Abstract
A human immunodeficiency virus type 1 (HIV-1) vaccine that induces potent immune responses in the gastrointestinal mucosa would be highly desirable. Here we show that attenuated recombinant Listeria monocytogenes, administered orally utilizing its natural route of infection, induces potent mucosal as well as systemic immune responses in mice. Moreover, these responses can be boosted efficiently with replication-incompetent adenoviral vectors. L. monocytogenes elicited more potent simian immunodeficiency virus (SIV) Gag-specific CD8+ T lymphocyte responses in mucosal compartments than DNA vaccines.
TEXT
The gastrointestinal mucosa serves as the major site for human immunodeficiency virus type 1 (HIV-1) replication and for the destruction of CD4+ T lymphocytes (1, 2). Moreover, HIV-1 transmission typically occurs across mucosal surfaces. Therefore, efforts to develop an HIV-1 vaccine capable of providing protective immunity at the mucosal portal of entry will likely prove critical.
A highly attenuated recombinant Listeria monocytogenes strain, LmddBsdal (referred to here as rLm), was previously developed as a vaccine vector by deleting the two essential genes, dal and dat, required for cell wall synthesis. The deletion of these genes was partially complemented by introducing the Bacillus subtilis dal gene into the vector, thus retaining attenuation (3). rLm, when administered orally, followed by a booster with a replication-incompetent recombinant adenovirus serotype 5 (rAd5) vector, has been shown to induce robust antigen (Ag)-specific cellular immune responses in systemic and mucosal inductive sites (4, 5). However, the elucidation of L. monocytogenes vector-induced cellular immunity in the effector sites of the gastrointestinal mucosa remains unclear, and the vector's potency compared with that of DNA vaccine priming remains to be determined. In this study, we examined the magnitude, kinetics, memory phenotypes, and anatomic distribution of the Ag-specific CD8+ T lymphocytes induced in the gut mucosa by an rLm priming/recombinant Ad (rAd) boosting regimen. Furthermore, we directly compared the priming effects of rLm and DNA vectors on the ability to induce cellular immune responses at mucosal sites.
We initially performed a dose escalation study to determine the optimal dose of rLm expressing simian immunodeficiency virus strain mac239 (SIVmac239) Gag (rLm.SIVgag). Naive C57BL/6 mice (n = 8/group) were intragastrically (i.g.) administered with 108 CFU, 109 CFU, 1010 CFU, or 1011 CFU rLm.SIVgag at week 0. No clinical adverse effects were observed (data not shown). Four weeks later, 4 mice from each group were boosted intramuscularly (i.m.) with 107 virus particles (vp) of rAd5.SIVgag (rLm alone, n = 4/group; rLm-rAd5, n = 4/group). Animals were bled weekly, and vaccine-elicited CD8+ T lymphocyte responses specific for the dominant SIV Gag AL11 epitope (AAVKNWMTQTL, H2-Db) (6) were monitored by tetramer binding assays (7, 8). As shown in Fig. 1A, the mean percentage of AL11-specific CD8+ T lymphocytes peaked at 2 weeks following rLm vector immunization, ranging between 0.30 and 0.73% CD8+ T lymphocytes. rLm at 1010 CFU induced the highest-frequency responses among the doses tested at week 2 (Fig. 1A, inset). When mice were boosted with rAd5 expressing SIV Gag, robust AL11+CD8+ T lymphocytes were detected at weeks 6 to 8, indicating that rLm-primed immune responses were effectively boosted by rAd5.
Fig 1.
