. The GCD10 subunit of yeast eIF-3 binds the methyltransferase-like domain of the 126 and 183 kDa replicase proteins of tobacco mosaic virus in the yeast two-hybrid system. J. Gen. Virol. 81:1587–1591 [DOI] [PubMed] [Google Scholar]
- 12. Mizumoto H, Iwakawa HO, Kaido M, Mise K, Okuno T. 2006. Cap-independent translation mechanism of red clover necrotic mosaic virus RNA2 differs from that of RNA1 and is linked to RNA replication. J. Virol. 80:3781–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanz MA, Castello A, Carrasco L. 2007. Viral translation is coupled to transcription in Sindbis virus-infected cells. J. Virol. 81:7061–7068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanz MA, Castello A, Ventoso I, Berlanga JJ, Carrasco L. 2009. Dual mechanism for the translation of subgenomic mRNA from Sindbis virus in infected and uninfected cells. PLoS One 4:e4772 doi:10.1371/journal.pone.0004772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller S, Krijnse-Locker J. 2008. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 6:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beauchemin C, Boutet N, Laliberte JF. 2007. Visualization of the interaction between the precursors of VPg, the viral protein linked to the genome of turnip mosaic virus, and the translation eukaryotic initiation factor iso 4E in planta. J. Virol. 81:775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beauchemin C, Laliberte JF. 2007. The poly(A) binding protein is internalized in virus-induced vesicles or redistributed to the nucleolus during turnip mosaic virus infection. J. Virol. 81:10905–10913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thivierge K, Cotton S, Dufresne PJ, Mathieu I, Beauchemin C, Ide C, Fortin MG, Laliberte JF. 2008. Eukaryotic elongation factor 1A interacts with turnip mosaic virus RNA-dependent RNA polymerase and VPg-Pro in virus-induced vesicles. Virology 377:216–225 [DOI] [PubMed] [Google Scholar]
- 19. Dufresne PJ, Thivierge K, Cotton S, Beauchemin C, Ide C, Ubalijoro E, Laliberte JF, Fortin MG. 2008. Heat shock 70 protein interaction with turnip mosaic virus RNA-dependent RNA polymerase within virus-induced membrane vesicles. Virology 374:217–227 [DOI] [PubMed] [Google Scholar]
- 20. Wang A, Krishnaswamy S. 2012. Eukaryotic translation initiation factor 4E-mediated recessive resistance to plant viruses and its utility in crop improvement. Mol. Plant Pathol. 13:795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonzalo P, Reboud JP. 2003. The puzzling lateral flexible stalk of the ribosome. Biol. Cell 95:179–193 [DOI] [PubMed] [Google Scholar]
- 22. Wool IG, Chan YL, Gluck A, Suzuki K. 1991. The primary structure of rat ribosomal proteins P0, P1, and P2 and a proposal for a uniform nomenclature for mammalian and yeast ribosomal proteins. Biochimie 73:861–870 [DOI] [PubMed] [Google Scholar]
- 23. Bailey-Serres J, Vangala S, Szick K, Lee CH. 1997. Acidic phosphoprotein complex of the 60S ribosomal subunit of maize seedling roots. Components and changes in response to flooding. Plant Physiol. 114:1293–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szick K, Springer M, Bailey-Serres J. 1998. Evolutionary analyses of the 12-kDa acidic ribosomal P-proteins reveal a distinct protein of higher plant ribosomes. Proc. Natl. Acad. Sci. U. S. A. 95:2378–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanchez-Madrid F, Vidales FJ, Ballesta JP. 1981. Effect of phosphorylation on the affinity of acidic proteins from Saccharomyces cerevisiae for the ribosomes. Eur. J. Biochem. 114:609–613 [DOI] [PubMed] [Google Scholar]
- 26. Tsurugi K, Ogata K. 1985. Evidence for the exchangeability of acidic ribosomal proteins on cytoplasmic ribosomes in regenerating rat liver. J. Biochem. 98:1427–1431 [DOI] [PubMed] [Google Scholar]
- 27. Zinker S, Warner JR. 1976. The ribosomal proteins of Saccharomyces cerevisiae. Phosphorylated and exchangeable proteins. J. Biol. Chem. 251:1799–1807 [PubMed] [Google Scholar]
- 28. Aruna K, Chakraborty T, Rao PN, Santos C, Ballesta JP, Sharma S. 2005. Functional complementation of yeast ribosomal P0 protein with Plasmodium falciparum P0. Gene 357:9–17 [DOI] [PubMed] [Google Scholar]
- 29. Kouyanou S, Santos C, Koliaraki V, Ballesta JP. 2003. Protein BmP0 from the silkworm Bombyx mori can be assembled and is functional in the Saccharomyces cerevisiae ribosomal stalk in the absence of the acidic P1 and P2 proteins. Gene 314:173–179 [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez-Gabriel MA, Remacha M, Ballesta JP. 2000. The RNA interacting domain but not the protein interacting domain is highly conserved in ribosomal protein P0. J. Biol. Chem. 275:2130–2136 [DOI] [PubMed] [Google Scholar]