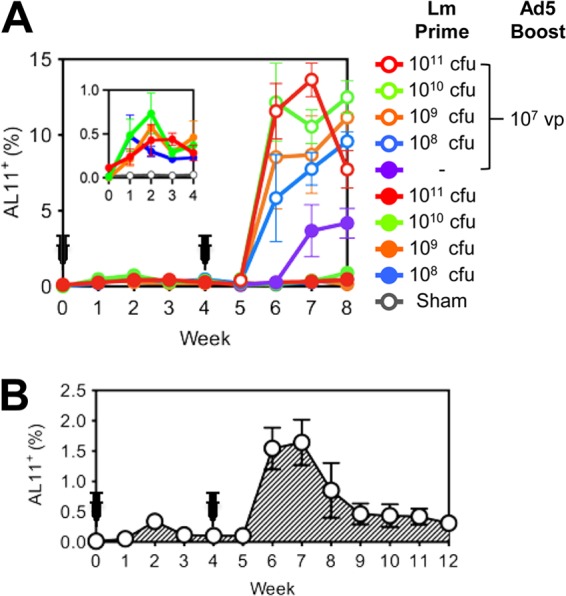
rLm.SIVgag immunogenicity in mice. (A) Groups of C57BL/6 mice (n = 8/group) received oral escalating doses of rLm.SIVgag (0 or 108 to 1011 CFU) (Lm Prime) and were then boosted i.m. with rAd5.SIVgag (0 or 107 vp; n = 4/group) (Ad5 Boost) 4 weeks later. The inset shows the AL11-specific CD8+ T lymphocyte responses at weeks 0 to 4 in a smaller scale. (B) C57BL/6 mice (n = 4) received orally two consecutive doses of 1010 CFU rLm.SIVgag at weeks 0 and 4. Animals were bled at the indicated time points, and AL11-specific CD8+ T lymphocyte responses were measured by tetramer binding assays. Error bars indicate standard errors of the means (SE).
To characterize the rLm vector-elicited immune responses further, naive C57BL/6 mice were injected i.g. twice with 1010 CFU rLm.SIVgag at weeks 0 and 4 (Fig. 1B). The AL11+ CD8+ T lymphocyte responses following the second rLm immunization were 1.5 to 1.6% at weeks 6 and 7, more than 5-fold higher than the peak response following priming, suggesting that prior exposure to L. monocytogenes does not abrogate immunogenicity (9). When assessed for the functionality of T lymphocyte responses by intracellular cytokine staining, a single administration of rLm.SIVgag induced mean percentages of gamma interferon (IFN-γ)-producing CD8+ and CD4+ T lymphocytes of 0.5% and 0.1%, respectively, in response to SIV Gag peptide pools, measured at week 2 (data not shown).
We next evaluated the CD8+ T lymphocyte responses in multiple systemic and mucosal compartments, including peripheral blood, spleen, inguinal lymph nodes (ILN), mesenteric lymph nodes (MLN), Peyer's patches (PP), and the intraepithelial lymphocyte (IEL) and the lamina propria lymphocyte (LPL) populations of both the small bowel and the large bowel (10). C57BL/6 mice (n = 8/group) received escalating doses of rLm.SIVgag (0 or 108 to 1011 CFU), and 4 weeks later, half of the animals were boosted i.m. with 107 vp rAd5.SIVgag (n = 4/group). Figure 2 shows the AL11-specific CD8+ T lymphocyte responses 4 weeks after rLm priming (left panel) and 4 weeks after rAd5 boosting (right panel). The kinetics of the responses were similar in all anatomic compartments evaluated. Four weeks after the priming with rLm, mean peak AL11+ CD8+ T lymphocyte responses in all measured compartments were significantly higher in the groups receiving 1010 CFU and 109 CFU than in that receiving 108 CFU (one-way analysis of variance [ANOVA] with Dunnett's posttest). Broad responses were detected in multiple mucosal surfaces at magnitudes comparable to those observed in peripheral blood. Four weeks after the rAd5 boost, animals primed with 1010 CFU rLm exhibited significantly higher AL11+ CD8+ T lymphocyte responses in the peripheral blood, spleen, ILN, and most of the gut mucosal compartments (IEL and LPL of the small bowel, IEL of the large bowel) than those in the rAd5-alone group, thus showing that rLm-primed responses could be effectively boosted by a heterologous vector (one-way ANOVA with Dunnett's posttest). The increased magnitude of CD8+ T lymphocyte responses observed in the peripheral blood, spleen, and LPL of the small bowel following the rAd5 boost is consistent with our previous observations with rAd vectors (7, 8). Moreover, mucosal CD8+ T lymphocyte responses in LPL appeared durable and were still detected 39 weeks after the Ad5 boost (rLm/Ad5, 0.81% [mean]; Ad5 alone, 0.22%) (data not shown).
Fig 2.
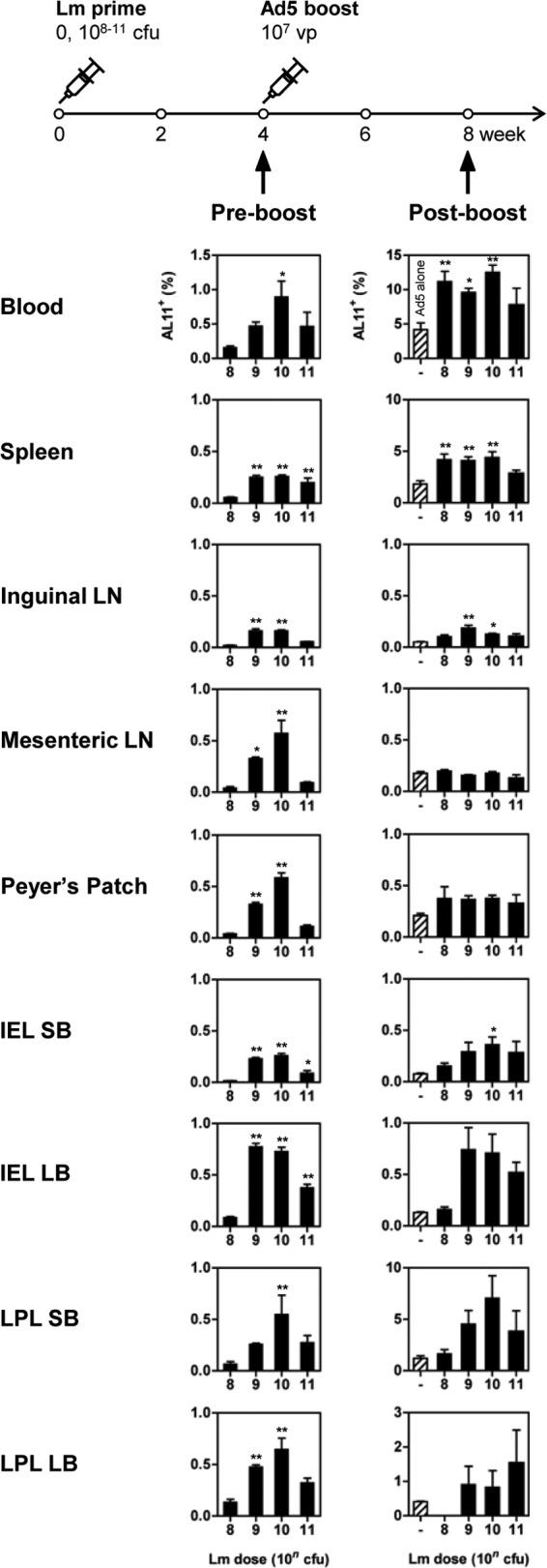
CD8+ T lymphocyte responses induced in the periphery and gastrointestinal mucosa by escalating doses of rLm.SIVgag. Groups of C57BL/6 mice (n = 8/group) received oral escalating doses of rLm.SIVgag (0 or 108 to 1011 CFU). Four weeks later, half the animals in each group were boosted i.m. with rAd5.SIVgag (107 vp; n = 4/group). AL11-specific responses were measured at weeks 4 (preboosting) and 8 (postboosting). Error bars, ±SE. One-way ANOVA with Dunnett's post hoc analysis (*, P < 0.05; **, P < 0.01) was used to compare the percentages of AL11+ CD8+ T lymphocytes in groups receiving 109 to 1011 CFU rLm.SIVgag with those of groups receiving 108 CFU rLm.SIVgag (left panel) or to compare groups primed with rLm.SIVgag and boosted with rAd5.SIVgag with those receiving rAd5.SIVgag alone (right panel). SB, small bowel; LB, large bowel.