- 31. Remacha M, Jimenez-Diaz A, Bermejo B, Rodriguez-Gabriel MA, Guarinos E, Ballesta JP. 1995. Ribosomal acidic phosphoproteins P1 and P2 are not required for cell viability but regulate the pattern of protein expression in Saccharomyces cerevisiae. Mol. Cell. Biol. 15:4754–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Remacha M, Jimenez-Diaz A, Santos C, Briones E, Zambrano R, Rodriguez Gabriel MA, Guarinos E, Ballesta JP. 1995. Proteins P1, P2, and P0, components of the eukaryotic ribosome stalk. New structural and functional aspects. Biochem. Cell Biol. 73:959–968 [DOI] [PubMed] [Google Scholar]
- 33. Huang C, Mandava CS, Sanyal S. 2010. The ribosomal stalk plays a key role in IF2-mediated association of the ribosomal subunits. J. Mol. Biol. 399:145–153 [DOI] [PubMed] [Google Scholar]
- 34. Martinez-Azorin F, Remacha M, Ballesta JP. 2008. Functional characterization of ribosomal P1/P2 proteins in human cells. Biochem. J. 413:527–534 [DOI] [PubMed] [Google Scholar]
- 35. Hafren A, Hofius D, Ronnholm G, Sonnewald U, Makinen K. 2010. HSP70 and its cochaperone CPIP promote potyvirus infection in Nicotiana benthamiana by regulating viral coat protein functions. Plant Cell 22:523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams AJ, Werner-Fraczek J, Chang IF, Bailey-Serres J. 2003. Regulated phosphorylation of 40S ribosomal protein S6 in root tips of maize. Plant Physiol. 132:2086–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eskelin K, Suntio T, Hyvarinen S, Hafren A, Makinen K. 2010. Renilla luciferase-based quantitation of potato virus A infection initiated with Agrobacterium infiltration of N. benthamiana leaves. J. Virol. Methods 164:101–110 [DOI] [PubMed] [Google Scholar]
- 38. Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133:462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. 2007. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104:34–41 [DOI] [PubMed] [Google Scholar]
- 40. Eskelin K, Hafren A, Rantalainen KI, Makinen K. 2011. Potyviral VPg enhances viral RNA translation and inhibits reporter mRNA translation in planta. J. Virol. 85:9210–9221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Helliwell C, Waterhouse P. 2003. Constructs and methods for high-throughput gene silencing in plants. Methods 30:289–295 [DOI] [PubMed] [Google Scholar]
- 42. Johansen LK, Carrington JC. 2001. Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol. 126:930–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elkon K, Skelly S, Parnassa A, Moller W, Danho W, Weissbach H, Brot N. 1986. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc. Natl. Acad. Sci. U. S. A. 83:7419–7423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Szick-Miranda K, Bailey-Serres J. 2001. Regulated heterogeneity in 12-kDa P-protein phosphorylation and composition of ribosomes in maize (Zea mays L.). J. Biol. Chem. 276:10921–10928 [DOI] [PubMed] [Google Scholar]
- 45. Santos C, Ballesta JP. 1994. Ribosomal protein P0, contrary to phosphoproteins P1 and P2, is required for ribosome activity and Saccharomyces cerevisiae viability. J. Biol. Chem. 269:15689–15696 [PubMed] [Google Scholar]
- 46. Dunoyer P, Thomas C, Harrison S, Revers F, Maule A. 2004. A cysteine-rich plant protein potentiates potyvirus movement through an interaction with the virus genome-linked protein VPg. J. Virol. 78:2301–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borden KL, CampbellDwyer EJ, Carlile GW, Djavani M, Salvato MS. 1998. Two RING finger proteins, the oncoprotein PML and the arenavirus Z protein, colocalize with the nuclear fraction of the ribosomal P proteins. J. Virol. 72:3819–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lo KY, Li Z, Bussiere C, Bresson S, Marcotte EM, Johnson AW. 2010. Defining the pathway of cytoplasmic maturation of the 60S ribosomal subunit. Mol. Cell 39:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krokowski D, Tchorzewski M, Boguszewska A, McKay AR, Maslen SL, Robinson CV, Grankowski N. 2007. Elevated copy number of L-A virus in yeast mutant strains defective in ribosomal stalk. Biochem. Biophys. Res. Commun. 355:575–580 [DOI] [PubMed] [Google Scholar]
- 50. Funkhouser AW, Schultz DE, Lemon SM, Purcell RH, Emerson SU. 1999. Hepatitis A virus translation is rate-limiting for virus replication in MRC-5 cells. Virology 254:268–278 [DOI] [PubMed] [Google Scholar]