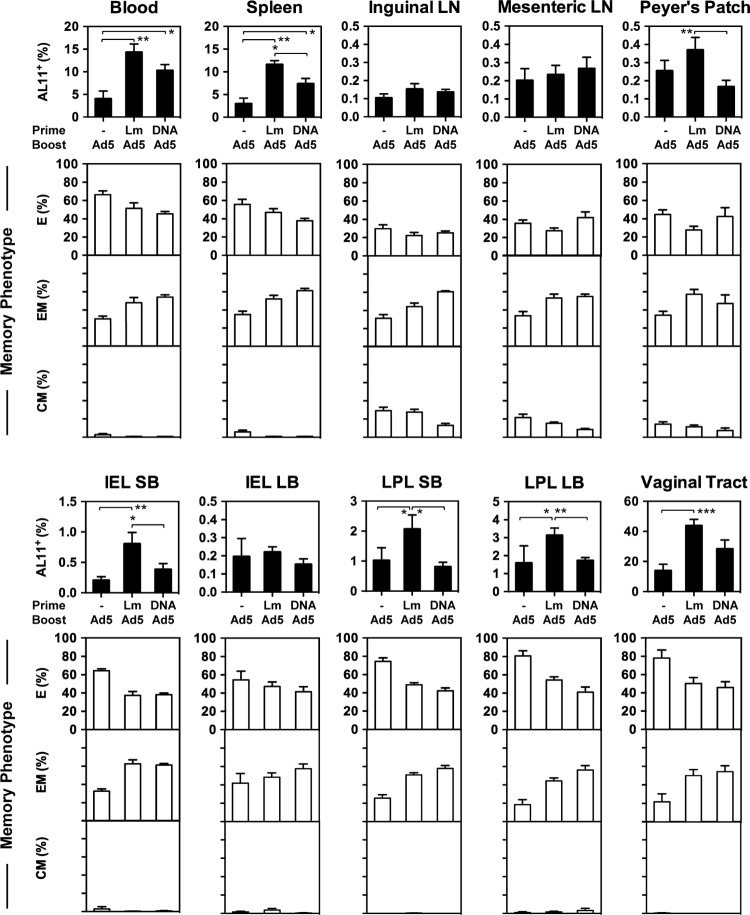
Next, we directly compared the priming effects of rLm and DNA vectors for a subsequent rAd5 boost. A recent study in mice showed that DNA vaccines delivered intramuscularly by in vivo electroporation were able to induce antigen-specific CD8+ T lymphocyte responses in the peripheral blood, spleen, and LPL of the small bowel (11). As shown in Fig. 3, naive C57BL/6 mice (n = 8/group) received either 1010 CFU rLm.SIVgag i.g. or 50 μg plasmid cytomegalovirus combined with SIV Gag (pCMV.SIVgag) i.m. and then were boosted i.m. with 107 vp rAd5.SIVgag 4 weeks later. Animals receiving rAd5.SIVgag alone served as the control (n = 8/group). At week 7, the magnitude, memory phenotype, and distribution of vaccine-elicited AL11-specific CD8+ T lymphocyte responses in multiple anatomic sites were determined using Db/AL11 tetramer binding assays. rLm.SIVgag induced significantly higher AL11+ CD8+ T lymphocyte responses than DNA.SIVgag in the spleen, Peyer's Patches, IEL and LPL of the small bowel, and LPL of the large bowel and showed a trend in other compartments (two-sided t test). Remarkably, in the vaginal mucosa, mean responses were 43.7% for rLm/rAd5-vaccinated animals, while the rAd5-alone and DNA-rAd5 groups induced mean responses of 13.9% and 28.3%, respectively. Thus, the rLm vector-primed AL11+ CD8+ T lymphocyte responses were broadly distributed and involved multiple peripheral and mucosal sites. In contrast, DNA vaccine-primed responses were more narrowly restricted, suggesting that CD8+ T lymphocytes generated by DNA vaccines have a more restricted pattern of trafficking to other compartments. In particular, the rLm/rAd5 regimen was more potent than the DNA/rAd5 regimen for inducing immune responses in the small bowel and vaginal mucosa. Consistently with our previous reports (8), peripheral and mucosal AL11-specific CD8+ T lymphocytes were predominantly effector memory cells (CD44+ CD62L− CD127+), especially in the rLm- or DNA-primed groups, while central memory phenotypes (CD44+ CD62L+ CD127+) were concentrated in the systemic and mucosal inductive lymphoid tissues.