- 51. Simoes EA, Sarnow P. 1991. An RNA hairpin at the extreme 5′ end of the poliovirus RNA genome modulates viral translation in human cells. J. Virol. 65:913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cherry S, Doukas T, Armknecht S, Whelan S, Wang H, Sarnow P, Perrimon N. 2005. Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes Dev. 19:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Basso J, Dallaire P, Charest PJ, Devantier Y, Laliberte JF. 1994. Evidence for an internal ribosome entry site within the 5′ non-translated region of turnip mosaic potyvirus RNA. J. Gen. Virol. 75(Part 11):3157–3165 [DOI] [PubMed] [Google Scholar]
- 54. Gallie DR. 2001. Cap-independent translation conferred by the 5′ leader of tobacco etch virus is eukaryotic initiation factor 4G dependent. J. Virol. 75:12141–12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Niepel M, Gallie DR. 1999. Identification and characterization of the functional elements within the tobacco etch virus 5′ leader required for cap-independent translation. J. Virol. 73:9080–9088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang C, Zhang C, Dittman JD, Whitham SA. 2009. Differential requirement of ribosomal protein S6 by plant RNA viruses with different translation initiation strategies. Virology 390:163–173 [DOI] [PubMed] [Google Scholar]
- 57. Martin MT, Garcia JA. 1991. Plum pox potyvirus RNA replication in a crude membrane fraction from infected Nicotiana clevelandii leaves. J. Gen. Virol. 72(Part 4):785–790 [DOI] [PubMed] [Google Scholar]
- 58. Schaad MC, Jensen PE, Carrington JC. 1997. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 16:4049–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carvalho CM, Santos AA, Pires SR, Rocha CS, Saraiva DI, Machado JP, Mattos EC, Fietto LG, Fontes EP. 2008. Regulated nuclear trafficking of rpL10A mediated by NIK1 represents a defense strategy of plant cells against virus. PLoS Pathog. 4:e1000247 doi:10.1371/journal.ppat.1000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brendel C, Rehbein M, Kreienkamp HJ, Buck F, Richter D, Kindler S. 2004. Characterization of Staufen 1 ribonucleoprotein complexes. Biochem. J. 384:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jonson L, Vikesaa J, Krogh A, Nielsen LK, Hansen T, Borup R, Johnsen AH, Christiansen J, Nielsen FC. 2007. Molecular composition of IMP1 ribonucleoprotein granules. Mol. Cell. Proteomics 6:798–811 [DOI] [PubMed] [Google Scholar]
- 62. Yacoub A, Kelley MR, Deutsch WA. 1996. Drosophila ribosomal protein PO contains apurinic/apyrimidinic endonuclease activity. Nucleic Acids Res. 24:4298–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang TS, Wei T, Laliberte JF, Wang A. 2010. A host RNA helicase-like protein, AtRH8, interacts with the potyviral genome-linked protein, VPg, associates with the virus accumulation complex, and is essential for infection. Plant Physiol. 152:255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grangeon R, Cotton S, Laliberte JF. 2010. A model for the biogenesis of turnip mosaic virus replication factories. Commun. Integr. Biol. 3:363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cotton S, Grangeon R, Thivierge K, Mathieu I, Ide C, Wei T, Wang A, Laliberte JF. 2009. Turnip mosaic virus RNA replication complex vesicles are mobile, align with microfilaments, and are each derived from a single viral genome. J. Virol. 83:10460–10471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vuorinen AL, Gammelgard E, Auvinen P, Somervuo P, Dere S, Valkonen JPT. 2010. Factors underpinning the responsiveness and higher levels of virus resistance realised in potato genotypes carrying virus-specific R genes. Ann. Appl. Biol. 157:229–241 [Google Scholar]
- 67. Leonard S, Viel C, Beauchemin C, Daigneault N, Fortin MG, Laliberte JF. 2004. Interaction of VPg-Pro of turnip mosaic virus with the translation initiation factor 4E and the poly(A)-binding protein in planta. J. Gen. Virol. 85:1055–1063 [DOI] [PubMed] [Google Scholar]
- 68. Khan MA, Miyoshi H, Gallie DR, Goss DJ. 2008. Potyvirus genome-linked protein, VPg, directly affects wheat germ in vitro translation: interactions with translation initiation factors eIF4F and eIFiso4F. J. Biol. Chem. 283:1340–1349 [DOI] [PubMed] [Google Scholar]
- 69. Coller J, Parker R. 2004. Eukaryotic mRNA decapping. Annu. Rev. Biochem. 73:861–890 [DOI] [PubMed] [Google Scholar]
- 70. Jacobson A, Peltz SW. 1996. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu. Rev. Biochem. 65:693–739 [DOI] [PubMed] [Google Scholar]
- 71. Beckham CJ, Parker R. 2008. P bodies, stress granules, and viral life cycles. Cell Host Microbe 3:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]