Fig 3.
Comparison of rLm and DNA as priming vaccine vectors for a subsequent rAd5 boosting. Groups of C57BL/6 mice (n = 8/group) received 1010 CFU rLm.SIVgag orally or 50 μg pCMV.SIVgag i.m. and were boosted i.m. with 107 vp rAd5.SIVgag 4 weeks later. Animals that received rAd5.SIVgag alone served as the control (n = 8). At week 7, the magnitude and distribution of vaccine-elicited AL11-specific CD8+ T lymphocyte responses in multiple anatomic sites were determined by Db/AL11 tetramer binding assays. The memory phenotype of the tetramer-positive population was determined based on the expression of CD62L and CD127. Error bars, ±SE. Two-sided t tests were used to compare the means of the results for the rLm-rAd5 group with those for the DNA-rAd5 group (*, P < 0.05; **, P < 0.01). CM, central memory; EM, effector memory; E, effector.
To determine the generalizability of the observations, we also evaluated the capacity of rLm-primed responses to be boosted by rAd26, an alternative serotype of adenovirus that has recently been shown to provide protective immunity in rhesus macaques challenged with SIV (12, 13). We utilized a low, marginally immunogenic dose of rAd26 (6) to better define the priming effects of the rLm vector. As shown in Fig. 4A, naive C57BL/6 mice received 1010 CFU rLm.SIVgag i.g. (n = 8/group) or a sham dose (n = 8/group) and were boosted i.m. with 107 vp rAd26.SIVgag at week 4. Like rAd5, rAd26 effectively boosted AL11-specific CD8+ T lymphocyte responses in multiple anatomic compartments, including the gastrointestinal and vaginal mucosas (two-sided t test). Moreover, the rLm/rAd5 and rLm/rAd26 regimens elicited CD8+ T lymphocytes with a trend toward lower PD-1 (14, 15) expression (not statistically significant) than with Ad5-alone regimens, suggestive of a less exhausted phenotype (Fig. 4B).
Fig 4.
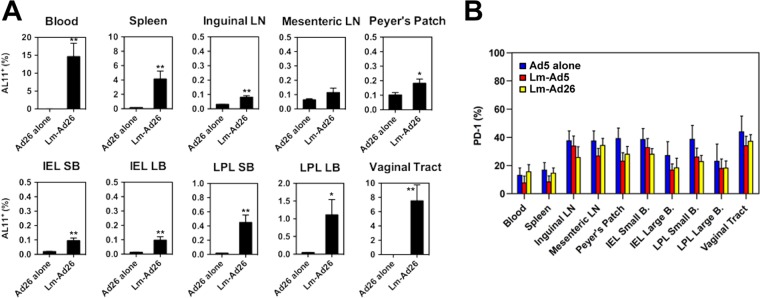
Induction of systemic and mucosal CD8+ T lymphocyte responses by an rLm priming/rAd26 boosting regimen. C57BL/6 mice received either 1010 CFU rLm.SIVgag orally (n = 8) or a sham dose (n = 8). Four weeks later, both groups received 107 vp rAd26.SIVgag i.m. (A) Three weeks after the boosting, AL11-specific CD8+ T lymphocyte responses in multiple anatomic sites were determined by tetramer binding assays. Error bars, ±SE. *, P < 0.05; **, P < 0.01 (two-sided t test). (B) Expression levels of PD-1 were measured and compared among groups of animals receiving rAd5 alone (blue bars), rLm/rAd5 (red bars), or rLm/rAd26 (yellow bars) at week 7.
In summary, our studies demonstrate that priming with the attenuated recombinant Listeria monocytogenes vector followed by boosting with the replication-incompetent rAd5 or rAd26 vectors results in potent antigen-specific CD8+ T lymphocyte responses in multiple peripheral and mucosal sites. rLm/rAd5 immunization induced significantly higher AL11-specific CD8+ T lymphocyte responses than rAd5 alone. Moreover, rLm proved more effective than DNA vaccines for priming, particularly for the induction of mucosal responses. These data suggest that rLm is an attractive priming vector that can be a useful component of a priming-boosting strategy involving other viral vectors, such as adenoviral vectors (6, 16), for the induction of mucosal immunity. In addition, it should be emphasized that its oral delivery route may be very practical for resource-poor settings.
ACKNOWLEDGMENTS
We thank A. Cruz, N. Simmons, D. Kaufman, D. Lynch, and P. Abbink for their generous advice and technical assistance.
We acknowledge support from the National Institutes of Health (grants AI060354, AI078526, AI078779, and AI096040) and the Ragon Institute of MGH, MIT, and Harvard. The authors declare no financial conflicts of interest.
Footnotes
Published ahead of print 6 February 2013
REFERENCES
- 1. Pasetti MF, Simon JK, Sztein MB, Levine MM. 2011. Immunology of gut mucosal vaccines. Immunol. Rev. 239:125–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Wijk F, Cheroutre H. 2009. Intestinal T cells: facing the mucosal immune dilemma with synergy and diversity. Semin. Immunol. 21:130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Z, Zhao X, Higgins DE, Frankel FR. 2005. Conditional lethality yields a new vaccine strain of Listeria monocytogenes for the induction of cell-mediated immunity. Infect. Immun. 73:5065–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Z, Zhang M, Zhou C, Zhao X, Iijima N, Frankel FR. 2008. Novel vaccination protocol with two live mucosal vectors elicits strong cell-mediated immunity in the vagina and protects against vaginal virus challenge. J. Immunol. 180:2504–2513 [DOI] [PubMed] [Google Scholar]
- 5. Zhao X, Zhang M, Li Z, Frankel FR. 2006. Vaginal protection and immunity after oral immunization of mice with a novel vaccine strain of Listeria monocytogenes expressing human immunodeficiency virus type 1 gag. J. Virol. 80:8880–8890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abbink P, Lemckert AAC, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJE, Barouch DH. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaufman DR, Bivas-Benita M, Simmons NL, Miller D, Barouch DH. 2010. Route of adenovirus-based HIV-1 vaccine delivery impacts the phenotype and trafficking of vaccine-elicited CD8+ T lymphocytes. J. Virol. 84:5986–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaufman DR, Liu J, Carville A, Mansfield KG, Havenga MJE, Goudsmit J, Barouch DH. 2008. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J. Immunol. 181:4188–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whitney JB, Mirshahidi S, Lim S-Y, Goins L, Ibegbu CC, Anderson DC, Raybourne RB, Frankel FR, Lieberman J, Ruprecht RM. 2011. Prior exposure to an attenuated Listeria vaccine does not reduce immunogenicity: preclinical assessment of the efficacy of a Listeria vaccine in the induction of immune responses against HIV. J. Immune Based Ther. Vaccines 9:2 doi:10.1186/1476-8518-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brandtzaeg P, Kiyono H, Pabst R, Russell MW. 2008. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 1:31–37 [DOI] [PubMed] [Google Scholar]
- 11. Shoji M, Katayama K, Tachibana M, Tomita K, Sakurai F, Kawabata K, Mizuguchi H. 2012. Intramuscular DNA immunization with in vivo electroporation induces antigen-specific cellular and humoral immune responses in both systemic and gut-mucosal compartments. Vaccine 30:7278–7285 [DOI] [PubMed] [Google Scholar]
- 12. Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EM, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687 [DOI] [PubMed] [Google Scholar]
- 15. Wherry EJ. 2011. T cell exhaustion. Nat. Immunol. 131:492–499 [DOI] [PubMed] [Google Scholar]
- 16. Teigler JE, Iampietro MJ, Barouch DH. 2012. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J. Virol. 86:9590–9598 [DOI] [PMC free article] [PubMed] [Google Scholar]